Abstract
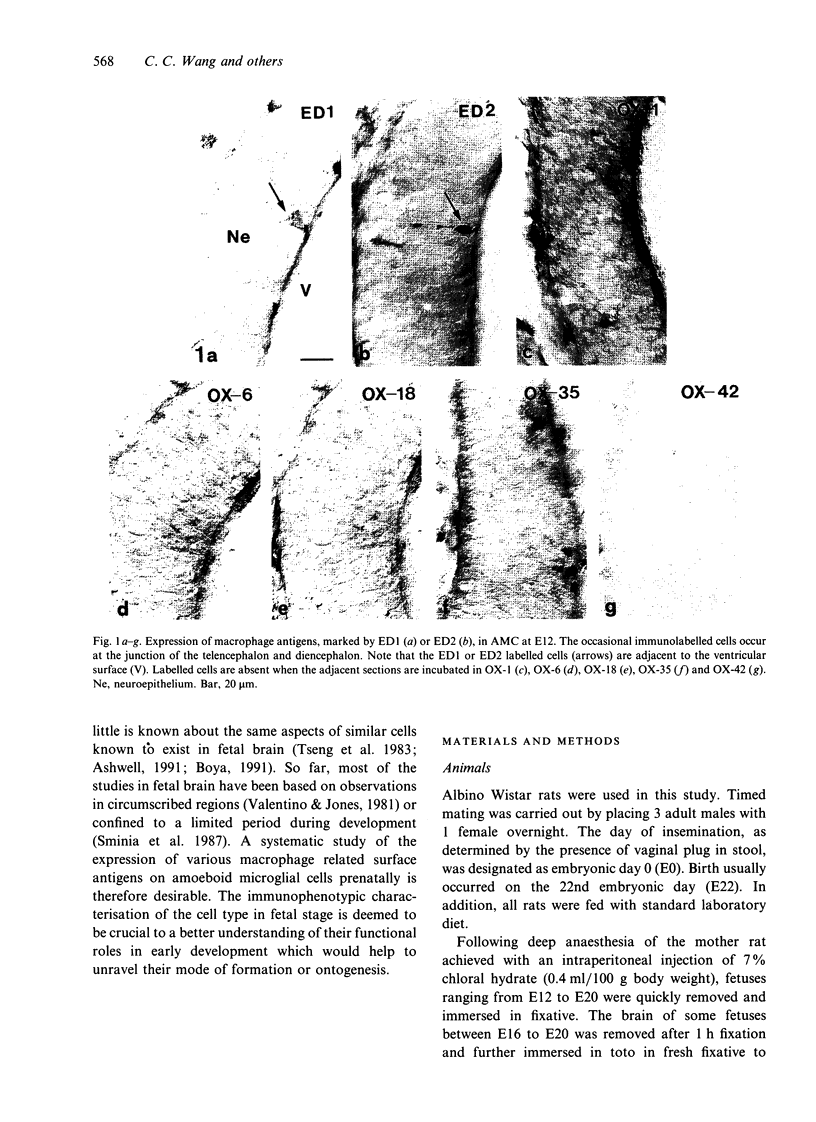
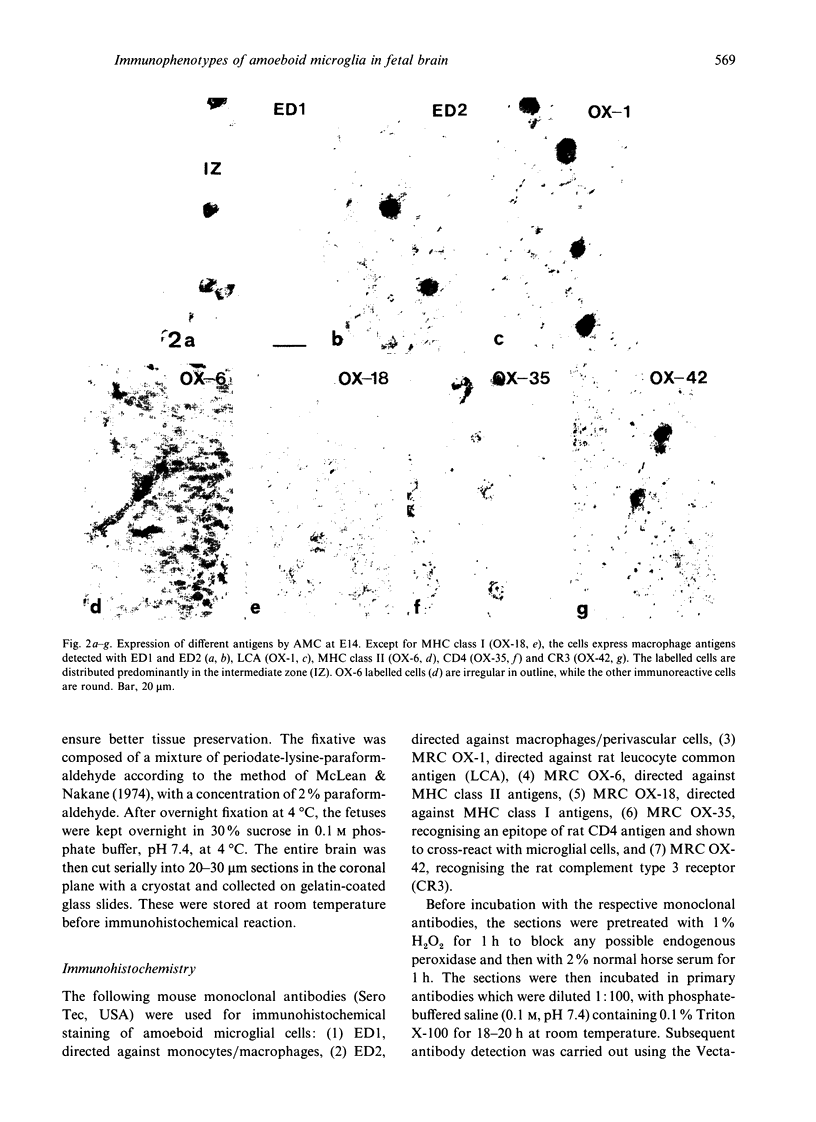
The present study examined the expression of different antigens in amoeboid microglial cells (AMC) in fetal rat brain extending from 12 to 20 d postconception (E12-E20) using a panel of monoclonal antibodies which recognised the major histocompatibility complex (MHC) class I (OX-18) and class II (OX-6) antigens, leucocyte common antigen (OX-1), CD4 receptor (OX-35), complement type 3 receptor (OX-42) or macrophage antigens of unknown function (ED1 and ED2). Of the above-mentioned antigens, ED1 and ED2-labelled AMC were observed in the neuroepithelia as early as embryonic day 12 (E12); other antigens were not detected at this stage. At E14, except for MHC class I antigen, all other antigens were expressed by AMC distributed predominantly in the developing white matter. At E16, AMC in the intermediate zone lateral to the striatum were endowed with all the above-mentioned antigens including MHC class I. At E18, the immunoreactivities of AMC stained with OX-6, OX-18, OX-35 and OX-42 antigens were noticeably reduced when compared with those cells at E16. At E20, amoeboid microglial cells exhibited full complement of antigen expression similar to those cells at E16; some of the labelled cells emitted a variable number of cytoplasmic processes. It is suggested that the successive and differential expression of various macrophage related antigens on AMC in fetal brain is related to the specific requirement of local environment in different stages of development.
Full text
PDF
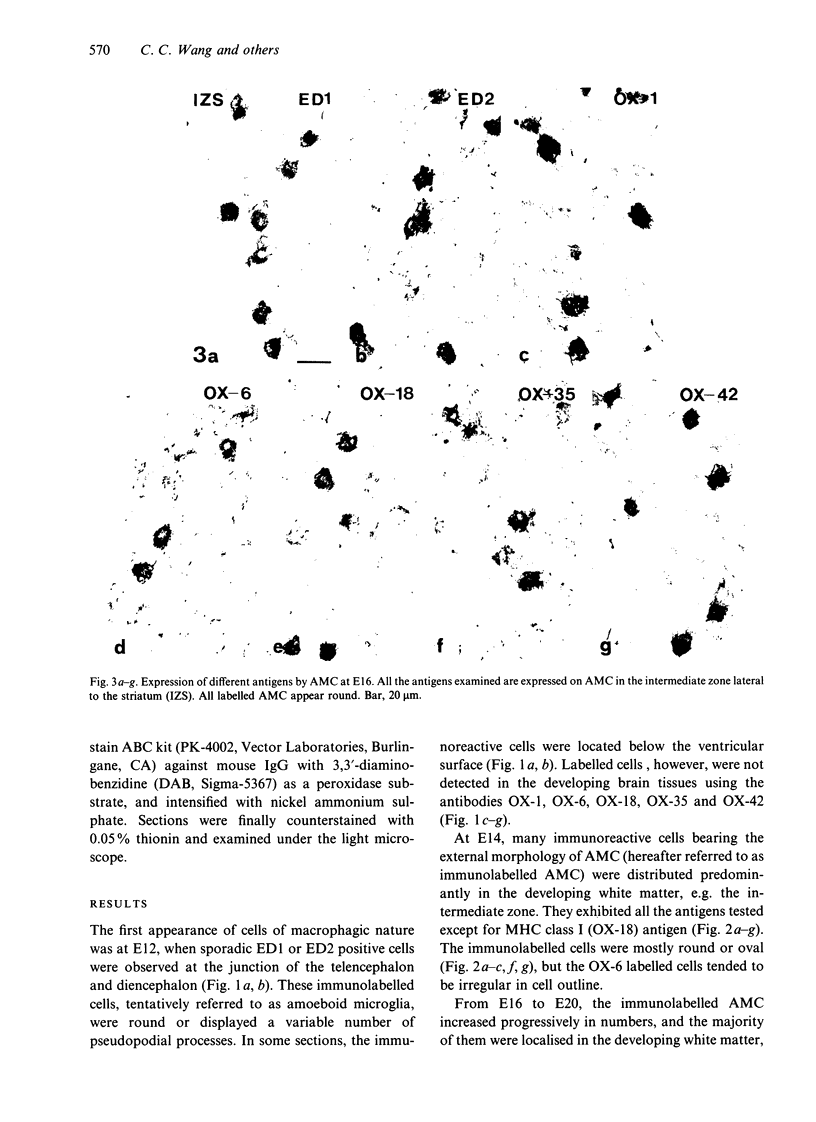
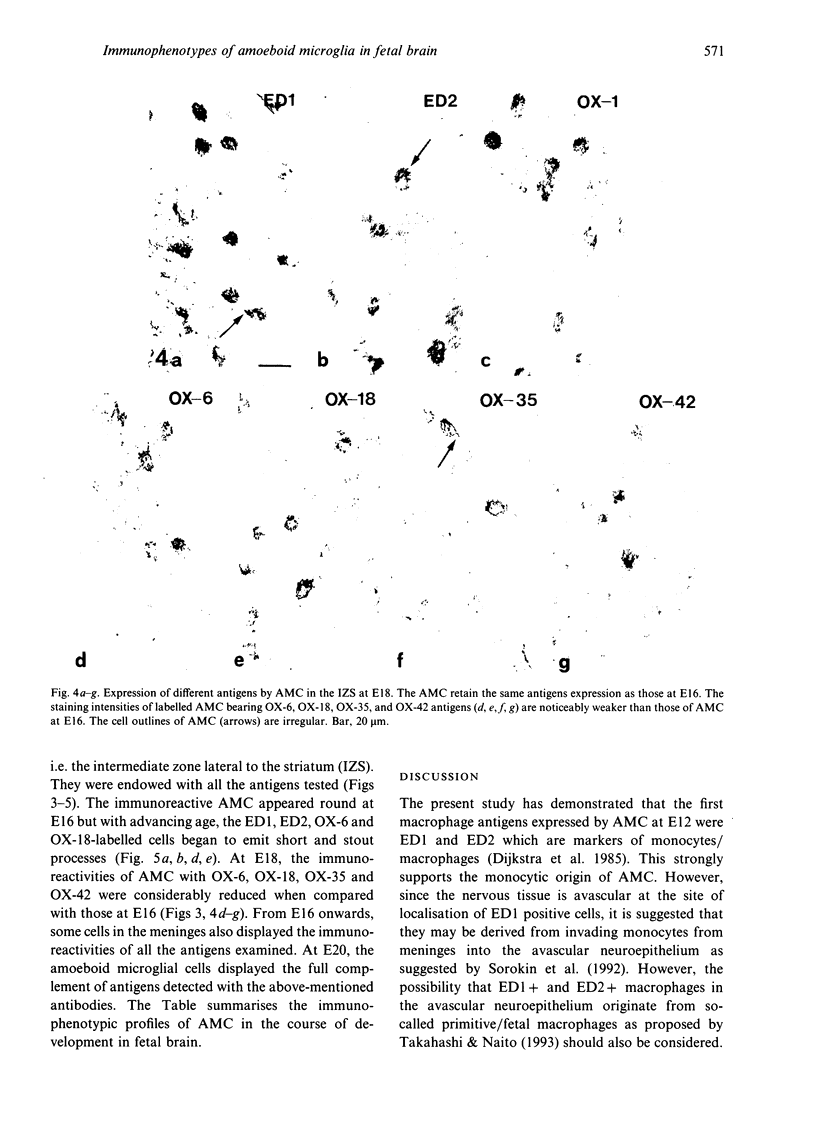
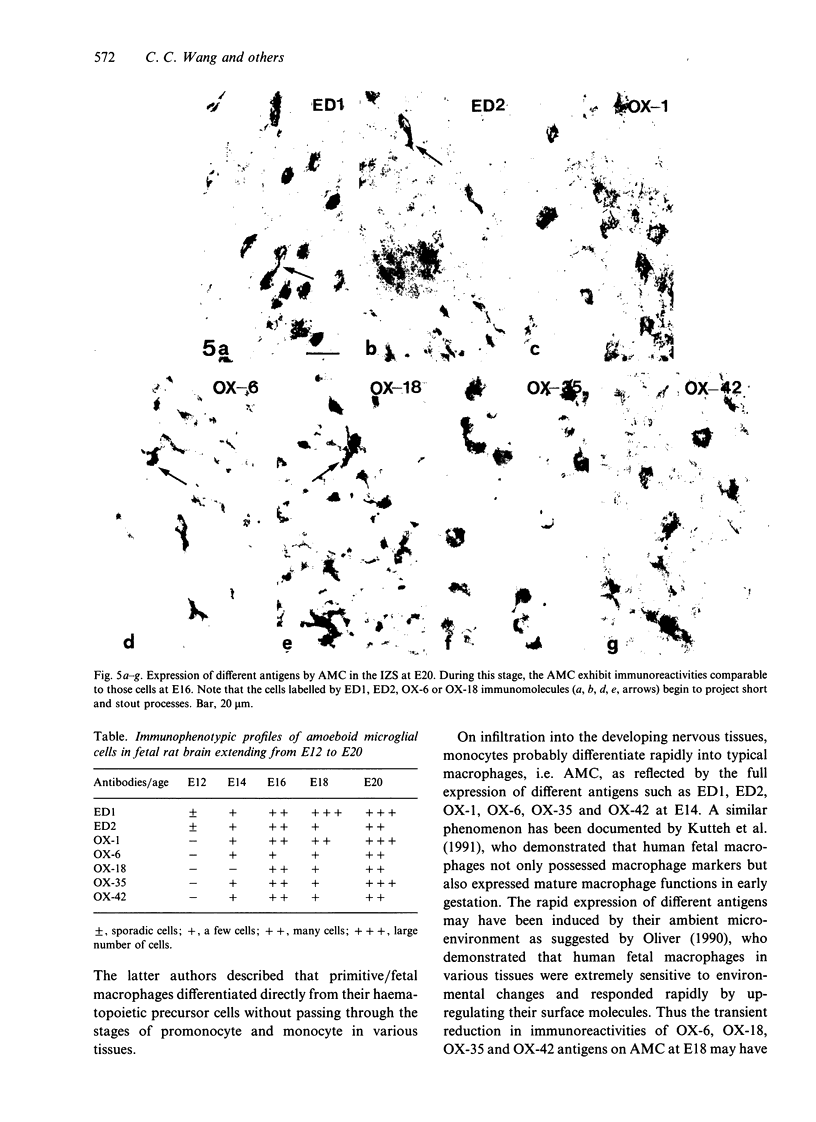


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Brain Res Dev Brain Res. 1991 Jan 15;58(1):1–12. doi: 10.1016/0165-3806(91)90231-7. [DOI] [PubMed] [Google Scholar]
- Banati R. B., Graeber M. B. Surveillance, intervention and cytotoxicity: is there a protective role of microglia? Dev Neurosci. 1994;16(3-4):114–127. doi: 10.1159/000112098. [DOI] [PubMed] [Google Scholar]
- Boya J., Calvo J. L., Carbonell A. L., Borregon A. A lectin histochemistry study on the development of rat microglial cells. J Anat. 1991 Apr;175:229–236. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Flaris N. A., Densmore T. L., Molleston M. C., Hickey W. F. Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia. 1993 Jan;7(1):34–40. doi: 10.1002/glia.440070108. [DOI] [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kiefer R., Schoen S. W., Kreutzberg G. W. New expression of myelomonocytic antigens by microglia and perivascular cells following lethal motor neuron injury. J Neuroimmunol. 1990 May;27(2-3):121–132. doi: 10.1016/0165-5728(90)90061-q. [DOI] [PubMed] [Google Scholar]
- Kutteh W. H., Rainey W. E., Carr B. R. Glucocorticoids inhibit lipopolysaccharide-induced production of tumor necrosis factor-alpha by human fetal Kupffer cells. J Clin Endocrinol Metab. 1991 Aug;73(2):296–301. doi: 10.1210/jcem-73-2-296. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Kaur C., Wong W. C. Expression of major histocompatibility complex and leukocyte common antigens in amoeboid microglia in postnatal rats. J Anat. 1991 Aug;177:117–126. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Kaur L. C., Yick T. Y., Wong W. C. Immunocytochemical localization of CR3 complement receptors with OX-42 in amoeboid microglia in postnatal rats. Anat Embryol (Berl) 1990;182(5):481–486. doi: 10.1007/BF00178913. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Wong W. C. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993 Jan;7(1):9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Milligan C. E., Cunningham T. J., Levitt P. Differential immunochemical markers reveal the normal distribution of brain macrophages and microglia in the developing rat brain. J Comp Neurol. 1991 Dec 1;314(1):125–135. doi: 10.1002/cne.903140112. [DOI] [PubMed] [Google Scholar]
- Milligan C. E., Levitt P., Cunningham T. J. Brain macrophages and microglia respond differently to lesions of the developing and adult visual system. J Comp Neurol. 1991 Dec 1;314(1):136–146. doi: 10.1002/cne.903140113. [DOI] [PubMed] [Google Scholar]
- Oliver A. M. Macrophage heterogeneity in human fetal tissue. Fetal macrophages. Clin Exp Immunol. 1990 Jun;80(3):454–459. doi: 10.1111/j.1365-2249.1990.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988 Jun;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Modulation of CD4 antigen on macrophages and microglia in rat brain. J Exp Med. 1987 Oct 1;166(4):1138–1143. doi: 10.1084/jem.166.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna M. P., Lampson L. A. Immune modulation within the brain: recruitment of inflammatory cells and increased major histocompatibility antigen expression following intracerebral injection of interferon-gamma. J Neuroimmunol. 1991 Nov;34(2-3):121–132. doi: 10.1016/0165-5728(91)90121-m. [DOI] [PubMed] [Google Scholar]
- Sminia T., de Groot C. J., Dijkstra C. D., Koetsier J. C., Polman C. H. Macrophages in the central nervous system of the rat. Immunobiology. 1987 Jan;174(1):43–50. doi: 10.1016/S0171-2985(87)80083-9. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P., Hoyt R. F., Jr, Blunt D. G., McNelly N. A. Macrophage development: II. Early ontogeny of macrophage populations in brain, liver, and lungs of rat embryos as revealed by a lectin marker. Anat Rec. 1992 Apr;232(4):527–550. doi: 10.1002/ar.1092320410. [DOI] [PubMed] [Google Scholar]
- Steiniger B., van der Meide P. H. Rat ependyma and microglia cells express class II MHC antigens after intravenous infusion of recombinant gamma interferon. J Neuroimmunol. 1988 Aug;19(1-2):111–118. doi: 10.1016/0165-5728(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Naito M. Development, differentiation, and proliferation of macrophages in the rat yolk sac. Tissue Cell. 1993 Jun;25(3):351–362. doi: 10.1016/0040-8166(93)90077-x. [DOI] [PubMed] [Google Scholar]
- Tseng C. Y., Ling E. A., Wong W. C. Light and electron microscopic and cytochemical identification of amoeboid microglial cells in the brain of prenatal rats. J Anat. 1983 Jun;136(Pt 4):837–849. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Vass K., Lassmann H. Intrathecal application of interferon gamma. Progressive appearance of MHC antigens within the rat nervous system. Am J Pathol. 1990 Oct;137(4):789–800. [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., McKimm-Breschkin J. L., Schrader J. W. Interferon-gamma induces the expression of H-2 and Ia antigens on brain cells. J Neuroimmunol. 1985 Feb-Mar;7(5-6):255–278. doi: 10.1016/s0165-5728(84)80026-0. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Wen C. Y., Shieh J. Y., Ling E. A. A quantitative and morphometric study of the transformation of amoeboid microglia into ramified microglia in the developing corpus callosum in rats. J Anat. 1992 Dec;181(Pt 3):423–430. [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Wen C. Y., Shieh J. Y., Ling E. A. Down-regulation of membrane glycoprotein in amoeboid microglia transforming into ramified microglia in postnatal rat brain. J Neurocytol. 1994 Apr;23(4):258–269. doi: 10.1007/BF01275530. [DOI] [PubMed] [Google Scholar]
- Xu J., Ling E. A. Expression of major histocompatibility complex class II antigen on amoeboid microglial cells in early postnatal rat brain following intraperitoneal injections of lipopolysaccharide. Exp Brain Res. 1994;100(2):287–292. doi: 10.1007/BF00227198. [DOI] [PubMed] [Google Scholar]
- Xu J., Ling E. A. Upregulation and induction of major histocompatibility complex class I and II antigens on microglial cells in early postnatal rat brain following intraperitoneal injections of recombinant interferon-gamma. Neuroscience. 1994 Jun;60(4):959–967. doi: 10.1016/0306-4522(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Xu J., Ling E. A. Upregulation and induction of surface antigens with special reference to MHC class II expression in microglia in postnatal rat brain following intravenous or intraperitoneal injections of lipopolysaccharide. J Anat. 1994 Apr;184(Pt 2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Yamada M., Wakabayashi K., Ikuta F. Endothelial fenestrae in the rat fetal cerebrum. Brain Res Dev Brain Res. 1988 Dec 1;44(2):211–219. doi: 10.1016/0165-3806(88)90219-2. [DOI] [PubMed] [Google Scholar]