Abstract
Mitochondrial biogenesis requires the expression of genes encoded by both the nuclear and mitochondrial genomes. However, aside from a handful transcription factors regulating specific subsets of mitochondrial genes, the overall architecture of the transcriptional control of mitochondrial biogenesis remains to be elucidated. The mechanisms coordinating these two genomes are largely unknown. We performed a targeted RNAi screen in developing eyes with reduced mitochondrial DNA content, anticipating a synergistic disruption of tissue development due to impaired mitochondrial biogenesis and mitochondrial DNA (mtDNA) deficiency. Among 638 transcription factors annotated in the Drosophila genome, 77 were identified as potential regulators of mitochondrial biogenesis. Utilizing published ChIP-seq data of positive hits, we constructed a regulatory network revealing the logic of the transcription regulation of mitochondrial biogenesis. Multiple transcription factors in core layers had extensive connections, collectively governing the expression of nearly all mitochondrial genes, whereas factors sitting on the top layer may respond to cellular cues to modulate mitochondrial biogenesis through the underlying network. CG1603, a core component of the network, was found to be indispensable for the expression of most nuclear mitochondrial genes, including those required for mtDNA maintenance and gene expression, thus coordinating nuclear genome and mtDNA activities in mitochondrial biogenesis. Additional genetic analyses validated YL-1, a transcription factor upstream of CG1603 in the network, as a regulator controlling CG1603 expression and mitochondrial biogenesis.
Research organism: D. melanogaster
Introduction
Mitochondria respiration, carried out by the electron transport chain (ETC) complexes, converts the energy in chemical fuels to the electrochemical potential across the mitochondrial inner membrane (Δψm) that drives the synthesis of ATP. Deficient ETC not only impairs energy metabolism, but may also disrupt cellular redox balance and various biosynthetic pathways (Shen et al., 2022; Spinelli and Haigis, 2018), and is associated with various human diseases (Gorman et al., 2016). Mitochondria are under dual genetic control. Their own genome, mitochondrial DNA (mtDNA) encodes 13 core subunits of ETC, alongside 2 rRNAs and 22 tRNAs that are required for the translation of these protein-coding genes inside the mitochondrial matrix (Chen et al., 2019).
The majority of more than 1000 mitochondrial proteins including the remaining ETC subunits, factors for mtDNA replication and transcription, and mitochondrial ribosomal proteins are all encoded on the nuclear genome (Hock and Kralli, 2009; Taylor and Turnbull, 2005). The mitochondrial transcription factor A (TFAM) compacts mtDNA into nucleoids and is a key regulator of mtDNA copy number (Alam et al., 2003; Scarpulla, 2008). TFAM, together with other auxiliary factors including mtTFB1 and mtTFB2, promotes the transcription of mtDNA by mitochondrial RNA polymerase (POLRMT) into long polycistronic precursor RNAs, which are further processed into individual RNAs (Chen et al., 2019; Falkenberg et al., 2002). POLRMT can also generate short RNA oligos, owing to its exoribonuclease activity, to prime mtDNA replication by polymerase γ (Liu et al., 2022). Nuclear-encoded mitochondrial proteins are synthesized in the cytoplasm and imported into mitochondria (Wiedemann and Pfanner, 2017). Hence, mitochondrial biogenesis is influenced by the abundance and activities of mitochondrial translocases as well. The intricate interplay between mitochondrial and nuclear-encoded components demands coordinated activities of these two genomes, to maintain the efficiency and the integrity of oxidative phosphorylation system and other critical mitochondrial processes (Hock and Kralli, 2009; Scarpulla, 2008).
Mitochondrial respiration, particularly, the contents and the activity of ETC, is fine-tuned to cope with the developmental and tissue-specific metabolic demands (Fernández-Vizarra et al., 2011). Various transcriptional cascades have emerged as effective and adaptable mechanisms regulating ETC biogenesis. The nuclear respiration factors, NRF1 and NRF2, activate the expression of many nuclear-encoded ETC subunits and genes essential for mtDNA replication and transcription (Scarpulla, 2008). This regulation allows NRFs to indirectly control the expression of mtDNA-encoded genes, and hence coordinate the activities of both genomes in ETC biogenesis. The peroxisome proliferator-activated receptors (PPARs), upon activation by diverse lipid ligands, induce the expression of nuclear genes in fatty acids oxidation pathway (Berger and Moller, 2002). Another family of nuclear receptors, the estrogen-related receptors (ERRs) regulate nuclear genes involved in oxidative phosphorylation, including ETCs and the citric acid (Deblois and Giguère, 2011). Notably, all forementioned transcription factors (TFs) share a common co-activator, PPARγ co-activator-1α (PGC-1α), that directly stimulates the basal transcriptional machinery (Finck and Kelly, 2007; Hock and Kralli, 2009; Scarpulla et al., 2012). PGC-1α and related co-activators are dynamically regulated in responses to various physiological or environmental cues, to adjust metabolic program and energy metabolism accordingly (Hock and Kralli, 2009; Scarpulla et al., 2012). Members of PPARs or ERRs families often show tissue-specific expression and regulate subsets of mitochondrial genes (Deblois and Giguère, 2011; Hock and Kralli, 2009). However, given the large number and diverse evolution origins of mitochondrial genes (Kurland and Andersson, 2000; Rath et al., 2021), the current understanding of transcriptional regulation of mitochondrial biogenesis appears incomplete. Additionally, mechanisms coordinating nuclear genome and mtDNA activities in ETC biogenesis remain unclear.
Recently, the modERN (model organism Encyclopedia of Regulatory Networks) project generated genome-wide binding profiles of a large set of TFs in Caenorhabditis elegans and Drosophila melanogaster (Kudron et al., 2018). The global mapping of TF-DNA interactions could potentially be applied to identify the transcriptional network governing mitochondrial biogenesis. However, the DNA binding profiles of TFs have their limitations in understanding the true biological functions of TFs in gene regulation (Jiang and Mortazavi, 2018). Gene expression is, in most cases, subject to the combined influences of multiple TFs. Additionally, an individual TF may have either activating or repressive roles based on the local chromatin environment (Jiang and Mortazavi, 2018). Furthermore, despite the substantial progress in bioinformatics analyses, the interpretation of genome-wide omics data still has its limitations due to a lack of robust statistical algorithms, variations in biological contexts, and intrinsic experimental variations (Angelini and Costa, 2014; Zhou et al., 2016). The integration of the DNA binding profiles with functional genetic and genomic studies is ideal to study gene expression regulations (Jiang and Mortazavi, 2018; Park, 2009).
The Drosophila eye is an excellent model for genetic analyses due to its ease of assessment and minimal impact on other physiological processes in the presence of developmental abnormalities. The cell proliferation and differentiation during eye development require robust mitochondrial respiration, and adult eyes are severely disrupted by mutations affecting nuclear ETC subunits or the mitochondrial translation apparatus (Liao et al., 2006; Owusu-Ansah et al., 2008). We previously developed a genetic scheme to generate mtDNA deficiency by expressing a mitochondrially targeted restriction enzyme (Chen et al., 2015; Xu et al., 2008). In this study, we performed an RNAi screen, targeting 638 TFs annotated in the Drosophila genome, in the presence of mtDNA deficiency in developing eyes. We recovered 77 TFs, RNAi against which had synergistic effects with the mtDNA deficiency in causing the small-eye phenotype. We further followed up on CG1603, one of the strongest hits from the initial modifier screen and revealed that it was essential for coordinating the nuclear genome and mtDNA for ETC biogenesis. Additional network analyses on the recovered hits using published DNA binding profiles illustrated potential regulatory connections and a complex hierarchy of the transcription regulations on mitochondrial biogenesis. The combination of genetic and bioinformatic analyses also facilitated the identification of YL-1 as an upstream regulator of CG1603.
Results
The design of a genetic modifier screen for genes regulating mtDNA maintenance and expression
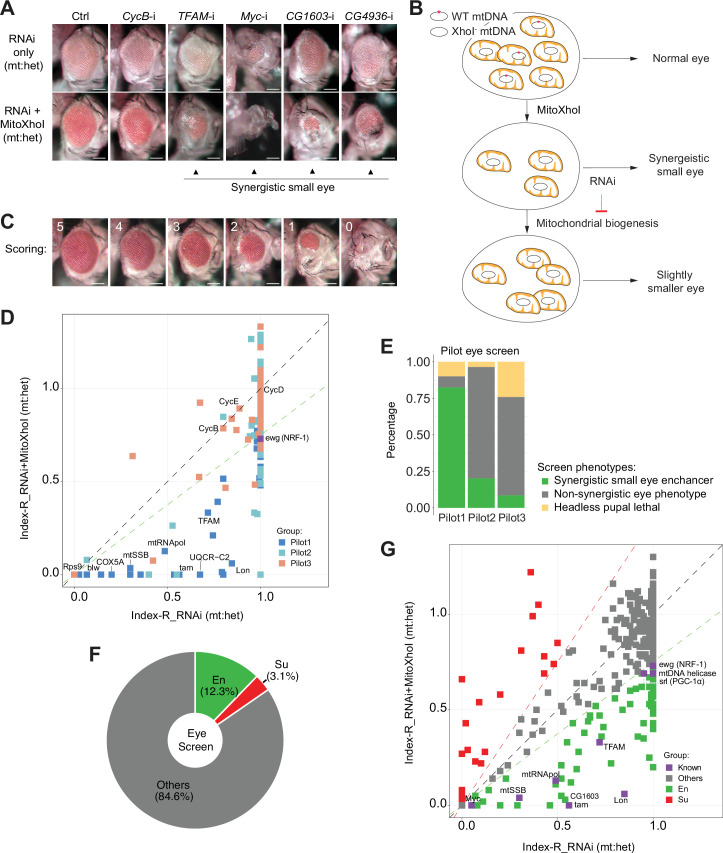
Mutations on nuclear-encoded ETC genes block cell cycle and disrupt the differentiation and morphogenesis of developing eyes (Mandal et al., 2005; Owusu-Ansah et al., 2008). RNAi against tfam or myc, which promote the expression of ETC genes encoded on mtDNA and the nuclear genome, respectively, also reduced adult eye size (Figure 1A). These observations prompted us to use fly eye as a model to identify TFs regulating ETC biogenesis. However, inhibitions of any genes essential for cell viability, proliferation, or differentiation, exemplified by RNAi targeting a mitotic cyclin, CycB (Figure 1A), would disrupt eye development. Therefore, assaying eye morphology alone is not sufficient to enrich candidates regulating ETC biogenesis.
Figure 1. A genetic modifier screen identifying transcription factors regulating electron transport chain (ETC) biogenesis.
(A) Representative images of adult eye of the control RNAi (Ctrl) and RNAi of selected genes tested in the eye screen, including CycB RNAi (CycB-i), TFAM RNAi (TFAM-i), Myc RNAi (Myc-i), CG1603 RNAi (CG1603-i), and CG4936 RNAi (CG4936-i). The upper panel shows eyes from RNAi-only offspring, and lower panel displays eyes from RNAi+MitoXhoI offspring cultured at the same condition. Arrowheads indicate the synergistic small-eye phenotype resulting from the combination of gene knockdown and the mitochondrial DNA (mtDNA) deficiency caused by mitoXhoI in the background of heteroplasmic mtDNAs. Scale bars: 100 µm. (B) Schematic of the genetic modifier screen methodology (see text for details). (C) Representative images illustrating the scoring of eye size. Scale bars: 100 µm. (D) A plot illustrating the calling of positive hits in the pilot screen. Each datapoint represents the Index-R of RNAi (X values) or RNAi+MitoXhoI flies (Y values) for each gene belonging to the different groups (see (E) and Supplementary file 1 for details). Genes with datapoints below the gray diagonal dash line exhibited a synergistic effect when combining their RNAi with mtDNA deficiency suggesting a potential role in regulating ETC biogenesis. The datapoint for ewg, the fly homolog of NRF-1, is labeled in purple. The green dashed line of slope 0.75 outlines the threshold for calling out positive hits based on ewg’s performance in the screen. (E) Graph summarizing the pilot screen of nuclear-encoded genes, demonstrating the efficacy of this screen in identifying genes involved in mitochondrial ETC biogenesis. Pilot group 1 (Pilot1) has 40 genes that are either nuclear-encoded ETC subunits or related to mtDNA maintenance and gene expression (Mito-EBR). Pilot2 has 84 genes involved in other mitochondrial processes. Pilot3 has 58 essential genes from other cellular components. (F) Graph summarizing the percentages of synergistic enhancers (En) and suppressors (Su) identified in the screen (see (G) and Supplementary file 1 for details). (G) A plot illustrating the calling of positive hits in the screen of transcription factor (TF) genes. Factors that are known to be involved in mitochondrial or ETC biogenesis are marked in purple (Known). The green dashed line outlines the threshold for calling out synergistic enhancers (En, green square). The red dashed line of slope 1.5 outlines the threshold for calling out suppressors (Su, red square).
The mitochondrial genome of wild type (wt) D. melanogaster contains a single XhoI site. The expression of a mitochondrially targeted restriction enzyme, XhoI (MitoXhoI) in Drosophila ovary effectively selects for escaper progeny carrying mtDNA mutations that abolish the XhoI site (Xu et al., 2008). In a heteroplasmic background containing both wt and XhoI-resistant genome (XhoI-), the expression of MitoXhoI can effectively remove the wt genome and hence generate mtDNA deficiency (Chen et al., 2015). As a result, the adult eyes were slightly smaller than the control (Figure 1A). Considering that mtDNA encodes core components of ETC, we reasoned that inhibiting a gene related to ETC biogenesis would have a synergistic effect with the mtDNA deficiency on eye development, and the combination of these two genetic manipulations should lead to a stronger disruption of eye development than either of these conditions individually (Figure 1A and B). On this basis, we devised a scheme of modifier screen in eye for genes involved in ETC biogenesis (Figure 1B).
The RNAi modifier screen identifying TFs regulating ETC biogenesis
To assess the efficacy of this scheme, we carried out a pilot RNAi screen, covering 124 nuclear-encoded mitochondrial genes and 58 non-mitochondrial genes annotated in various cellular processes (Figure 1C–E and Supplementary file 1). In practice, male flies carrying a UAS-IR transgene were crossed with Sco/CyO, mitoXhoI; eyeless-GAL4 heteroplasmic female flies (carrying both wt and XhoI- mtDNA). This cross generated two groups of offspring, RNAi-only and RNAi together with MitoXhoI expression (RNAi+MitoXhoI) that were cultured in the same vial, thereby minimizing any potential discrepancy caused by environmental factors. Most RNAi flies survived to adult stage but had reduced eye size. A few RNAi flies were lethal at the pupae stage, due to a lack of head capsule that is derived from the eye-antenna disc.
For most genes tested in the pilot screen, eyes of RNAi+MitoXhoI flies were smaller than the corresponding RNAi-only flies. To rule out a simple additive effect between RNAi and MitoXhoI expression, we carried out additional analyses to semi-quantify a potential synergy between RNAi intervention and mtDNA deficiency caused by MitoXhoI expression. The eye size of progeny was arbitrarily scored on a scale from 0 to 5 (Figure 1C). The indexes of eye size reduction (Index-R) of RNAi and RNAi+MitoXhoI flies were calculated by normalizing the mean eye size scores of each genotype to the corresponding values of control RNAi or control RNAi+MitoXhoI, respectively, and were subsequently plotted against each other on a linear graph (Figure 1D). If an RNAi intervention had a synergistic effect with mtDNA deficiency, it would lie below the diagonal line. We also included ewg, the fly homolog of NRF-1, in the pilot screen to set the threshold for calling out positive hits. Of total 40 genes that are related to ETC biogenesis (Mito-EBR) including ETC subunits, mitochondrial protein import and membrane insertion machinery, ETC assembly factors, and proteins related to the expression of mtDNA-encoded ETC subunits, 82.5% (33 genes) emerged as enhancers (Figure 1E). The proportions of synergistic enhancers were much lower in the group of genes involved in other mitochondrial processes (20.2%) or the group of other essential genes not related to mitochondria (8.6%), indicating the efficacy of this modifier screen in enriching genes related to ETC biogenesis (Figure 1E).
To understand transcriptional regulations of ETC biogenesis, we screened 1264 RNAi lines that cover 638 genes annotated as transcriptional regulators in the Drosophila genome. A total of 77 enhancers were identified (Figure 1F and G and Supplementary file 1), including all known factors involved in ETC biogenesis such as Myc, TFAM (Scarpulla, 2008; Wang et al., 2019). We also recovered 20 suppressors, of which, eyes of RNAi+MitoXhoI flies were larger than the corresponding RNAi-only flies.
Regulatory network of mitochondrial biogenesis
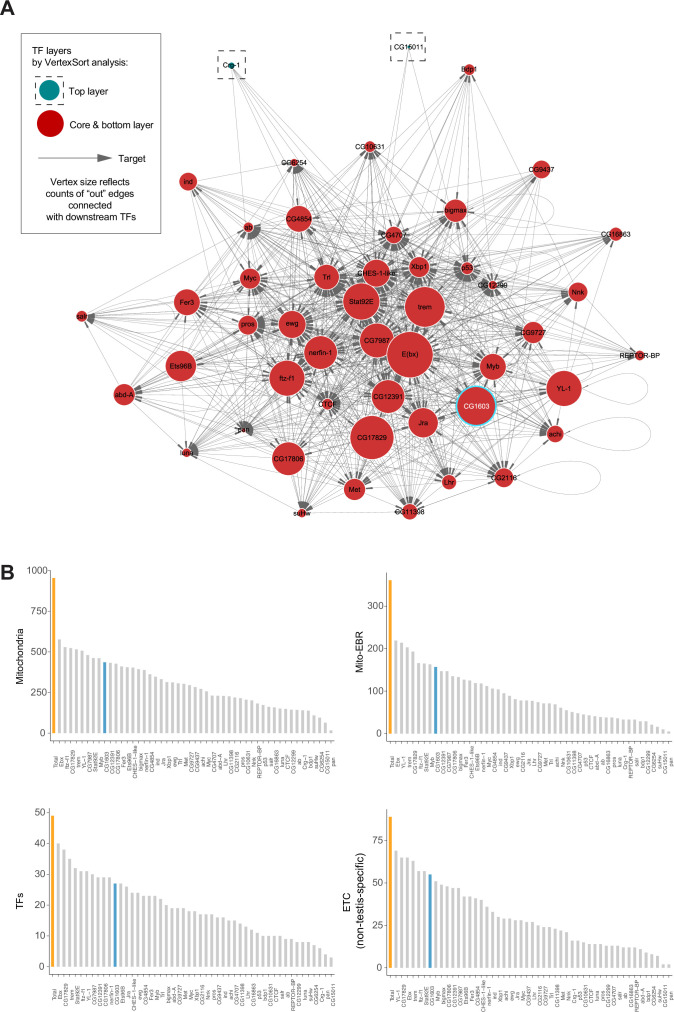
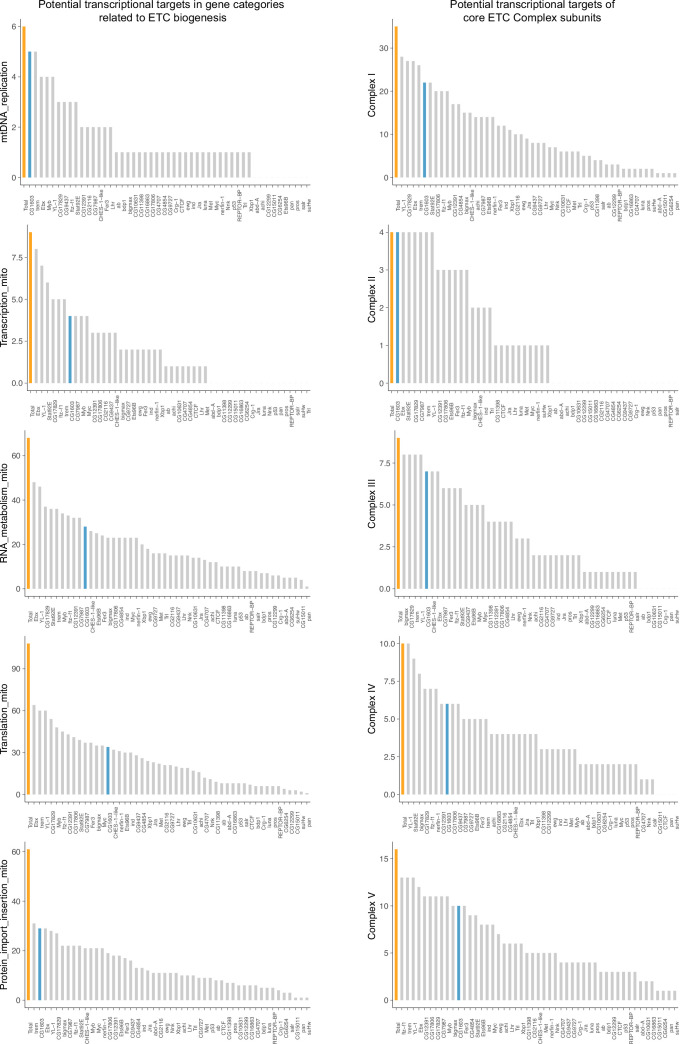
Among 77 TFs identified in the initial modifier screen, 49 TFs have ChIP-seq data available in modERN (Kudron et al., 2018). To further understand the transcriptional regulation of ETC biogenesis, we performed the network analysis on these 49 TFs using the ‘vertex sort’ algorithm (Jothi et al., 2009), and constructed a regulatory network (Figure 2A and Supplementary file 2). All 49 TFs had binding sites on the promoter region of at least one nuclear mitochondrial gene (Figure 2B, Figure 2—figure supplement 1, and Supplementary file 3). Respectively, 89% nuclear-encoded mitochondrial genes (851), including nearly all Mito-EBR genes (Supplementary file 3), were bound by at least one TF. Given that 28 hits were not included in the analyses due to a lack of ChIP-seq data, the actual coverage of total 77 TFs on nuclear mitochondrial genes would be more comprehensive. Six TFs bound to more than half of mitochondrial genes (Figure 2B, Figure 2—figure supplement 1, and Supplementary file 3). However, not a single TF covered all mitochondrial genes, or all genes in a specific mitochondrial process, which is consistent with the diverse evolution origin of mitochondrial genes (Kurland and Andersson, 2000). It also indicates that there is no such a ‘master’ regulator controlling all aspects of mitochondrial genesis. Forty-seven TFs were identified as strongly connected components due to their extensive connections and were classified in the core or bottom layer of the hierarchical structure, suggesting complex co-regulations and potential redundancy among these TFs in controlling mitochondrial biogenesis (Figure 2A and Supplementary file 2). Through this interconnected network, every single node can link to all 851 mitochondrial genes in the coverage. Two TFs, Crg-1 and CG15011, were identified as the top-layer TFs with no upstream regulators in the network (Figure 2A and Supplementary file 2). Crg-1 is a circadian- regulated gene (Rouyer et al., 1997). CG15011 is an X-box binding protein and had a binding profile similar to another X-box binding protein, Xbp1 (Supplementary file 3), a stress respondent and regulator (Acosta-Alvear et al., 2007). These top-layer TFs may sense physiological oscillations and stresses to modulate mitochondrial biogenesis through the underlying network. Additionally, YL-1 and E(bx), two components in the middle layer, are involved in chromatin remodeling (Liang et al., 2016; Wysocka et al., 2006), suggesting a potential regulation of mitochondrial biogenesis at the chromatin level.
Figure 2. Regulatory network of mitochondrial biogenesis.
(A) The transcriptional regulatory network of nuclear-encoded mitochondrial genes. (B) Bar graphs illustrating the promoter binding profiles of 49 synergistic enhancer transcription factors (TFs) within different groups of genes (nuclear-encoded mitochondrial genes, Mito-EBR genes, 49 synergistic enhancer TFs, and electron transport chain [ETC] genes). The number of genes in each group (orange) and the number of these bound by CG1603 (cyan) were highlighted.
Figure 2—figure supplement 1. The promoter binding profiles of the enhancer transcription factors (TFs) in different mitochondrial biogenesis-related groups.
CG1603 regulates ETC gene expression and mitochondrial biogenesis
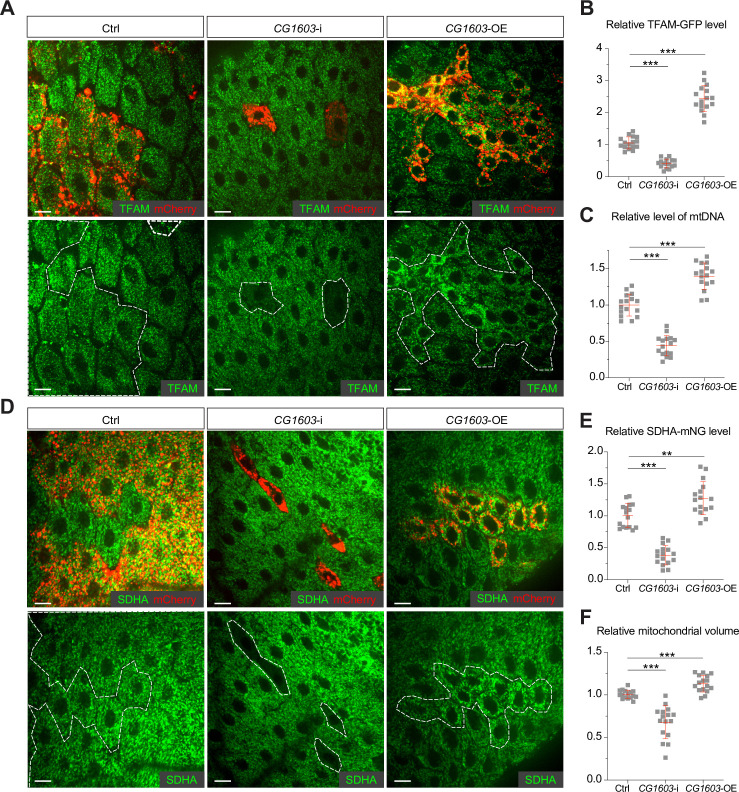
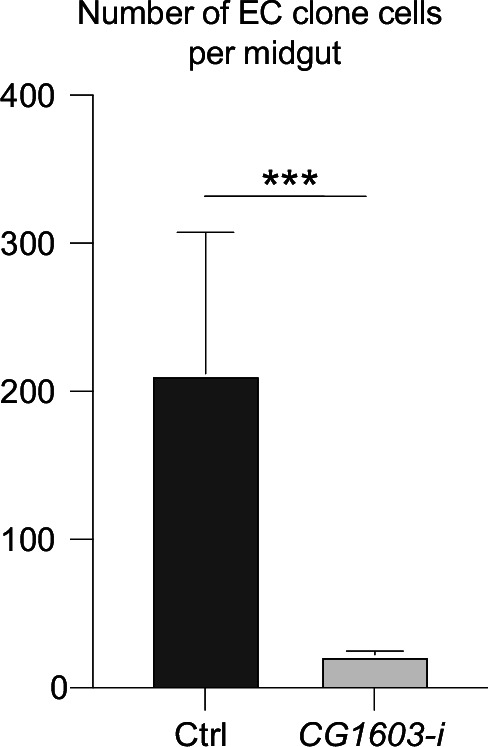
To validate the efficacy of this integrated genetic and bioinformatic approach, we next followed up on CG1603, one of the strongest hits from the primary screen (Figure 1A and G) and exhibited binding to a diverse array of genes associated with ETC biogenesis (Figure 2B, Figure 2—figure supplement 1, and Supplementary file 3). CG1603 RNAi slightly reduced eye size. However, the combination of CG1603 RNAi with MitoXhoI expression in the heteroplasmic background resulted in markedly smaller eyes, indicating a clear synergy between the inhibition of CG1603 and the mtDNA deficiency. We next asked whether CG1603 was involved in mtDNA maintenance. The Drosophila midgut is essentially a monolayer epithelium, composed of intestine stem cells, enteroblasts, enteroendocrine cells, and enterocytes (EC). The large, flattened EC allow high-resolution imaging of mitochondria and mitochondrial nucleoids. Additionally, the simple organization and distinct cell types, containing both proliferative and terminally differentiated cells, render the midgut an ideal model to evaluate the impact of mitochondrial disruptions on cell proliferation and differentiation (Zhang et al., 2020). We used a ‘flip-out’ method to activate CG1603 RNAi in a subset of cells (Prober and Edgar, 2000; Zhang et al., 2020), and imaged TFAM-GFP (Zhang et al., 2016), a marker for mitochondrial nucleoids in midgut clones. Both the total TFAM-GFP level and the number of mtDNA nucleoids (TFAM puncta) were markedly reduced in CG1603 RNAi clones (Figure 3A–C), suggesting that CG1603 is necessary for maintaining the steady-state level of mtDNA. We constructed an SDHA-mNG reporter line by inserting the mNeonGreen (mNG) cDNA in-frame, downstream of the endogenous locus of SDHA, a subunit of ETC Complex II that is entirely encoded by the nuclear genome. SDHA-mNG level was notably reduced in CG1603 RNAi clones (Figure 3D and E), suggesting that CG1603 is also required for the expression of nuclear-encoded ETC subunits. Different from TFAM-GFP that marks mitochondrial nucleoids and appears as puncta in mitochondria (Chen et al., 2020), SDHA-mNG uniformly diffused in the mitochondrial matrix (Figure 3D). By quantifying the total volume of SDHA-mNG positive voxels in the 3D rendering, we found that the total mitochondrial volume was also reduced in CG1603 RNAi clones (Figure 3F). Collectively, these results demonstrate that CG1603 regulates the expression of genes essential for both ETC function and mitochondrial biogenesis. CG1603 RNAi produced very few EC clones (Figure 3A, D, Figure 3—figure supplement 1), consistent with the notion that mitochondrial respiration is necessary for ISCs’ differentiation (Zhang et al., 2020).
Figure 3. CG1603 promotes electron transport chain (ETC) gene expression and mitochondrial biogenesis.
(A, D) Representative images of control RNAi (Ctrl), CG1603 RNAi (CG1603-i), and CG1603 overexpression (CG1603-OE) midgut enterocytes (EC) clones with endogenously expressed TFAM-GFP (A) or SDHA-mNG (D) visualized in green. Clones were labeled by mCherry red and compared with wild type (wt) neighbors. White dashed lines aided in illustrating clones. Scale bars: 10 µm. (B, C, E, F) Quantification of the relative TFAM-GFP level (B), the relative levels of mtDNA (C), the relative SDHA-mNG level (E), and the relative mitochondrial volume (F) in the EC clones to their wt neighbors. n=16 from 8 midguts for each group, error bar: SD. Two-tailed Student's t-test, **: p<0.01, ***: p<0.001.
Figure 3—figure supplement 1. CG1603 RNAi led to fewer enterocytes (EC) clone cells.
CG1603 regulates cell growth and differentiation
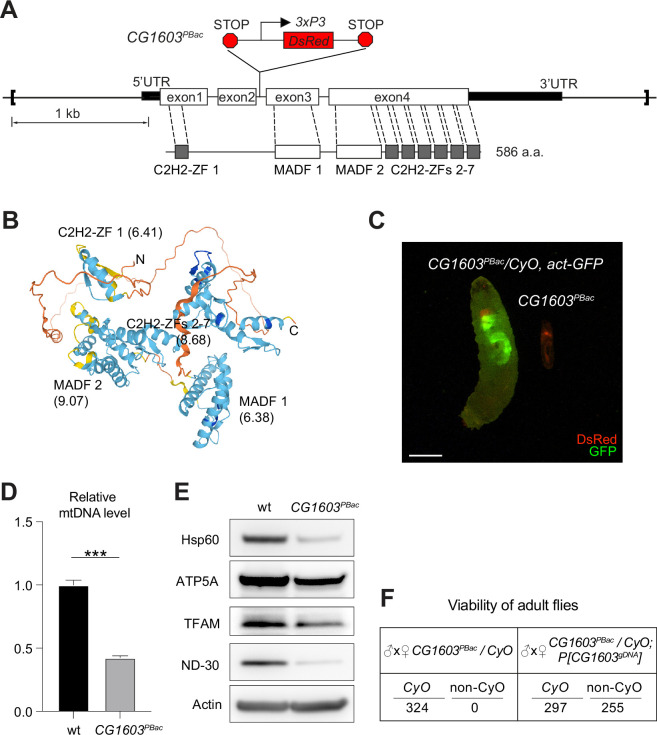
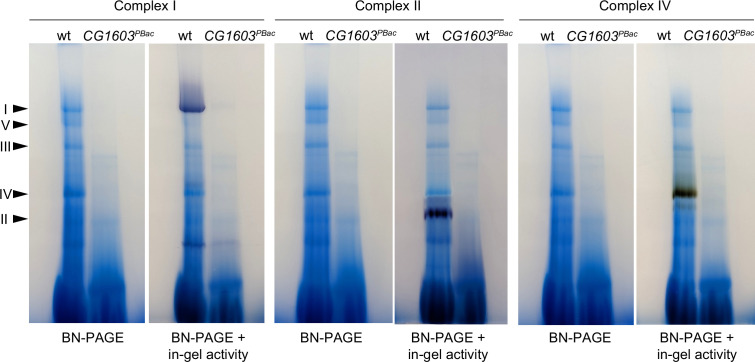
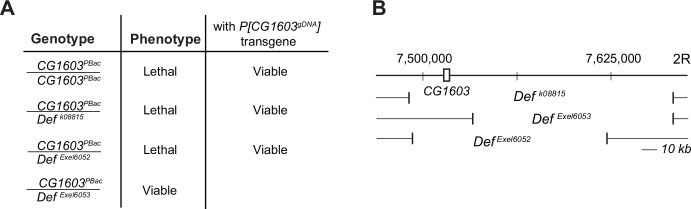
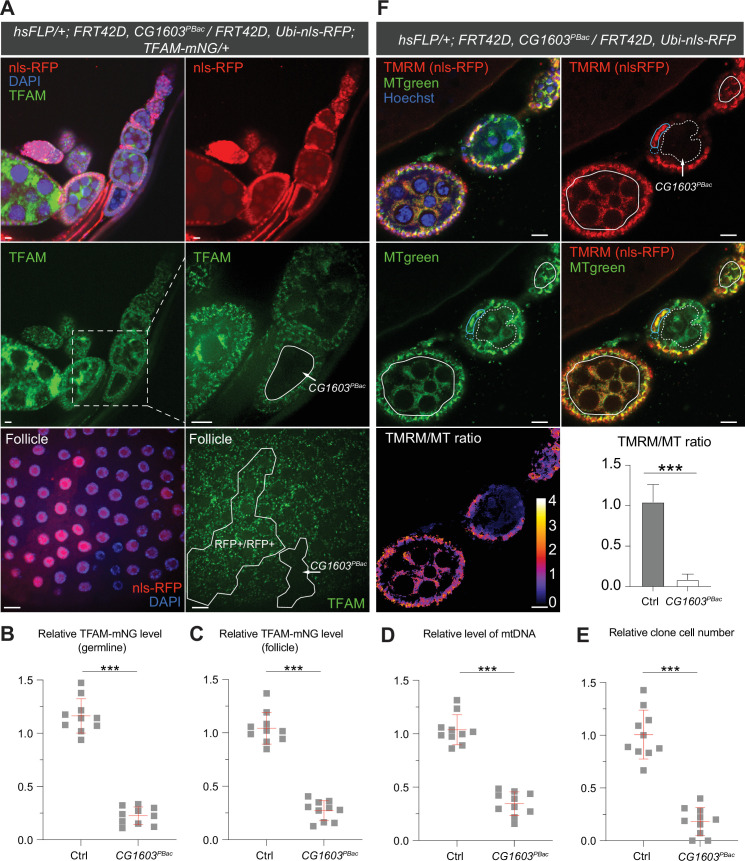
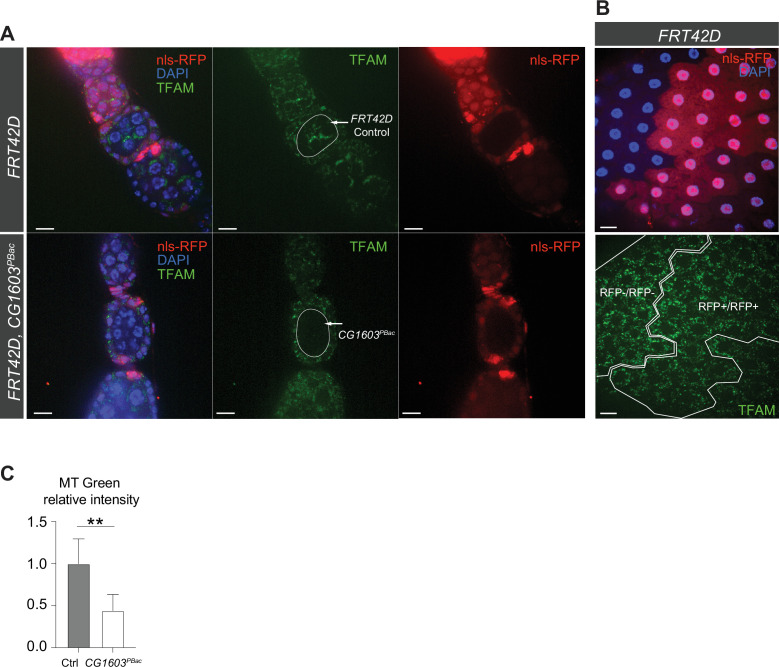
CG1603 encodes a C2H2 zinc finger (C2H2-ZF) protein. It has one C2H2-ZF at its N-terminus, followed by two MADF (myb/SANT-like domain in Adf-1) domains, and six additional zinc fingers at the C-terminus (Figure 4A and B). A PiggyBac transgene, PBac[SAstopDSRed]LL06826, is inserted between the exons 2 and 3 of CG1603 locus. This modified PiggyBac mutator transgene contains splicing donors and stop codons in all three reading frames (Schuldiner et al., 2008), and thereby would disrupt the translation of the full-length CG1603 protein. Homozygous PBac[SAstopDSRed]LL06826 was lethal, arrested at the second instar larval stage and eventually died after 10days (Figure 4C). Both the steady-state level of mtDNA and total mitochondrial mass assessed by the levels of several mitochondrial proteins were reduced in these larvae (Figure 4D and E), as well as the integrities and activities of ETC complexes (Figure 4—figure supplement 1). The lethality of this PiggyBac transgene was mapped to a genomic region spanning the CG1603 locus (Figure 4—figure supplement 2). Importantly, a P[CG1603gDNA] transgene that covers the genomic region of CG1603 fully rescued its viability (Figure 4A and F). These results demonstrate that the lethality of PBac[SAstopDSRed]LL06826 was caused by the loss of function of CG1603, and we hence named it CG1603PBac thereafter. Using FLP/FRT-mediated recombination, we generated homozygous CG1603PBac mutant clones in both germline and follicle cells in adult ovaries. Consistent with the results of ‘flip-out’ RNAi experiments in the midgut, both the total TFAM level and the number of mtDNA nucleoids, visualized by an endogenously expressed TFAM-mNG reporter, were significantly reduced in CG1603PBac clones (Figure 5A–D, Figure 5—figure supplement 1A and B). In most CG1603PBac clones, TFAM-mNG puncta were hardly observed, demonstrating an essential role of CG1603 in mtDNA maintenance. Compared to twin clones, CG1603PBac follicle cell clones contained significantly fewer cells, and these cells were smaller, indicating that CG1603 promotes both cell growth and cell proliferation (Figure 5A and E). CG1603PBac egg chambers were also notably small, even smaller than the adjacent anterior egg chambers that are at earlier developmental stages in the same ovariole (Figure 5A). We assessed Δψm using the ratiometric imaging of TMRM and MitoTracker Green (Zhang et al., 2019). Δψm was nearly abolished in CG1603PBac clones with reduced MitoTracker Green staining (Figure 5F and Figure 5—figure supplement 1C). All together, these observations demonstrate that CG1603 promotes mitochondrial biogenesis and is essential for ETC biogenesis.
Figure 4. CG1603 gene model, product, mutant, and the genomic DNA transgene.
(A) Schematic representation of CG1603 genomic locus, showing the CG1603 transcript (5’ and 3’UTR in black bar and four exons in white), its protein product (586 amino acids in length, and characterized by seven C2H2-ZF and two MADF domains), the CG1603PBac mutant allele (with a PiggyBac insertion in the second intron, which is marked by fluorescent DsRed driven by an eye-specific 3xP3 promoter and flanked by stop codons in all three reading frames terminating translation through downstream), and the genomic region (in square brackets, from 955 bp upstream of the CG1603 5’UTR to 656 bp downstream of CG1603 3’UTR) used for the P[CG1603gDNA] transgene. (B) Predicted 3D structure of the CG1603 protein by AlphaFold. Labels indicate the N- and C-terminus, as well as the specific protein domains along with their predicted isoelectric point (pI). (C) Images of CG1603PBac/CyO, Act-GFP, and homozygous CG1603PBac larvae cultured together at 25°C, day 4 after egg laying. Green: GFP; red: DsRed. Scale bars: 1 mm. (D) Relative mitochondrial DNA (mtDNA) levels in CG1603PBac mutant larvae to wild type (wt) control. n=3, error bar: SD. Two-tailed Student's t-test, ***: p<0.001. (E) Western blots of mitochondrial proteins in CG1603PBac mutant larvae to wt control. (F) P[CG1603gDNA] restored viability of CG1603PBac flies. The number of progenies for each genotype is listed.
Figure 4—figure supplement 1. The integrities and activities of electron transport chain (ETC) complexes were reduced in CG1603PBac mutant.
Figure 4—figure supplement 2. Adult viability phenotypes of combinations of CG1603PBac mutant, P[CG1603gDNA] transgene, and deficiency chromosomes.
Figure 5. Clonal analyses confirmed CG1603’s role in mitochondrial biogenesis and activity.
(A) Representative images of CG1603PBac mutant germline (top and middle panel) and follicle (bottom panel) clones in late-stage egg chambers of adult ovaries with endogenously expressed TFAM-mNG visualized in green. Homozygous mutant clones lacked RFP and were compared with either flanking RFP-positive cysts (germline) or homozygous wild type (wt) twin (follicle). White dashed lines aided in illustrating clones. The wt (RFP+/RFP+) follicle clone showed markedly higher RFP intensity than the heterozygous (RFP+/RFP-) cells, as shown in Figure 5—figure supplement 1B. Red: nls-RFP; blue: DAPI. Scale bars: 10 μm. (B) Quantification of the relative TFAM-mNG level in the homozygous FRT42D control and CG1603PBac mutant germline clone in the early-stage egg chamber to the adjacent anterior RFP-positive cyst within the same ovariole. As shown in Figure 5—figure supplement 1A. n=10 for each group, error bar: SD. Two-tailed Student's t-test, ***: p<0.001. (C–E) Quantification of the relative TFAM-mNG level (C), the relative levels of mtDNA (D), and the relative clone cell number (E) in the homozygous FRT42D control and CG1603PBac mutant follicle clones to their wt twins. n=10 for each group, error bar: SD. Two-tailed Student's t-test, ***: p<0.001. (F) TMRM/MitoTracker Green (MT) ratiometric live imaging and quantification of ovarioles containing homozygous CG1603PBac mutant germline clones (highlighted by white dashed lines). Notably, in contrast to flanking control cysts (highlighted by white lines), Δψm was almost absent in mutant clones. Please note that compared to TMRM, nls-RFP signal was too low to be detected in ratiometric imaging. Nonetheless, the nls-RFP was readily detected in control cysts, but not in homozygous CG1603PBac clones, via visual observation, as depicted in (A), Figure 5—figure supplement 1A and B. A twin pair of follicle clones in the same egg chamber were also highlighted (cyan line for control and cyan dashed line for homozygous CG1603PBac mutant). The MT intensity was reduced in both the germline and follicle CG1603PBac clones, compared to germ cells in adjacent egg chambers and follicle cells in the same egg chamber, respectively. Quantification with background correction for MT intensity in germline clones is shown in Figure 5—figure supplement 1C. Blue: Hoechst. Scale bars: 10 µm. n=8, error bar: SD. Two-tailed Student's t-test, ***: p<0.001.
Figure 5—figure supplement 1. Clonal analyses confirmed CG1603’s role in mitochondrial biogenesis.
CG1603 is a TF regulating nuclear mitochondrial gene expression
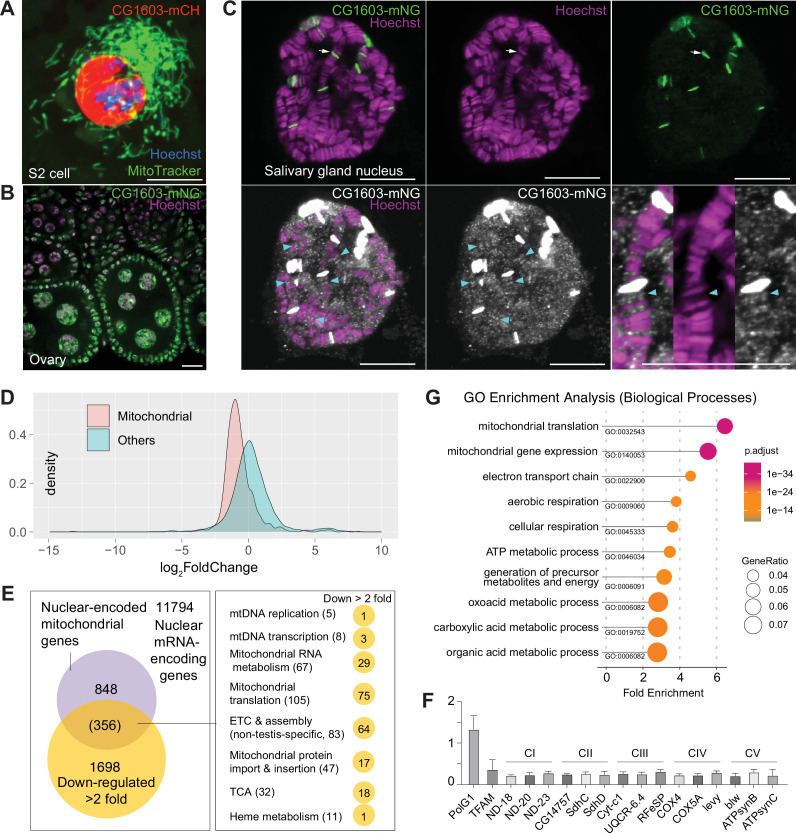
CG1603 protein exclusively localized to the nucleus when expressed in cultured cells (Figure 6A). We generated a transgene expressing CG1603-mNG fusion protein by inserting mNeonGreen cDNA into the endogenous locus of CG1603. CG1603-mNG localized to nuclei in ovaries (Figure 6B) and directly bound to polytene chromosomes in the salivary gland (Figure 6C). Notably, CG1603-mNG was highly enriched on less condensed chromatin regions that had weak Hoechst staining (Figure 6C). We performed RNA sequencing (RNA-seq) in larvae to uncover potential targets of CG1603. Between wt and CG1603PBac larvae, total 7635 genes were differentially expressed, including 86% nuclear-encoded mitochondrial genes (Figure 6D and Supplementary file 4; Supplementary file 5). Nearly half of nuclear-encoded mitochondrial genes were among 1698 genes that were reduced by more than two-fold in CG1603 mutant (Figure 6E and Supplementary file 5a), including many structural subunit genes of all five ETC complexes (Supplementary file 5b), some of which were further confirmed by quantitative real-time PCR (Figure 6F). Gene Ontology (GO) enrichment analyses on these 1698 genes also revealed that all top 10 significantly enriched biological processes were related to mitochondria, including ‘mitochondrial translation’, ‘mitochondrial gene expression’, ‘electron transport chain’, ‘aerobic respiration’, ‘cellular respiration’, and ‘ATP metabolic process’ (Figure 6G).
Figure 6. CG1603 localizes in the nucleus and is essential for regulating nuclear mitochondrial gene expression.
(A–B) Representative images showing the nuclear localization of CG1603 protein in cultured S2 cells (A) and adult ovary (B). Green: MitoTracker Green in S2 cell, and CG1603-mNG in tissues; red: CG1603-mCH; blue and magenta: Hoechst. Scale bars: 10 μm. (C) Representative images showing bindings of endogenously expressed CG1603 proteins to less condensed euchromatin regions in the polytene chromosomes of a salivary gland. High-intensity CG1603-mNG bands were visualized in green in the upper panel and indicated by arrows, and low-intensity bands were pesudo-colored in white in theblower panel and indicated by arrow heads. The images of the lower panel were same as these in the upper panel, but digitally enhanced. Magenta: Hoechst. Scale bars: 10 μm. (D) Density plot illustrating the distribution of expression changes of the nuclear-encoded mitochondrial and non-mitochondrial genes in CG1603PBac mutant. (E) Graph illustrating the overlap between nuclear-encoded mitochondrial genes and differentially expressed genes (DEGs) that down-regulated >2-fold, as well as the distribution of the overlapped genes in different mitochondrial function categories. (F) Relative mRNA levels of several electron transport chain (ETC) biogenesis-related genes in CG1603PBac mutant larvae to control, measured by real-time PCR. n=3, error bar: SD. (G) Gene Ontology (GO) enrichment analyses of DEGs that down-regulated >2-fold. The top 10 enriched biological processes are shown.
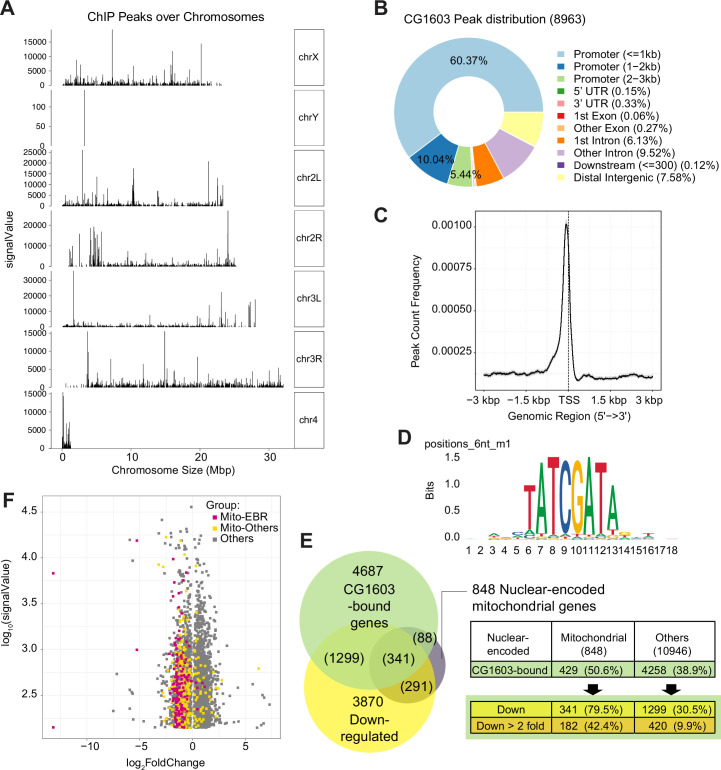
CG1603 had 8963 binding sites (peaks) distributed over all four chromosomes (Figure 7A and Supplementary file 6). A subset of peaks showed high intensity evaluated by signalValue (Figure 7A and Supplementary file 6), which may correspond to these high-intensity CG1603-mNG bands on the polytene chromosomes of the salivary gland (Figure 6C). Most CG1603 binding sites (6799) were found at promoter regions, close to the transcription start site (Figure 7B and C and Supplementary file 6), which is a key feature of a typical TF. Using the RSAT ‘peak-motifs’ tool (Thomas-Chollier et al., 2012), an 8bp palindromic sequence, ‘TATCGATA’ emerged as the most prevalent CG1603 binding motif (Figure 7D and Supplementary file 7). CG1603 bound to the genomic regions of 50% nuclear-encoded mitochondrial genes, and among these genes, 79.5% were down-regulated in the CG1603PBac mutant (Figure 7E and Supplementary file 6), indicating a great accordance between ChIP data and RNA-seq results. Most nuclear-encoded mitochondrial genes that were both bound by CG1603 and down-regulated in CG1603 mutant were ETC genes or related to ETC biogenesis (Figure 7F and Supplementary file 6). Collectively, CG1603 appears to be essential for mitochondrial biogenesis and coordinates the expression of both nuclear and mtDNA genes in ETC biogenesis.
Figure 7. ChIP analysis identified nuclear mitochondrial genes that may be directly regulated by CG1603.
(A) CG1603 ChIP peaks over all chromosomes. (B) Genomic distribution of CG1603 peaks. (C) Average profile of CG1603 peaks binding to transcription start site (TSS) regions. (D) Representative binding motif discovered with CG1603 ChIP peaks. (E) The number of nuclear-encoded mitochondrial and non-mitochondrial genes bound by CG1603, and the overlapping down-regulated differentially expressed genes (DEGs) in each group. (F) Scatterplot illustrating the signalValue of CG1603 ChIP peaks (y-axis) and log2 fold change in expression of DEGs between CG1603PBac mutant and control (x-axis). Mito-EBR: genes related to electron transport chain (ETC) biogenesis and maintenance, including ETC subunits and assembly factors, mitochondrial DNA (mtDNA) replication and transcription, mitochondrial RNA metabolism and translation, as well as mitochondrial protein import and membrane insertion machinery.
The integrated approach identifies YL-1 as an upstream regulator of CG1603
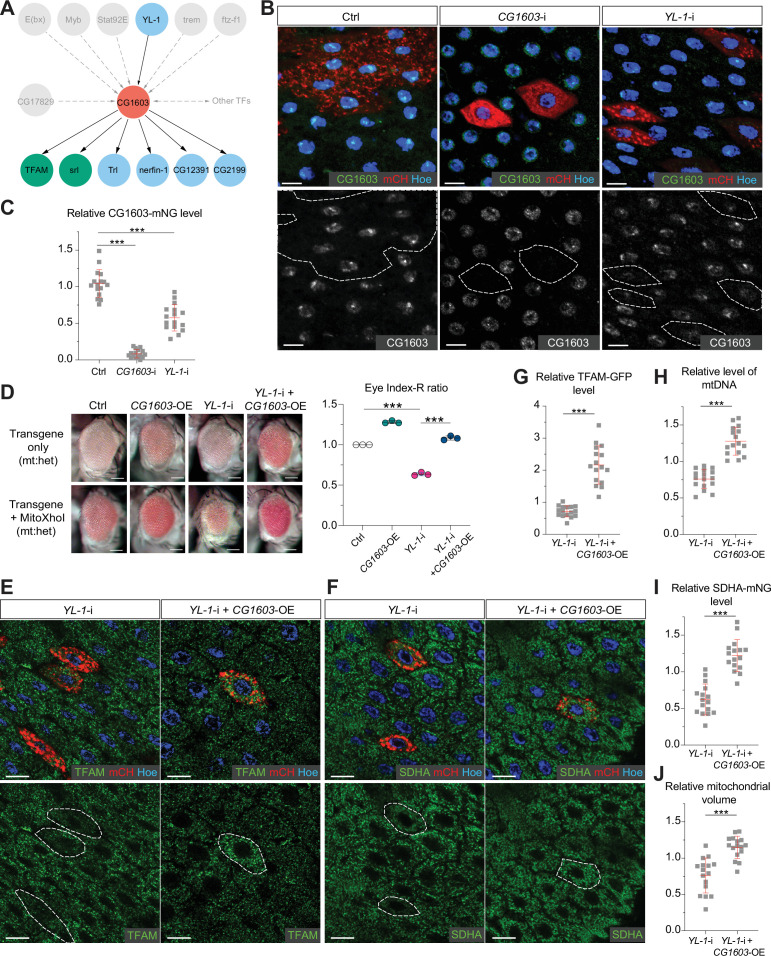
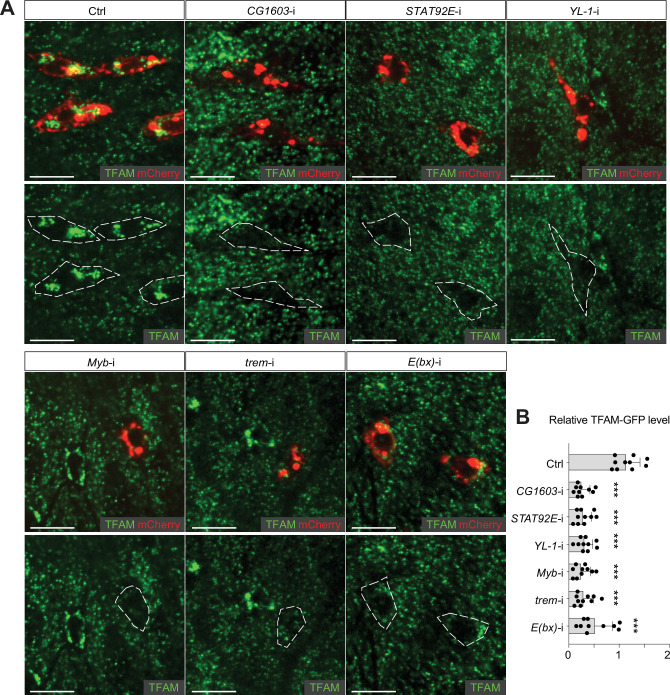
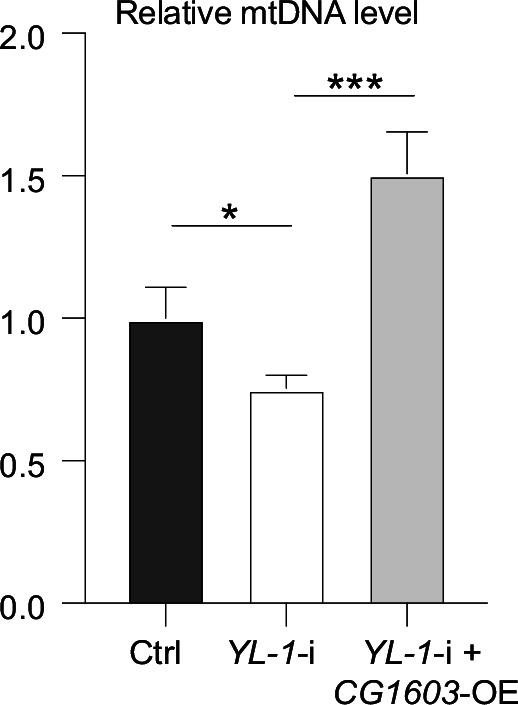
In the network analyses, CG1603 was positioned in the middle layer, linked to seven TFs above and six TFs below by integrating the RNA-seq result with ChIP-seq data (Figure 8A). Through these TFs below, CG1603 may indirectly control the expression of 2230 genes, including 291 mitochondrial genes down-regulated in CG1603PBac but not bound by CG1603 (Figure 7E). Using the ‘flip-out’ RNAi system in the midgut, we found that among seven TFs upstream of CG1603 in the network, E(bx), YL-1, trem, STAT92E, and Myb were also required for maintaining TFAM levels (Figure 8—figure supplement 1). To further verify their potential roles in regulating CG1603, we performed RNAi against these genes in midgut clones carrying CG1603-mNG reporter. Only YL-1 RNAi clones displayed a marked reduction of CG1603 protein compared with neighboring cells (Figure 8B and C). Furthermore, overexpression of CG1603 restored the reduced eye size, TFAM-GFP, SDHA-mNG, and mtDNA levels caused by YL-1 RNAi (Figure 8D–J and Figure 8—figure supplement 2). These results indicate that YL-1 is indeed an upstream regulator of CG1603, and through which to regulate ETC biogenesis.
Figure 8. YL-1 is an upstream regulator of CG1603.
(A) Schematic graph illustrating the CG1603 upstream and downstream (co-)TFs (transcription factors) involved in regulating mitochondrial electron transport chain (ETC) biogenesis, inferred from ChIP-seq, RNA-seq, and genetics data. (B) Representative images of control RNAi (Ctrl), CG1603 RNAi (CG1603-i), and YL-1 RNAi (YL-1-i) midgut enterocytes (EC) clones with endogenously expressed CG1603-mNG visualized in green or white. Clones were labeled by mCherry red and compared with wild type (wt) neighbors. White dashed lines aided in illustrating clones. Blue: Hoechst. Scale bars: 10 μm. (C) Quantification of the relative CG1603-mNG level in the EC clones to their wt neighbors. n=16 from 8 midguts for each group, error bar: SD. Two-tailed Student's t-test, ***: p<0.001. (D) Representative eye image and Index-R ratio (RNAi+mitoXhoI/RNAi-only) of adult flies with indicated genotypes. Three biological repeats were performed for each group, error bar: SD. Two-tailed Student's t-test, ***: p<0.001. Scale bars: 100 µm. (E–F) Representative images of YL-1 RNAi (YL-1-i) and YL-1 RNAi+CG1603 overexpression (YL-1-i+CG1603 OE) midgut EC clones with endogenously expressed TFAM-GFP (E) or SDHA-mNG (F) visualized in green. Clones were labeled by mCherry red and compared with wt neighbors. Blue: Hoechst. Scale bars: 10 µm. (G–J) Quantification of the relative TFAM-GFP level (G), the relative levels of mtDNA (H), the relative SDHA-mNG level (I), and the relative mitochondrial volume (J) in the EC clones to their wt neighbors. n=16 from 8 midguts for each group, error bar: SD. Two-tailed Student's t-test, ***: p<0.001.
Figure 8—figure supplement 1. TFAM levels in the RNAi midgut clones of transcription factors (TFs) upstream of CG1603 in the network.
Figure 8—figure supplement 2. Overexpression of CG1603 restored the reduced mitochondrial DNA (mtDNA) level caused by YL-1 RNAi in the eye discs.
Discussion
The dual genetic control of mitochondria presents a fundamental challenge: how are the nuclear genome and mtDNA coordinated to ensure the efficiency and the integrity of oxidative phosphorylation system and other critical mitochondrial processes? In Drosophila ovary, the mitochondrial A-kinase-anchor-protein, MDI promotes the translation of a subset of nuclear mitochondrial proteins by cytosolic ribosomes on the mitochondrial outer membrane (Zhang et al., 2016). MDI’s targets are predominantly ETC subunits and proteins essential for mitochondrial genome maintenance and gene expression (Zhang et al., 2019). This mechanism coordinates the nuclear and mitochondrial genomes to augment the ETC biogenesis that takes place in differentiating germ cells (Wang et al., 2019; Wang et al., 2023). Cytosolic and mitochondrial translation are up-regulated in concert to boost ETC biogenesis in budding yeast undergoing a metabolic shift from glycolysis to oxidative phosphorylation (Couvillion et al., 2016), further supporting the synchronized expression of ETC components from dual genetic origins at the translational level. Nevertheless, nuclear-encoded mitochondrial ETC subunits often exhibited a concordant expression pattern at the RNA level (Eisen et al., 1998), and mtDNA-encoded ETC RNAs consistently exhibited similar trends, albeit with a more gradual increase compared to their nuclear-encoded counterparts accompanying the metabolic shift (Couvillion et al., 2016). These observations suggest a potential coordination at the transcriptional level as well. We uncovered a zinc finger protein encoded by the CG1603 locus as a core regulator in a transcription network regulating mitochondrial biogenesis. CG1603 promoted the expression of more than half of nuclear-encoded mitochondrial proteins, and the inhibition of CG1603 severely reduced mitochondrial mass and mtDNA contents. CG1603 targets were highly enriched in nuclear-encoded ETC subunits and essential factors required for mtDNA genome maintenance and gene expression. Thus, CG1603 not only promotes mitochondrial biogenesis in general, but also affords a transcriptional coordination of the nuclear and mitochondrial genomes in ETC biogenesis.
The modifier screen in the developing eyes took advantage of the mtDNA deficiency resulted from the expression of MitoXhoI in a heteroplasmic background. Besides 77 enhancers, we also recovered 20 suppressors, of which ‘RNAi+MitoXhoI’ flies had larger eyes than ‘RNAi-only’ (Figure 1G and Supplementary file 1). Knockdown of these genes alone severely reduced eye size (Figure 1G and Supplementary file 1). Noteworthy, five of them were lethal due to the lack of head capsule that is developed from the eye antenna disc, but the viability of these RNAi flies was restored by MitoXhoI expression. Given that MitoXhoI expression also disrupts eye development, it is perplexing that the combination of RNAi and MitoXhoI expression, two genetic conditions causing the same phenotype, led to a milder phenotype. Perhaps, mtDNA deficiency caused by MitoXhoI expression triggers a retrograde signal, which boosts cellular stress responses and thereby mitigates the cell growth defects in these RNAi backgrounds.
The CG1603 belongs to a large family of C2H2-ZF TFs that contains 272 genes in the Drosophila genome (https://flybase.org/reports/FBgg0000732.html). It has one N-terminus C2H2-ZF, followed by two MADFs and a cluster of six C2H2-ZFs at the C-terminus (Figure 4A). In addition to the C2H2-ZF cluster, which predominantly mediates sequence-specific DNA binding (Persikov et al., 2015; Wolfe et al., 2000), C2H2-ZF TFs often possess additional N-terminal protein-protein interaction domains, such as KRAB, SCAN, and BTB/POZ domains in vertebrates, ZAD and BTB/POZ in Drosophila, for binding to transcription co-regulators (Fedotova et al., 2017; Perez-Torrado et al., 2006; Sobocińska et al., 2021). These interactions allow them to either activate or repress gene expression. CG1603 binds to the genomic regions of 4687 genes in the Drosophila genome. Among these genes, 602 and 562 genes were, respectively, decreased or increased more than two folds in CG1603 mutant (Supplementary file 6). Thus, CG1603 is likely a dual-function TF, capable of both activating and repressing transcription depending on the chromosomal environment of its targets. Ying Yang 1, a well-characterized dual-function TF in mammals, contains both a transcription activation domain and a repression domain, in addition to four C2H2-ZFs at its C-terminus (Verheul et al., 2020). The N-terminus C2H2-ZF and the first MADF domain of CG1603 are negatively charged, and hence have low probability of binding to DNA that is also negatively charged (Figure 4B). In the predicted 3D structure of CG1603, the positively charged C-terminal zinc fingers and MADF-2 domain cluster in the center, while the negatively charged N-terminal C2H2-ZF and the MADF-1 extend in opposite directions (Figure 4B), resembling the domain arrangement of Ying Yang 1 (Verheul et al., 2020). MADF domains share significant similarity with Myb/SANT domains that may bind to either DNA or proteins (Maheshwari et al., 2008). Some MADF domains, due to their negative charge, have been proposed to interact with positively charged histone tails, similar to the Myb/SANT domain in a well-known chromatin remodeler ISWI, suggesting a potential role in chromatin remodeling (Maheshwari et al., 2008). Notably, some chromatin remodelers possess tandem Myb/SANT domains that can directly interact with histones and DNA or histone remodeling enzymes like ISWI and SMRT (Boyer et al., 2004). It is plausible that CG1603 may play a role in chromatin remodeling directly or by recruiting nucleosome remodeling factors to its binding sites, thereby modulating gene transcription in those regions.
Notably, CG1603 had no impact on the expression of one-third of its binding genes (Supplementary file 6), highlighting that DNA binding profiling alone is not sufficient to predict the function of a TF. Nonetheless, the network analyses on TFs’ ChIP-seq data allowed us to construct a potential regulatory network among these TFs, which subsequently served as a blueprint for genetic analyses to verify potential regulations. Total seven TFs were upstream of CG1603 in the network and emerged as positive hits in the initial screen in the eye. RNAi against five of them led to reduced TFAM levels in the midgut, while the other two had no noticeable phenotype, suggesting that these two TFs may regulate mitochondrial biogenesis in a tissue-specific manner. Only YL-1 was confirmed to act upstream of CG1603 based on the genetic epistasis analysis, further indicating the necessity of combining genomic, bioinformatic, and genetic analyses to gain more reliable and comprehensive understanding on transcriptional regulations. YL-1 is one of DNA binding subunits of the SRCAP complex, which is essential for histone H2A.Z incorporation and replacement (Liang et al., 2016). Recently, it has been shown that both SRCAP complex and H2A.Z are necessary for the transcription of nuclear-encoded mitochondrial genes (Lowden et al., 2021; Xu et al., 2021). Our work offers a mechanistic insight into how CG1603 and its upstream regulator, YL-1, may regulate mitochondrial biogenesis at nucleosome and chromatin levels. Currently, most known transcription paradigms controlling mitochondrial biogenesis are centered on TFs and co-activators that stabilize or directly stimulate the core transcription machinery. The YL-1 to CG1603 cascade may represent a previously underappreciated layer of transcriptional regulation on ETC biogenesis and could act in concert with NRF1 and other TFs to coordinate both the nuclear and mitochondrial genomes in ETC biogenesis.
Materials and methods
Key resources table.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Drosophila melanogaster) | CG1603 | GenBank | FLYB:FBgn0033185 | |
| Genetic reagent (D. melanogaster) | w1118 | Bloomington Drosophila Stock Center | BDSC: 3605; RRID:BDSC_3605 |
|
| Genetic reagent (D. melanogaster) | Heteroplasmic Sco/CyO, UAS-mitoXhoI; eyeless-GAL4 | This paper | See Materials and methods, Section Fly genetics | |
| Genetic reagent (D. melanogaster) | RNAi stains used for genetic screen | Bloomington Drosophila Stock Center; Vienna Drosophila Resource Center | See ‘ID’, ‘SYMBOL’, and ‘Stock #’ column in Supplementary file 1 | Stock # begins with ‘v’ is from VDRC, otherwise BDSC |
| Genetic reagent (D. melanogaster) | UAS-Luciferase | Bloomington Drosophila Stock Center | BDSC:35788 | |
| Genetic reagent (D. melanogaster) | TFAM‐GFP | PMID:27053724 | ||
| Genetic reagent (D. melanogaster) | hsFLP | Bloomington Drosophila Stock Center | BDSC:7 | |
| Genetic reagent (D. melanogaster) | Act >CD2>GAL4, UAS-mCD8::mCherry | This paper | See Materials and methods, Section Fly genetics | |
| Genetic reagent (D. melanogaster) | PBac[SAstopDsRed]LL06826 | Kyoto Drosophila Stock Center | Kyoto:141919 | |
| Genetic reagent (D. melanogaster) | CyO, act-GFP | Bloomington Drosophila Stock Center | BDSC:4533 | |
| Genetic reagent (D. melanogaster) | FRT42D | Bloomington Drosophila Stock Center | BDSC:1802 | |
| Genetic reagent (D. melanogaster) | FRT42D, Ubi-nls-RFP | Bloomington Drosophila Stock Center | BDSC:35496 | |
| Genetic reagent (D. melanogaster) | Defk08815 | Bloomington Drosophila Stock Center | BDSC:10818 | |
| Genetic reagent (D. melanogaster) | DefExel6052 | Bloomington Drosophila Stock Center | BDSC:7534 | |
| Genetic reagent (D. melanogaster) | DefExel6053 | Bloomington Drosophila Stock Center | BDSC:7535 | |
| Genetic reagent (D. melanogaster) | UASz-CG1603 | This paper | See Materials and methods, Section Transgenic flies | |
| Genetic reagent (D. melanogaster) | P[CG1603gDNA] | This paper | See Materials and methods, Section Transgenic flies | |
| Genetic reagent (D. melanogaster) | SDHA-mNeonGreen | This paper | See Materials and methods, Section Transgenic flies | |
| Genetic reagent (D. melanogaster) | TFAM-mNeonGreen | This paper | See Materials and methods, Section Transgenic flies | |
| Genetic reagent (D. melanogaster) | CG1603-Halo-mNeonGreen | This paper | See Materials and methods, Section Transgenic flies | |
| Cell line (D. melanogaster) | S2 | Drosophila Genomics Resource Center | FLYB:FBtc0000181; RRID:CVCL_Z992 | |
| Antibody | Anti-Actin (Mouse monoclonal) | MilliporeSigma | Cat# MAB1501; RRID:AB_2223041 |
WB (1:1000) |
| Antibody | Anti-ATP5A (Mouse monoclonal) | abcam | Cat# 15H4C4; RRID:AB_301447 |
WB (1:2000) |
| Antibody | Anti-ND30 (Mouse monoclonal) | abcam | Cat# 17D95; | WB (1:1000) |
| Antibody | Anti-TFAM (Rabbit polyclonal) | PMID:35449456 | WB (1:1000) | |
| Antibody | Anti-HSP60 (Rabbit polyclonal) | Cell Signaling | Cat# 4870; RRID:AB_2295614 |
WB (1:1000) |
| Antibody | Anti-rabbit IgG, HRP-linked (Goat polyclonal) | Cell Signaling | Cat# 7074; RRID:AB_2099233 |
WB (1:2000) |
| Antibody | Anti-mouse IgG, HRP-linked (Horse polyclonal) | Cell Signaling | Cat# 7076; RRID:AB_330924 |
WB (1:2000) |
| Recombinant DNA reagent | pIB-CG1603-mCherry (plasmid) | This paper | See Materials and methods, Section Cell culture and gene expression | |
| Recombinant DNA reagent | pIB/V5-His (plasmid) | Thermo Fisher Scientific | Cat# V802001 | |
| Sequence-based reagent | Primers for real-time PCR | This paper | Listed in Supplementary file 8 | |
| Commercial assay or kit | Effectene Transfection Reagent | QIAGEN | Cat# 301425 | |
| Commercial assay or kit | NativePAGE Sample Prep Kit | Thermo Fisher Scientific | Cat# BN2008 | |
| Commercial assay or kit | Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Cat# 23225 | |
| Commercial assay or kit | NativePAGE Running Buffer Kit | Thermo Fisher Scientific | Cat# BN2007 | |
| Commercial assay or kit | DNeasy Blood & Tissue Kit | QIAGEN | Cat# 69504 | |
| Commercial assay or kit | RNeasy Mini Kit | QIAGEN | Cat# 74104 | |
| Commercial assay or kit | SuperScript VILO cDNA Synthesis Kit | Thermo Fisher Scientific | Cat# 11754050 | |
| Commercial assay or kit | PowerTrack SYBR Green Master Mix | Thermo Fisher Scientific | Cat# A46012 | |
| Chemical compound, drug | TRIzol | Thermo Fisher Scientific | Cat# 15596026 | |
| Chemical compound, drug | DAPI | Thermo Fisher Scientific | Cat# D1306 | |
| Chemical compound, drug | Hoechst 33342 | Thermo Fisher Scientific | Cat# H1399 | |
| Chemical compound, drug | TMRM | Thermo Fisher Scientific | Cat# I34361 | |
| Chemical compound, drug | MitoTracker Green | Thermo Fisher Scientific | Cat# M7514 | |
| Software, algorithm | Imaris | Oxford Instruments | See Materials and methods, Section Imaging analyses | |
| Software, algorithm | Fiji/ImageJ | NIH | See Materials and methods, Section Imaging analyses | |
| Software, algorithm | FastQC | Babraham Bioinformatics | See Materials and methods, Section RNA-seq analysis | |
| Software, algorithm | STAR | PMID:23104886 | See Materials and methods, Section RNA-seq analysis | |
| Software, algorithm | HTseq | PMID:35311944 | See Materials and methods, Section RNA-seq analysis | |
| Software, algorithm | DESeq2 | PMID:25516281 | See Materials and methods, Section RNA-seq analysis | |
| Software, algorithm | clusterProfiler | PMID:22455463 | See Materials and methods, Section RNA-seq analysis | |
| Software, algorithm | BWA | PMID:19451168 | See Materials and methods, Section ChIP-seq computational analysis | |
| Software, algorithm | Samtools | PMID:19505943 | See Materials and methods, Section ChIP-seq computational analysis | |
| Software, algorithm | MACS2 | PMID:18798982 | See Materials and methods, Section ChIP-seq computational analysis | |
| Software, algorithm | ChIPseeker | PMID:25765347 | See Materials and methods, Section ChIP-seq computational analysis | |
| Software, algorithm | VertexSort | PMID:19690563 | See Materials and methods, Section ChIP-seq computational analysis | |
| Software, algorithm | RSAT | PMID:22836136 | See Materials and methods, Section ChIP-seq computational analysis | |
| Software, algorithm | SMART | PMID:10592234 | See Materials and methods, Section Prediction of protein domains, isoelectric point, net charge, and structure | |
| Software, algorithm | AlphaFold | PMID:34265844 | See Materials and methods, Section Prediction of protein domains, isoelectric point, net charge, and structure |
Fly genetics
Flies were maintained on standard cornmeal medium at 25°C, unless otherwise stated. Heteroplasmic lines that contain~50% XhoI-resistant mt:CoIT300I genome (Hill et al., 2014) were maintained at 18°C. The heteroplasmic w1118; Sco/CyO, UAS-mitoXhoI; eyeless-GAL4 females were crossed with different RNAi lines to generate male offspring for assessing adult eye morphology. RNAi lines used in the screen were obtained from the Bloomington Drosophila Stock Center (BDSC), or Vienna Drosophila Resource Center, and listed in Supplementary file 1. UAS-Luciferase (BDSC#35788) was used as the transgene control. TFAM‐GFP reporter line was described previously (Zhang et al., 2016). Act >CD2>GAL4, UAS-mCD8::mCherry, and hsFLP (BDSC#7) were used to generate ‘flip-out’ clones in midguts. We found that the leakage expression of flippase at 22°C was sufficient to induce ‘flip-out’ clones. PBac[SAstopDsRed]LL06826 (Kyoto#141919) was obtained from Kyoto Drosophila Stock Center, and backcrossed to w1118 for six generations before phenotypic analyses. A fluorescent ‘CyO, act-GFP’ (BDSC#4533) was used for selecting homozygous mutant larvae. PBac[SAstopDsRed]LL06826 was recombined with FRT42D (BDSC #1802) to generate FRT42D, CG1603PBac, which was crossed with hs-flp; FRT42D, Ubi-nls-RFP (derived from BDSC#35496) for generating mitotic clones in ovaries (Laws and Drummond-Barbosa, 2015). Briefly, 0- to 2-day-old females were transferred along with sibling males to the Kimwipe-semi-covered vials, then passed to 37°C water bath, heat shocked for 1 hr, twice daily, for 3 consecutive days. The clones were assessed 7–10days after the final heat shock. Defk08815(BDSC#10818), DefExel6052(BDSC#7534), and DefExel6053(BDSC#7535) were obtained from BDSC.
Cell culture and gene expression
S2 cells from Drosophila Genomics Resource Center (DGRC) were cultured as previously described Zhang et al., 2015 following the online instruction (DRSC, https://fgr.hms.harvard.edu/fly-cell-culture). Briefly, cells were maintained in Schneider’s medium (Thermo Fisher Scientific) with 10% heat inactivated fetal bovine serum (FBS, Thermo Fisher Scientific) and 1% penicillin-streptomycin (Thermo Fisher Scientific) at 27°C. Effectene Transfection Reagent (QIAGEN) was used for plasmids transfection following the manufacturer’s instructions. For expression in S2 cells, the coding sequence of CG1603 was cloned into a pIB vector (Thermo Fisher Scientific), with an mCherry coding sequence fused at the 3' end.
Transgenic flies
UASz-CG1603 plasmid was generated by inserting CG1603 coding sequence between the Acc65I and XbaI sites of pUASz1.0 (https://dgrc.bio.indiana.edu//stock/1431; RRID:DGRC_1431). UASz-CG1603 was inserted into either attP2 or attP40 (Bestgene Inc) using PhiC31 integrase-mediated site-specific transformation, to generate transgenes on third and second chromosome, respectively.
The DNA fragment spanning CG1603 genomic region was amplified by PCR and subcloned into a pattB vector (https://dgrc.bio.indiana.edu//stock/1420; RRID:DGRC_1420). The resulted plasmid was inserted into attP2 site (Bestgene Inc) using PhiC31 integrase-mediated site-specific transformation to generate the transgene P[CG1603gDNA].
SDHA-mNeonGreen reporter line was generated using a previously published method (Wang et al., 2019). The targeting cassette comprising of 1kb genomic DNA fragment upstream of SDHA stop codon, mNeonGreen coding sequence, a fragment containing GMR-Hid flanked by two FRT sites, and 1kb genomic DNA fragment downstream of SDHA stop codon was inserted into a pENTR vector to make the homology donor construct. This donor construct and an SDHA chiRNA construct (gRNA-SDHA recognizes GTAGACATCCGTACGAGTGA[TGG]) were injected into the Vasa-Cas9 expressing embryos (BDSC#51323). G0 adults were crossed with w1118 files (BDSC#3605), and progeny with small-eye phenotype were selected as candidates due to the expression of GMR-Hid. To remove the GMR-Hid cassette, the SDHA-mNeon-GMR-Hid flies were crossed with nos-Gal4; UASp-FLP. The F1 progeny with the genotype of nos-Gal4/SDHA-mNeon-GMR-Hid; UASp-FLP/+ were selected and crossed with Sco/CyO. The F2 flies of SDHA-mNeon/CyO with normal white eyes were selected and maintained.
For TFAM-mNeonGreen and CG1603-Halo-mNeonGreen knock-In lines, the targeting cassette comprising of 1kb genomic DNA fragment upstream of the stop codon, either mNeonGreen or Halo-mNeonGreen coding sequence, and 1kb genomic DNA fragment downstream of the stop codon was inserted into pOT2 vector to generate the donor constructs. Each donor construct and the corresponding chiRNA construct (gRNA for TFAM: ATGATTTGTGAATTATGTGATGG; gRNA for CG1603: GGAATGAACTCTCGCCTTGAGGG) were injected into Vasa-Cas9 expressing embryos (BDSC#51323 or BDSC#51324). G0 adults were crossed with w1118 files, and the progeny carrying the mNeonGreen insertions were screened by PCR. Primers for TFAM-mNeonGreen are GCTCGCTGATCAACAAAGTC and GGTGGACTTCAGGTTTAACTCC. Primers for CG1603-mNeonGreen are AGTGCGAGTTCCTCAGT-GTG and CGCCCAGGACTTCCACATAA.
RNA-seq analysis
For bulk RNA-seq analysis, total RNA was extracted from wt and CG1603 mutant larvae (48hr after egg laying) by TRIzol (Thermo Fisher Scientific) following the standard protocol. Three samples were used for each genotype. Poly (A) capture libraries were generated at the DNA Sequencing and Genomics Core, NHLBI, NIH. RNA-seq was performed with using an NovaSeq 6000 (Illumina) and 100bp paired-end reads were generated at the DNA Sequencing and Genomics Core, NHLBI, NIH. Sequencing data were analyzed following the Bioinformatics Pipeline of mRNA Analysis, NCI, NIH. After quality assessment of FASTQ files using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc), paired-end reads were aligned against D. melanogaster reference genome (Dmel6) using a two-pass method with STAR (v2.7.9a) (Dobin et al., 2013). Gene-level read counts were produced by HTseq (v0.11.4) (Putri et al., 2022). Differential expression analysis at the gene level was carried out using DESeq2 open-source R package (Love et al., 2014) with an FDR cut-off of 0.05. GO enrichment analysis was performed using clusterProfiler R package (Yu et al., 2012) with the log2 fold change cut-off>1and <–1 for up-regulated and down-regulated genes, respectively. A density plot was generated by ggplot2 R package (https://ggplot2.tidyverse.org). Drosophila mitochondrial genes and subgroups were referenced against a modified MitoCarta 3.0 inventory (Rath et al., 2021; Wang et al., 2019).
ChIP-seq computational analysis
ChIP-seq reads in FASTQ format and the narrowPeak output files for each candidate TF were downloaded from ENCODE Project open resource (https://www.encodeproject.org; Kudron et al., 2018). ChIP-seq reads were aligned to the D. melanogaster reference genome (Dmel6) using BWA (v0.7.17) (Li and Durbin, 2009). SAM files were sorted and compressed into BAM format with Samtools (v1.16.1) (Li et al., 2009). Replicates were merged by Picard tools (v2.27.3, https://broadinstitute.github.io/picard) using lenient criteria, and all alignments with an MAPQ value less than 20 were removed. Lags prediction and peak-calling were done with MACS2 (v2.2.7.1) Zhang et al., 2008 following the ENCODE TF ChIP pipeline with IDR analysis performed for consistency analysis (https://github.com/mforde84/ENCODE_TF_ChIP_pipeline; mforde84, 2016). Peak annotation and analysis of profile of ChIP peaks binding to TSS regions were performed with ChIPseeker R package (Yu et al., 2015). Transcription network was analyzed and visualized with VertexSort (Jothi et al., 2009) and igraph (https://igraph.org) R packages, respectively, and ChIP peaks of each TF identified in the gene promoter regions (<2kb) were used for analyses. CG1603 binding motif discovery was done using online integrated pipeline ‘peak-motifs’ of RSAT tools (https://rsat.france-bioinformatique.fr/rsat/peak-motifs_form.cgi; Thomas-Chollier et al., 2012).
Imaging analyses
Imaging analyses were performed as previously described (Zhang et al., 2020) using the Zeiss Axio Observer equipped with a Perkin Elmer spinning disk confocal system or a Zeiss LSM880 confocal system. Tissues were dissected out and rinsed in room temperature Schneider’s medium (Thermo Fisher Scientific) supplied with 10% heat inactivated FBS (Thermo Fisher Scientific), and then used for either direct imaging or further staining and fixation. For live imaging, a Zeiss incubation system was used to maintain proper temperature and humidity. Live tissues were mounted with medium on the coverslip in a custom-made metal frame and then covered with a small piece of Saran wrap before imaging. For tissue fixation, PBS containing 4% PFA was used, followed by three washes with PBS. Hoechst 33342 and DAPI (5μg/ml, Thermo Fisher Scientific) incubation in PBS for 5 min was used for nuclear staining of live tissues and fixed tissues, respectively. The image processing and quantification were performed by Volocity (Perkin Elmer, for image acquisition), Zen (Zeiss, for image acquisition), Imaris (Oxford Instruments, https://imaris.oxinst.com/, for 3D surface, voxels, and intensity statistics), and Fiji/ImageJ software (NIH, https://fiji.sc/, for image processing and statistics) based on the previously published methods (Liu et al., 2022; Wang et al., 2023). The relative level of TFAM-GFP or TFAM-mNeonGreen, or relative SDHA-mNeonGreen level, was calculated as the ratio of the mean fluorescence intensity in the RNAi or mutant clone to that of its neighboring control, with background correction. The relative CG1603-mNG level was calculated as the ratio of the mean nuclear fluorescence intensity in the RNAi clone to that of its neighboring control, with background correction. The relative level of mtDNA was determined by calculating the ratio of the TFAM-GFP or TFAM-mNeonGreen puncta number in the RNAi or mutant clone, standardized by clone volume, to that of its neighboring control. The relative mitochondrial volume was calculated as the ratio of the total SDHA-GFP positive voxels with local contrast in the RNAi clone, standardized by clone volume, to that of its neighboring control.
Mitochondrial membrane potential was detected using a protocol adopted from a previous study (Zhang et al., 2020; Zhang et al., 2019). Briefly, after dissection, adult ovaries were incubated in the Schneider’s medium containing TMRM (200nM, Thermo Fisher Scientific) and MitoTracker Green (200nM, Thermo Fisher Scientific) for 20min at room temperature, rinsed with PBS for three times, and then imaged live within 1hr. TMRM and MitoTracker signal intensities were quantified and ratiometric images were generated using Fiji/ImageJ software (NIH). Mitochondrial membrane potential was computed as the ratio of the mean intensity of TMRM to MitoTracker fluorescence with background correction.
Western blot
Protein extracts from wt and CG1603 mutant larvae tissues (48hr after egg laying) were prepared using the RIPA buffer (MilliporeSigma) with Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific), 5mM NaF (MilliporeSigma), and 1mM Na3VO4 (MilliporeSigma). Western blot was performed using a XCell SureLock Mini-Cell and XCell II Blot Module (Thermo Fisher Scientific). Samples were electrophoresed under a reducing condition on NuPAGE 4–12% Bis-Tris Mini Protein Gels (Thermo Fisher Scientific). Proteins on the gel were transferred to a polyvinylidene difluoride membrane (Thermo Fisher Scientific). The membranes were blocked with 5% BSA or non-fat milk (MilliporeSigma) in TBST (Tris buffered saline with 0.1% Tween-20). After a series of washes and incubations with primary antibodies, TBST, and secondary antibodies, the immunoreactivity was visualized using SuperSignal West Dura Chemiluminescent Substrate (Thermo Fisher Scientific) and Amersham ImageQuant 800 system (Cytiva). The antibodies used were: Mouse anti-Actin antibody (C4, MAB1501, MilliporeSigma), Mouse anti-ATP5A antibody (15H4C4, abcam), Mouse anti-ND30 antibody (17D95, abcam), rabbit anti-TFAM antibody (Liu et al., 2022), rabbit anti-HSP60 antibody (#4870, Cell Signaling), Anti-rabbit IgG, HRP-linked Antibody (#7074, Cell Signaling), and Anti-mouse IgG, HRP-linked Antibody (#7076, Cell Signaling).
Blue native PAGE and in-gel activity assays of ETC complexes
Mitochondria from fly larvae were isolated by homogenization and differential centrifugation following previous protocol (Chen et al., 2015). Solubilized protein samples from isolated mitochondria were prepared with NativePAGE Sample Prep Kit (Thermo Fisher Scientific) and the concentrations were determined by Pierce BCA protein assay (Thermo Fisher Scientific). Blue native PAGE was performed using NativePAGE 4–16% Bis-Tris gels and NativePAGE Running Buffer Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. 60µg proteins for each sample were used. For in-gel activity assays, gels were incubated with one of the following solutions: Complex I buffer (5mM Tris-HCl pH 7.4; 0.1mg/ml NADH; 2.5mg/ml Nitro Blue Tetrazolium), Complex II buffer (5mM Tris-HCl pH 7.4; 20mM sodium succinate; 0.2mM phenazine methasulfate; 2.5mg/ml Nitro Blue Tetrazolium) or Complex IV buffer (50mM sodium phosphate pH 7.2; 0.05% 3,3'-diaminobenzidine tetrahydrochloride, 50µM horse heart cytochrome c) at room temperature for hours, stopped by fixation with 50% methanol and 10% acetic acid for 30min and washed with 10% acetic acid. All chemicals from MilliporeSigma.
Quantitative real-time PCR
Total genomic DNAs or RNAs were isolated using the DNeasy Blood & Tissue Kit (QIAGEN) and RNeasy Mini Kit (QIAGEN), respectively, following the manufacturer’s instructions. cDNAs were synthesized by the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific). Real-time PCRs were performed in triplicate using the PowerTrack SYBR Green Master Mix (Thermo Fisher Scientific), MicroAmpOptical 96-Well Reaction Plate with Barcode (Thermo Fisher Scientific), and QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific). Primers for amplifying mtDNA and nuclear DNA, as well as for measuring gene expression levels are listed in Supplementary file 8. The relative mtDNA levels of fly larvae or eye discs were measured in three biological replicates for each group using total DNAs extracted from 20 larvae or eye discs. The relative mRNA levels of ETC genes were measured in three biological replicates for each group using total RNAs extracted from 20 larvae.
Prediction of protein domains, isoelectric point, net charge, and structure
Protein domains were predicted via SMART (Schultz et al., 2000). Protein domain isoelectric point and net charge were predicted using bioinformatic toolbox, Prot pi (https://www.protpi.ch/Calculator/ProteinTool). Protein 3D structure was predicted by AlphaFold (Jumper et al., 2021).
Statistical analysis
Two-tailed Student’s t-test was used for statistical analysis. The difference was considered statistically significant when p<0.05. Results are represented as mean ± SD of the number of determinations.
Acknowledgements
We thank BDSC, Vienna Drosophila Resource Center, and Kyoto Drosophila Genomics and Genetics Resources for various fly lines; NHLBI Light Microscope Core and NHLBI DNA Sequencing and Genomics Core for technical assistance. This work is supported by NHLBI Intramural Research Program.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Hong Xu, Email: hong.xu@nih.gov.
Hugo J Bellen, Baylor College of Medicine, United States.
Benoit Kornmann, University of Oxford, United Kingdom.
Funding Information
This paper was supported by the following grant:
National Heart, Lung, and Blood Institute to Hong Xu.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing – original draft, Project administration, Writing – review and editing.
Data curation, Formal analysis, Investigation.
Data curation, Formal analysis, Investigation, Methodology.
Resources.
Resources, Investigation, Methodology.
Investigation.
Resources.
Conceptualization, Resources, Data curation, Supervision, Funding acquisition, Validation, Visualization, Methodology, Writing – original draft, Project administration, Writing – review and editing.
Additional files
(a) List of differentially expressed nuclear-encoded genes in CG1603 PBac mutant flies compared to controls. (b) Summary of nuclear-encoded electron transport chain (ETC) genes that down-regulated more than twofold in CG1603PBac mutant.
Data availability
Sequencing data have been deposited in GEO under accession code GSE282638.All data generated or analyzed during this study are included in the manuscript and supporting files; source data files have been provided for Figures 1-8.
The following dataset was generated:
Zhang F, Xu H. 2024. CG1603 regulation of nuclear-encoded mitochondrial gene expression. NCBI Gene Expression Omnibus. GSE282638
The following previously published datasets were used:
modENCODEProject 2016. TF ChIP-seq_ab. ENCODE. ENCSR140RMO
modENCODEProject 2019. TF ChIP-seq_abd-A. ENCODE. ENCSR609JDR
modENCODEProject 2016. TF ChIP-seq_achi. ENCODE. ENCSR959SWC
modENCODEProject 2016. TF ChIP-seq_bdp1. ENCODE. ENCSR504AYW
modENCODEProject 2018. TF ChIP-seq_bigmax. ENCODE. ENCSR224YOT
modENCODEProject 2016. TF ChIP-seq_CG10631. ENCODE. ENCSR751FSX
modENCODEProject 2016. TF ChIP-seq_CG11398. ENCODE. ENCSR486MQI
modENCODEProject 2018. TF ChIP-seq_CG12299. ENCODE. ENCSR028DHQ
modENCODEProject 2018. TF ChIP-seq_CG12391. ENCODE. ENCSR483VVZ
modENCODEProject 2018. TF ChIP-seq_CG15011. ENCODE. ENCSR608VFH
modENCODEProject 2019. TF ChIP-seq_CG1603. ENCODE. ENCSR680NFF
modENCODEProject 2016. TF ChIP-seq_CG16863. ENCODE. ENCSR848GYF
modENCODEProject 2018. TF ChIP-seq_CG17806. ENCODE. ENCSR118TAH
modENCODEProject 2019. TF ChIP-seq_CG17829. ENCODE. ENCSR810MLF
modENCODE 2018. TF ChIP-seq_CG2116. ENCODE. ENCSR456UZW
modENCODEProject 2018. TF ChIP-seq_CG4707. ENCODE. ENCSR302AQS
modENCODEProject 2016. TF ChIP-seq_CG4854. ENCODE. ENCSR860FUA
modENCODEProject 2017. TF ChIP-seq_CG6254. ENCODE. ENCSR233UXG
modENCODEProject 2018. TF ChIP-seq_CG7987. ENCODE. ENCSR681RZY
modENCODEProject 2018. TF ChIP-seq_CG9437. ENCODE. ENCSR965XSS
modENCODEProject 2017. TF ChIP-seq_CG9727. ENCODE. ENCSR624PKN
modENCODEProject 2016. TF ChIP-seq_CHES-1-like. ENCODE. ENCSR791TWT
modENCODEProject 2016. TF ChIP-seq_Crg-1. ENCODE. ENCSR596YNC
modENCODEProject 2017. TF ChIP-seq_CTCF. ENCODE. ENCSR661BEZ
modENCODEProject 2016. TF ChIP-seq_E(bx) ENCODE. ENCSR559AJG
modENCODEProject 2019. TF ChIP-seq_Ets96B. ENCODE. ENCSR572JUN
modENCODEProject 2017. TF ChIP-seq_ewg. ENCODE. ENCSR444JKK
modENCODEProject 2018. TF ChIP-seq_Fer3. ENCODE. ENCSR367SBM
modENCODEProject 2016. TF ChIP-seq_ftz-f1. ENCODE. ENCSR741KZZ
modENCODEProject 2016. TF ChIP-seq_ind. ENCODE. ENCSR486EPD
modENCODEProject 2016. TF ChIP-seq_Jra. ENCODE. ENCSR471GSA
modENCODEProject 2017. TF ChIP-seq_Lhr. ENCODE. ENCSR272DEA
modENCODEProject 2016. TF ChIP-seq_luna. ENCODE. ENCSR770AUN
modENCODEProject 2016. TF ChIP-seq_Met. ENCODE. ENCSR363SHZ
modENCODEProject 2016. TF ChIP-seq_Myb. ENCODE. ENCSR393AKW
modENCODEProject 2017. TF ChIP-seq_Myc. ENCODE. ENCSR191VCQ
modENCODEProject 2019. TF ChIP-seq_nerfin-1. ENCODE. ENCSR335NNR
modENCODEProject 2018. TF ChIP-seq_Nnk. ENCODE. ENCSR165KWP
modENCODEProject 2016. TF ChIP-seq_p53. ENCODE. ENCSR808XNJ
modENCODEProject 2017. TF ChIP-seq_pan. ENCODE. ENCSR033IIP
modENCODEProject 2019. TF ChIP-seq_pros. ENCODE. ENCSR682YQM
modENCODEProject 2017. TF ChIP-seq_REPTOR-BP. ENCODE. ENCSR271ZJI
modENCODEProject 2016. TF ChIP-seq_salr. ENCODE. ENCSR042XCV
modENCODEProject 2019. TF ChIP-seq_Stat92E. ENCODE. ENCSR290OJD
modENCODEProject 2017. TF ChIP-seq_su(Hw) ENCODE. ENCSR761TCG
modENCODEProject 2019. TF ChIP-seq_trem. ENCODE. ENCSR104XOO
modENCODEProject 2016. TF ChIP-seq_Trl. ENCODE. ENCSR629WQT
modENCODEProject 2016. TF ChIP-seq_Xbp1. ENCODE. ENCSR698IEF
modENCODEProject 2017. TF ChIP-seq_YL-1. ENCODE. ENCSR396LMJ
References
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Molecular Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Alam TI, Kanki T, Muta T, Ukaji K, Abe Y, Nakayama H, Takio K, Hamasaki N, Kang D. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Research. 2003;31:1640–1645. doi: 10.1093/nar/gkg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini C, Costa V. Understanding gene regulatory mechanisms by integrating ChIP-seq and RNA-seq data: statistical solutions to biological problems. Frontiers in Cell and Developmental Biology. 2014;2:51. doi: 10.3389/fcell.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annual Review of Medicine. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Latek RR, Peterson CL. The SANT domain: a unique histone-tail-binding module? Nature Reviews. Molecular Cell Biology. 2004;5:158–163. doi: 10.1038/nrm1314. [DOI] [PubMed] [Google Scholar]
- Chen Z, Qi Y, French S, Zhang G, Covian Garcia R, Balaban R, Xu H. Genetic mosaic analysis of a deleterious mitochondrial DNA mutation in Drosophila reveals novel aspects of mitochondrial regulation and function. Molecular Biology of the Cell. 2015;26:674–684. doi: 10.1091/mbc.E14-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang F, Xu H. Human mitochondrial DNA diseases and Drosophila models. Journal of Genetics and Genomics = Yi Chuan Xue Bao. 2019;46:201–212. doi: 10.1016/j.jgg.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang ZH, Zhang G, Bleck CKE, Chung DJ, Madison GP, Lindberg E, Combs C, Balaban RS, Xu H. Mitochondrial DNA segregation and replication restrict the transmission of detrimental mutation. The Journal of Cell Biology. 2020;219:e201905160. doi: 10.1083/jcb.201905160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillion MT, Soto IC, Shipkovenska G, Churchman LS. Synchronized mitochondrial and cytosolic translation programs. Nature. 2016;533:499–503. doi: 10.1038/nature18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois G, Giguère V. Functional and physiological genomics of estrogen-related receptors (ERRs) in health and disease. Biochimica et Biophysica Acta. 2011;1812:1032–1040. doi: 10.1016/j.bbadis.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. PNAS. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nature Genetics. 2002;31:289–294. doi: 10.1038/ng909. [DOI] [PubMed] [Google Scholar]
- Fedotova AA, Bonchuk AN, Mogila VA, Georgiev PG. C2H2 zinc finger proteins: the largest but poorly explored family of higher eukaryotic transcription factors. Acta Naturae. 2017;9:47–58. [PMC free article] [PubMed] [Google Scholar]
- Fernández-Vizarra E, Enríquez JA, Pérez-Martos A, Montoya J, Fernández-Silva P. Tissue-specific differences in mitochondrial activity and biogenesis. Mitochondrion. 2011;11:207–213. doi: 10.1016/j.mito.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM. Mitochondrial diseases. Nature Reviews. Disease Primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- Hill JH, Chen Z, Xu H. Selective propagation of functional mitochondrial DNA during oogenesis restricts the transmission of a deleterious mitochondrial variant. Nature Genetics. 2014;46:389–392. doi: 10.1038/ng.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annual Review of Physiology. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- Jiang S, Mortazavi A. Integrating ChIP-seq with other functional genomics data. Briefings in Functional Genomics. 2018;17:104–115. doi: 10.1093/bfgp/ely002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothi R, Balaji S, Wuster A, Grochow JA, Gsponer J, Przytycka TM, Aravind L, Babu MM. Genomic analysis reveals a tight link between transcription factor dynamics and regulatory network architecture. Molecular Systems Biology. 2009;5:294. doi: 10.1038/msb.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudron MM, Victorsen A, Gevirtzman L, Hillier LW, Fisher WW, Vafeados D, Kirkey M, Hammonds AS, Gersch J, Ammouri H, Wall ML, Moran J, Steffen D, Szynkarek M, Seabrook-Sturgis S, Jameel N, Kadaba M, Patton J, Terrell R, Corson M, Durham TJ, Park S, Samanta S, Han M, Xu J, Yan K-K, Celniker SE, White KP, Ma L, Gerstein M, Reinke V, Waterston RH. The ModERN Resource: Genome-wide binding profiles for hundreds of Drosophila and Caenorhabditis elegans transcription factors. Genetics. 2018;208:937–949. doi: 10.1534/genetics.117.300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland CG, Andersson SG. Origin and evolution of the mitochondrial proteome. Microbiology and Molecular Biology Reviews. 2000;64:786–820. doi: 10.1128/MMBR.64.4.786-820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws KM, Drummond-Barbosa D. Genetic mosaic analysis of stem cell lineages in the Drosophila Ovary. Methods in Molecular Biology. 2015;1328:57–72. doi: 10.1007/978-1-4939-2851-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S. The sequence alignment/map format and samtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Shan S, Pan L, Zhao J, Ranjan A, Wang F, Zhang Z, Huang Y, Feng H, Wei D, Huang L, Liu X, Zhong Q, Lou J, Li G, Wu C, Zhou Z. Structural basis of H2A.Z recognition by SRCAP chromatin-remodeling subunit YL1. Nature Structural & Molecular Biology. 2016;23:317–323. doi: 10.1038/nsmb.3190. [DOI] [PubMed] [Google Scholar]
- Liao TSV, Call GB, Guptan P, Cespedes A, Marshall J, Yackle K, Owusu-Ansah E, Mandal S, Fang QA, Goodstein GL, Kim W, Banerjee U. An efficient genetic screen in Drosophila to identify nuclear-encoded genes with mitochondrial function. Genetics. 2006;174:525–533. doi: 10.1534/genetics.106.061705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen Z, Wang ZH, Delaney KM, Tang J, Pirooznia M, Lee DY, Tunc I, Li Y, Xu H. The PPR domain of mitochondrial RNA polymerase is an exoribonuclease required for mtDNA replication in Drosophila melanogaster. Nature Cell Biology. 2022;24:757–765. doi: 10.1038/s41556-022-00887-y. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowden C, Boulet A, Boehler NA, Seecharran S, Rios Garcia J, Lowe NJ, Liu J, Ong JLK, Wang W, Ma L, Cheng AH, Senatore A, Monks DA, Liu B-H, Leary SC, Cheng H-YM. Homeostatic control of nuclear-encoded mitochondrial gene expression by the histone variant H2A.Z is essential for neuronal survival. Cell Reports. 2021;36:109704. doi: 10.1016/j.celrep.2021.109704. [DOI] [PubMed] [Google Scholar]
- Maheshwari S, Wang J, Barbash DA. Recurrent positive selection of the Drosophila hybrid incompatibility gene Hmr. Molecular Biology and Evolution. 2008;25:2421–2430. doi: 10.1093/molbev/msn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Developmental Cell. 2005;9:843–854. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- mforde84 ENCODE_TF_ChIP_pipeline. swh:1:rev:e2c7dc403d6d44a8eb667c20b9bf45e091d27134Software Heritage. 2016 https://archive.softwareheritage.org/swh:1:dir:5639bfed7102cc98642baf0f340782b309fcb56d;origin=https://github.com/mforde84/ENCODE_TF_ChIP_pipeline;visit=swh:1:snp:df73736416d2b812d0676475f5f6436bedad3f3d;anchor=swh:1:rev:e2c7dc403d6d44a8eb667c20b9bf45e091d27134
- Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nature Genetics. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nature Reviews. Genetics. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torrado R, Yamada D, Defossez PA. Born to bind: the BTB protein-protein interaction domain. BioEssays. 2006;28:1194–1202. doi: 10.1002/bies.20500. [DOI] [PubMed] [Google Scholar]
- Persikov AV, Wetzel JL, Rowland EF, Oakes BL, Xu DJ, Singh M, Noyes MB. A systematic survey of the Cys2His2 zinc finger DNA-binding landscape. Nucleic Acids Research. 2015;43:1965–1984. doi: 10.1093/nar/gku1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–446. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Putri GH, Anders S, Pyl PT, Pimanda JE, Zanini F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics. 2022;38:2943–2945. doi: 10.1093/bioinformatics/btac166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S, Sharma R, Gupta R, Ast T, Chan C, Durham TJ, Goodman RP, Grabarek Z, Haas ME, Hung WHW, Joshi PR, Jourdain AA, Kim SH, Kotrys AV, Lam SS, McCoy JG, Meisel JD, Miranda M, Panda A, Patgiri A, Rogers R, Sadre S, Shah H, Skinner OS, To T-L, Walker MA, Wang H, Ward PS, Wengrod J, Yuan C-C, Calvo SE, Mootha VK. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Research. 2021;49:D1541–D1547. doi: 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouyer F, Rachidi M, Pikielny C, Rosbash M. A new gene encoding A putative transcription factor regulated by the Drosophila circadian clock. The EMBO Journal. 1997;16:3944–3954. doi: 10.1093/emboj/16.13.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiological Reviews. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends in Endocrinology and Metabolism. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner O, Berdnik D, Levy JM, Wu JS, Luginbuhl D, Gontang AC, Luo L. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Developmental Cell. 2008;14:227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Copley RR, Doerks T, Ponting CP, Bork P. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Research. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Pender CL, Bar-Ziv R, Zhang H, Wickham K, Willey E, Durieux J, Ahmad Q, Dillin A. Mitochondria as cellular and organismal signaling hubs. Annual Review of Cell and Developmental Biology. 2022;38:179–218. doi: 10.1146/annurev-cellbio-120420-015303. [DOI] [PubMed] [Google Scholar]
- Sobocińska J, Molenda S, Machnik M, Oleksiewicz U. KRAB-ZFP transcriptional regulators acting as oncogenes and tumor suppressors: an overview. International Journal of Molecular Sciences. 2021;22:2212. doi: 10.3390/ijms22042212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nature Cell Biology. 2018;20:745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nature Reviews. Genetics. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Chollier M, Darbo E, Herrmann C, Defrance M, Thieffry D, van Helden J. A complete workflow for the analysis of full-size ChIP-seq (and similar) data sets using peak-motifs. Nature Protocols. 2012;7:1551–1568. doi: 10.1038/nprot.2012.088. [DOI] [PubMed] [Google Scholar]
- Verheul TCJ, van Hijfte L, Perenthaler E, Barakat TS. The Why of YY1: Mechanisms of transcriptional regulation by yin yang 1. Frontiers in Cell and Developmental Biology. 2020;8:592164. doi: 10.3389/fcell.2020.592164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZH, Liu Y, Chaitankar V, Pirooznia M, Xu H. Electron transport chain biogenesis activated by a JNK-insulin-Myc relay primes mitochondrial inheritance in Drosophila. eLife. 2019;8:e49309. doi: 10.7554/eLife.49309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZH, Zhao W, Combs CA, Zhang F, Knutson JR, Lilly MA, Xu H. Mechanical stimulation from the surrounding tissue activates mitochondrial energy metabolism in Drosophila differentiating germ cells. Developmental Cell. 2023;58:2249–2260. doi: 10.1016/j.devcel.2023.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N, Pfanner N. Mitochondrial machineries for protein import and assembly. Annual Review of Biochemistry. 2017;86:685–714. doi: 10.1146/annurev-biochem-060815-014352. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annual Review of Biophysics and Biomolecular Structure. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, Wu C, Allis CD. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- Xu H, DeLuca SZ, O’Farrell PH. Manipulating the metazoan mitochondrial genome with targeted restriction enzymes. Science. 2008;321:575–577. doi: 10.1126/science.1160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Yao J, Shi Y, Yi H, Zhao W, Lin X, Yang Z. The SRCAP chromatin remodeling complex promotes oxidative metabolism during prenatal heart development. Development. 2021;148:dev199026. doi: 10.1242/dev.199026. [DOI] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of chip-seq (MACS) Genome Biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Qi Y, Zhou K, Zhang G, Linask K, Xu H. The cAMP phosphodiesterase Prune localizes to the mitochondrial matrix and promotes mtDNA replication by stabilizing TFAM. EMBO Reports. 2015;16:520–527. doi: 10.15252/embr.201439636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Gucek M, Xu H. The mitochondrial outer membrane protein MDI promotes local protein synthesis and mtDNA replication. The EMBO Journal. 2016;35:1045–1057. doi: 10.15252/embj.201592994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang ZH, Liu Y, Chen Y, Sun N, Gucek M, Zhang F, Xu H. PINK1 inhibits local protein synthesis to limit transmission of deleterious mitochondrial DNA mutations. Molecular Cell. 2019;73:1127–1137. doi: 10.1016/j.molcel.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Pirooznia M, Xu H. Mitochondria regulate intestinal stem cell proliferation and epithelial homeostasis through FOXO. Molecular Biology of the Cell. 2020;31:1538–1549. doi: 10.1091/mbc.E19-10-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Sherwood B, Ji H. Computational prediction of the global functional genomic landscape: applications, methods, and challenges. Human Heredity. 2016;81:88–105. doi: 10.1159/000450827. [DOI] [PMC free article] [PubMed] [Google Scholar]