Abstract
Background/Objectives: Tendinopathy is a condition associated with pain and limited function. While upper and lower extremity tendinopathies may have different functional implications, there have been a number of reports supporting different patterns of dysfunction in pain processing and inhibition. The purpose of this scoping review was to examine the methods across studies examining pain processing in patients with upper and lower extremity tendinopathy. Methods: Five electronic databases (PubMed, Scopus, CINAHL, the Cochrane Library, and SPORTDiscus) and gray literature sources were searched from inception through 15 April 2024, using appropriate keywords and relevant synonyms. Results: In total, 3219 titles were retrieved from the searches, with 43 studies retained for final inclusion. Of the 43 studies, 22 were specific to upper extremity tendinopathies, 19 were specific to lower extremity tendinopathies, and 2 studies included mixed samples. Physical testing to detect nervous system sensitization was most commonly performed using pressure pain thresholds. Although infrequent, questionnaire instruments were used mostly to include the central sensitization inventory. Substantial variation was noted across studies in mode of testing and instruments used, while patient demographics and inclusion criteria were not clearly reported in many instances. Thirty-one studies (72%) reported nervous system sensitization or dysfunction in tendinopathy, while 13 (28%) did not. Conclusions: While the difference between pain processing in tendinopathy is likely multifactorial, the results of this review identified substantial variability in methodology used and reporting in tendon pain research. As inconsistency in evidence can limit clinical guidance, efforts to standardize tendinopathy pain research appear warranted.
Keywords: pain assessment, physical therapy, tendon, sensitization
1. Introduction
Tendinopathy is a common musculoskeletal diagnosis associated with pain and loss of function [1]. Epidemiological studies report high incidence and prevalence rates of tendinopathies in the upper [2,3] and lower extremities [4,5]. Despite substantial research related to best practices in managing tendinopathy, positive long-term outcomes are not consistently seen. With patellar tendinopathy, nearly one-third of athletes with the condition do not return to sports after six months [6], while one study reported 53% of athletes with the condition retired from sport completely [7]. In a large longitudinal study of soccer players, 27% of all Achilles tendinopathies were recurrent injuries, indicating an incomplete initial recovery [8]. Without treatment, greater trochanteric pain syndrome (GTPS) persisted in 45% of patients at 11-year follow-up and may contribute to secondary conditions such as hip osteoarthritis [9]. Nearly one-third of patients with lateral elbow tendinopathy [10,11] and half of patients with rotator cuff tendinopathy have persistent pain more than one year after initial onset despite intervention [12]. The lack of improvement and negative impact of tendon pain on long-term function warrants additional investigation into optimal evaluation and management of these conditions.
Localized tendon pain aggravated by activity and relieved with relative rest is consistent with a nociceptive mechanism for pain [13]. However, the central nervous system (CNS) demonstrates plasticity in pathological states, and sustained peripheral nociceptive input may lead to the development of altered peripheral and central pain processing [14]. This phenomenon, known as sensitization, is defined by the International Association for the Study of Pain (IASP) as “increased responsiveness of nociceptive neurons to their normal input and/or recruitment of a response to normally subthreshold inputs” [15] and could be an underlying mechanism for prolonging an individual’s pain experience [16]. While multiple input and processing mechanisms are involved in a patient’s pain experience, the identification of peripheral or central pain processing to inform the selection of interventions (e.g., education, electrical stimulation, exercise, manual therapy, pharmaceuticals, etc.) may be beneficial in improving an individual’s level of function [13,16]. Identification of pain processing dysfunction is particularly important with tendinopathy, as lateral elbow and shoulder studies found the presence of nervous system sensitization at baseline may be associated with poorer long-term outcomes [17,18,19,20]. Therefore, evaluating the predominant pain mechanism(s) involved in tendon pain may be warranted.
Previous reviews revealed an association between enhanced CNS sensitization as measured by quantitative sensory testing (QST) and upper extremity tendinopathies, whereas lower extremity tendinopathies may be predominantly peripheral nociceptive pain states [21,22]. While differing tendon characteristics may play a role in the conflicting results, it is also possible that variability of assessment methodologies between studies contributes to the inconsistency. Partly because of heterogeneity among studies, international experts saw the need to recently form consensus statements on the terminology, suggested reporting, and core health-related domains (among others) associated with tendinopathy research [23,24,25]. Inconsistencies in tendinopathy research study design, methods, modalities examined, and self-report instruments make synthesizing conclusions on altered pain processing mechanisms difficult. A lack of consistency in methodology and reporting also impairs the ability of researchers to perform meta-analyses or similar synthesized judgments to guide clinical practice. The evaluation of testing strategies and reporting for tendon pain research has not been critically evaluated and synthesized. Determining the degree of variation across studies evaluating tendon pain is an important step in closing current knowledge gaps.
A thorough examination of methodology, one of the primary indications for performing a scoping review [26], in tendinopathy research is warranted to understand the strength of current recommendations and guide research. Therefore, the primary purpose of this scoping review was to examine the variability of methodology across studies examining pain processing in patients with tendinopathy. Additionally, this project sought to identify gaps in available evidence to inform future tendinopathy pain studies.
2. Materials and Methods
This scoping review was registered on the Open Science Framework (OSF) website and is available online: https://doi.org/10.17605/OSF.IO/59FNS. Reporting followed guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR).
2.1. Data Sources and Searches
A comprehensive and systematic computerized search of the electronic databases PubMed, Scopus, CINAHL, the Cochrane Library, and SPORTDiscus was conducted to identify articles relevant to the clinical question. All articles from database inception through 15 April 2024 were considered for inclusion. With the assistance of a research librarian, key search terms and various combinations of synonyms related to the concepts of tendinopathy and pain processing were entered into each database. Specific search strategies are presented in Supplementary File S1. The reference lists of eligible articles were manually examined for key review articles and additional relevant studies. Finally, clinical trial registries, Google, and the Open Grey database were scanned for pertinent work not captured otherwise.
2.2. Eligibility Criteria
Studies were considered in this review if they related to the clinical question and had a full-text report published in the English language in a peer-reviewed journal. For inclusion, the study population needed to have a clinical diagnosis of tendinopathy, and testing had to incorporate a subjective and/or objective clinical assessment tool for alterations in pain processing. Assessment strategies to detect peripheral and/or central sensitization and involved pain mechanisms (e.g., nociceptive, neuropathic, nociplastic) were included. Dysfunction in pain processing was dependent on the instrument used (i.e., reduced thermal or pain thresholds, depressed conditioned pain modulation [CPM], higher scores on pain questionnaires). Subacromial impingement and GTPS, conditions most commonly attributed to tendon pathology [27,28], were included if the study authors clarified no other condition was likely. Studies were excluded if they were published in abstract form only, were not in the English language or reasonably translated to English, participants did not have a clinical diagnosis of tendinopathy, or testing could not detect peripheral and/or central pain processing or inhibition. Study protocols, intervention studies, case reports with less than 10 participants, and animal studies were also excluded.
2.3. Study Selection
Two reviewers independently screened the titles and abstracts for eligibility through the web-based software platform Covidence using the criteria determined a priori. After the preliminary search of the above databases, any article that included clinical assessment of pain processing in tendinopathy was retained for further analysis. In cases where details of the study methods were unclear, the study’s corresponding author was contacted for additional information. Studies were retained for further analysis if both reviewers voted for inclusion. Amongst those articles in which disagreement occurred (i.e., one vote to include, one vote to exclude), a third author blinded to previous voting made the final decision for inclusion. After full-text articles were obtained, two reviewers independently evaluated the study for appropriateness. If a unanimous decision regarding inclusion was not obtained based on the two reviewers’ decisions, a third author blinded to previous voting was consulted for a final vote. In each case of disagreement at any stage, a majority (i.e., 2 reviewers to 1) vote guided the decision to include or exclude a study.
2.4. Data Extraction and Reporting
Data from studies were extracted to standardized electronic forms using Excel based on available information in the published article. Grouping of data was subclassified into 3 thematic areas after concept mapping was completed by the research team: (1) participant demographics and reporting, (2) physical testing used, and (3) self-reported or questionnaire-based testing used. For demographic data, the following information was extracted: the presence of clear inclusion and exclusion criteria, the diagnostic criteria used for tendinopathy, sample size, participant age, participant sex, participant body mass index (BMI), whether participants were taking medications, whether participants had a single site of pain, and for comparative studies, whether there were significant between-group differences. While more extensive data were retrieved from each study (Supplementary File S2), for purposes of describing results, frequencies were captured to more easily highlight comparisons between pain mechanism testing strategies for upper and lower extremity tendinopathy. The presence of nervous system sensitization or dysfunction, as determined by the study’s statement of results, was also noted based on testing modalities and study design.
2.5. Quality Assessment
Individual study quality was not assessed for this scoping review. Instead, this study focused on the relevance of variation in testing modalities and reporting characteristics in tendon pain or possible evidence gaps. For study quality and risk of bias for many of the included studies, readers are referred to previously published works [21,22].
3. Results
3.1. Selection of Studies
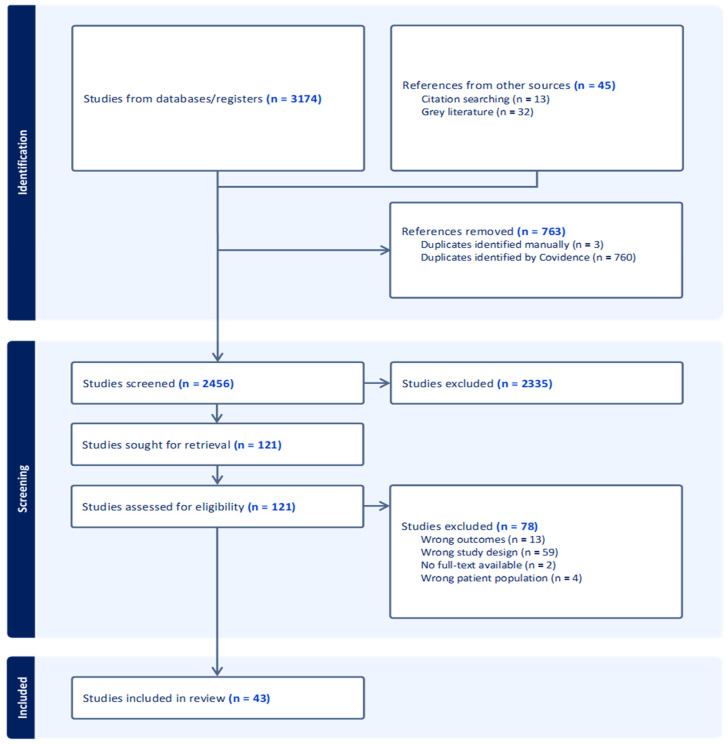
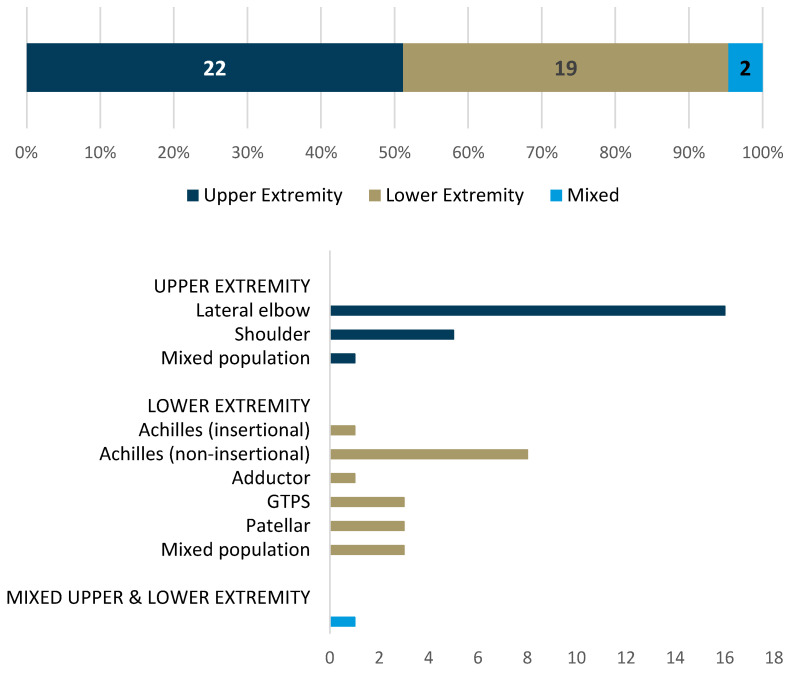
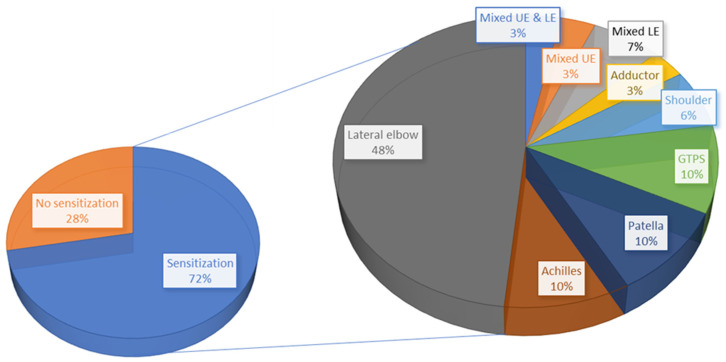
In total, 3219 titles were retrieved from the searches. After the removal of duplicates, 2456 studies were screened, of which 43 studies were retained for final inclusion in this scoping review (Figure 1). Of the included studies, 32 (75%) used case–control designs [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60], 9 (21%) used cross-sectional designs [61,62,63,64,65,66,67,68,69], 1 (2%) was a cohort study [70], and 1 (2%) was a post hoc analysis [71]. A breakdown of the number of studies by the specific tendon researched is presented in Figure 2. In total, included studies reported on 3728 participants, of which 2646 had a clinical diagnosis related to tendinopathy while 1082 participants were used for comparison.
Figure 1.
PRISMA flow diagram.
Figure 2.
Number and proportion of included studies specific to each tendon.
3.2. Study Reporting of Demographics
Selected demographic data extraction variables were based on the ICON consensus statement [24] and items likely to affect pain perception (Table 1, File S2). Each of the studies (100%) clearly reported sample size, age, and sex of participants. The diagnostic criteria, inclusion criteria, and exclusion criteria were clearly reported in 95.3%, 88.4%, and 90.7% of studies, respectively. In comparative studies (n = 33), no significant between-group differences at baseline were reported by 18 studies (54.5%). BMI was calculated and reported in 53.5% of included studies. Importantly, while most studies stated recent corticosteroid injection was an exclusion criterion, only 30.2% of studies clearly stated that pain medication usage was not allowed by participants. Studies frequently excluded individuals with systematic pathology or chronic pain conditions (e.g., rheumatoid arthritis and fibromyalgia) and possible referred pain (e.g., cervical radiculopathy or surgery in the assessment of lateral elbow tendinopathy); however, no study (0%) explicitly noted that symptomatic participants had to have a single site of symptoms at the involved tendon to be included. No substantial differences were noted in demographic reporting between upper and lower extremity studies, except for BMI, which was reported by 40.9% and 68.4%, respectively.
Table 1.
Number of studies clearly reporting relevant demographic information, n (%).
| Tendon Studied | Inclusion Criteria | Exclusion Criteria | Diagnostic Criteria | Sample Size | Age | Gender | BMI | Medication Usage | Single Pain Site | No Between-Group Differences * |
|---|---|---|---|---|---|---|---|---|---|---|
| Total studies (n = 43) | 38 (88.4%) | 39 (90.7%) | 41 (95.3%) |
43 (100%) | 43 (100%) | 43 (100%) | 23 (53.5%) | 13 (30.2%) |
0 (0%) |
18/33 (54.5%) |
| UE studies (n = 22) | 20 (90.9%) | 21 (95.5%) | 20 (90.9%) |
22 (100%) | 22 (100%) | 22 (100%) | 9 (40.9%) | 6 (27.3%) |
0 (0%) |
10/19 (52.6%) |
| Lateral elbow (n = 16) | 15 (94.8%) | 16 (100%) |
15 (94.8%) |
16 (100%) | 16 (100%) | 16 (100%) | 7 (43.8%) | 3 (18.8%) |
0 (0%) |
8/14 (57.1%) |
| Shoulder (n = 5) | 5 (100%) |
5 (100%) |
4 (80%) |
5 (100%) | 5 (100%) | 5 (100%) | 1 (20%) |
3 (60%) |
0 (0%) |
2/5 (40%) |
| Mixed sample (n = 1) | 0 (0%) | 0 (0%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 0/1 (0%) | 0 (0%) | NA |
| LE studies (n = 19) | 17 (89.5%) | 17 (89.5%) | 19 (100%) | 19 (100%) | 19 (100%) | 19 (100%) | 13 (68.4%) | 7 (36.8%) | 0 (0%) | 7/13 (53.8%) |
| Achilles (n = 9) | 9 (100%) | 9 (100%) | 9 (100%) | 9 (100%) | 9 (100%) | 9 (100%) | 6 (66.7%) | 2 (22.2%) | 0 (0%) | 3/6 (50%) |
| Adductor (n = 1) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0/1 (0%) |
| GTPS (n = 3) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | 1/2 (50%) |
| Patellar (n = 3) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 0 (0%) | 2/3 (66.7%) |
| Mixed sample (n = 3) | 1 (33.3%) | 1 (33.3%) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 1 (33.3%) | 1 (33.3%) | 0 (0%) | 1/1 (100%) |
| Mixed UE/LE studies (n = 2) | 2 (100%) |
2 (100%) |
2 (100%) |
2 (100%) | 1 (50%) | 0 (0%) |
0 (0%) |
1 (100%) |
2 (100%) | 1/1 (100%) |
Abbreviations: BMI—body mass index; GTPS—greater trochanteric pain syndrome; LE—lower extremity; UE—upper extremity. * Indicates studies that specifically reported no between-group differences; does not include studies without a comparison group.
3.3. Physical Examination Testing Modalities Used
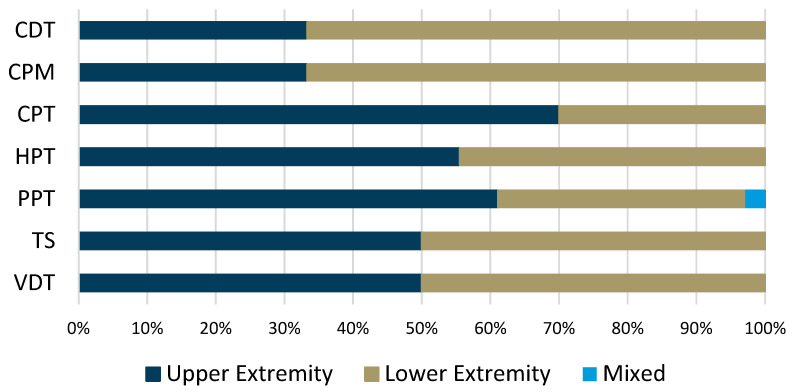
Of the 43 included studies, 39 (90.7%) used some form of physical testing measure to detect sensitization (Table 2). The proportion of physical testing modality use based on the tendon location can be seen in Figure 3. Four testing modalities were used by at least 20% of studies: pressure pain thresholds (PPT), cold pain thresholds (CPT), heat pain thresholds (HPT), and CPM. PPT were most commonly assessed, performed in 35 of the studies using physical testing (89.7%), and were employed in 22 upper extremity tendon studies compared to 13 lower extremity tendon studies. Twelve of the thirty-five studies (34.3%) examining PPT did not perform bilateral testing to include a remote site. Of the 12 studies, 8 examined upper extremity tendinopathies compared to 4 examining lower extremity tendinopathies. Five of the studies tested unilaterally and locally only (two upper extremity studies, three lower extremity studies), while seven studies tested bilaterally but not at a remote site (six upper extremity studies, one lower extremity study).
Table 2.
Number of studies utilizing specific physical examination assessments of pain, n (%).
| Tendon Studied | Dysfunction Detected |
CDT | CPM | CPT | HPT | PPT | TS | VDT |
|---|---|---|---|---|---|---|---|---|
| Total studies (n = 39) | 28 (71.9%) |
4 (10.3%) |
9 (23.1%) |
10 (25.6%) | 9 (23.1%) |
35 (89.7%) | 6 (15.4%) |
4 (10.3%) |
| Upper extremity studies (n = 22) | 18 (90%) | 2 (9.1%) | 3 (13.6%) | 7 (31.8%) | 5 (22.7%) | 22 (100%) | 3 (13.6%) | 2 (9.1%) |
| Lateral elbow (n = 16) | 15 (93.8%) | 1 (6.3%) | 2 (12.5%) | 5 (31.3%) | 4 (25%) | 16 (100%) | 2 (12.5%) | 2 (12.5%) |
| Shoulder (n = 5) | 2 (40%) | 1 (20%) | 1 (20%) | 1 (20%) | 1 (20%) | 5 (100%) | 1 (20%) | 0 (0%) |
| Mixed sample (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) |
| Lower extremity studies (n = 16) | 10 (62.5%) | 2 (12.5%) | 6 (37.5%) | 3 (18.8%) | 4 (25%) | 13 (81.3%) | 3 (18.8%) | 2 (12.5%) |
| Achilles (n = 8) | 3 (37.5%) | 0 (0%) | 4 (50%) | 0 (0%) | 1 (12.5%) | 5 (62.5%) | 1 (12.5%) | 0 (0%) |
| Adductor (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) |
| GTPS (n = 3) | 3 (100%) | 0 (0%) | 2 (66.7%) | 1 (33.3%) | 1 (33.3%) | 3 (100%) | 2 (66.7%) | 0 (0%) |
| Patellar (n = 3) | 3 (100%) | 1 (33.3%) | 0 (0%) | 1 (33.3%) | 1 (33.3%) | 3 (100%) | 0 (0%) | 1 (33.3%) |
| Mixed sample (n = 1) | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | 1 (100%) | 1 (100%) | 0 (0%) | 1 (100%) |
| Mixed studies (n = 1) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) |
Abbreviations: CDT—cold detection threshold; CPM—conditioned pain modulation; CPT—cold pressure threshold; GTPS—greater trochanteric pain syndrome; HPT—heat pressure threshold; PPT—pressure pain threshold; TS—temporal summation; VDT—vibration detection threshold.
Figure 3.
Proportion of studies using specific quantitative sensory testing instruments based on the type of tendon studied. Abbreviations: CDT—cold detection threshold; CPM—conditioned pain modulation; CPT—cold pressure threshold; HPT—heat pressure threshold; PPT—pressure pain threshold; TS—temporal summation; VDT—vibration detection threshold.
After PPT, CPT was the most commonly employed QST measure (10 studies). Testing for CPT was skewed towards upper extremity studies (n = 7, 70%), while only three lower extremity studies (30%) completed this assessment. Of the 10 studies, 3 (one upper extremity, two lower extremity) did not complete a bilateral assessment: 1 (upper extremity) was local only, 1 (lower extremity) was local and a remote elbow site, and 1 (lower extremity) was not clearly reported. HPT was assessed in nine studies (five upper extremity and four lower extremity). Similarly to CPT, some inconsistency was noted with unilateral versus bilateral testing. Three of the four (75%) lower extremity studies did not report a bilateral assessment, while five of the five upper extremity studies did report.
CPM was examined in nine studies. There was a skew towards being more used in lower extremity studies (n = 6, 66.7%) as compared to upper extremity studies (n = 3, 33.3%). Similar methodology and clear reporting were noted in studies using CPM.
Nervous system sensitization was detected using physical testing instruments in approximately 72% of all studies, but less frequently in the lower extremity (62.5% of the time) compared to the upper extremity (90%). When considering PPT, taken locally and/or at a remote site, 18 of 22 studies (81.2%) examining upper extremity tendons found sensitization (i.e., reduced thresholds compared to comparison groups), whereas 9 of 13 studies (69.2%) examining lower extremity tendons found sensitization. Of the 10 studies using CPT, 7 found evidence of sensitization, which included 5 of the 7 (71.4%) upper extremity studies and 2 of the 3 (66.7%) lower extremity studies. Similarly, sensitization was identified in four of five of the upper extremity studies and three of four of the lower extremity studies incorporating HPT. Dysfunction was found in six of nine (66.7%) studies using CPM; two of three (66.7%) were upper extremity studies, and four of six (66.7%) were lower extremity studies.
3.4. Self-Reported Instruments and Questionnaires Used
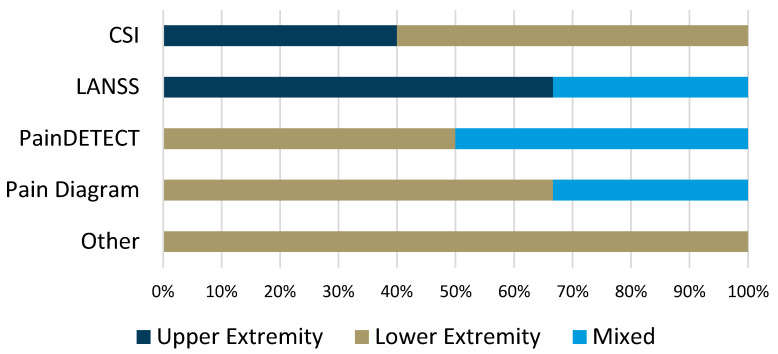
Of the included studies, a relatively small proportion (n = 11, 25.6%) used self-reported outcomes or questionnaires to detect nervous system sensitization. The most commonly used instrument was the central sensitization inventory (CSI), which was used in five studies, two (40%) examining upper extremity tendons and three (60%) examining lower extremity tendons (Table 3). The proportion of studies using questionnaires or self-reports by tendon location is presented in Figure 4. More lower extremity tendon studies (n = 7, 63.6%) compared to upper extremity tendons (n = 3, 27.3%) used questionnaires, while one study (9.1%) had a mixed population. Dysfunction was detected in seven (63.6%) of the studies using questionnaires, which often employed a cross-sectional design.
Table 3.
Number of studies utilizing patient-reported questionnaire assessments of pain, n (%).
| Tendon Studied | Dysfunction Detected |
CSI | LANSS | PainDETECT | SSS | WPI | Pain Diagram |
Other |
|---|---|---|---|---|---|---|---|---|
| Total studies (n = 11) | 7 (63.6%) | 5 (45.5%) | 3 (27.2%) | 2 (18.2%) | 0 (0%) | 0 (0%) | 3 (27.2%) | 1 (9.1%) |
| Upper extremity studies (n = 3) | 2 (66.7%) | 2 (66.7%) | 2 (66.7%) | 0 (0% | 2 (66.7%) | 2 (66.7%) | 1 (33.3%) | 0 (0%) |
| Lateral elbow (n = 2) | 1 (50%) | 2 (100%) | 2 (100%) | 0 (0%) | 2 (100%) | 2 (100%) | 0 (0%) | 0 (0%) |
| Shoulder (n = 0) | NA | NA | NA | NA | NA | NA | NA | NA |
| Mixed sample (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) |
| Lower extremity studies (n = 7) | 4 (57.1%) | 3 (42.9%) | 0 (0%) | 2 (28.6%) | 0 (0%) | 0 (0%) | 2 (28.6%) | 1 (14.3%) |
| Achilles (n = 1) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) |
| Adductor (n = 0) | NA | NA | NA | NA | NA | NA | 0 (0%) | NA |
| GTPS (n = 2) | 2 (100%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (50%) | 0 (0%) |
| Patellar (n = 2) | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (50%) |
| Mixed sample (n = 2) | 2 (100%) | 1 (50%) | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mixed studies (n = 1) | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Abbreviations: CSI—central sensitization inventory; GTPS—greater trochanteric pain syndrome; LANSS—Leeds assessment of neuropathic pain symptoms and signs; SSS—symptom severity scale; WPI—widespread pain index.
Figure 4.
Proportion of studies using self-reported outcome or questionnaire instruments according to the type of tendon studied. Abbreviations: CSI—central sensitization inventory; LANSS—Leeds assessment of neuropathic pain symptoms and signs.
3.5. Presence of Nervous System Sensitization or Dysfunction
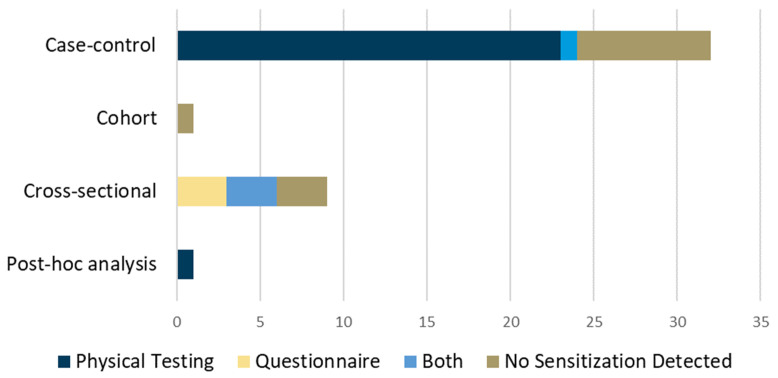
A primary intent of the included articles was to evaluate pain processing and mechanisms in persons with tendinopathy. Of the 43 included studies, 72% of studies (n = 31) detected peripheral and/or central sensitization in some capacity, while 28% (n = 12) did not. A tendon-specific breakdown of the studies detecting sensitization is presented in Figure 5. Lateral elbow tendinopathy was the most commonly studied tendon and most frequently associated with nervous system sensitization. The presence of sensitization in tendinopathy was also categorized by the study design (Figure 6).
Figure 5.
Percentage of studies detecting sensitization (left) including a breakdown of proportional tendon-specific contributions (right).
Figure 6.
Number of studies, by design, detecting sensitization in tendon pain and the type of instrument used.
4. Discussion
This scoping review sought to examine the methodological variation among studies examining pain mechanisms in tendinopathy. Based on results of the review, PPT is the most used testing modality to detect the presence of sensitization in individual with tendinopathy, questionnaire instruments were infrequently used, and participant reporting is an area requiring more specificity. Additionally, the variation in testing strategies and methodology may play a role in the conflicting results related to pain processing in patients with tendinopathy.
Our review found substantial procedural variation and a lack of clear reporting of QST protocols found across studies. Methodological rigor with the inclusion of matched controls for major demographics (e.g., age, sex, BMI) is essential to understand the sample studied, but particularly for comparing groups. Almost half of the studies comparing groups at baseline either had significant differences or did not report differences between groups. While significant between-group differences may be more relevant if studies are adequately powered for such analysis, comparing groups with different baseline characteristics makes comparison of conclusions impractical. Similarly to previous trials related to exercise for tendinopathy [72], inconsistent participant characteristic reporting was found in studies examining pain processing in tendinopathy. Only 53.5% of the studies included in this review calculated and reported BMI. Incomplete or missing participant demographics, including BMI, can limit interpretation and comparison to other studies. For example, multiple studies have identified a correlation between adiposity and pain [73,74], with one study identifying obese individuals to have more pressure pain sensitivity as compared to thermal sensitivity, indicating different testing modalities may have different results for different body morphologies [75]. Few studies (30.2%) explicitly stated that pain medication usage was not allowed. If participants were taking pain medication, their ability to perceive painful stimuli would be altered, seemingly invalidating the ability to detect differences between persons with or without a condition. Similarly, no study explicitly noted that participants with tendinopathy were excluded if they had any symptoms outside of the specific tendon of interest. While studies attempted to reduce the possibility of referred pain from the spine, studies did not clarify that participants were free of pain reports in any part of the body. This can challenge the ability to differentiate peripheral nociceptive pain from neuropathic from centrally mediated pain, particularly if other pain sites are not recorded and reported. Multi-site pain is a common complaint in persons with musculoskeletal pain and can be more common than single-site reports for many with chronic pain [76,77]. Without recognition of whether participants had pain in other areas, accurate comparisons between individuals with single-site pain or no pain become difficult.
In our review, upper extremity tendinopathies demonstrated high rates of the presence of centralized pain through QST compared to lower extremity tendinopathies, which aligns with previous studies that have compared these diagnoses [21,22]. Hypotheses for discrepancies within findings have previously highlighted differences in the unique functional demands of upper and lower extremity tendons, making direct comparison difficult [78]. While classically referred to for their recreational connotations of ‘tennis elbow’ or ‘golfer’s elbow’, elbow tendinopathy is often related to several occupations [79,80]. Musculoskeletal pain can contribute to substantial burden as it relates to vocational activities, including a considerable psychological impact [81]. Manual jobs and high physical strain at work also carry a negative prognostic relationship with lateral elbow and rotator cuff tendinopathy [82,83]. Alternatively, while patellar and Achilles tendinopathies can occur in the general population, they are frequently seen in athletes and recreationally active individuals [84,85]. Importantly, pain processing in athletes may be different than in non-athletes. For recreationally active individuals, and high-level athletes in particular, pain thresholds are often higher [86,87], and regular exercise can have a protective effect against the centralization of pain [88]. The negative impact of upper and lower extremity tendinopathy on function may contribute to the higher proportion of nervous system sensitization in the upper versus lower extremity seen in this and previous reviews [21,22].
In the studies reviewed, PPT was the most common physical testing modality used across studies. When comparing PPT of upper and lower extremity tendons, the administration of testing should be considered. Reliability and accuracy of testing require consistent testing positions. When testing PPT for tendinopathy, testing is typically conducted at the site of maximal pain. Using the Achilles tendon as an example, while it is perhaps performed but rarely reported, consistent angles of ankle dorsiflexion would be required to ensure appropriate and comparable tendon deformation given a consistent rate of application. Alternatively, a consistent resting position of elbow flexion is more easily achieved to allow exposure to the common extensor tendon, making elbow testing seemingly easier than the ankle. While a direct comparison should be considered with caution, the reliability of pressure algometry may be better for testing lateral elbow versus patellar tendinopathy [89,90]. A lack of standardized testing positions for different tendons likely contributes to the variability seen among the results of the pain assessment studies. The lack of consistency across studies seen in this review (e.g., lack of remote site algometry assessment, inconsistent bilateral thermal assessment) makes comparisons between upper and lower extremity tendinopathy sensitization challenging. Also, if a local site is solely tested, the presence of secondary hyperalgesia becomes difficult to detect, limiting the ability to accurately differentiate peripheral versus central sensitization [14]. It is also important to recognize different QST measures assess unique characteristics of pain processing and inhibition. For example, CPM assesses endogenous pain inhibition, TS can identify enhanced facilitation, while thermal and PPT findings may represent peripheral, central, or a mixed presentation of sensitization. However, normative QST data for the tendinopathy population does not yet exist, which would seemingly make standard and consistent testing essential. Recognizing the purpose of each tool and understanding the available evidence for QST findings in the tendinopathy population is helpful in applying appropriate clinical assessment and management strategies.
Questionnaires have been developed with the proposed purpose of identifying neuropathic and centrally mediated pain syndromes. These questionnaires can be useful to identify phenotypic profiles in a large sample of individuals and do so rapidly with low investigator and participant burden [91]. However, the psychometric properties of commonly used questionnaires have been called into question [92], and in some cases may represent psychological constructs more so than nociceptive function [93]. Also, while researchers may have provided specific instructions to participants completing the questionnaires, the instruments have not been previously validated in the tendinopathy population, making their utility unclear. This review found that when utilized, questionnaires were typically a part of cross-sectional study designs. Sensitization was detected with questionnaires in two-thirds of the cross-sectional studies, as compared to one of the case–control designs. Without a comparison sample of persons without the condition, or even persons with the condition and multiple pain sites, using these instruments with reportedly questionable psychometric properties warrants additional consideration.
Previous studies have examined pain mechanisms involved in tendinopathy [21,22]. This scoping review is unique to previous studies in that the methodological approaches across studies evaluating pain mechanisms in tendinopathy have not been examined. As noted within this review, participant characteristics were not clearly identified to appropriately determine their baseline and ability to report pain (e.g., resting intensity, pain sites, medication usage). Similarly, the mode of testing should be examined as it relates to pain mechanism conclusions. While PPT was commonly used, it may be a less effective modality in some populations, and tendon pain may be reported differently depending on the assessment used and tendon tested. While contemporary pain science research has positively impacted the quality of care delivered to individuals with musculoskeletal pain, much remains unknown. Physiologic and psychologic changes may create a pain mechanistic network or signature of sorts, particularly in the chronic pain population [94]. Identifying the variables and mechanisms associated with a pain condition can be useful in selecting appropriate treatment strategies. Using manual therapy as an example, there is moderate-quality evidence supporting effectiveness in improving mechanical hyperalgesia, whereas low-quality evidence supports its use for improving temporal summation or CPM [95]. These findings suggest certain interventions may have an effect on a specific aspect of pain, and pain detection strategies may need to change based on the mechanism involved. As additional findings regarding pain processing and inhibition in tendinopathy are presented, clinicians may be able to provide more precise management approaches.
Despite its novelty and potential benefit in guiding future research, this scoping review does carry a number of limitations. With all systematic, scoping, and narrative reviews, it is possible relevant works were not retained for inclusion. The search was conducted with a research librarian, using multiple electronic databases and a rigorous blinded screening process; however, recommendations and synthesized results may change with additional studies. Some may question the absence of study quality assessment. The primary focus of this review was to examine the variability of methods across studies, and therefore quality assessment of the included studies was not undertaken. Additionally, while variation in methodology and reporting may affect outcomes and conclusions, no explanatory research analysis was performed as part of this study. The quantitative impact of methodology on tendinopathy pain research is unclear.
Given the suboptimal outcomes in up to half of the individuals with tendinopathy [7,12], more research is needed. This scoping review highlights key areas of opportunity for developing stronger research methodology related to tendon pain. Differences across studies based on testing modality, testing methodology, and participant demographics may influence the conclusions drawn and capacity to make comparative statements across upper and lower extremity tendinopathies. Future study design and methodology should be guided by ICON consensus statements and reported thoroughly for reproducibility. Exploration of methodological standardization and instrument validation in the tendinopathy population should be considered. Studies using QST could follow previously established protocols [96]. Ideally, samples should be systematically and specifically described, adequately powered, and data appropriately analyzed. Statistically significant differences should be scrutinized for biologic and clinical relevance. In doing so, accurate conclusions can be made related to the predominant pain mechanism(s) involved in various tendinopathies, in turn allowing for more thorough understanding and targeted intervention strategies. With improved methodology in tendon pain research, clinicians can more confidently use evidence conclusions to effectively manage patients with tendinopathy, a condition often associated with protracted and incomplete recovery. If clear conclusions made from consistent methodology identify impairments or dysfunction (or lack thereof) in pain processing, treatment may be inclusive of interventions to address the appropriate mechanisms and pathways.
5. Conclusions
Musculoskeletal pain, including tendinopathy, is complex and multifactorial. Numerous instruments have been used to detect pain processing mechanisms clinically and in research settings. Tendon pain, while typically presenting as a focal location of symptoms, can be associated with altered central pain processing potentially contributing to poor long-term outcomes. Based on this scoping review, there appears to be variance in the methodology used to detect pain processing in studies evaluating upper and lower extremity tendinopathies. Consistent and thorough participant reporting and standardized procedures may minimize methodological variation to allow researchers and clinicians to draw appropriate conclusions across studies.
Acknowledgments
The authors would like to acknowledge Ruth Chimenti for her guidance and content expertise during the conceptualization and development of this project.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13247592/s1, File S1: Search String and Results; File S2: Data Extraction.
Author Contributions
Conceptualization, D.J., A.P., J.S. and J.M.; methodology, D.J., A.P., J.S. and J.M.; software, D.J.; validation, D.J., A.P., J.S. and J.M.; formal analysis, D.J., A.P., J.S. and J.M.; investigation, D.J., A.P., J.S. and J.M.; resources, D.J.; data curation, D.J.; writing—original draft preparation, D.J., A.P., J.S. and J.M.; writing—review and editing, D.J., A.P., J.S. and J.M.; visualization, D.J.; supervision, D.J.; project administration, D.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was deemed exempt for formal institutional review due to the study design using previously published data.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are provided within the manuscript and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Millar N.L., Silbernagel K.G., Thorborg K., Kirwan P.D., Galatz L.M., Abrams G.D., Murrell G.A.C., McInnes I.B., Rodeo S.A. Tendinopathy. Nat. Rev. Dis. Primers. 2021;7:1. doi: 10.1038/s41572-020-00234-1. [DOI] [PubMed] [Google Scholar]
- 2.Ostör A.J.K., Richards C.A., Prevost A.T., Speed C.A., Hazleman B.L. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology. 2005;44:800–805. doi: 10.1093/rheumatology/keh598. [DOI] [PubMed] [Google Scholar]
- 3.Degen R.M., Conti M.S., Camp C.L., Altchek D.W., Dines J.S., Werner B.C. Epidemiology and Disease Burden of Lateral Epicondylitis in the USA: Analysis of 85,318 Patients. HSS J. 2018;14:9–14. doi: 10.1007/s11420-017-9559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riel H., Lindstrom C.F., Rathleff M.S., Jensen M.B., Olesen J.L. Prevalence and incidence rate of lower-extremity tendinopathies in a Danish general practice: A registry-based study. BMC Musculoskelet. Disord. 2019;20:239. doi: 10.1186/s12891-019-2629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albers I.S., Zwerver J., Diercks R.L., Dekker J.H., Van den Akker-Scheek I. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: A cross sectional study. BMC Musculoskelet. Disord. 2016;17:16. doi: 10.1186/s12891-016-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook J.L., Purdam C.R. The challenge of managing tendinopathy in competing athletes. Br. J. Sports Med. 2014;48:506–509. doi: 10.1136/bjsports-2012-092078. [DOI] [PubMed] [Google Scholar]
- 7.Kettunen J.A., Kvist M., Alanen E., Kujala U.M. Long-term prognosis for jumper’s knee in male athletes. A prospective follow-up study. Am. J. Sports Med. 2002;30:689–692. doi: 10.1177/03635465020300051001. [DOI] [PubMed] [Google Scholar]
- 8.Gajhede-Knudsen M., Ekstrand J., Magnusson H., Maffulli N. Recurrence of Achilles tendon injuries in elite male football players is more common after early return to play: An 11-year follow-up of the UEFA Champions League injury study. Br. J. Sports Med. 2013;47:763–768. doi: 10.1136/bjsports-2013-092271. [DOI] [PubMed] [Google Scholar]
- 9.Bicket L., Cooke J., Knott I., Fearon A. The natural history of greater trochanteric pain syndrome: An 11-year follow-up study. BMC Musculoskelet. Disord. 2021;22:1048. doi: 10.1186/s12891-021-04935-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binder A.I., Hazleman B.L. Lateral humeral epicondylitis--a study of natural history and the effect of conservative therapy. Br. J. Rheumatol. 1983;22:73–76. doi: 10.1093/rheumatology/22.2.73. [DOI] [PubMed] [Google Scholar]
- 11.Bot S.D., van der Waal J.M., Terwee C.B., van der Windt D.A., Bouter L.M., Dekker J. Course and prognosis of elbow complaints: A cohort study in general practice. Ann. Rheum. Dis. 2005;64:1331–1336. doi: 10.1136/ard.2004.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Windt D.A., Koes B.W., Boeke A.J., Devillé W., De Jong B.A., Bouter L.M. Shoulder disorders in general practice: Prognostic indicators of outcome. Br. J. Gen. Pract. 1996;46:519–523. [PMC free article] [PubMed] [Google Scholar]
- 13.Vardeh D., Mannion R.J., Woolf C.J. Toward a Mechanism-Based Approach to Pain Diagnosis. J. Pain. 2016;17((Suppl. S9)):50. doi: 10.1016/j.jpain.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latremoliere A., Woolf C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Association for the Study of Pain IASP Terminology. [(accessed on 11 November 2024)]. Available online: https://www.iasp-pain.org/resources/terminology/
- 16.Chimenti R.L., Frey-Law L.A., Sluka K.A. A Mechanism-Based Approach to Physical Therapist Management of Pain. Phys. Ther. 2018;98:302–314. doi: 10.1093/ptj/pzy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coombes B.K., Bisset L., Vicenzino B. Management of Lateral Elbow Tendinopathy: One Size Does Not Fit All. J. Orthop. Sports Phys. Ther. 2015;45:938–949. doi: 10.2519/jospt.2015.5841. [DOI] [PubMed] [Google Scholar]
- 18.Roh Y.H., Gong H.S., Baek G.H. The Prognostic Value of Pain Sensitization in Patients with Lateral Epicondylitis. J. Hand Surg. Am. 2019;44:250.e1–250.e7. doi: 10.1016/j.jhsa.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Littlewood C., May S., Walters S. Epidemiology of rotator cuff tendinopathy: A systematic review. Shoulder Elbow. 2013;5:256–265. doi: 10.1111/sae.12028. [DOI] [Google Scholar]
- 20.Gwilym S.E., Oag H.C.L., Tracey I., Carr A.J. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J. Bone Jt. Surg. Br. 2011;93:498–502. doi: 10.1302/0301-620X.93B4.25054. [DOI] [PubMed] [Google Scholar]
- 21.Rio E., Sandler J., Cheng K., Moseley G.L., Cook J., Girdwood M. Sensory Processing in People with and Without Tendinopathy: A Systematic Review with Meta-analysis of Local, Regional, and Remote Sites in Upper- and Lower-Limb Conditions. J. Orthop. Sports Phys. Ther. 2021;51:12–26. doi: 10.2519/jospt.2021.9417. [DOI] [PubMed] [Google Scholar]
- 22.Plinsinga M.L., Brink M.S., Vicenzino B., van Wilgen C.P. Evidence of Nervous System Sensitization in Commonly Presenting and Persistent Painful Tendinopathies: A Systematic Review. J. Orthop. Sports Phys. Ther. 2015;45:864–875. doi: 10.2519/jospt.2015.5895. [DOI] [PubMed] [Google Scholar]
- 23.Scott A., Squier K., Alfredson H., Bahr R., Cook J.L., Coombes B., de Vos R.-J., Fu S.N., Grimaldi A., Lewis J.S., et al. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br. J. Sports Med. 2020;54:260–262. doi: 10.1136/bjsports-2019-100885. [DOI] [PubMed] [Google Scholar]
- 24.Rio E.K., Auliffe S.M., Kuipers I., Girdwood M., Alfredson H., Bahr R., Cook J.L., Coombes B., Fu S.N., Grimaldi A., et al. ICON PART-T 2019-International Scientific Tendinopathy Symposium Consensus: Recommended standards for reporting participant characteristics in tendinopathy research (PART-T) Br. J. Sports Med. 2020;54:627–630. doi: 10.1136/bjsports-2019-100957. [DOI] [PubMed] [Google Scholar]
- 25.Vicenzino B., de Vos R.-J., Alfredson H., Bahr R., Cook J.L., Coombes B.K., Fu S.N., Silbernagel K.G., Grimaldi A., Lewis J.S., et al. ICON 2019-International Scientific Tendinopathy Symposium Consensus: There are nine core health-related domains for tendinopathy (CORE DOMAINS): Delphi study of healthcare professionals and patients. Br. J. Sports Med. 2020;54:444–451. doi: 10.1136/bjsports-2019-100894. [DOI] [PubMed] [Google Scholar]
- 26.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littlewood C., Moffatt M., Maher N., Irving G. Current and future advances in practice: Tendinopathies of the shoulder. Rheumatol. Adv. Pract. 2023;7:rkad086. doi: 10.1093/rap/rkad086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speers C.J., Bhogal G.S. Greater trochanteric pain syndrome: A review of diagnosis and management in general practice. Br. J. Gen. Pract. 2017;67:479–480. doi: 10.3399/bjgp17X693041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alburquerque-Sendín F., Camargo P.R., Vieira A., Salvini T.F. Bilateral myofascial trigger points and pressure pain thresholds in the shoulder muscles in patients with unilateral shoulder impingement syndrome: A blinded, controlled study. Clin. J. Pain. 2013;29:478–486. doi: 10.1097/AJP.0b013e3182652d65. [DOI] [PubMed] [Google Scholar]
- 30.Bisset L., Carty M., Smith A. Unilateral Lateral Epicondylalgia Shows a Pro-nociceptive Pain Profile: A Case-control Observational Study. Clin. J. Pain. 2018;34:954–959. doi: 10.1097/AJP.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 31.Coombes B.K., Bisset L., Vicenzino B. Thermal hyperalgesia distinguishes those with severe pain and disability in unilateral lateral epicondylalgia. Clin. J. Pain. 2012;28:595–601. doi: 10.1097/AJP.0b013e31823dd333. [DOI] [PubMed] [Google Scholar]
- 32.Drew M.K., Lovell G., Palsson T.S., Chiarelli P.E., Osmotherly P.G. Do Australian Football players have sensitive groins? Players with current groin pain exhibit mechanical hyperalgesia of the adductor tendon. J. Sci. Med. Sport. 2016;19:784–788. doi: 10.1016/j.jsams.2015.12.516. [DOI] [PubMed] [Google Scholar]
- 33.Eckenrode B.J., Kietrys D.M., Stackhouse S.K. Pain Sensitivity in Chronic Achilles Tendinopathy. Int. J. Sports Phys. Ther. 2019;14:945–956. doi: 10.26603/ijspt20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez-Carnero J., Fernandez-de-las-Penas C., de la Llave-Rincon A.I., Ge H.Y., Arendt-Nielsen L. Bilateral myofascial trigger points in the forearm muscles in patients with chronic unilateral lateral epicondylalgia: A blinded, controlled study. Clin. J. Pain. 2008;24:802–807. doi: 10.1097/AJP.0b013e31817bcb79. [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Carnero J., Fernández-de-las-Peñas C., Sterling M., Souvlis T., Arendt-Nielsen L., Vicenzino B. Exploration of the extent of somato-sensory impairment in patients with unilateral lateral epicondylalgia. J. Pain. 2009;10:1179–1185. doi: 10.1016/j.jpain.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Carnero J., Fernandez-de-Las-Penas C., de la Llave-Rincon A.I., Ge H.Y., Arendt-Nielsen L. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: A blinded, controlled study. Clin. J. Pain. 2009;25:555–561. doi: 10.1097/AJP.0b013e3181a68a040. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Carnero J., Fernandez-de-Las-Penas C., de la Llave-Rincon A.I., Ge H.Y., Arendt-Nielsen L. Prevalence of and referred pain from myofascial trigger points in the forearm muscles in patients with lateral epicondylalgia. Clin. J. Pain. 2007;23:353–360. doi: 10.1097/AJP.0b013e31803b3785. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-de-Las-Penas C., Ortega-Santiago R., Ambite-Quesada S., Jimenez-Garci A.R., Arroyo-Morales M., Cleland J.A. Specific mechanical pain hypersensitivity over peripheral nerve trunks in women with either unilateral epicondylalgia or carpal tunnel syndrome. J. Orthop. Sports Phys. Ther. 2010;40:751–760. doi: 10.2519/jospt.2010.3331. [DOI] [PubMed] [Google Scholar]
- 39.French H.P., Jong C.C., McCallan M. Do features of central sensitisation exist in Greater Trochanteric Pain Syndrome (GTPS)? A case control study. Musculoskelet. Sci. Pract. 2019;43:6–11. doi: 10.1016/j.msksp.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Garnevall B., Rabey M., Edman G. Psychosocial and personality factors and physical measures in lateral epicondylalgia reveal two groups of “tennis elbow” patients, requiring different management. Scand. J. Pain. 2013;4:155–162. doi: 10.1016/j.sjpain.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Haik M.N., Evans K., Smith A., Henríquez L., Bisset L. People with musculoskeletal shoulder pain demonstrate no signs of altered pain processing. Musculoskelet. Sci. Pract. 2019;39:32–38. doi: 10.1016/j.msksp.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Hidalgo-Lozano A., Fernández-de-las-Peñas C., Alonso-Blanco C., Ge H., Arendt-Nielsen L., Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: A blinded, controlled study. Exp. Brain Res. 2010;202:915–925. doi: 10.1007/s00221-010-2196-4. [DOI] [PubMed] [Google Scholar]
- 43.Jespersen A., Amris K., Graven-Nielsen T., Arendt-Nielsen L., Bartels E.M., Torp-Pedersen S., Bliddal H., Danneskiold-Samsoe B. Assessment of pressure-pain thresholds and central sensitization of pain in lateral epicondylalgia. Pain Med. 2013;14:297–304. doi: 10.1111/pme.12021. [DOI] [PubMed] [Google Scholar]
- 44.Kregel J., van Wilgen C.P., Zwerver J. Pain assessment in patellar tendinopathy using pain pressure threshold algometry: An observational study. Pain Med. 2013;14:1769–1775. doi: 10.1111/pme.12178. [DOI] [PubMed] [Google Scholar]
- 45.Lim E.C., Sterling M., Pedler A., Coombes B.K., Vicenzino B. Evidence of spinal cord hyperexcitability as measured with nociceptive flexion reflex (NFR) threshold in chronic lateral epicondylalgia with or without a positive neurodynamic test. J. Pain. 2012;13:676–684. doi: 10.1016/j.jpain.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Lim E.C.W., Sterling M., Vicenzino B. Chronic Lateral Epicondylalgia Does Not Exhibit Mechanical Pain Modulation in Response to Noxious Conditioning Heat Stimulus. Clin. J. Pain. 2017;33:932–938. doi: 10.1097/AJP.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 47.Mkumbuzi N.S., Mafu T.S., September A.V., Posthumus M., Collins M. Conditioned pain modulation is not altered in recreational athletes with Achilles tendinopathy. Transl. Sports Med. 2021;4:147–153. doi: 10.1002/tsm2.201. [DOI] [Google Scholar]
- 48.Paul T.M., Soo Hoo J., Chae J., Wilson R.D. Central hypersensitivity in patients with subacromial impingement syndrome. Arch. Phys. Med. Rehabil. 2012;93:2206–2209. doi: 10.1016/j.apmr.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plinsinga M.L., Meeus M., Brink M., Heugen N., van Wilgen P. Evidence of Widespread Mechanical Hyperalgesia but Not Exercise-Induced Analgesia in Athletes with Mild Patellar Tendinopathy Compared with Pain-Free Matched Controls: A Blinded Exploratory Study. Am. J. Phys. Med. Rehabil. 2021;100:946–951. doi: 10.1097/PHM.0000000000001673. [DOI] [PubMed] [Google Scholar]
- 50.Plinsinga M.L., Coombes B.K., Mellor R., Vicenzino B. Individuals with Persistent Greater Trochanteric Pain Syndrome Exhibit Impaired Pain Modulation, as well as Poorer Physical and Psychological Health, Compared with Pain-Free Individuals: A Cross-Sectional Study. Pain Med. 2020;21:2964–2974. doi: 10.1093/pm/pnaa047. [DOI] [PubMed] [Google Scholar]
- 51.Plinsinga M.L., van Wilgen C.P., Brink M.S., Vuvan V., Stephenson A., Heales L.J., Mellor R., Coombes B.K., Vicenzino B.T. Patellar and Achilles tendinopathies are predominantly peripheral pain states: A blinded case control study of somatosensory and psychological profiles. Br. J. Sports Med. 2018;52:284–291. doi: 10.1136/bjsports-2016-097163. [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro I.L., Camargo P.R., Alburquerque-Sendín F., Madeleine P., Fernández-de-Las-Peñas C., Salvini T.F. Topographical pressure pain sensitivity maps of the shoulder region in individuals with sub-acromial pain syndrome. Man. Ther. 2015;20:20. doi: 10.1016/j.math.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Rio E.K., Ellis R.F., Henry J.M., Falconer V.R., Kiss Z.S., Girdwood M.A., Cook J.L., Gaida J.E. Don’t Assume the Control Group Is Normal-People with Asymptomatic Tendon Pathology Have Higher Pressure Pain Thresholds. Pain Med. 2018;19:2267–2273. doi: 10.1093/pm/pny117. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-Ruiz B., Fernandez-de-Las-Penas C., Ortega-Santiago R., Arendt-Nielsen L., Madeleine P. Topographical pressure and thermal pain sensitivity mapping in patients with unilateral lateral epicondylalgia. J. Pain. 2011;12:1040–1048. doi: 10.1016/j.jpain.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Sara L.K., Gutsch S.B., Bement M.H., Hunter S.K. Plantar Flexor Weakness and Pain Sensitivity Cannot Be Assumed in Midportion Achilles Tendinopathy. Exerc. Sport Mov. 2023;1:1–7. doi: 10.1249/ESM.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skinner I.W., Debenham J.R., Krumenachera S., Bulsarab M.K., Wand B.M. Chronic mid portion Achilles tendinopathy is not associated with central sensitisation. Pain Rehabil. J. Physiother. Pain Assoc. 2014;2014:34–40. [Google Scholar]
- 57.Slater H., Arendt-Nielsen L., Wright A., Graven-Nielsen T. Sensory and motor effects of experimental muscle pain in patients with lateral epicondylalgia and controls with delayed onset muscle soreness. Pain. 2005;114:118–130. doi: 10.1016/j.pain.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Tompra N., Van Dieën J.H., Coppieters M.W. Central pain processing is altered in people with Achilles tendinopathy. Br. J. Sports Med. 2016;50:1004–1007. doi: 10.1136/bjsports-2015-095476. [DOI] [PubMed] [Google Scholar]
- 59.Vallance P., Crowley L., Vicenzino B., Malliaras P. Contralateral mechanical hyperalgesia and altered pain modulation in men who have unilateral insertional Achilles tendinopathy: A cross-sectional study. Musculoskelet. Sci. Pract. 2021;52:102353. doi: 10.1016/j.msksp.2021.102353. [DOI] [PubMed] [Google Scholar]
- 60.Wilgen C.P., Konopka K.H., Keizer D., Zwerver J., Dekker R. Do patients with chronic patellar tendinopathy have an altered somatosensory profile?—A Quantitative Sensory Testing (QST) study. Scand. J. Med. Sci. Sports. 2013;23:149–155. doi: 10.1111/j.1600-0838.2011.01375.x. [DOI] [PubMed] [Google Scholar]
- 61.Cancela-Cilleruelo I., Rodríguez-Jiménez J., Fernández-de-Las-Peñas C., Arendt-Nielsen L., Arias-Buría J.L. Sensitization-associated and neuropathic-associated symptoms in patients with unilateral lateral elbow tendinopathy: An exploratory study. Physiother. Theory Pract. 2024;40:2522–2529. doi: 10.1080/09593985.2023.2264384. [DOI] [PubMed] [Google Scholar]
- 62.Corrigan P., Hornsby S., Pohlig R.T., Willy R.W., Cortes D.H., Silbernagel K.G. Tendon loading in runners with Achilles tendinopathy: Relations to pain, structure, and function during return-to-sport. Scand. J. Med. Sci. Sports. 2022;32:1201–1212. doi: 10.1111/sms.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrer-Peña R., Muñoz-Garcıa D., Calvo-Lobo C., Fernandez-Carnero J. Pain expansion and severity reflect central sensitization in primary care patients with greater trochanteric pain syndrome. Pain Med. 2019;20:961–970. doi: 10.1093/pm/pny199. [DOI] [PubMed] [Google Scholar]
- 64.Palaniswamy V., Ng S.-K., Manickaraj N., Ryan M., Yelland M., Rabago D., Bisset L. Relationship between ultrasound detected tendon abnormalities, and sensory and clinical characteristics in people with chronic lateral epicondylalgia. PLoS ONE. 2018;13:e0205171. doi: 10.1371/journal.pone.0205171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pienimaki T., Siira P., Vanharanta H. Widespread pain in chronic epicondylitis. Eur. J. Pain. 2011;15:921–927. doi: 10.1016/j.ejpain.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Rio E.K., Rabusin C.L., Munteanu S.E., Docking S.I., Perrott M., Couch J., Murphy M.C., Girdwood M. Where is Your Pain? Achilles Tendinopathy Pain Location on Loading Is Different to Palpation, Imaging and Recall Location. J. Orthop. Sports Phys. Ther. 2024;54:86–94. doi: 10.2519/jospt.2023.12131. [DOI] [PubMed] [Google Scholar]
- 67.Wheeler P.C. Nearly half of patients with chronic tendinopathy may have a neuropathic pain component, with significant differences seen between different tendon sites: A prospective cohort of more than 300 patients. BMJ Open Sport Exerc. Med. 2022;8:e001297. doi: 10.1136/bmjsem-2021-001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wheeler P.C. Up to a quarter of patients with certain chronic recalcitrant tendinopathies may have central sensitisation: A prospective cohort of more than 300 patients. Br. J. Pain. 2019;13:137–144. doi: 10.1177/2049463718800352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wheeler P.C. Neuropathic pain may be common in chronic lower limb tendinopathy: A prospective cohort study. Br. J. Pain. 2017;11:16–22. doi: 10.1177/2049463716680560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy M.C., Rio E.K., Chivers P., Debenham J., Docking S.I., Travers M., Gibson W. Do people with unilateral mid-portion Achilles tendinopathy who participate in running-related physical activity exhibit a meaningful conditioned pain modulation (CPM) effect: A pilot study. J. Sci. Med. Sport. 2021;24:441–447. doi: 10.1016/j.jsams.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 71.Coombes B.K., Bisset L., Vicenzino B. Cold hyperalgesia associated with poorer prognosis in lateral epicondylalgia: A 1-year prognostic study of physical and psychological factors. Clin. J. Pain. 2015;31:30–35. doi: 10.1097/AJP.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 72.Auliffe S.M., Korakakis V., Hilfiker R., Whiteley R., O’Sullivan K. Participant characteristics are poorly reported in exercise trials in tendinopathy: A systematic review. Phys. Ther. Sport. 2021;48:43–53. doi: 10.1016/j.ptsp.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Stone A.A., Broderick J.E. Obesity and pain are associated in the United States. Obesity (Silver Spring) 2012;20:1491–1495. doi: 10.1038/oby.2011.397. [DOI] [PubMed] [Google Scholar]
- 74.Walsh T.P., Arnold J.B., Evans A.M., Yaxley A., Damarell R.A., Shanahan E.M. The association between body fat and musculoskeletal pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2018;19:233. doi: 10.1186/s12891-018-2137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tashani O.A., Astita R., Sharp D., Johnson M.I. Body mass index and distribution of body fat can influence sensory detection and pain sensitivity. Eur. J. Pain. 2017;21:1186–1196. doi: 10.1002/ejp.1019. [DOI] [PubMed] [Google Scholar]
- 76.Kamaleri Y., Natvig B., Ihlebaek C.M., Bruusgaard D. Localized or widespread musculoskeletal pain: Does it matter? Pain. 2008;138:41–46. doi: 10.1016/j.pain.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Carnes D., Parsons S., Ashby D., Breen A., Foster N.E., Pincus T., Vogel S., Underwood M. Chronic musculoskeletal pain rarely presents in a single body site: Results from a UK population study. Rheumatology. 2007;46:1168–1170. doi: 10.1093/rheumatology/kem118. [DOI] [PubMed] [Google Scholar]
- 78.Mc Auliffe S., Whiteley R., Malliaras P., O’Sullivan K. Central sensitisation in different tendinopathies: Are we comparing apples and oranges? Br. J. Sports Med. 2019;53:142–143. doi: 10.1136/bjsports-2017-098863. [DOI] [PubMed] [Google Scholar]
- 79.Sanders T.L., Jr., Maradit Kremers H., Bryan A.J., Ransom J.E., Smith J., Morrey B.F. The epidemiology and health care burden of tennis elbow: A population-based study. Am. J. Sports Med. 2015;43:1066–1071. doi: 10.1177/0363546514568087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker-Bone K., Palmer K.T., Reading I., Coggon D., Cooper C. Occupation and epicondylitis: A population-based study. Rheumatology. 2011;51:305–310. doi: 10.1093/rheumatology/ker228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buck R., Wynne-Jones G., Varnava A., Main C.J., Phillips C.J. Working with Musculoskeletal Pain. Rev. Pain. 2009;3:6–10. doi: 10.1177/204946370900300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haahr J.P., Andersen J.H. Prognostic factors in lateral epicondylitis: A randomized trial with one-year follow-up in 266 new cases treated with minimal occupational intervention or the usual approach in general practice. Rheumatology. 2003;42:1216–1225. doi: 10.1093/rheumatology/keg360. [DOI] [PubMed] [Google Scholar]
- 83.Herberts P., Kadefors R., Högfors C., Sigholm G. Shoulder pain and heavy manual labor. Clin. Orthop. Relat. Res. 1984:166–178. doi: 10.1097/00003086-198412000-00022. [DOI] [PubMed] [Google Scholar]
- 84.Colbert L.H., Hootman J.M., Macera C.A. Physical activity-related injuries in walkers and runners in the aerobics center longitudinal study. Clin. J. Sport Med. 2000;10:259–263. doi: 10.1097/00042752-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Malliaras P., Cook J., Purdam C., Rio E. Patellar Tendinopathy: Clinical Diagnosis, Load Management, and Advice for Challenging Case Presentations. J. Orthop. Sports Phys. Ther. 2015;45:887–898. doi: 10.2519/jospt.2015.5987. [DOI] [PubMed] [Google Scholar]
- 86.Tesarz J., Schuster A.K., Hartmann M., Gerhardt A., Eich W. Pain perception in athletes compared to normally active controls: A systematic review with meta-analysis. Pain. 2012;153:1253–1262. doi: 10.1016/j.pain.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Pettersen S.D., Aslaksen P.M., Pettersen S.A. Pain Processing in Elite and High-Level Athletes Compared to Non-athletes. Front. Psychol. 2020;11:1908. doi: 10.3389/fpsyg.2020.01908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sluka K.A., O’Donnell J.M., Danielson J., Rasmussen L.A. Regular physical activity prevents development of chronic pain and activation of central neurons. J. Appl. Physiol. 2013;114:725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heales L., Hill C.E., Kean C.O., Stanton R. Within- and between-session test-retest reliability of pressure pain threshold in individuals with lateral elbow tendinopathy. J. Sci. Med. Sport. 2021;24:S79–S80. doi: 10.1016/j.jsams.2021.09.197. [DOI] [Google Scholar]
- 90.van Wilgen P., van der Noord R., Zwerver J. Feasibility and reliability of pain pressure threshold measurements in patellar tendinopathy. J. Sci. Med. Sport. 2011;14:477–481. doi: 10.1016/j.jsams.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Attal N., Bouhassira D., Baron R. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 2018;17:456–466. doi: 10.1016/S1474-4422(18)30071-1. [DOI] [PubMed] [Google Scholar]
- 92.Mathieson S., Maher C.G., Terwee C.B., Folly de Campos T., Lin C.C. Neuropathic pain screening questionnaires have limited measurement properties. A systematic review. J. Clin. Epidemiol. 2015;68:957–966. doi: 10.1016/j.jclinepi.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 93.Adams G.R., Gandhi W., Harrison R., van Reekum C.M., Wood-Anderson D., Gilron I., Salomons T.V. Do “central sensitization” questionnaires reflect measures of nociceptive sensitization or psychological constructs? A systematic review and meta-analyses. Pain. 2023;164:1222–1239. doi: 10.1097/j.pain.0000000000002830. [DOI] [PubMed] [Google Scholar]
- 94.Giordano R., Arendt-Nielsen L., Gerra M.C., Kappel A., Østergaard S.E., Capriotti C., Dallabona C., Petersen K.K.-S. Pain mechanistic networks: The development using supervised multivariate data analysis and implications for chronic pain. Pain. 2024 doi: 10.1097/j.pain.0000000000003410. [DOI] [PubMed] [Google Scholar]
- 95.Martínez-Pozas O., Sánchez-Romero E.A., Beltran-Alacreu H., Arribas-Romano A., Cuenca-Martínez F., Villafañe J.H., Fernández-Carnero J. Effects of Orthopedic Manual Therapy on Pain Sensitization in Patients with Chronic Musculoskeletal Pain: An Umbrella Review with Meta-Meta-analysis. Am. J. Phys. Med. Rehabil. 2023;102:879–885. doi: 10.1097/PHM.0000000000002239. [DOI] [PubMed] [Google Scholar]
- 96.Rolke R., Baron R., Maier C., Tölle T.R., Treede D.R., Beyer A., Binder A., Birbaumer N., Birklein F., Bötefür I.C., et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are provided within the manuscript and in the Supplementary Materials.