Simple Summary
Insulin-like peptides are known to be crucial endocrine hormones that influence various physiological processes, including growth and reproduction in insects. However, the specific roles of insulin-like peptides in the reproduction of natural enemy insects remain to be known. Coccinella septempunctata is an effective biological control agent and it is of great biocontrol significance to study the functions of insulin-like peptide genes in female reproduction of this natural predator. In this study, we cloned two insulin-like peptide genes and analyzed their functions in female C. septempunctata. It was found that silencing these insulin-like peptide genes resulted in significant down-regulation of ovarian development-related genes, leading to a prolonged pre-oviposition period, decreased fecundity, and reduced hatching rates of female C. septempunctata. These findings confirm the regulatory functions of these insulin-like peptide genes in female C. septempunctata reproduction and enhance our understanding of peptide hormones in natural enemy insects, contributing to improved biological pest control strategies.
Keywords: insulin-like peptides, insulin pathway, female reproduction, Coccinella septempunctata
Abstract
Insulin-like peptides (ILPs) are important peptide hormones in insects, particularly involved in regulating physiological processes such as growth, development, and reproduction. However, the specific roles of ILPs in the reproduction of natural enemy insects remain unknown. In this study, two ILP genes, CsILP1 and CsILP2, were cloned and their functions were analyzed in female Coccinella septempunctata L. (Coleoptera: Coccinellidae). The open reading frames (ORFs) of CsILP1 and CsILP2 were 384 bp and 357 bp, respectively. The expression of CsILP1 increased on the 6th day after eclosion, reaching its peak on the 12th day, while CsILP2 levels showed a significant increase on the 6th day and then stabilized. In different tissues, CsILP1 was highly expressed in ovaries, while CsILP2 predominated in elytra. Injection of dsRNA targeting CsILP1 and CsILP2 resulted in the down-regulation of insulin pathway genes. The relative expression of ovarian development-related genes Vasa, G2/M, and Vg was reduced by 82.50%, 89.55%. and 96.98% in dsCsILP1-treated females, and by 42.55%, 91.36%, and 55.63% in dsCsILP2-treated females. Furthermore, substantial decreases in 14-day fecundity were observed, with reductions of 89.99% for dsCsILP1 and 83.45% for dsCsILP2. These results confirm the regulatory functions of CsILP1 and CsILP2 in female C. septempunctata reproduction.
1. Introduction
Insulin-like peptides (ILPs) are bioactive polypeptides in insects that act as endocrine hormones, playing a critical role in maintaining physiological homeostasis [1,2,3,4]. For insects, ILP was first identified in Bombyx mori, and it was designated as bombyxin [5,6,7]. Subsequently, more than 30 ILPs were identified in B. mori [8,9,10]. Up to now, eight ILPs have been found in Drosophila melanogaster [2,3], seven in Anopheles gambiae, five in Anopheles stephensi [11], eleven in Acyrthosiphon pisum [12], and five in Leptinotarsa decemlineata [13]. Different ILPs show different levels of expression in various tissues [4,10]. For instance, in adult D. melanogaster, ILP2 was most highly expressed in the brain, while ILP3 was in the midgut muscle, ILP6 was in the fat body, ILP7 was in the abdominal neuromeres and ILP8 was in the ovary [4,14]. In Aedes aegypti, the ILPs 1, 3, 4, 7, and 8 were specifically expressed in the brain, ILP2 in the ovary, ILP5 in the carcass, and ILP6 in the fat body [15]. In B. mori, bombyxins A-G showed high expression in the brain [10], bombyxin-Y in the fat body and ovary [9,16,17], bombyxin-Z was highly expressed in follicular cells, and bombyxin-X exhibited the highest expression in the fat body [9]. This tissue-specific expression observed among ILPs contributes to the functional diversity in the regulation of growth, development, metabolism, and reproduction [2,10,18].
ILPs regulate insect reproduction mainly through the insulin pathway [19,20,21,22,23]. In the insulin pathway, ILPs act as upstream regulatory factors, binding to the insulin receptor (InR) and phosphorylating it. The phosphorylated InR further binds to and phosphorylates the insulin receptor substrate (IRS). The phosphorylated IRS transmits the signal downstream via phosphoinositide 3-kinase (Pi3k) [19]. Pi3k consists of a regulatory subunit (Pi3k-R) and a catalytic subunit (Pi3k-C). Phosphorylated IRS binds to Pi3k-R, activating Pi3k-C, which in turn phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3). When the concentration of PIP3 accumulates to a certain level, it activates downstream protein kinases AKT [22]. Activated AKT cascades downstream effector proteins to regulate cell growth, development, and differentiation [24]. Among the downstream effector proteins related to reproduction, protein G2/mitotic-specific cyclin-B (G2/M) is a cell-cycle regulatory protein that mediates the cells from interphase to division. Since vigorous reproduction in insects is associated with active cell division, G2/M is regarded as an indicator of reproductive system development [25]. Protein VASA is a member of the DEAD-box family, and it functions in regulating cell proliferation. VASA is widely expressed in insect germ cells, and required for oogenesis [26]. Vitellogenin (Vg) is a glucose–lipid complex protein with high molecular weight [27]. The synthesis of protein Vg in the fat body determines the vitellogenesis [27,28]. Therefore, the expression levels of Vasa, Vg, and G2/M are often used to assess ovarian development at the molecular level [25,26,27,28].
Coccinella septempunctata L. (Coleoptera: Coccinellidae) is a natural predator of many pests such as aphids, spider mites, and scale insects [29,30]. It is characterized by strong reproduction, long lifespan, and wide distribution [31,32]. As an effective biological control agent, its reproductive capacity has been heavily researched [33,34]. Insect reproduction is regulated by various endocrine hormones [27,35,36,37], especially juvenile hormone (JH) and peptide hormones [38]. JH has been proven to be necessary for female ovarian maturation [37,38,39], while the role of peptide hormones like ILPs remains unclear. In this study, two ILP genes (named CsILP1 and CsILP2) were cloned from C. septempunctata, and their expression profiles and functions in female reproduction were verified. These results will enhance our understanding of the molecular mechanisms by which peptide hormones regulate the reproduction of natural enemy insects, and promote biological control of pests.
2. Materials and Methods
2.1. Insects
Seven-spot ladybird beetles (C. septempunctata) were captured from wheat fields (38°82′ N, 115°45′ E) at Hebei Agriculture University, Baoding, Hebei, China. Subsequent generations of larvae and adults were reared under conditions of (24 ± 1) °C and (60 ± 5)% relative humidity with a 16:8 h light/dark photoperiod, and fed on Megoura japonica Matsumura. Larvae were reared singly in 4 cm diameter Petri dishes. Newly emerged adults were paired, and each couple was transferred into a 180 mL clear plastic cup.
M. japonica was reared for generations in the Insect Physiology and Toxicology Laboratory of Hebei Agricultural University under conditions of (23 ± 1) °C and (50 ± 5)% relative humidity. They were fed on fresh pea seedlings [40].
2.2. RNA Isolation and cDNA Cloning of CsILP1 and CsILP2
Total RNA was isolated from the whole body of a 10-day-old female adult with the Total RNA Extraction Kit (Tiangen, Beijing, China) following the instructions. RNA contamination and degradation were monitored on 0.8% agarose gel electrophoresis. The concentration of RNA was examined by ultra-micro spectrophotometer MD2000C (Biofuture, Beijing, China). RNA samples (>1 μg, 28S:18S ≥ 1.0, OD260/280 = 1.8–2.2, OD260/230 ≥ 2.0) were used to synthesize cDNA using RT mix with DNase All-in-One (Suzhou, US EVERBRIGHT, China).
From the unpublished transcriptome database of C. septempunctata, we identified two differently expressed insulin-like peptides, namely CsILP1 and CsILP2. Primers for amplification of CsILP1-ORF and CsILP2-ORF were designed using Primer Premier 6.0 software (Table S1). The sequences of CsILP1 and CsILP2 were PCR-amplified following protocol: 95 °C 3 min, (95 °C 15 s, 55 °C 15 s, 72 °C 3 min) *35 cycles, 72 °C 5 min, 4 °C hold, using 2× High-fidelity PCR Master Mix (Sangon, Shanghai, China). The PCR products with the expected size were cut from the gels, cloned into a pEasy-Blunt Zero Cloning vector (Transgen, Beijing, China), transferred into Trans1-T1 phage Resistant Chemically Competent cells, and then sequenced.
2.3. Bioinformatics Analysis
The ORFs of CsILP1 or CsILP2 were determined by NCBI Open Reading Frame Finder (https://www.ncbi.nlm.nih.gov/orffinder, accessed on 25 January 2024). The RNA sequences were translated to amino acid sequences by the Expasy Translate tool (https://web.expasy.org/translate/, accessed on 21 April 2024). Conserved Domains were searched by NCBI Conserved Domains Search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 21 April 2024). The physical and chemical parameters were given by the Expasy ProtParam tool (https://web.expasy.org/protparam/, accessed on 21 April 2024). The presence of signal peptides was predicted by PredictProtein (https://predictprotein.org/, accessed on 16 May 2024). The amino acid sequences of ILPs in various insect species were searched from the NCBI database. The genomic contexts were predicted by NCBI Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 23 May 2024). DNAMAN 8.0 and MEGA7.0 software were used for sequence alignment. The Swiss Model (https://swissmodel.expasy.org/, accessed on 23 May 2024) was used for protein tertiary structure prediction. The ESPript 3.x (https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi, accessed on 23 May 2024) was used to edit sequence alignment results.
2.4. Expression Profiling Analysis of CsILP1 and CsILP2
To determine the expression profiles of CsILP1 and CsILP2 on different days after female eclosion, female adults were collected randomly on days 2, 4, 6, 8, 10, 12, and 14 after eclosion randomly. For each time point, 1 individual was treated as 1 replicate, and 4 biological replicates were set (a total of 28 individuals were used). To study the expression profiles of CsILP1 and CsILP2 in different tissues, we dissected the head, muscle, fat body, elytra, gut, and ovary of 10-day-old female adults. Each sample weighed 20 mg (each dissected from 20 females), with 4 biological replicates for each tissue.
Total RNA isolation and cDNA synthesis of each sample was performed as described above, and the cDNA concentration was diluted to 120–150 ng/μL for RT-qPCR. The primers for RT-qPCR were designed by Primer Premier 6.0, and 16S ribosomal RNA (16S rRNA) and β-actin were selected as reference genes [41,42]. RT-qPCR was performed using 2*SYBR Green qPCR Master Mix (Suzhou, UElandy, China) on a Bio-Rad CFX machine (Bio-Rad, Hercules, CA, USA). The reaction volumes contained 10 μL of 2*SYBR Green qPCR Master Mix, 8 μL of ddH2O, 0.5 μL of each primer (10 μm), and 1 μL of cDNAs, and followed protocol: 95 °C for 2 min, followed by 40 cycles of 95 °C for 10 s, 58 °C for 15 s, and 72 °C for 15 s, then 95 °C for 1 min, 50 °C for 1 min, and 65 °C for 5 s. Technical replicates were performed 3 times.
2.5. RNA Interference Experiment
RNA Interference (RNAi) was applied to study the function of CsILP1 and CsILP2 in regulating the reproduction of female C. septempunctata, and dsGFP (green fluorescent protein) was used as the control [23]. The primers of dsRNA templates (dsCsILP1, dsCsILP2, and dsGFP, Table S2) were designed using Primer Premier 6.0 including a T7 promoter sequence. The dsRNA was synthesized according to the instructions of the T7 RiboMAX™ Express RNAi System kit (Promega, Shanghai, China), and then the dsRNA concentration was diluted to 1000 ng/μL. Afterward, 0.5 μL of dsRNA was injected into each abdomen of 4-day-old female adults (1:1 paired with male adults) using the 5 μL microinjector (Hamilton, Shanghai, China). For each treatment, 30 females were injected in 1 replication, from which 3 individuals were collected at 1, 3, and 5 days after injection to test the silencing effects of target genes, and 3 biological replicates were conducted. To further test the specificity of dsRNA, 3 individuals were collected at 3 days after injection. The relative expression levels of CsILP2 after injection of dsCsILP1 and levels of CsILP1 after injection of dsCsILP2 were measured and 3 biological replicates were conducted (a total of 270 female individuals were injected and 81 individuals were used to test the silencing effects and specificity of dsRNA at random).
In addition, to determine the function of CsILP1 and CsILP2, female adults were selected 3 days after interference. The expressions of downstream genes in the insulin pathway InR, IRS, Pi3k-R, Pi3k-C, and AKT were tested. The expressions of genes Vasa, Vg, and G2/M, reflecting the developmental status of the female reproductive system, were also measured. The primers for RT-qPCR were designed by Primer Premier 6.0 (Table S3), and 16S rRNA and β-actin were selected as reference genes [41,42]. For each treatment, 3 samples were set for 1 replicate and this was repeated 3 times (a total of 27 individuals were tested).
Then, 3 days after interference, female adults were dissected to observe the ovary status, 3 females were used for each treatment, and this was repeated 3 times (a total of 27 females were dissected). After interference, the pre-oviposition period, 14-day fecundity, and the color of eggs laid in the first spawning were recorded, 8 couples were set for 1 replicate and repeated 3 times [40,43]. For each treatment, the hatching rate was recorded, with 100 eggs collected in 1 sample, 4 samples for 1 replicate, and repeated 3 times.
2.6. Statistical Analysis
Relative expression of genes was calculated by 2−∆∆Ct (Ct value is the number of qPCR cycles). IBM SPSS Statistics V21.0 was used for statistical analysis. For the RNAi experiment, the interference efficiency and specificity of dsRNA were analyzed by Student’s t-test. Other data were analyzed using one-way analysis of variance (ANOVA), with multiple comparisons conducted using Tukey’s HSD (p < 0.05). All data were shown as mean ± standard error (SE).
3. Results
3.1. Sequence Analysis of CsILP1 and CsILP2
The cDNA sequences of CsILP1 and CsILP2 were cloned and submitted to GenBank with accession Nos. OR656512 and OR656513, respectively. Both gene CsILP1 and CsILP2 were located on chromosome 3 (NC_058191.1), gene CsILP1 has three exons and gene CsILP2 has two exons.
The ORF of CsILP1 was 384 bases, encoding a protein of 127 amino acids with a predicted molecular weight (MW) of 14.51 kDa and a theoretical isoelectric point (pI) of 8.34. The ORF of CsILP2 was 357 bases, and it encoded a protein of 118 amino acids with MW of 13.28 kDa and a pI of 6.68. The predicted CsILP1 and CsILP2 protein sequences both contained a Sec/SPI signal peptide (1-27aa) and a conserved IlGF_like superfamily domain (pfam00049), including B-chain, linker (C-peptide), and A-chain. For CsILP1, the domain spans 37-125aa, and for CsILP2, it spans 38-117aa (Figure 1).
Figure 1.
Gene sequences of CsILP1 (A) and CsILP2 (B) and the corresponding protein sequence from C. septempunctata. Signal peptides were “—” underlined, B chains were framed by green squares, C peptides were framed by red squares, A chains were framed by blue squares. An asterisk indicates the stop codon. Conserved domains were in red font. The characteristic cysteines of insulin-like sequences were highlighted in green.
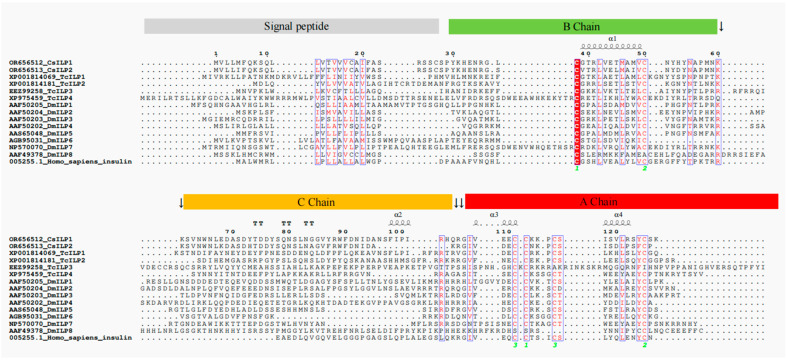
Multiple sequence alignment (Figure 2) showed that CsILP1 and CsILP2 were highly conserved, with 79.53% identity. For CsILP1 and CsILP2, the two cysteine residues (-C-) in the B chain and the four cysteine residues (-C-) in the A chain are highly conserved across all proteins, including four sequences derived from Tribolium castaneum (TcILP1, TcILP2b, TcILP3, and TcILP4), eight sequences derived from D. melanogaster (DmILP1, DmILP2b, DmILP3, DmILP4, DmILP5, DmILP6, DmILP7, and DmILP8), and one sequence derived from humans (Homo sapiens insulin). At the C-terminal end of the B chain of CsILP1 and CsILP2, there are typical C-peptide cleavage sites occupied by two consecutive lysines (-K-K-). However, at the N-terminus of the A chain, CsILP2 has a typical C-peptide cleavage site composed of two consecutive arginine and/or lysine residues (-K-R-). In CsILP1, the lysine (K) at this position is replaced by glutamine (Q) (-Q-R-), and the -K-K- site appears at the eighth and ninth amino acid residues following this.
Figure 2.
Multiple sequence alignment of CsILP1 and CsILP2 from female C. septempunctata with other insulin superfamily proteins. Sequences TcILP1, TcILP2b, TcILP3, and TcILP4 were derived from T. castaneum. Sequences DmILP1, DmILP2b, DmILP3, DmILP4, DmILP5, DmILP6, DmILP7, and DmILP8 were derived from D. melanogaster. The sequence name was preceded by GenBank accession number. Sequence Homo sapiens insulin was derived from humans. The canonical cleavage sites of the C-peptide are marked by black arrows. The common sequence is highlighted in red font. The green numbers represent the sites of the disulfide bonds, and the same numbers indicate they will be connected together after the cleavage of the C peptide.
3.2. Expression Profiling of CsILP1 and CsILP2
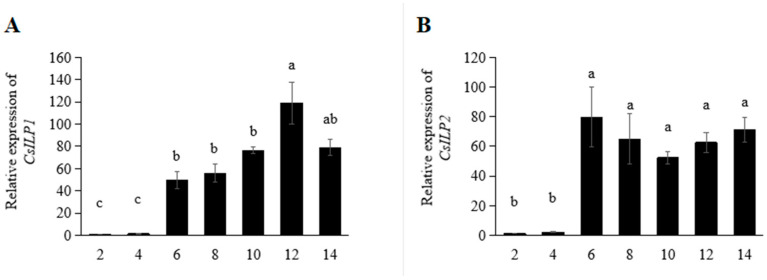
The expression profiles of genes CsILP1 and CsILP2 at different days after eclosion are shown in Figure 3. For CsILP1, the expression level increased sharply on the 6th day, remained stable from the 6th to the 10th day, and peaked on the 12th day (F = 23.671; df = 6, 27; p < 0.001). The relative expression of the CsILP2 gene significantly increased on the 6th day after eclosion and then remained stable (F = 9.013; df = 6, 27; p < 0.001).
Figure 3.
Mean (±SE) expression levels of CsILP1 (A) and CsILP2 (B) in C. septempunctata at different days (2, 4, 6, 8, 10, 12, and 14 days after eclosion). Different letters represented significant differences (ANOVA, Tukey’s HSD, α = 0.05).
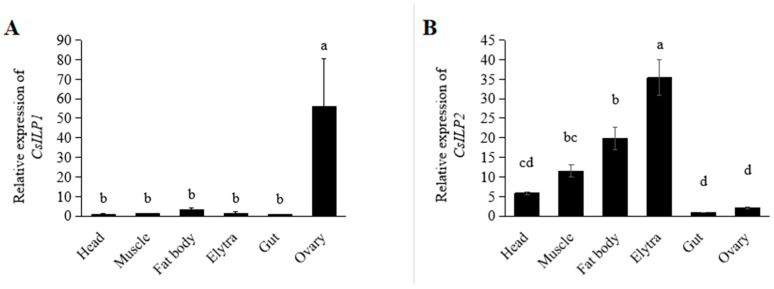
Tissue-specific expression profiles are shown in Figure 4. Both CsILP1 and CsILP2 were expressed in all tissues. CsILP1 had the highest transcription levels in the ovary (F = 5.046; df = 5, 23; p = 0.005). CsILP2 was the highest expressed in the elytra, followed by the fat body, and the lowest in the gut and ovary (F = 33.570; df = 5, 23; p < 0.001).
Figure 4.
Mean (±SE) expression levels of CsILP1 (A) and CsILP2 (B) in different tissues of C. septempunctata. Different letters indicated significant differences (ANOVA, Tukey’s HSD, α = 0.05).
3.3. Functional Analysis of CsILP1 and CsILP2 by RNAi
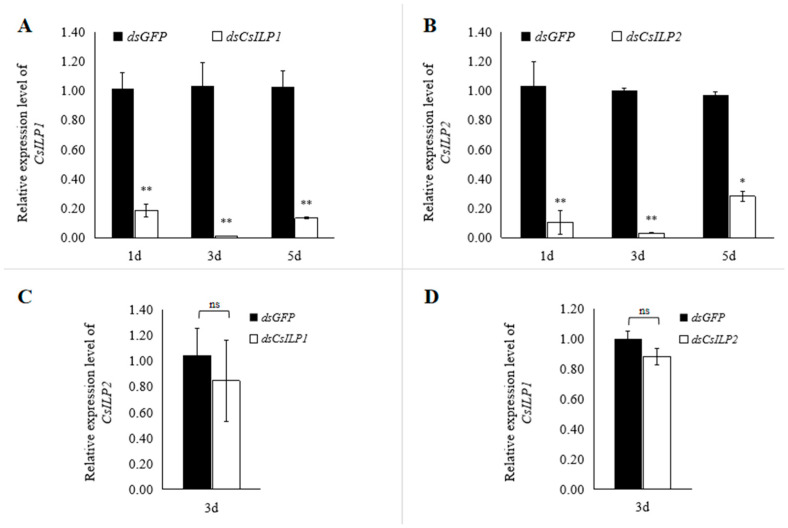
To investigate the function of CsILP1 and CsILP2 in the reproductive process, females were injected with dsCsILP1 or dsCsILP2, with dsGFP used as a control. The silencing efficiencies of CsILP1 and CsILP2 were analyzed by RT-qPCR after dsRNA injection. Results showed that, compared with the dsGFP control, the relative expression of CsILP1 decreased by 82.02%, 99.06%, and 87.16% on days 1, 3, and 5 after dsCsILP1 injection, respectively (day 1, t = 6.283, df = 4, p = 0.003; day 3, t = 5.695, df = 4, p = 0.005; day 5, t = 7.381, df = 4, p = 0.002, Figure 5A). Similarly, the relative expression of CsILP2 decreased by 87.91%, 96.75%, and 78.67% on days 1, 3, and 5 after dsCsILP2 injection, respectively (day 1, t = 14.426, df = 4, p < 0.001; day 3, t = 8.166, df = 4, p = 0.001; day 5, t = 4.088, df = 4, p = 0.015, Figure 5B). Compared to dsGFP control, there was no significant difference in the relative expression of CsILP2 after dsCsILP1 interference (t = 0.539, df = 4, p = 0.618), and vice versa (t = 1.710, df = 4, p = 0.162).
Figure 5.
Silencing efficiency of CsILP1 (A) and CsILP2 (B) after dsRNA Interference in C. septempunctata and the specificity of dsRNA (C,D). Bars represented the mean ± SE, asterisk and “ns” indicate significant differences (Student’s t-test, * p < 0.05, ** p < 0.01).
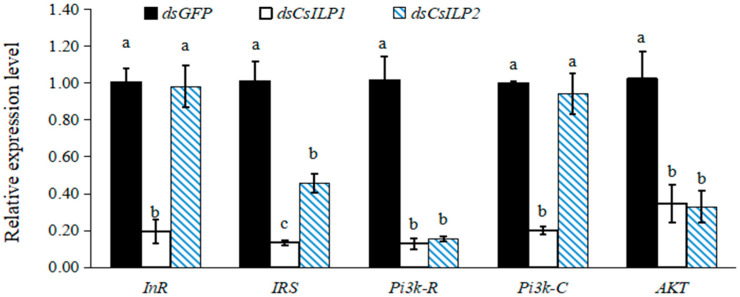
Silencing CsILP1 and CsILP2 affected the expression of insulin pathway genes (Figure 6). Compared with the dsGFP control, the relative expression levels of downstream genes InR, IRS, Pi3k-R, Pi3k-C, and AKT decreased significantly by 80.95%, 87.77%, 88.58%, 79.96%, and 67.57%, respectively, on the third day post-dsCsILP1 injection. For dsCsILP2 injection, the relative expression levels of InR and Pi3k-C showed no significant difference, while IRS, Pi3k-R, and AKT expression decreased significantly by 55.45%, 85.97%, and 69.50%, respectively. Notably, the relative expression level of IRS in the dsCsILP1 group was significantly lower than that in the dsCsILP2 group, and there was no significant difference in the relative expression levels of Pi3k-R and AKT between the dsCsILP1 and dsCsILP2 groups (InR, F = 28.626, df = 2, 8, p = 0.001; IRS, F = 42.947, df = 2, 8, p = 0.000; Pi3k-R, F = 42.217, df = 2, 8, p = 0.000; Pi3k-C, F = 48.054, df = 2, 8, p = 0.000; AKT, F = 11.832, df = 2, 8, p = 0.008).
Figure 6.
Mean (±SE) relative expression levels of insulin pathway genes in C. septempunctata 3 days after treatment with dsGFP control, dsCsILP1 and dsCsILP2. Different letters represented significant differences in the same gene (ANOVA, Tukey’s HSD, α = 0.05).
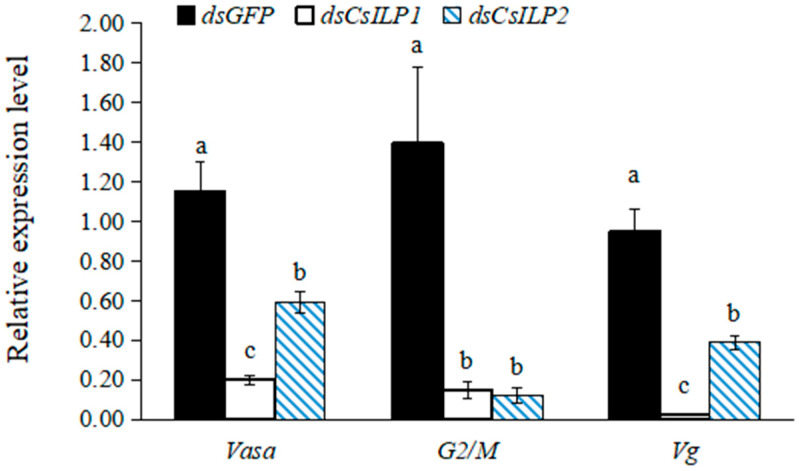
Expression of reproductive-related genes after CsILP1 and CsILP2 silencing was shown in Figure 7. Compared with dsGFP control, the expressions of Vasa, G2/M, and Vg decreased significantly by 82.50%, 89.55%, and 96.98%, respectively, on the third day after dsCsILP1 injection. In the dsCsILP2 injection group, the expression of Vasa, G2/M, and Vg decreased significantly by 42.55%, 91.36%, and 55.63%, respectively. Furthermore, the expressions of Vasa and Vg in dsCsILP1 treatment were significantly lower than those in dsCsILP2 treatment (Vasa, F = 21.337, df = 2, 8, p = 0.002; G2/M, F = 7.890, df = 2, 8, p = 0.021; Vg, F = 36.101, df = 2, 8, p < 0.001).
Figure 7.
Mean (±SE) relative expression levels of reproduction-related genes in C. septempunctata after 3 days of treatment with dsGFP control, dsCsILP1 and dsCsILP2. Different letters represented significant differences in the same gene (ANOVA, Tukey’s HSD, α = 0.05).
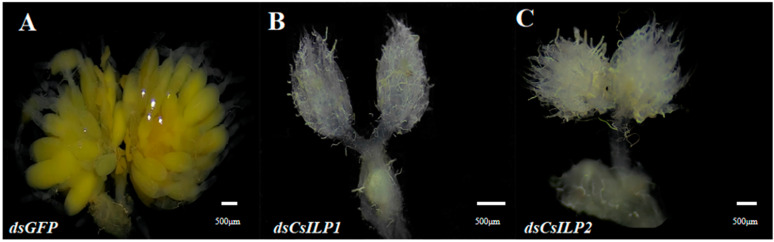
The ovarian development of female adults of C. septempunctata after CsILP1 and CsILP2 silencing is shown in Figure 8. Three days post-injection, the ovaries in the dsGFP control matured with yolk deposition (Figure 8A). The ovaries in the dsCsILP1 (Figure 8B) and dsCsILP2 (Figure 8C) treatment groups remained immature, with no yolk deposition observed.
Figure 8.
The ovarian development of female C. septempunctata 3 days after treatment with dsGFP control (A); dsCsILP1 (B) and dsCsILP2 treatment (C).
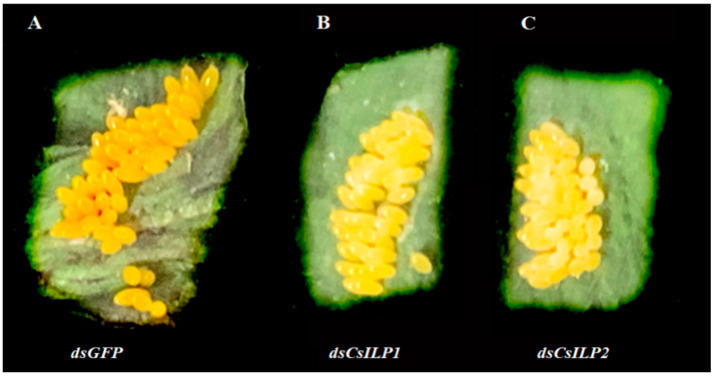
The effects of CsILP1 and CsILP2 silencing on the color of eggs are shown in Figure 9. The color of eggs laid in the first spawning by female adults C. septempunctata in dsCsILP1 (Figure 9B) or dsCsILP2 (Figure 9C) treatment group appeared notably lighter than that in the dsGFP control group (Figure 9A).
Figure 9.
Eggs laid in the first spawning by female in C. septempunctata in dsGFP control group (A); dsCsILP1 (B) and dsCsILP2 treatment (C).
The effects of CsILP1 and CsILP2 silencing on the pre-oviposition, 14-day fecundity, and hatching rate are shown in Table 1. Compared with the dsGFP control, the pre-oviposition of female adults in the dsCsILP1 and dsCsILP2 treatment groups were significantly prolonged by 5.58 days and 4.54 days, respectively (F = 94.297; df = 2, 71; p = 0.048). The total 14-day fecundity was significantly lower in the dsCsILP1 and dsCsILP2 treatment groups, reduced by 89.99% and 83.45%, respectively (F = 88.020; df = 2, 71; p < 0.001), with no significant difference between the dsCsILP1 and dsCsILP2 treatment groups. The hatching rate for the dsCsILP1 (83.11%) and dsCsILP2 (82.44%) treatment groups was significantly lower than that of the dsGFP control group (90.11%), with no significant difference between the two treatment groups (Table 1; F = 15.239, df = 2, 35, p < 0.001).
Table 1.
Effects of CsILP1 and CsILP2 silencing on reproduction of female adults C. septempunctata.
| Treatments | Pre-Oviposition (Days) | 14 Days Fecundity (Eggs per Female) | Hatching Rate (%) |
|---|---|---|---|
| dsGFP | 10.17 ± 0.29 c | 296.79 ± 22.54 a | 90.11 ± 0.93 a |
| dsCsILP1 | 15.75 ± 0.36 a | 29.71 ± 8.83 b | 83.11 ± 1.23 b |
| dsCsILP2 | 14.71 ± 0.26 b | 49.13 ± 13.03 b | 82.44 ± 1.50 b |
Note: data were mean ± SE; different letters indicated significant differences between treatments (ANOVA, Tukey’s HSD, α = 0.05).
4. Discussion
Insect ILPs belong to the insulin superfamily proteins (ISPs), which include insulin, insulin-like growth factor (IGF), relaxin, ILPs, etc. [44]. These ISPs are structurally conserved across divergent taxa, with the ILP gene encoding a propeptide that includes a signal peptide, a B-chain, a C-peptide linker, and an A-chain. Following the cleavage of the C-peptide, the B- and A-chains, linked by three disulfide bonds (formed by six cysteine residues), convert into an activated form [44]. In this study, two ILP genes, CsILP1 and CsILP2, were cloned from C. septempunctata. The predicted protein sequences for both CsILP1 and CsILP2 contain a signal peptide and a conservative domain of IlGF_like superfamily (including a B-chain, a C-peptide, and an A-chain).
Multiple sequence alignment showed the cysteine residue sites are conserved across all sequences. CsILP2 has two typical C-peptide cleavage sites at the C-terminus of the B-chain and the N-terminus of the A-chain, each occupied by pairs of consecutive arginine and/or lysine residues (-K-K- and -K-R-). However, at the N-terminus of the A chain in CsILP1, the lysine (K) is replaced by glutamine (Q) (-Q-R-). The cleavage of the C-peptide is crucial for the structure and function of ISPs, and any mutation at the cleavage site may affect protein function [44]. Therefore, mutations in the cleavage site of c-peptide in CsILP1 might have some influence on its function.
On different days after female eclosion, the expression of CsILP1 sharply increased on the 6th day and reached its peak on the 12th day, while the expression of CsILP2 significantly increased on the 6th day and then remained stable. Previous research found that female C. septempunctata deposited yolk on the 6th to 8th day after eclosion, and reached the spawning peak around the 12th day. This consistency suggests that the expression dynamics of CsILP1 and CsILP2 may be closely related to the reproductive dynamics of C. septempunctata [39].
In most insects, ILPs are expressed in a variety of tissues, including the ovary, gut, fat body, brain, hemolymph, and carcass [4,12,45]. This diversity in tissue-specific expression often suggests functional differences among them. For instance, in B. mori, bombyxins A-G, secreted by brain neurosecretory cells, regulate nutrient-dependent growth, and metabolism, while the high expression of Bombyx IGF-like peptide (BIGFLP) in the fat body shows its function on ovarian development [4,10,17,46]. In D. melanogaster, DmILP8, which is highly expressed in the ovary, showed a clear reproductive relevance, and it was proposed to be a relaxin-like peptide and have gonadotropic functions [14,47,48,49,50]. Furthermore, the expression levels of ILPs are influenced by various environmental factors including temperature, circadian rhythm [51], pesticides [52], and nutritional conditions [48]. Many hormones and growth factors present in the elytra can alter body color and size in response to environmental changes, which in turn affects the growth, development, and reproduction of insects [53,54]. Therefore, we speculate that the expression of ILPs in the elytra may be related to environmental response. In this research, CsILP1 was highly expressed in the ovaries and CsILP2 in the elytra. These tissue-specific expressions of CsILP1 and CsILP2 indicate their relevance to reproduction and development, highlighting the importance of further investigating their mechanisms of action.
The functions of ILPs in regulating the female reproduction are common in many insects, such as D. melanogaster [14], A. aegypti [55], T. castaneum [56], Spodoptera litura [57], Chrysopa septempunctata [38], Propylea japonica [23], Colaphellus bowringi [58], Chrysopa pallens [36], Chilo suppressalis [59], and Adelphocoris suturalis [20]. In this study, females treated with dsCsILP1 and dsCsILP2 showed prolonged preoviposition periods, reduced 14-day fecundity, decreased hatching rates, and delayed ovarian development. At the molecular level, the expressions of reproduction-related genes Vasa, Vg, and G2/M were down-regulated in both treatment groups. These observations indicated that CsILP1 and CsILP2 have a regulatory role in the reproductive process of C. septempunctata. The reproductive development of insects involves oogenesis and vitellogenesis [60]. Gene Vasa is essential for oogenesis, Vg for vitellogenesis, and G2/M for the germ cell cycle [25,26,27,28,61]. Currently, research on female insect reproductive development mainly focuses on vitellogenesis [58,62], with less emphasis on oogenesis and the germ cell cycle. Investigating the differential regulatory effects of ILPs on oogenesis and vitellogenesis in female C. septempunctata will deepen our understanding of the regulatory mechanisms underlying insect ovarian development.
ILPs regulate the reproduction of insects primarily through insulin pathways [4,17,20]. In this study, silencing CsILP1 led to significant downregulation of downstream genes InR, IRS, Pi3k-R, Pi3k-C, and AKT. Silencing CsILP2 resulted in significant downregulation of IRS, Pi3k-R, and AKT genes, while InR and Pi3k-C were not significantly affected. This suggests that CsILP1 and CsILP2 differentially affect the mRNA expression level of genes in the insulin pathway, and their specific mode of action in female reproduction remains to be further investigated. In the insulin pathway, the specific binding of ILPs and InR is a key transmembrane signal. InR is a receptor tyrosine kinase. The structures and functions of InRs vary in insects [60,63,64,65]. In N. lugens (Stål), two types of InRs, InR1 and InR2, have been found to play opposite roles in ovarian development regulation [65]. InR1 and InR2 have also been found in T. castaneum regulating reproductive development through different mechanisms [64]. In D. melanogaster, a leucine-rich repeat G protein-coupled receptor (LGR), a homolog of relaxin receptor, mediates the regulation of DmILP8 in female reproduction [14,47,49]. Therefore, further explorations to clarify the interaction between different ILPs and different receptors are expected to deepen our understanding of the insulin pathway. Furthermore, genes in the insulin pathway can also regulate insect reproduction by interacting with other hormone pathways or nutrition pathways, such as the target of rapamycin (TOR) nutritional pathway and JH pathway [66,67,68,69]. Further exploration of the interaction mechanisms among the insulin pathway, JH pathway, and TOR pathway in regulating insect reproduction is of great significance for elucidating the endocrine regulation of insect reproduction.
In this study, CsILP1 and CsILP2 were cloned from C. septempunctata, and their expression patterns and roles in ovarian development and fecundity were investigated. These results clarified the role of CsILP1 and CsILP2 in female C. septempunctata reproduction, providing a basis for further studies on the molecular mechanisms involved. In the wild, C. septempunctata obtain nutrition from various small insects. Therefore, a deeper investigation into the interactions between the insulin pathway, TOR nutrient pathway and the JH pathway in reproductive regulation is of significant scientific importance for enhancing the application efficiency of releasing C. septempunctata in fields.
Acknowledgments
We are grateful to anonymous reviewers for providing comments that improved this paper.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15120981/s1. Table S1: the primers for cDNA Cloning of CsILP1 and CsILP2. Table S2: the primers for RNA Interference. Table S3: the primers for RT-qPCR.
Author Contributions
S.F.: conceptualization, data curation, formal analysis, methodology, validation, and writing—original draft; D.W.: conceptualization, methodology, project administration, supervision, and writing—review and editing; Q.Q.: methodology, project administration, and writing—review and editing; K.C.: formal analysis and investigation; W.Z.: methodology and investigation; Y.H.: conceptualization, supervision, and writing—review and editing. All authors have agreed to be held accountable for the work performed herein. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The raw data of the cDNA sequences of CsILP1 and CsILP2 were submitted to GenBank with accession Nos. OR656512 and OR656513.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32102194), the Hebei Natural Science Foundation (C2022204009), the Hebei Natural Science Foundation for Young Scholars (C2022204003), the Science Research Project of Hebei Education Department (QN2024136), and the Earmarked Fund for Hebei Modern Agro-industry Technology Research System (HBCT2024170205, HBCT2024110207).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wu Q., Brown M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 2.Nassel D.R., Liu Y., Luo J. Insulin/IGF signaling and its regulation in Drosophila. Gen. Comp. Endocrinol. 2015;221:255–266. doi: 10.1016/j.ygcen.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Nassel D.R., Vanden B.J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016;73:271–290. doi: 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowanski S., Walkowiak-Nowicka K., Winkiel M., Marciniak P., Urbanski A., Pacholska-Bogalska J. Insulin-Like Peptides and Cross-Talk With Other Factors in the Regulation of Insect Metabolism. Front. Physiol. 2021;12:701203. doi: 10.3389/fphys.2021.701203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami A., Iwami M., Nagasawa H., Suzuki A., Ishizaki H. Structure and organization of four clustered genes that encode bombyxin, an insulin-related brain secretory peptide of the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA. 1989;86:6843–6847. doi: 10.1073/pnas.86.18.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagasawa H., Kataoka H., Isogai A., Tamura S., Suzuki A., Ishizaki H., Mizoguchi A., Fujiwara Y., Suzuki A. Amino-terminal amino Acid sequence of the silkworm prothoracicotropic hormone: Homology with insulin. Science. 1984;226:1344–1345. doi: 10.1126/science.226.4680.1344. [DOI] [PubMed] [Google Scholar]
- 7.Nagasawa H., Kataoka H., Isogai A., Tamura S., Suzuki A., Mizoguchi A., Fujiwara Y., Suzuki A., Takahashi S.Y., Ishizaki H. Amino acid sequence of a prothoracicotropic hormone of the silkworm Bombyx mori. Proc. Natl. Acad. Sci. USA. 1986;83:5840–5843. doi: 10.1073/pnas.83.16.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo H., Ino M., Suzuki A., Ishizaki H., Iwami M. Multiple gene copies for bombyxin, an insulin-related peptide of the silkmoth Bombyx mori: Structural signs for gene rearrangement and duplication responsible for generation of multiple molecular forms of bombyxin. J. Mol. Biol. 1996;259:926–937. doi: 10.1006/jmbi.1996.0370. [DOI] [PubMed] [Google Scholar]
- 9.Aslam A.F., Kiya T., Mita K., Iwami M. Identification of novel bombyxin genes from the genome of the silkmoth Bombyx mori and analysis of their expression. Zool. Sci. 2011;28:609–616. doi: 10.2108/zsj.28.609. [DOI] [PubMed] [Google Scholar]
- 10.Mizoguchi A., Okamoto N. Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: Discovery, structure, secretion and function. Front. Physiol. 2013;4:217. doi: 10.3389/fphys.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquez A.G., Pietri J.E., Smithers H.M., Nuss A., Antonova Y., Drexler A.L., Luckhart S. Insulin-like peptides in the mosquito Anopheles stephensi: Identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 2011;173:303–312. doi: 10.1016/j.ygcen.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huygens C., Ribeiro L.M., Gaget K., Duport G., Peignier S., De Groef S., Callaerts P. Evolutionary diversification of insulin-related peptides (IRPs) in aphids and spatiotemporal distribution in Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2022;141:103670. doi: 10.1016/j.ibmb.2021.103670. [DOI] [PubMed] [Google Scholar]
- 13.Fu K.Y., Zhu T.T., Guo W.C., Ahmat T., Li G.Q. Knockdown of a putative insulin-like peptide gene LdILP2 in Leptinotarsa decemlineata by RNA interference impairs pupation and adult emergence. Gene. 2016;581:170–177. doi: 10.1016/j.gene.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Veenstra J.A. Arthropod IGF, relaxin and gonadulin, putative orthologs of Drosophila insulin-like peptides 6, 7 and 8, likely originated from an ancient gene triplication. PeerJ. 2020;8:e9534. doi: 10.7717/peerj.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling L., Raikhel A.S. Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2021;118:e2023470118. doi: 10.1073/pnas.2023470118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto N., Yamanaka N., Satake H., Saegusa H., Kataoka H., MizoguchI A. An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J. 2009;276:1221–1232. doi: 10.1111/j.1742-4658.2008.06859.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujinaga D., Shiomi K., Yagi Y., Kataoka H., Mizoguchi A. An insulin-like growth factor-like peptide promotes ovarian development in the silkmoth Bombyx mori. Sci. Rep. 2019;9:18446. doi: 10.1038/s41598-019-54962-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veenstra J.A. Differential expression of some termite neuropeptides and insulin/IGF-related hormones and their plausible functions in growth, reproduction and caste determination. PeerJ. 2023;11:e15259. doi: 10.7717/peerj.15259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuen A.C., Hillion K.H., Wang R., Amoyel M. Germ cells commit somatic stem cells to differentiation following priming by PI3K/Tor activity in the Drosophila testis. PLoS Genet. 2021;17:e1009609. doi: 10.1371/journal.pgen.1009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue H., Huang X., Chang G., Ma W., Hull J.J., Chen L. Reproductive capacity in Adelphocoris suturalis (Hemiptera: Miridae) is regulated by the insulin signaling pathway. Pestic. Biochem. Physiol. 2022;187:105195. doi: 10.1016/j.pestbp.2022.105195. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S., Ray K. Somatic PI3K activity regulates transition to the spermatocyte stages in Drosophila testis. J. Biosci. 2017;42:285–297. doi: 10.1007/s12038-017-9678-5. [DOI] [PubMed] [Google Scholar]
- 22.Chen J.J., Liu X.X., Guo P.H., Teets N.M., Zhou J.C., Chen W.B., Luo Q.Z., Kanjana N., Li Y.Y., Zhang L.S. Regulation of forkhead box O transcription factor by insulin signaling pathway controls the reproductive diapause of the lady beetle, Coccinella septempunctata. Int. J. Biol. Macromol. 2024;258:128104. doi: 10.1016/j.ijbiomac.2023.128104. [DOI] [PubMed] [Google Scholar]
- 23.Huangfu N., Zhu X., Wang L., Zhang K., Li D., Chen L., Cui J. Insulin receptor substrate-1 (IRS1) regulates oogenesis and vitellogenesis in Propylea japonica by mediating the FOXO transcription factor expression, independent of JH and 20E signaling pathways. J. Agric. Food Chem. 2023;71:300–310. doi: 10.1021/acs.jafc.2c07433. [DOI] [PubMed] [Google Scholar]
- 24.Mensah L.B., Goberdhan D., Wilson C. mTORC1 signalling mediates PI3K-dependent large lipid droplet accumulation in Drosophila ovarian nurse cells. Biol. Open. 2017;6:563–570. doi: 10.1242/bio.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng W.M., Althauser C., Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–4746. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- 26.Dehghani M., Lasko P. Multiple functions of the dead-box helicase vasa in drosophila oogenesis. Results Probl. Cell Differ. 2017;63:127–147. doi: 10.1186/s13071-023-05747-8. [DOI] [PubMed] [Google Scholar]
- 27.Roy S., Saha T.T., Zou Z., Raikhel A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018;63:489–511. doi: 10.1146/annurev-ento-020117-043258. [DOI] [PubMed] [Google Scholar]
- 28.Han H., Han S., Qin Q., Chen J., Wang D., He Y.Z. Molecular Identification and Functional Characterization of Vitellogenin Receptor From Harmonia axyridis (Coleoptera: Coccinellidae) J. Econ. Entomol. 2022;115:325–333. doi: 10.1093/jee/toab224. [DOI] [PubMed] [Google Scholar]
- 29.Xue Y., Bahlai C.A., Frewin A., Sears M.K., Schaafsma A.W., Hallett R.H. Predation by Coccinella septempunctata and Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Homoptera: Aphididae) Environ. Entomol. 2009;38:708–714. doi: 10.1603/022.038.0322. [DOI] [PubMed] [Google Scholar]
- 30.Farooq M.U., Qadri H., Khan M.A. Aphid species affect foraging behavior of Coccinella septempunctata (Coccinellidae: Coleoptera) Pak. J. Biol. Sci. 2017;20:160–164. doi: 10.3923/pjbs.2017.160.164. [DOI] [PubMed] [Google Scholar]
- 31.Lopes P.C., Souza P., Santos J., Borges C.E., Araujo F., Martins J.C., Silva R. Spatiotemporal distribution of Schizaphis graminum (Rondani) and its natural enemy Coccinella septempunctata (Linnaeus) in graniferous sorghum crops. Braz. J. Biol. 2022;84:e261972. doi: 10.1590/1519-6984.261972. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y., Zhi J.R., Li F.L., Li W.H., Zhou Y.H. Improving the artificial diet for adult of seven spotted ladybird beetle Coccinella septempunctata L. (Coleoptera: Coccinellidae) with orthogonal design. Bull. Entomol. Res. 2018;108:337–343. doi: 10.1017/S0007485317000797. [DOI] [PubMed] [Google Scholar]
- 33.Jalali M.A., Reitz S., Mehrnejad M.R., Ranjbar F., Ziaaddini M. Food utilization, development, and reproductive performance of Coccinella septempunctata (Coleoptera: Coccinellidae) feeding on an aphid or psylla prey species. J. Econ. Entomol. 2019;112:571–576. doi: 10.1093/jee/toy424. [DOI] [PubMed] [Google Scholar]
- 34.Turnipseed R.K., Ugine T.A., Losey J.E. Egg predation by the introduced lady beetle, Coccinella septempunctata (Coleoptera: Coccinellidae), lowers mortality but raises relative risk for the native lady beetle, Coccinella novemnotata. PLoS ONE. 2015;10:e118493. doi: 10.1371/journal.pone.0118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalid M.Z., Ahmad S., Ngegba P.M., Zhong G. Role of endocrine system in the regulation of female insect reproduction. Biology. 2021;10:614. doi: 10.3390/biology10070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han B., Zhang T., Feng Y., Liu X., Zhang L., Chen H., Mao J. Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 2020;123:104049. doi: 10.1016/j.jinsphys.2020.104049. [DOI] [PubMed] [Google Scholar]
- 37.Han H., Feng Z.Y., Han S.P., Chen J., Wang D., He Y.Z. Molecular identification and functional characterization of Methoprene-Tolerant (Met) and Kruppel-Homolog 1 (Kr-h1) in Harmonia axyridis (Coleoptera: Coccinellidae) J. Econ. Entomol. 2022;115:334–343. doi: 10.1093/jee/toab252. [DOI] [PubMed] [Google Scholar]
- 38.Zhang T.T., Zhang G.C., Zeng F.F., Liu C.Y., Mao J.J. Insulin-like peptides regulate vitellogenesis and oviposition in the green lacewing, Chrysopa septempunctata. Bull. Entomol. Res. 2017;107:148–154. doi: 10.1017/S0007485316000742. [DOI] [PubMed] [Google Scholar]
- 39.Fu Y.L., Chen Z.H. The concentration of juvenile hormone in female adults of Coccinella septempunctata during ovarian development. Acta Entomol. Sin. 1984;27:268–274. [Google Scholar]
- 40.Cheng Z., Qin Q.J., Wang D., Han S.P., Zhang S., He Y.Z. Sublethal and transgenerational effects of exposures to the thiamethoxam on the seven-spotted lady beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae) Ecotoxicol. Environ. Saf. 2022;243:114002. doi: 10.1016/j.ecoenv.2022.114002. [DOI] [PubMed] [Google Scholar]
- 41.Lu J., Yang C., Zhang Y., Pan H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insects: A systematic review. Front. Physiol. 2018;9:1560. doi: 10.3389/fphys.2018.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C., Preisser E.L., Zhang H., Liu Y., Dai L., Pan H., Zhou X. Corrigendum: Selection of reference genes for RT-qPCR analysis in Coccinella septempunctata to assess un-intended effects of RNAi transgenic plants. Front. Plant. Sci. 2016;7:1835. doi: 10.3389/fpls.2016.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng Y., Yu Y., Li W., Li F. Influence of artificial diets on biological characteristics and digestive enzymes of Coccinella septempunctata L. J. Insect Sci. 2023;23:7. doi: 10.1093/jisesa/iead022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao S.S., Kundapura S.V., Dey D., Palaniappan C., Sekar K., Kulal A., Ramagopal U.A. Cumulative phylogenetic, sequence and structural analysis of Insulin superfamily proteins provide unique structure-function insights. Mol. Inform. 2024;43:e202300160. doi: 10.1002/minf.202300160. [DOI] [PubMed] [Google Scholar]
- 45.Dai Y., Li X., Ding J., Liang Z., Guo R., Yi T., Liu W. Molecular and expression characterization of insulin-like signaling in development and metabolism of Aedes albopictus. Parasit. Vectors. 2023;16:134. doi: 10.1186/s13071-023-05747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okamoto N., Yamanaka N., Endo Y., Kataoka H., Mizoguchi A. Spatiotemporal patterns of IGF-like peptide expression in the silkmoth Bombyx mori predict its pleiotropic actions. Gen. Comp. Endocrinol. 2011;173:171–182. doi: 10.1016/j.ygcen.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Gontijo A.M., Garelli A. The biology and evolution of the Dilp8-Lgr3 pathway: A relaxin-like pathway coupling tissue growth and developmental timing control. Mech. Dev. 2018;154:44–50. doi: 10.1016/j.mod.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Semaniuk U., Strilbytska O., Malinovska K., Storey K.B., Vaiserman A., Lushchak V., Lushchak O. Factors that regulate expression patterns of insulin-like peptides and their association with physiological and metabolic traits in Drosophila. Insect Biochem. Mol. Biol. 2021;135:103609. doi: 10.1016/j.ibmb.2021.103609. [DOI] [PubMed] [Google Scholar]
- 49.Liao S., Nassel D.R. Drosophila insulin-like peptide 8 (DILP8) in ovarian follicle cells regulates ovulation and metabolism. Front. Endocrinol. 2020;11:461. doi: 10.3389/fendo.2020.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H., Luo X., Li N., Liu T., Zhang J. Insulin-like peptide 8 (Ilp8) regulates female fecundity in flies. Front. Cell Dev. Biol. 2023;11:1103923. doi: 10.3389/fcell.2023.1103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi S.T., Kobayashi R., Tomita J., Kume K. The regulation of circadian rhythm by insulin signaling in Drosophila. Neurosci. Res. 2022;183:76–83. doi: 10.1016/j.neures.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Wang N., Wang Z., Gong S., Zhang Y., Xue C. Sublethal concentration of λ-cyhalothrin inhibits insulin-like peptides and leads to reproductive toxicity in Chrysoperla sinica. Insect Sci. 2024. Online ahead of print . [DOI] [PubMed]
- 53.Umbers K.D., Fabricant S.A., Gawryszewski F.M., Seago A.E., Herberstein M.E. Reversible colour change in Arthropoda. Biol. Rev. Camb. Philos. Soc. 2014;89:820–848. doi: 10.1111/brv.12079. [DOI] [PubMed] [Google Scholar]
- 54.Li J.X., Tian Z., Liu X.F., Li B., An H.M., Brent C.S., Wang J.L., Wang X.P., Liu W. Juvenile hormone regulates the photoperiodic plasticity of elytra coloration in the ladybird Harmonia axyridis. Mol. Ecol. 2023;32:2884–2897. doi: 10.1111/mec.16896. [DOI] [PubMed] [Google Scholar]
- 55.Riehle M.A., Fan Y., Cao C., Brown M.R. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: Expression, cellular localization, and phylogeny. Peptides. 2006;27:2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 56.Sheng Z., Xu J., Bai H., Zhu F., Palli S.R. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 2011;286:41924–41936. doi: 10.1074/jbc.M111.269845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan X., Pei Y., Zhang C., Huang Y., Chen L., Wei L., Chen X. Effect of insulin receptor on juvenile hormone signal and fecundity in Spodoptera litura (F.) Insects. 2022;13:701. doi: 10.3390/insects13080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian Z., Guo S., Zhu F., Liu W., Wang X.P. Targeting coat protein II complex genes via RNA interference inhibits female adult feeding and reproductive development in the cabbage beetle Colaphellus bowringi. Pest. Manag. Sci. 2022;78:2141–2150. doi: 10.1002/ps.6836. [DOI] [PubMed] [Google Scholar]
- 59.Wu S., Tang Y., Su S., Ding W., He H., Xue J., Li Y. RNA interference knockdown of insulin receptor inhibits ovarian development in Chilo suppressalis. Mol. Biol. Rep. 2022;49:11765–11773. doi: 10.1007/s11033-022-07948-3. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z., Tan D., Wang F., Guo S., Liu J., Cuthbertson A., Sang W. Insulin peptides and their receptors regulate ovarian development and oviposition behavior in Diaphorina citri. Insect Sci. 2023;30:95–108. doi: 10.1111/1744-7917.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mouawad R., Himadewi P., Kadiyala D., Arnosti D.N. Selective repression of the Drosophila cyclin B promoter by retinoblastoma and E2F proteins. Biochim. Biophys. Acta Gene Regul. Mech. 2020;1863:194549. doi: 10.1016/j.bbagrm.2020.194549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y.Y., Chen J.J., Liu M.Y., He W.W., Reynolds J.A., Wang Y.N., Zhang L.S. Enhanced degradation of juvenile hormone promotes reproductive diapause in the predatory ladybeetle Coccinella Septempunctata. Front. Physiol. 2022;13:877153. doi: 10.3389/fphys.2022.877153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castro-Arnau J., Marin A., Castells M., Ferrer I., Maestro J.L. The expression of cockroach insulin-like peptides is differentially regulated by physiological conditions and affected by compensatory regulation. J. Insect Physiol. 2019;114:57–67. doi: 10.1016/j.jinsphys.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Sang M., Li C., Wu W., Li B. Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene. 2016;585:196–204. doi: 10.1016/j.gene.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 65.Xue W.H., Liu Y.L., Jiang Y.Q., He S.F., Wang Q.Q., Yang Z.N., Xu H.J. Molecular characterization of insulin-like peptides in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) Insect Mol. Biol. 2020;29:309–319. doi: 10.1111/imb.12636. [DOI] [PubMed] [Google Scholar]
- 66.Lu K., Chen X., Liu W.T., Zhou Q. TOR pathway-mediated juvenile hormone synthesis regulates nutrient-dependent female reproduction in Nilaparvata lugens (Stal) Int. J. Mol. Sci. 2016;17:438. doi: 10.3390/ijms17040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perez-Hedo M., Rivera-Perez C., Noriega F.G. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Insect Biochem. Mol. Biol. 2013;43:495–500. doi: 10.1016/j.ibmb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhai Y., Sun Z., Zhang J., Kang K., Chen J., Zhang W. Activation of the TOR signalling pathway by glutamine regulates insect fecundity. Sci. Rep. 2015;5:10694. doi: 10.1038/srep10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng W., Wu F., Ye Y., Li T., Zhang Z., Zhang H. Small GTPase Rab40C is up regulated by 20-hydroxyecdysone and insulin pathways to regulate ovarian development and fecundity. Insect Sci. 2022;29:1583–1600. doi: 10.1111/1744-7917.13026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of the cDNA sequences of CsILP1 and CsILP2 were submitted to GenBank with accession Nos. OR656512 and OR656513.