Abstract
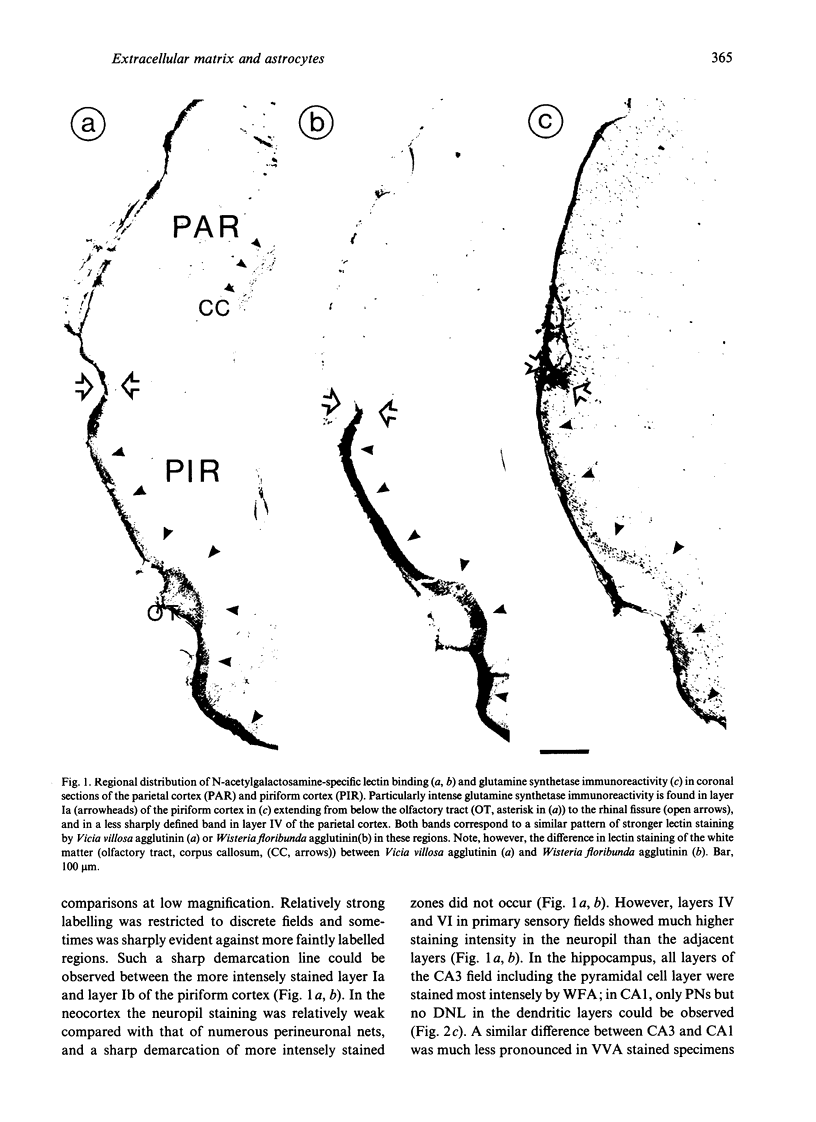
Extracellular matrix proteoglycans have previously been revealed by immunocytochemical and lectin-histochemical methods as distinct perineuronal nets in the microenvironment of different types of neurons, but also as a diffuse stain throughout the neuropil in region-dependent patterns. Ultrastructural investigations of perineuronal nets in subcortical regions have demonstrated glycan components in the close vicinity of astrocyte processes, suggesting that the extracellular matrix contributes differentially to the glianeuron interface. In the present study the spatial relationship of extracellular matrix components and astrocytes was characterised at the regional and cellular level by lectin histochemistry (soybean agglutinin, Vicia villosa agglutinin, Wisteria floribunda agglutinin) and antiglutamine synthetase immunocytochemistry in the rat neocortex and hippocampus. In most cortical areas layer-specific patterns of diffuse neuropil staining revealed by the lectins could also be recognised after glutamine synthetase (GS) immunostaining. In double-labelling experiments GS-immunoreactive astrocyte processes were found to reach lectin-stained perineuronal nets. GS-immunoreactivity was often parallelled but did not coincide with the lectin label completely, but was observed to form net-like structures similar to the perineuronal lectin staining. Using immunocytochemistry with anti-GS perineuronal, net-like structures were demonstrated on certain parvalbumin-immunopositive neurons which are known to be ensheathed by lectin-stained perineuronal nets. It was evident that a single neuron may receive net-like contacts from several astrocytes and that a single astrocyte can contribute to perineuronal nets on more than one neuron. The findings support the view that N-acetylgalactosamine-containing extracellular matrix molecules and astrocytic processes are topically associated to a high degree. Different proportions of both components may specify the individual neuronal micro-environment.
Full text
PDF

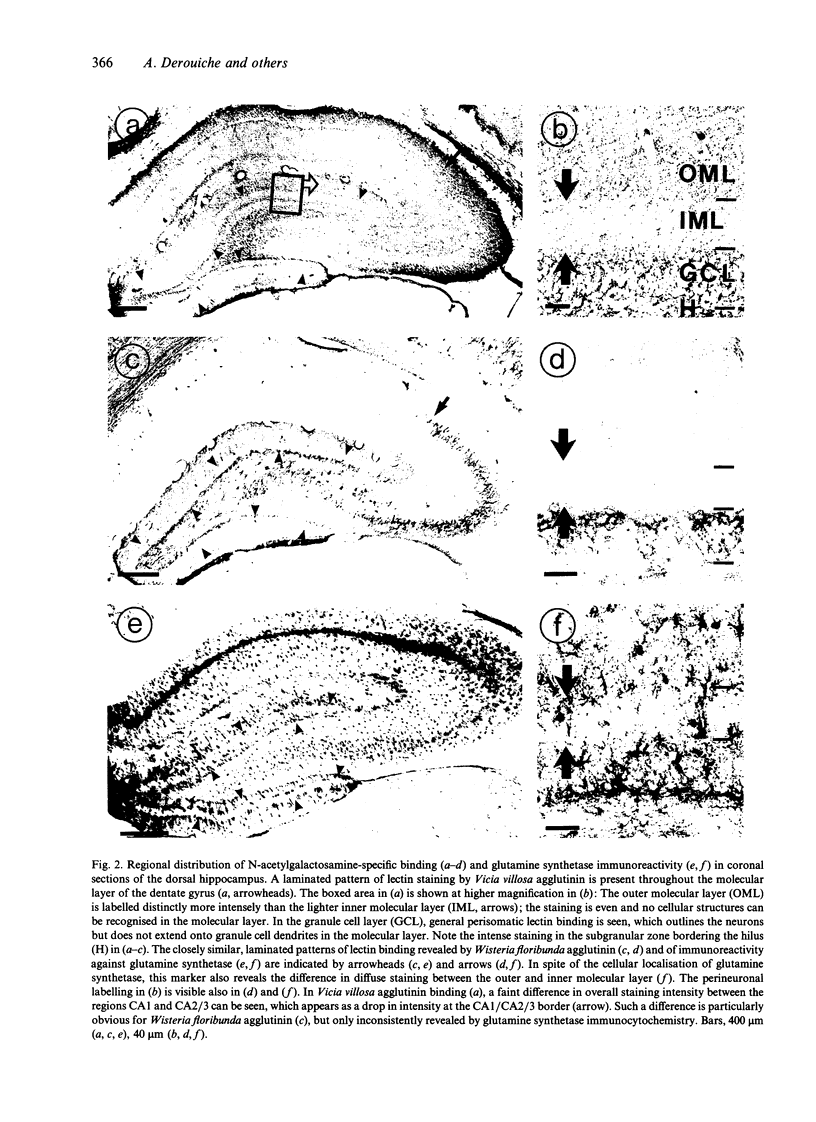






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolotto A., Rocca G., Canavese G., Migheli A., Schiffer D. Chondroitin sulfate proteoglycan surrounds a subset of human and rat CNS neurons. J Neurosci Res. 1991 Jun;29(2):225–234. doi: 10.1002/jnr.490290213. [DOI] [PubMed] [Google Scholar]
- Bignami A., Asher R., Perides G. Co-localization of hyaluronic acid and chondroitin sulfate proteoglycan in rat cerebral cortex. Brain Res. 1992 May 1;579(1):173–177. doi: 10.1016/0006-8993(92)90759-3. [DOI] [PubMed] [Google Scholar]
- Blümcke I., Eggli P., Celio M. R. Relationship between astrocytic processes and "perineuronal nets" in rat neocortex. Glia. 1995 Oct;15(2):131–140. doi: 10.1002/glia.440150205. [DOI] [PubMed] [Google Scholar]
- Brauer K., Brückner G., Leibnitz L., Werner L. Structural and cytochemical features of perineuronal glial nets in the rat brain. Acta Histochem. 1984;74(1):53–60. doi: 10.1016/S0065-1281(84)80026-4. [DOI] [PubMed] [Google Scholar]
- Brauer K., Werner L., Leibnitz L. Perineuronal nets of glia. J Hirnforsch. 1982;23(6):701–708. [PubMed] [Google Scholar]
- Brückner G., Brauer K., Härtig W., Wolff J. R., Rickmann M. J., Derouiche A., Delpech B., Girard N., Oertel W. H., Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993 Jul;8(3):183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- Brückner G., Delpech B., Delpech A., Girard N. Concentration of hyaluronectin and anionic glycoconjugates in perineuronal glial cell processes at GABAergic synapses of rat cerebellum. Acta Histochem Suppl. 1990;38:161–165. [PubMed] [Google Scholar]
- Brückner G., Seeger G., Brauer K., Härtig W., Kacza J., Bigl V. Cortical areas are revealed by distribution patterns of proteoglycan components and parvalbumin in the Mongolian gerbil and rat. Brain Res. 1994 Sep 26;658(1-2):67–86. doi: 10.1016/s0006-8993(09)90012-9. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Baier W., Schärer L., de Viragh P. A., Gerday C. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988 Apr;9(2):81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Blümcke I. Perineuronal nets--a specialized form of extracellular matrix in the adult nervous system. Brain Res Brain Res Rev. 1994 Jan;19(1):128–145. doi: 10.1016/0165-0173(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Conti F., Rustioni A., Petrusz P., Towle A. C. Glutamate-positive neurons in the somatic sensory cortex of rats and monkeys. J Neurosci. 1987 Jun;7(6):1887–1901. doi: 10.1523/JNEUROSCI.07-06-01887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche A., Frotscher M. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: evidence for transmitter degradation. Brain Res. 1991 Jun 28;552(2):346–350. doi: 10.1016/0006-8993(91)90103-3. [DOI] [PubMed] [Google Scholar]
- Hallermayer K., Hamprecht B. Cellular heterogeneity in primary cultures of brain cells revealed by immunocytochemical localization of glutamine synthetase. Brain Res. 1984 Mar 12;295(1):1–11. doi: 10.1016/0006-8993(84)90810-2. [DOI] [PubMed] [Google Scholar]
- Härtig W., Brauer K., Bigl V., Brückner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 1994 Jan 28;635(1-2):307–311. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- Härtig W., Brauer K., Brückner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992 Oct;3(10):869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Heizmann C. W. Selective staining of a population of parvalbumin-containing GABAergic neurons in the rat cerebral cortex by lectins with specific affinity for terminal N-acetylgalactosamine. Brain Res. 1989 Mar 27;483(1):158–163. doi: 10.1016/0006-8993(89)90048-6. [DOI] [PubMed] [Google Scholar]
- Lafarga M., Berciano M. T., Blanco M. The perineuronal net in the fastigial nucleus of the rat cerebellum. A Golgi and quantitative study. Anat Embryol (Berl) 1984;170(1):79–85. doi: 10.1007/BF00319461. [DOI] [PubMed] [Google Scholar]
- Naegele J. R., Barnstable C. J. Molecular determinants of GABAergic local-circuit neurons in the visual cortex. Trends Neurosci. 1989 Jan;12(1):28–34. doi: 10.1016/0166-2236(89)90153-7. [DOI] [PubMed] [Google Scholar]
- Naegele J. R., Katz L. C. Cell surface molecules containing N-acetylgalactosamine are associated with basket cells and neurogliaform cells in cat visual cortex. J Neurosci. 1990 Feb;10(2):540–557. doi: 10.1523/JNEUROSCI.10-02-00540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa F., Schulte B. A., Wu J. Y., Spicer S. S. GABAergic neurons of rodent brain correspond partially with those staining for glycoconjugate with terminal N-acetylgalactosamine. J Neurocytol. 1986 Jun;15(3):389–396. doi: 10.1007/BF01611440. [DOI] [PubMed] [Google Scholar]
- Norenberg M. D., Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979 Feb 2;161(2):303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Price J. L. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol. 1973 Jul 1;150(1):87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Streit W. J., Müller C. M. Postnatal development and localization of an N-acetylgalactosamine containing glycoconjugate associated with nonpyramidal neurons in cat visual cortex. J Comp Neurol. 1993 Mar 15;329(3):313–327. doi: 10.1002/cne.903290303. [DOI] [PubMed] [Google Scholar]
- Seeger G., Brauer K., Härtig W., Brückner G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience. 1994 Jan;58(2):371–388. doi: 10.1016/0306-4522(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976 Jun 1;167(3):285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Westrum L. E., Bakay R. A. Plasticity in the rat olfactory cortex. J Comp Neurol. 1986 Jan 8;243(2):195–206. doi: 10.1002/cne.902430205. [DOI] [PubMed] [Google Scholar]
- Witter M. P., Griffioen A. W., Jorritsma-Byham B., Krijnen J. L. Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci Lett. 1988 Feb 29;85(2):193–198. doi: 10.1016/0304-3940(88)90350-3. [DOI] [PubMed] [Google Scholar]
- Wouterlood F. G., Hrtig W. Calretinin-immunoreactivity in mitral cells of the rat olfactory bulb. Brain Res. 1995 Jun 5;682(1-2):93–100. doi: 10.1016/0006-8993(95)00326-l. [DOI] [PubMed] [Google Scholar]