Simple Summary
Ant gardens (AGs) constitute a sophisticated example of mutualism between ants and plants (myrmecochory), characterized by intricate interspecific relationships with vascular epiphytes. Our main aim was to characterize the epiphytes and their associated ants in the Tingana Reserve in San Martín, situated at 800 m. a.s.l. This unique and humid ecosystem, distinguished by its altitude and microclimate, is home to a diverse array of plant species, including aguaje (Mauritia flexuosa). The area falls within the distribution range of Neotropical AGs, and our results highlight the ecological significance of ant specificity in seed dispersal among the epiphytes of the Mauritia flexuosa peat swamp forest in Peruvian ecosystems.
Keywords: ant-plant interaction, Azteca, Camponotus, hymenoptera, parabiosis, phorophytes
Abstract
Mutualisms characterized by reciprocal benefits between species are a fundamental relationship of tropical ecosystems. Ant Gardens (AGs) represent an interesting ant-plant mutualism, involving specialized interactions between vascular epiphytes and ants. While this relationship has been extensively studied in various tropical regions, the available information on Peruvian ecosystems is limited. The objective of this study was to identify the ant and epiphyte species that constitute AGs. From February 2023 to January 2024, a study was conducted on two 50 × 10 m transects within the Mauritia flexuosa peat swamp forest, located within the Water Association Aguajal Renacal del Alto Mayo (ADECARAM) Tingana in San Martín, Peru. A total of 69 ant gardens were documented, comprising 18 phorophyte species, 19 epiphyte species, and three ant species. The results demonstrated that neither the height nor the diameter at breast height (DBH) of phorophytes exhibited a statistically significant correlation with the number of AGs per host. However, a positive correlation was observed between the length and width of the AGs and the number of ants per AG. The findings of this study contribute to the understanding of AG mutualism in Peruvian ecosystems.
1. Introduction
Tropical rainforests are characterized by complex and dynamic systems, in which biotic interactions are the primary determinants of ecosystem function and structure [1]. Within these ecological interactions, the mutualistic association between epiphytic species and ants demonstrates highly specialized coevolutionary processes [2]. The ant gardens (AGs) represent a highly intricate form of ant-plant mutualism [3], leading to physiological and morphological adaptations that allow taxa to thrive in competitive environments [3,4]. The new information of these interactions contributes to enhancing the understanding of biodiversity and ecological processes in tropical forests.
In this complex system, ants select seeds from specific epiphytes, primarily guided by distinctive nutritional coatings or pheromones of the plant species. These seeds have morphological adaptations (specialized structures or appendages) that facilitate their transport and dispersal by the ants [5]. When germinated, the seeds not only transform the nest into an enriched microhabitat, but also moreover provide constant resources to the ants [6]. This interaction is a mutualism in which epiphytes receive protection from herbivory and continuous nutrients from the nitrogen-rich excretions of ants [7]. In addition, ants obtain benefits from the nectar and microhabitat provided by epiphytes [8]. It is worth noting that certain epiphytes, such as bromeliads, develop specialized structures capable of accumulating water, thus providing an optimal environment for ants [9].
Specific environmental factors of tropical ecosystems favor the presence of AGs. In fact, the extensive coverage and wide branching of tree vegetation in tropical forests acts as a filter for solar radiation at different levels of the canopy [10], allowing the development of epiphytic species associated with AG. These plants do not require permanent or direct exposure to light, but they do need moderate levels of light to carry out photosynthesis efficiently [11]. Furthermore, good air circulation is essential [12], as it not only facilitates transpiration and reduces the risk of fungal diseases in epiphytes, but also benefits ant colonies [13]. Rainfall and rain cycles influence ant activity patterns and epiphyte growth [14], with periods of lower rainfall coinciding with reduced periods of ant foraging, and periods of higher rainfall favoring expansion of AGs [15,16]. This balance culminates in a beneficial exchange, in return for the shelter, the ants carry out an active defense of the plant against herbivores, eradicating competing organisms and even removing nearby plant growth that could overshadow their host plant [17,18].
Azteca, Camponotus, and Crematogaster are genus ants and are distributed throughout the Neotropics, from Mexico to Brazil [19,20,21]. These ants typically construct their nests in shrubs and trees belonging to the families Lauraceae, Melastomataceae, Orchidaceae, Moraceae, Urticaceae, and Rubiaceae [22]. They exhibit a robust defense mechanism against external agents, effectively preventing herbivory [23,24], they participate in the dispersal of seeds of epiphytic species [25] and maintain a nutrient-rich substrate by incorporating organic waste such as vertebrate excreta [26].
Despite the crucial function of AGs, there remains a significant knowledge gap regarding the dynamics and diversity of epiphytes that comprise them in tropical zones, particularly in the Andean–Amazonian piedmont of Peru. This emphasizes the necessity for in-depth information in this area. The objective of this research was to determine the richness of ants, the composition of epiphytes, and the diversity of phorophytes in AGs in areas with different levels of human disturbance in the Mauritia flexuosa peat swamp forest of San Martín State, Peru. This research will provide important insights into mutualistic relationships in tropical ecosystems, which will support biodiversity conservation efforts and add to the limited knowledge of ant gardens.
2. Materials and Methods
2.1. Study Area
Alto Mayo Valley’s Andean–Amazonian piedmont is located between the yungas of the eastern Peruvian Andes and the low-lying seasonally flooded area of Central Huallaga [27]. This transitional area (20–30% slope) is characterized by a humid subtropical climate with rainfall concentrated during a single wet season between October and April [28]. The life zone is humid subtropical, mean temperature and annual precipitation are 22.8 °C and 1265 mm, respectively [29]. The soils of the Alto Mayo flooded forest are characterized by peat deposits accumulated since the Quaternary [30].
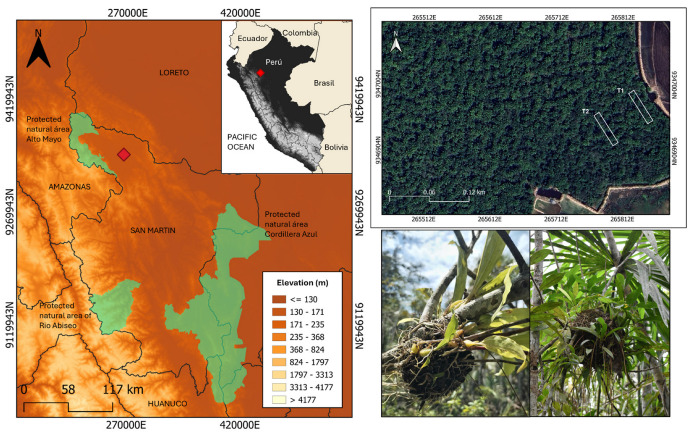
The study area was in ADECARAM Tingana (Water Association Aguajal Renacal del Alto Mayo, ecotourism; 05°54′17.9″ S, 77°07′07.5″ W) and was carried out from February 2023 to January 2024 in a Mauritia flexuosa peat swamp forest with frequent anthropic activity (selective extraction of wood and vanilla) [31]. In these peat swamp forests, palms dominate the canopy (>20 m). Additionally, we find pioneer trees and shrubs (Cecropia, Inga, Tococa, Miconia) being colonized by epiphytic plants [32] in territories that were recently deforested and that have gaps in the canopy, in addition to the presence of peat and Sphagnum moss covering the ground. The recurrent floods caused by the Huascayacu and Avisado rivers, close to Tingana, cause recurrent flooding within the territories. This restricts the distribution of species that are unable to adapt to these conditions (Figure 1).
Figure 1.
Study area in Andean–Amazonian piedmont (San Martín) and Mauritia flexuosa peat swamp forest, showing transect locations (up) and a typical garden tree.
2.2. Field Sampling
Two 50 × 10 m transects were used for sampling, with all ant gardens identified. The first transect (T1) was situated at the forest edge close to agricultural lands, including rice, coffee, cocoa, and banana plantations. The second transect (T2) was positioned 50 m into the forest, parallel to the first. As a consequence of selective deforestation, the edges of the forest are becoming less visible in these territories.
For each phorophyte containing AGs, the following protocol was implemented:
-
(i)
Photographic documentation of both the phorophytes and epiphytes;
-
(ii)
Triplicate sampling of phorophytes and epiphytes;
-
(iii)
Recording of dasometric variables for the phorophytes;
-
(iv)
Measuring the length, width, and height of the AGs from the ground;
-
(v)
Collecting samples of the associated ant species.
Moreover, 20 AGs were collected from both transects and transported to the laboratory for measurement and ant counting. Botanical nomenclature was based on W3-Tropicos (www.tropicos.org). The conservation status of recorded species was noted according to the Red List criteria and the Peruvian categorization of threatened flora species (Decreto Supremo N° 043–2006–AG). Ants associated with AGs were identified at the Entomology’s Department of the Natural History Museum at the Universidad Nacional Mayor de San Marcos, using keys from Longino [21,22,23,24,25,26,27,28,29,30,31,32,33], Mackay [34], and Feitosa and Dias [35]. Furthermore, the specimens were deposited at the Tropical Ecology and Data Analysis Laboratory at the Universidad Nacional Mayor de San Marcos.
2.3. Data Analysis
A one-way analysis of variance (ANOVA) was conducted to assess the significant effects of the two most common host species on epiphyte richness and the number of ant gardens (AGs) among the two most common hosts. A Pearson correlation was conducted to examine the relationship between the height and diameter at breast height (DBH) of the host trees and the number of AGs per host. The χ2 test was performed to determine if there were a significant difference for richness and abundance of epiphytes, and length and width of AGs between transects. A Pearson correlation was performed between the length and width of the AGs with 162 the abundance of ants per AGs. All statistical tests and correlation analyses were performed using the statistical software Statgraphics Centurion v.16.
Analysis of Similarities (ANOSIM) was performed using the Bray–Curtis distance (9999 permutations), considering the epiphytes composition species per transects and sorted the species composition using non-metric multidimensional scaling analysis (NMDS) with Bray–Curtis index. The data were analyzed using the gsankey library [36] to determine the preference of AG compositions over phorophytes in Tingana. The tripartite figure shows nodes representing species of epiphytes (left), ants (center) and host species (right). The NMDS and the tripartite figure were carried out with the Rstudio software version 2024.04.1+748 using the vegan packages developed by Oksanen et al. [37].
3. Results
3.1. Characterization of Phorophytes
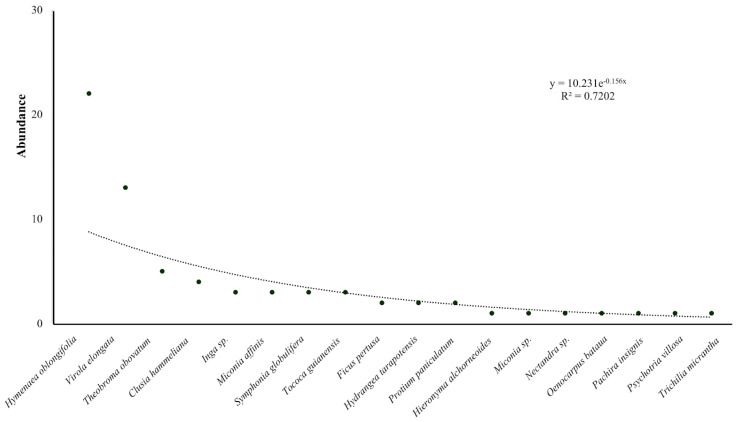
Eighty-nine AGs were found in 69 phorophytes belonging to 13 families, 17 genera, and 18 species (Table A1). Hymenaea oblongifolia, Virola elongata, and Theobroma obovatum were the most common phorophytes observed in Tingana, accounting for 64% of the observations (Figure 2). The botanical families Fabaceae, Myristicaceae, Melastomataceae, and Malvaceae were identified as the primary hosts for AG. The only palm species identified as a phorophyte was Oenocarpus bataua, with a single AG recorded at a height of 3 m.
Figure 2.
Dominance−diversity graph of phorophyte species in Tingana.
A total of 78% of phorophytes had only one AG, while 17.4% had two ant gardens. Hymenaea oblongifolia and Inga sp. accounted for 50% of the phorophytes hosting two AGs. The only phorophytes with four AGs were Theobroma obovatum and Tococa guianensis. Richness (T = 1.21743; df = 1; p = 0.23207) and number of AGs per phorophyte (T = 0.687798; df = 1; p = 0.496387) did not show significant differences between the two most common phorophytes. Phorophyte height (p = 0.9463; R = 0.008) and DBH (p = 0.7244; R = −0.04) were not significantly related to the number of AGs per host.
3.2. Characterization of Epiphytic Species
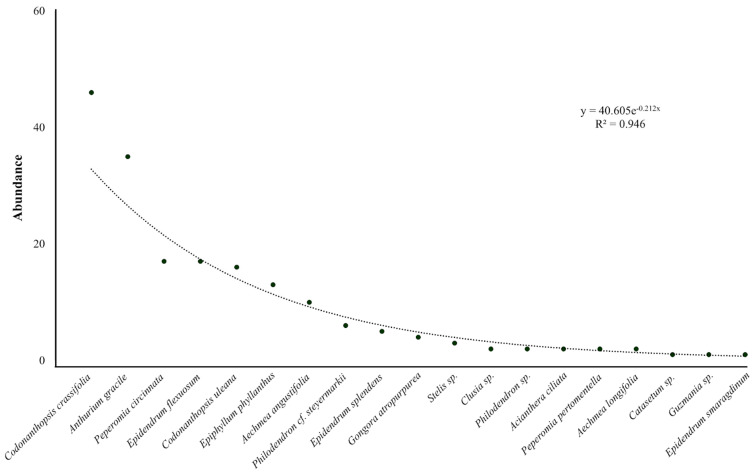
In the first and the second transect, 32 and 57 AGs, respectively, were recorded. A total of 180 epiphytic individuals were observed within the AGs, belonging to 19 species, 13 genera, and 7 families. The best represented families were Orchidaceae (seven species) and Araceae (three species), together accounting for 52.6% of the total richness. A total of 57.9% of the epiphytes were common to both transects. The most diverse genera were Epidendrum, Aechmea, Codonanthopsis, Peperomia, and Philodendron, and the most abundant species were Codonanthopsis crassifolia, Anthurium gracile, Epiphyllum phyllanthus, Epidendrum sp., Peperomia circinnata, and Codonanthopsis uleana (Figure 3 and Figure 4). The epiphytes A. gracile, C. crassifolia, and C. uleana were found in 62.5% of the AGs in the transects at the forest edge (T1) and in 94.7% of the AGs in the forest interior (T2), respectively.
Figure 3.
Dominance−diversity graph of epiphyte species in Tingana.
Figure 4.
Epiphyte species reported in AGs: (A,B) Epidendrum imatophyllum, (C) Acianthera lanceana, (D) Aechmea angustifolia, (E) Anthurium gracile, (F) Aechmea longifolia, and (G) Clusia sp.
The number of epiphytic species observed ranged from zero to five per AG. The abundance (T = −3.4677; df = 1; p < 0.001) and richness (T = −3.6200; df = 1; p = 0.000) of epiphyte species showed significant differences between the transects. On the contrary, the length (T =1.1037; df = 1; p = 0.2727) and width (T = −0.4761; df = 1; p = 0.6351) of the AGs showed no differences between transects but showed a positive correlation with the number of ants per AG (ength: R = 0.8579; p = 0.000; width: R = 0.9119; p < 0.001).
3.3. Characteristics of AGs
A total of 62% of the AGs were observed in understory areas (less than 5 m in height) dominated by shrub vegetation and gaps in the canopy. In Tingana, the understory AGs had low light exposure, protected by the foliage of higher plants. In other cases, the AGs were exposed to more light intensity (greater than 10 m; 12%), with only the upper foliage of the phorophyte providing shade (Figure 5A) being the most abundant family Gesneriaceae (47%). Structurally, AGs are located where the main phorophyte axis crosses a secondary branch (Figure 5B). They are formed by the accumulation of leaves that decompose to form this assembly. However, not all trees or plants are conducive to the development of AGs. The stems of palm trees do not attract ants to form an AG.
Figure 5.
(A) AGs with high exposure, (B) AGs at intersection of phorophyte branches, (C) AGs collapsed by excess weight, (D) AGs show erosion, and (E,F) AGs on young leaves of Mauritia flexuosa.
In some cases, AGs grow to over 1 m long and wide, becoming extremely heavy for the developing shrub or tree to support; the main branch breaks, causing the AG to fall to the wet ground (Figure 5C), even submerging. The AG is quickly abandoned, and the epiphytes are deprived of light, water, and nutrients, and exposed to stress conditions leading to loss of AGs biomass (Figure 5D). The dominance of Mauritia flexuosa at the study site allowed the recording of many regeneration and juvenile individuals of this species. In fact, small AGs with A. gracile as the dominant epiphyte were observed on the underside of the leaves of young palms (maximum height: 3.5 m). The short lifespan of these leaves, generally around six months, limits the period during which they can support the colony (AG), with abandoned anthills observed on dry juvenile leaves of M. flexuosa (Figure 5E,F).
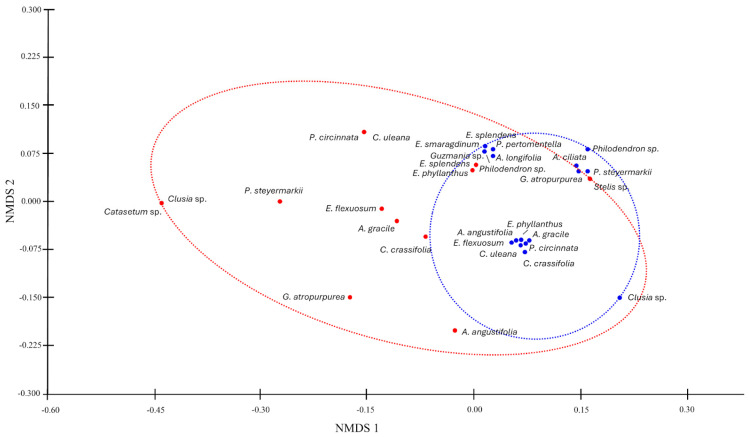
In the NMDS analysis, we observed that 12 epiphytic species were in both transects, forming a single assemblage with a stress level of 0.4076 (Figure 6; ANOSIM: R = 0.3316, p = 0.0001). This was evident from the proximity of the epiphytic species to one another, regardless of their respective transects. Most species in T2 were grouped within a blue ellipse, indicating a close relationship that suggests they may share common phorophytes. In contrast, the transect located at the edge of the agricultural perimeter (T1) exhibited isolated epiphytic species. For instance, Hieronyma alchorneoides served as a host for Clusia sp., Philodendron cf. steyermarkii, and Catasetum sp. These epiphytes are infrequently in the study area, which accounts for their considerable distance from their phorophyte.
Figure 6.
NMDS analysis of epiphyte species composition in AGs: comparing transects at forest edge (red ellipse) and interior forest (blue ellipse) of Mauritia flexuosa peat swamp forest in Tingana.
3.4. Ants Diversity and Interactions
Azteca instabilis (Smith, 1862), Camponotus femoratus (Fabricius, 1804), and Crematogaster levior (Longino, 2003) were the ant species identified in the Tingana AGs studied (Figure 7). Among these, A. instabilis was more abundant, with 62.9% of the total records, while C. femoratus was the least abundant with 16.9%. We documented three AGs that were uninhabited (abandoned nests), 83 AGs hosting a single ant species, and three AGs with two ant species. The phorophyte with the highest ant diversity, three species, was Hymenaea oblongifolia.
Figure 7.
Ant species in the AGs of Mauritia flexuosa peat swamp forest: (A,B) Crematogaster levior (C,D), Azteca instabilis, and (E,F) Camponotus femoratus.
Clusia hammeliana exclusively hosted C. levior, while Protium paniculatum, Ficus pertusa, and Symphonia globulifera were exclusively associated with C. femoratus. T. guianensis was the exclusive host for A. instabilis and no C. levior individuals were recorded on Theobroma obovatum. We encountered two ant species, C. femoratus and C. levior, that exhibited parabiosis within the phorophyte species Miconia affinis and F. pertusa, separately.
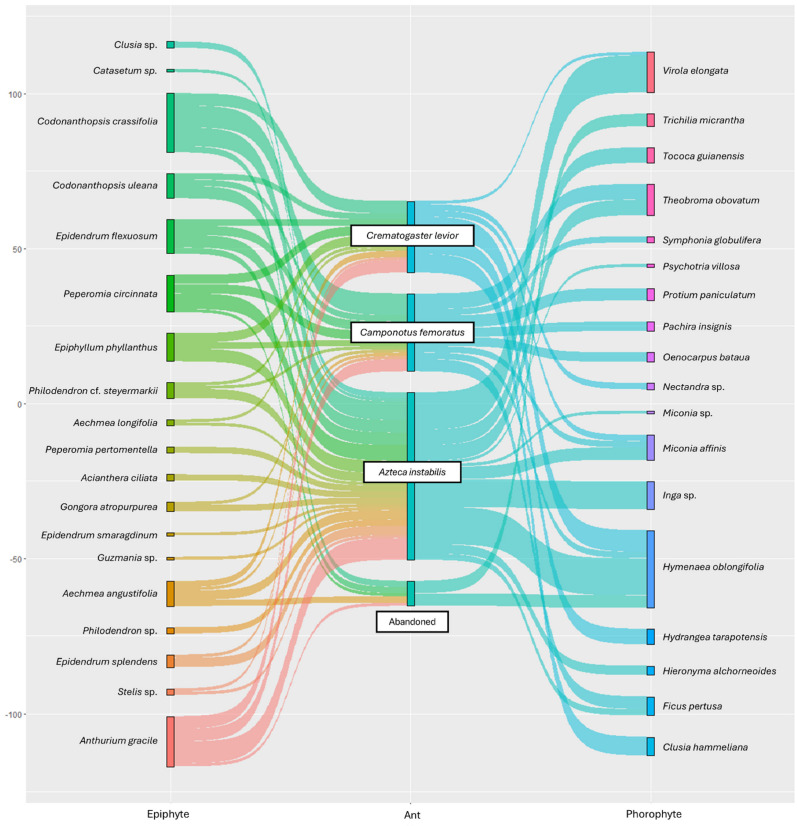
The complexity of the observed interactions between ants, their epiphytes, and phorophyte species is illustrated in a tripartite graph (Figure 8).
Figure 8.
Tripartite graph showing the associations among phorophytes, ant species, and epiphytic species in AGs.
The tripartite graph illustrates the intricate web of interactions between epiphytes, ants, and phorophytes within the studied ecosystem. It is evident that ants such as Crematogaster levior and Azteca instabilis form associations with a considerable variety of epiphytes, which indicates a pivotal role for these species in the sustenance of ant gardens. Furthermore, the presence of abandoned gardens suggests that some epiphytes have lost their interactions with ants, which could potentially impact their development by leaving them without the protection and cleaning benefits that these provide. Regarding phorophytes, trees such as Hymenanea oblongifolia and Virola elongata exhibit extensive associations, acting as recurrent hosts for diverse ant and epiphyte species. These observations indicate the presence of a complex ecological structure, characterised by the coexistence of generalist and specialist ants, with potential influences from resource availability and tree characteristics on association dynamics (Figure 8).
3.5. Conservation Status
The phorophytes F. pertusa, Hydrangea tarapotensis, Hymenaea oblongifolia, Miconia affinis, Miconia sp., Oenocarpus bataua, Pachira insignis, P. paniculatum, Psychotria villosa, S. globulifera, Theobroma obovatum, Tococa guianensis, Trichilia micrantha, and V. elongata, as well as the epiphyte E. phyllanthus, are all considered as being of ‘Least Concern’ on the IUCN Red List of Threatened Species. The epiphytes Epidendrum sp., Stelis sp., and Epidendrum splendens are included in CITES Appendix II. In contrast, none of the ant species is threatened or listed in CITES.
4. Discussion
4.1. Phorophytes
Phorophytes play a crucial role in the development of ant gardens [38]. The structural characteristics of trees, such as diameter at breast height (DBH) greater than 10 cm, bark type, and crown architecture, significantly influence both epiphyte colonization and garden formation [39,40]. In this regard, few studies mention the hosts of AGs, and Hymenaea oblongifolia, Virola elongata, and Theobroma obovatum (dominant in Tingana) have not been previously reported. We attribute this to habitat specialization in Tingana [27], which is highly restrictive for those species not adapted to flooded ecosystems [32]. Common hosts in other Neotropical territories include Miconia, Psychotria, and the Inga genus [19,41].
The prevalence of pioneer species, adapted to high-light and disturbed environments, has been observed in Tingana and other disturbed regions [42]. Campos et al. [43] have demonstrated rapid colonization of these areas by ants and epiphytes. In contrast, ecosystems with lower disturbance levels and denser canopy cover tend to harbor different phorophyte species, such as Tococa guianensis and Hymenaea oblongifolia. In these environments, symbiotic relationships between plants and ants develop, indicating high ecological stability [44].
The ability of the Hymenaea oblongifolia to adapt to fluctuating flood conditions is crucial for maintaining mutualistic relationships between ants and epiphytes. Pioneer species (Miconia sp., Miconia affinis, Symphonia globulifera, and Inga sp.) can benefit from both post-disturbance high-light periods and flood-induced wet conditions [45].
Approximately 60% of myrmecophyte flora species are categorized as pioneers, exhibiting high light demand and rapid growth [46,47,48], as observed in Tingana. Myrmecophyte species predominantly occur in areas with human-induced disturbances, particularly near riparian vegetation or water bodies. This observation aligns with findings from the study area, as reported by Quinteros-Gómez et al. [32].
In the study area, trees and shrubs with higher DBH and height did not show more epiphytic colonization, as expected [49,50]. This is mainly because the area is in a secondary forest with selective anthropogenic activity [27,31], where individuals with DBH greater than 60 cm are rare. These are the individuals that regularly concentrate on the highest richness and abundance of epiphyte species [51,52].
The genera Tococa and Hymenaea are notable AG hosts across diverse ecological contexts [53]. Tococa, an ombrophilous genus, is better adapted to growth in less disturbed environments with limited sun exposure. Its leaves, often featuring extrafloral nectaries, attract ants that provide protection from herbivores, establishing mutualistic relationships in more closed and humid habitats [54,55]. Conversely, Hymenaea species adapt to varied light conditions, including more open and bright environments [56,57]. Although typically found in seasonally flooded or pre-montane forests [58], these species exhibit ecological plasticity that enables them to serve as phorophytes in areas with greater solar exposure. This facilitates ant and epiphyte interactions in more open environments (T1) [59], promoting ant proliferation that benefits both phorophyte and epiphyte protection [60].
4.2. Epiphytic Flora
The high diversity of epiphytic angiosperms in ant gardens (AGs) observed in this study parallels that of other Neotropical ecosystems, including those in Tabasco and Chiapas, Mexico [20], French Guiana [61,62], Venezuelan rainforests [63], and central Amazonian forests of Brazil [64]. The families Araceae, Bromeliaceae, Gesneriaceae, Piperaceae, and Orchidaceae show the highest diversity in these areas, suggesting a general pattern in AG structure and composition throughout the tropics, where mutualistic interactions between ants and epiphytes play a crucial role in shaping biodiversity [65]. Forest edge (T1) AGs exhibited lower epiphyte diversity, likely due to extreme conditions at the agricultural border [66]. In these conditions, Codonanthopsis crassifolia and Anthurium gracile predominate, distinguished by their ability to thrive in environments with significant humidity fluctuations and survive extended drought periods without growth impairment [67,68]. In contrast, the forest interior (T2), with its more stable microclimatic conditions, supports greater epiphyte diversity [69].
Codonanthopsis crassifolia exemplifies species that have developed specialized structures for ant interactions [70]. Its seeds feature a lipid- and amino acid-rich exocarp that attracts Crematogaster ants, which transport them to their nests [8]. The ants consume only the external covering, leaving the viable seed in the nest for germination [71]. C. crassifolia also possesses extrafloral nectaries that provide ant nutrition [72], and in return, ants protect the plant from herbivores and other threats [73]. This mutualism benefits both parties: the epiphyte gains dispersal and enhanced development, while ants receive lipids, nectar, and fruits [70].
Peperomia pertomentella represents another characteristic AG species. It demonstrates remarkable colonization capability across different phorophytes [74], establishing itself in diverse arboreal microhabitats within the Tingana forest [75].
These characteristics make them essential components of AGs, particularly in less disturbed forests [76]. While epiphytes can survive temporarily without ants following nest death [8], several factors facilitate their continued existence, primarily the phorophyte’s structural integrity and the availability of nitrogen-rich, organic substrates [77,78].
4.3. Ants and Mutualism
The genus Azteca was documented as the most abundant in Tingana ant gardens (AGs). Azteca is renowned for its aggressive defensive behavior towards hosts [79] and its capacity to establish nests across diverse tree and shrub families [22,80], as evidenced in Tingana with Fabaceae and Myristicaceae. This behavior enables Azteca to monopolize food resources and reduce herbivory damage, thereby promoting plant growth [81]. Conversely, Crematogaster species, which frequently engage in mutualistic associations [38], have been observed in association with Hymenaea oblongifolia, valued for its durable wood and aromatic resin. These plants attract ants through extrafloral nectary secretions, establishing a mutually beneficial relationship [6,34,82].
Significant geographical variation exists in ant-plant symbiotic associations [83]. For instance, Tococa guianensis establishes associations with Azteca, while Camponotus femoratus interacts mainly with the pioneer species Symphonia globulifera, Theobroma obovatum and Miconia affinis found in T1, where most anthropogenic activity takes place.
Resource availability significantly influences ant presence in tropical ecosystems [84]. Host plants provide diverse resources, including habitat, structural support, thermal regulation, climatic protection, and nutrition [85]. Ants utilize these resources for nest establishment and growth [4,86], constructing nests from fecal material, wood fragments, and leaves [87].
The Miconia and Tococa genera produces domatia, specialized ant shelters [8,88], conferring selective advantages in herbivore protection and competition [89]. Domatia size and plant characteristics influence ant occupancy [88]. In Tococa guianensis, no evidence suggests that Azteca instabilis receives nutritional rewards for protection [90]. These ants incorporate seeds into their nests, enabling germination and root development that creates supporting structures on host plant branches [91]. A family-level mutualism between Bromeliaceae (Aechmea and Guzmania in Tingana) and Camponotus femoratus, documented in French Guiana [92], represents one of the most intricate flowering plant mutualisms, being obligatory for Bromeliaceae [93,94] in partially shaded areas.
4.4. Ecological Importance
Ant gardens (AGs) exemplify a remarkable form of ant-plant mutualism and play a vital role in ecosystem conservation [95]. In these relationships, epiphytes gain three primary benefits: seed dispersal, herbivore protection, and nutrient access [94]. These conditions ensure optimal assemblage development and prevent epiphyte desiccation [19].
The ecological impact of AGs is significant, as they modify environments and maintain ecological balance [96]. The integration of diverse epiphytes into ant nests enhances biodiversity and creates microhabitats that attract various organisms [97]. Ant’s shape microclimatic conditions through their complex structures [95] and function as biocontrol agents, protecting plants from potential herbivores and competing species [89]. Notably, ants (particularly Azteca instabilis) play a crucial role in host plant defense by killing or deterring leaf-cutting ants and removing lepidopteran and beetle eggs from plant surfaces [23,55,98]. These interactions underscore the importance of understanding tropical ecosystem dynamics and how ants, serving as biological indicators, provide insights into ecosystem health and resilience in response to natural and anthropogenic disturbances [99].
AG establishment under favorable conditions depends on multiple factors, including relative humidity, temperature, soil type, vegetation, light intensity, and canopy cover [6,100]. These factors contribute to diverse microclimate formation that facilitates AG development. However, extremely dense canopies can impede light penetration, adversely affecting plant-ant interactions and inhibiting nest formation [101]. Reduced light availability can compromise the functionality of ants and epiphytes not specifically adapted to such conditions. Environmental conditions significantly influence ant behavior [102] and their epiphyte selection based on reciprocal benefits [73]. Ants contribute not only to AG construction but also to maintenance, regularly incorporating new materials and repairing substrates to maintain optimal conditions and prevent epiphyte desiccation.
AG longevity is often limited [103], influenced by excessive growth of certain epiphytes (including A. longifolia, A. angustifolia, Philodendron cf. steyermarkii, C. uleana, and C. crassifolia). When AGs become too heavy for developing shrubs or trees to support, branches break and fall, leading to ant nest abandonment.
5. Conclusions
In the Alto Mayo Valley’s Andean–Amazonian piedmont, 18 phorophyte species harbor ant gardens (AGs). Of these phorophytes, 78% supported a single AG, while 17.4% contained two AGs. The number of AGs per phorophyte ranged from 1 to 4, with single AGs being most common (78%). The AGs were associated with 19 epiphytic species. Azteca instabilis, Camponotus femoratus, and Crematogaster levior were the ant species identified in the AGs. Two ant species, C. femoratus and C. levior, exhibited parabiosis within separate phorophyte species, Miconia affinis and Ficus pertusa.
The differences in community composition between the two transects demonstrate how local factors, particularly light availability and microclimatic conditions, influence epiphyte distribution patterns.
Acknowledgments
I would like to extend my gratitude to Anderson Cabrera, Bercelia Mestanza, Dino Cabrera, Emerson Cabrera, Juan Isuiza, and all the members of ADECARAM Tingana and the Don Pepito Farm in Tingana, without whom the investigation would not have been possible.
Appendix A
Table A1.
Data on phorophytes and their ant-gardens in a Mauritia flexuosa peat swamp forest in Tingana, Peru.
| Phorophytes | Ant Gardens | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Scientific Name | Height (m) | DBH (cm) | # AG | Ant Species | Epiphyte Species | Epiphyte Family | Height (cm) | Length (cm) | Width (cm) | |
| Melastomataceae | Miconia sp. | 2 | 5 | 1 | Azteca instabilis | 1 | a | 80 | 12 | 10 | |
| Clusiaceae | Symphonia globulifera L.f. | 7 | 30 | 1 | Camponotus femoratus | - | - | 550 | 15 | 20 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 9 | 43 | 1 | Azteca instabilis | 3 | b | 800 | 60 | 28 | |
| Phyllanthaceae | Hieronyma alchorneoides Allemão | 5 | 20.5 | 1 | Azteca instabilis | 2, 8, 9 | a, d, f | 200 | 29 | 22 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 5.5 | 21 | 1 | Azteca instabilis | 10 | d | 350 | 50 | 40 | |
| Melastomataceae | Miconia affinis DC. | 3.5 | 9.5 | 2 | Camponotus femoratus | Crematogaster levior | 1, 2 | a | 200 | 12 | 8 |
| Camponotus femoratus | Crematogaster levior | 1 | a | 100 | 7 | 8 | |||||
| Clusiaceae | Symphonia globulifera L.f. | 4 | 13.7 | 1 | Camponotus femoratus | 5 | d | 200 | 7 | 8 | |
| Clusiaceae | Symphonia globulifera L.f. | 6 | 15.5 | 1 | Camponotus femoratus | 5, 7 | d, e | 300 | 60 | 60 | |
| Melastomataceae | Miconia affinis DC. | 2 | 5.5 | 1 | Azteca instabilis | 1, 2, 5 | a, d | 150 | 13 | 12 | |
| Fabaceae | Hymenaea oblongifolia Huber | 8.5 | 24 | 1 | Azteca instabilis | 7, 12 | a, e | 350 | 35 | 98 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 9.5 | 23.5 | 1 | Crematogaster levior | 10 | d | 850 | 16 | 15 | |
| Melastomataceae | Miconia affinis DC. | 4.5 | 12.5 | 1 | Azteca instabilis | 1, 2, 11 | a, d | 100 | 15 | 12 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 11 | 37.5 | 1 | Azteca instabilis | 10 | d | 1000 | 30 | 25 | |
| Fabaceae | Hymenaea oblongifolia Huber | 10 | 30.5 | 1 | Azteca instabilis | 3, 11 | b, d | 700 | 30 | 22 | |
| Malvaceae | Pachira insignis (Sw.) Sw. exSavigny | 8.5 | 31 | 1 | Camponotus femoratus | 3, 7, 16 | b, d, e | 700 | 60 | 55 | |
| Fabaceae | Hymenaea oblongifolia Huber | 8 | 19.5 | 1 | Azteca instabilis | 7 | e | 600 | 11 | 10 | |
| Malvaceae | Theobroma obovatum Klotzsch ex Bernoulli | 9 | 24.5 | 1 | Azteca instabilis | 1, 5 | a, d | 250 | 21 | 20 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 8 | 21 | 1 | Azteca instabilis | 11 | d | 700 | 12 | 11 | |
| Fabaceae | Hymenaea oblongifolia Huber | 18 | 60.5 | 1 | Azteca instabilis | 7 | e | 1300 | 40 | 35 | |
| Lauraceae | Nectandra sp. | 3 | 9.8 | 1 | Crematogaster levior | 1, 7 | a, e | 200 | 25 | 20 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 1.5 | 6.5 | 1 | Crematogaster levior | - | - | 200 | 7 | 8 | |
| Malvaceae | Theobroma obovatum Klotzsch ex Bernoulli | 3.5 | 11 | 1 | Azteca instabilis | 5 | d | 250 | 11 | 7 | |
| Malvaceae | Theobroma obovatum Klotzsch ex Bernoulli | 13 | 46.4 | 4 | Azteca instabilis | 3, 4, 5 | b, c, d | 250 | 25 | 18 | |
| Azteca instabilis | 7 | e | 400 | 5 | 7 | ||||||
| Azteca instabilis | 1 | a | 700 | 12 | 10 | ||||||
| Azteca instabilis | 1 | a | 1100 | 25 | 20 | ||||||
| Malvaceae | Theobroma obovatum Klotzsch ex Bernoulli | 8 | 22.6 | 1 | Camponotus femoratus | 3, 5 | b, d | 700 | 35 | 30 | |
| Malvaceae | Theobroma obovatum Klotzsch ex Bernoulli | 9 | 22.4 | 2 | Camponotus femoratus | 7, 11 | d, e | 300 | 20 | 15 | |
| Camponotus femoratus | 6 | a | 400 | 21 | 18 | ||||||
| Moraceae | Ficus pertusa L. f. | 2.5 | 7 | 1 | Camponotus femoratus | Azteca instabilis | 7, 13 | e, g | 100 | 20 | 17 |
| Fabaceae | Hymenaea oblongifolia Huber | 12 | 46.7 | 1 | Azteca instabilis | 7 | e | 1100 | 45 | 32 | |
| Fabaceae | Hymenaea oblongifolia Huber | 11 | 25 | 1 | Azteca instabilis | 1, 7, 11 | a, d, e | 650 | 30 | 23 | |
| Fabaceae | Hymenaea oblongifolia Huber | 11 | 16 | 1 | Azteca instabilis | 7 | e | 1000 | 8 | 8 | |
| Fabaceae | Hymenaea oblongifolia Huber | 14 | 35.2 | 1 | Azteca instabilis | 7, 13 | e, g | 400 | 65 | 50 | |
| Fabaceae | Hymenaea oblongifolia Huber | 11 | 29.5 | 3 | Azteca instabilis | 1, 6, 7 | a, e | 700 | 40 | 32 | |
| Azteca instabilis | 1 | a | 600 | 12 | 17 | ||||||
| Azteca instabilis | 6 | e | 900 | 15 | 15 | ||||||
| Burseraceae | Protium paniculatum Engl. | 9 | 29 | 1 | Camponotus femoratus | 5, 7 | d, e | 700 | 25 | 14 | |
| Rubiaceae | Psychotria villosa Ruiz & Pav. | 2.5 | 5.8 | 1 | Azteca instabilis | 7 | e | 200 | 9 | 4 | |
| Burseraceae | Protium paniculatum Engl. | 8 | 19 | 1 | Camponotus femoratus | 1, 4, 7 | a, c, e | 300 | 60 | 50 | |
| Arecaceae | Oenocarpus bataua Mart. | 8 | 16.3 | 1 | Camponotus femoratus | 1, 4, 7 | a, c, e | 300 | 50 | 50 | |
| Melastomataceae | Tococa guianensis Aubl. | 3.5 | 8 | 4 | Azteca instabilis | 1, 6 | a, e | 100 | 9 | 7 | |
| Azteca instabilis | 6 | e | 100 | 12 | 11 | ||||||
| Azteca instabilis | 6 | e | 200 | 4 | 4 | ||||||
| Azteca instabilis | 1 | a | 250 | 30 | 70 | ||||||
| Meliaceae | Trichilia micrantha Benth. | 9 | 18 | 2 | Abandoned | 5, 7, 13 | d, e, g | 200 | 18 | 12 | |
| Abandoned | 1 | a | 500 | 15 | 11 | ||||||
| Fabaceae | Inga sp. | 10 | 25.2 | 1 | Azteca instabilis | 1, 4, 7, 12, 13 | a, c, e, g | 700 | 80 | 37 | |
| Melastomataceae | Tococa guianensis Aubl. | 3.5 | 9.4 | 1 | Azteca instabilis | 3, 9 | b, f | 200 | 18 | 17 | |
| Clusiaceae | Clusia hammeliana Pipoly | 18 | 34 | 1 | Crematogaster levior | 3, 5 | b, d | 600 | 80 | 70 | |
| Fabaceae | Hymenaea oblongifolia Huber | 4 | 12.8 | 2 | Azteca instabilis | 1, 14, 15 | a, d, g | 200 | 8 | 6 | |
| Azteca instabilis | 1 | a | 300 | 18 | 14 | ||||||
| Melastomataceae | Tococa guianensis Aubl. | 2 | 4.6 | 1 | Azteca instabilis | 4, 6 | c, e | 150 | 7 | 7 | |
| Fabaceae | Hymenaea oblongifolia Huber | 12 | 35.2 | 1 | Abandoned | 4, 5, 7, 13 | c, d, e, g | 400 | 113 | 97 | |
| Fabaceae | Hymenaea oblongifolia Huber | 4 | 8.6 | 1 | Azteca instabilis | 4, 5, 7 | c, d, e | 200 | 14 | 7 | |
| Fabaceae | Hymenaea oblongifolia Huber | 16 | 44.5 | 1 | Camponotus femoratus | 1, 7 | a, e | 1000 | 60 | 43 | |
| Fabaceae | Hymenaea oblongifolia Huber | 3 | 9.5 | 1 | Azteca instabilis | 4, 7, 13 | c, e, g | 300 | 18 | 15 | |
| Fabaceae | Inga sp. | 4 | 10.5 | 2 | Azteca instabilis | 1, 4, 6, 7 | a, c, e | 200 | 13 | 9 | |
| Azteca instabilis | 1, 16 | a, d | 300 | 6 | 6 | ||||||
| Hydrangeaceae | Hydrangea tarapotensis Briq. | 3 | 14 | 1 | Crematogaster levior | 6, 13 | e, g | 300 | 40 | 37 | |
| Fabaceae | Hymenaea oblongifolia Huber | 4 | 4.8 | 1 | Azteca instabilis | 7 | e | 300 | 26 | 23 | |
| Fabaceae | Hymenaea oblongifolia Huber | 3 | 27.9 | 2 | Crematogaster levior | 1, 7, 19 | a, e, g | 250 | 12 | 9 | |
| Crematogaster levior | 1 | a | 300 | 10 | 8 | ||||||
| Fabaceae | Hymenaea oblongifolia Huber | 21 | 66.9 | 2 | Crematogaster levior | 5, 6 | d, e | 1400 | 21 | 18 | |
| Crematogaster levior | 6 | e | 1100 | 40 | 32 | ||||||
| Hydrangeaceae | Hydrangea tarapotensis Briq. | 10 | 19.8 | 2 | Crematogaster levior | 3, 4, 7 | b, c, e | 400 | 100 | 80 | |
| Crematogaster levior | 7 | e | 600 | 16 | 14 | ||||||
| Myristicaceae | Virola elongata (Benth.) Warb. | 9 | 24.6 | 2 | Azteca instabilis | 3, 4, 7 | b, c, e | 400 | 40 | 28 | |
| Azteca instabilis | 7, 17, 19 | d, e, g | 800 | 23 | 20 | ||||||
| Fabaceae | Hymenaea oblongifolia Huber | 20 | 72 | 1 | Crematogaster levior | 3, 6 | b, e | 1500 | 135 | 118 | |
| Fabaceae | Hymenaea oblongifolia Huber | 10 | 53 | 1 | Crematogaster levior | 1, 4, 6 | a, c, e | 800 | 45 | 30 | |
| Fabaceae | Hymenaea oblongifolia Huber | 13 | 30.7 | 2 | Azteca instabilis | 3, 7, 13 | b, e, g | 800 | 12 | 14 | |
| Azteca instabilis | 4, 7 | c, e | 700 | 25 | 19 | ||||||
| Clusiaceae | Clusia hammeliana Pipoly | 24 | 69.7 | 1 | Crematogaster levior | 3, 5, 13 | b, d, g | 2200 | 18 | 12 | |
| Clusiaceae | Clusia hammeliana Pipoly | 4.5 | 69.7 | 1 | Crematogaster levior | 4, 5, 7 | c, d, e | 150 | 12 | 8.5 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 12 | 25.5 | 2 | Azteca instabilis | 5, 7, 16 | d, e | 600 | 25 | 18 | |
| Azteca instabilis | 1 | a | 700 | 20 | 13 | ||||||
| Myristicaceae | Virola elongata (Benth.) Warb. | 6 | 11.5 | 1 | Azteca instabilis | 1, 7 | a, e | 400 | 28 | 14 | |
| Fabaceae | Hymenaea oblongifolia Huber | 18 | 14.4 | 1 | Azteca instabilis | 7 | e | 400 | 45 | 33 | |
| Fabaceae | Hymenaea oblongifolia Huber | 18 | 56.8 | 1 | Azteca instabilis | 3, 7, 18 | b, e | 1200 | 60 | 52 | |
| Clusiaceae | Clusia hammeliana Pipoly | 18 | 61.8 | 1 | Crematogaster levior | 1, 5, 7 | a, d, e | 300 | 65 | 52 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 6 | 17.5 | 1 | Azteca instabilis | 1, 2 | a | 200 | 10 | 8 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 4 | 18.5 | 1 | Azteca instabilis | 7 | e | 250 | 15 | 11 | |
| Fabaceae | Inga sp. | 4 | 16 | 2 | Azteca instabilis | 1, 4, 7, 18 | a, c, e | 200 | 16 | 13 | |
| Azteca instabilis | 6, 7, 17 | d, e | 300 | 14 | 11 | ||||||
| Myristicaceae | Virola elongata (Benth.) Warb. | 5 | 14 | 1 | Azteca instabilis | 1, 7, 16 | a, d, e | 400 | 10 | 7 | |
| Myristicaceae | Virola elongata (Benth.) Warb. | 2.5 | 12.5 | 1 | Azteca instabilis | 1, 4, 6, 7 | a, c, e | 200 | 18 | 16 | |
| Moraceae | Ficus pertusa L. f. | 20 | 49 | 1 | Camponotus femoratus | 4, 6, 7 | c, e | 100 | 20 | 20 | |
Epiphyte species: (1) Anthurium gracile (Rudge) Schott, (2) Philodendron cf. steyermarkii G.S. Bunting, (3) Epiphyllum phyllanthus (L.) Haw, (4) Peperomia circinnata Link, (5) Epidendrum flexuosum G.Mey., (6) Codonanthopsis uleana (Fritsch) Chautems & Mat. Perret, (7) Codonanthopsis crassifolia (H.Focke) Chautems & Mat.Perret, (8) Catasetum sp., (9) Clusia sp., (10) Stelis sp., (11) Epidendrum splendens Schltr., (12) Philodendron sp., (13) Aechmea angustifolia Poepp. & Endl., (14) Guzmania sp., (15) Epidendrum smaragdinum Lindl., (16) Gongora atropurpurea Hook., (17) Acianthera ciliata (Knowles & Westc.) F.Barros & L.R.S.Guim., (18) Peperomia pertomentella Trel., and (19) Aechmea longifolia (Rudge) L.B.Sm. & M.A.Spencer. Botanic family of epiphyte species: (a) Araceae, (b) Cactaceae, (c) Piperaceae, (d) Orchidaceae, (e) Gesneriaceae, (f) Clusiaceae, and (g) Bromeliaceae.
Author Contributions
Writing—original draft preparation, review, and editing, Y.Q.-G., D.G.-T., J.M.-B., and O.M.-V.; Y.Q.-G., J.M.-B. and D.G.-T. conceived and designed the study. V.S.-L., S.O.-C., G.A.-I., and F.A.-A. coordinated the field work. J.M.-B., A.S.-I., F.A.-A., F.A.-R., S.O.-C., and E.J.-P. carried out the field work, counting, and collection of material. V.S.-L. and A.S.-I. carried out the georeferencing of the plots and the elaboration of maps for the fieldwork and the manuscript. E.J.-P., J.M.-B., A.S.-I., F.A.-R., F.A.-A., S.O.-C. and G.A.-I. participated in taxonomic determination. O.M.-V., F.A.-R., Y.Q.-G., J.S.-S., D.G.-T., E.J.-P. and V.S.-L. prepared the database and performed the statistical analyses. Y.Q.-G., J.S.-S., G.A.-I., O.M.-V. and J.M.-B. interpreted the results and wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data used in this study can be requested from the corresponding author via email: tavomonroyvilchis@gmail.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the Universidad Nacional Mayor de San Marcos—RR N° 011136-R-22 and project number B22140132.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Armenteras D., González T.M., Vergara L.K., Luque F.J., Rodríguez N., Bonilla M.A. Revisión del concepto de ecosistema como “unidad de la naturaleza” 80 años después de su formulación. Ecosistemas. 2016;25:83–89. doi: 10.7818/ECOS.2016.25-1.12. [DOI] [Google Scholar]
- 2.Johnson C.A., Smith G.P., Yule K., Davidowitz G., Bronstein J.L., Ferrière R. Coevolutionary transitions from antagonism to mutualism explained by the co-opted antagonist hypothesis. Nat. Commun. 2021;12:2867. doi: 10.1038/s41467-021-23177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomicki G., Janda M., Renner S.S. The assembly of ant-farmed gardens: Mutualism specialization following host broadening. Proc. Biol. Sci. 2017;284:20161759. doi: 10.1098/rspb.2016.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbara B., Leroy C., Orivel J., Dejean A., Delsinne T. Relaciones entre las hormigas y las plantas en los trópicos del Nuevo Mundo (Hormigas de Colombia) In: Fernández F., Guerrero R.J., Delsinne T., editors. Hormigas de Colombia. Universidad Nacional de Colombia; Bogotá, Colombia: 2019. pp. 203–253. [Google Scholar]
- 5.Del Toro I., Ribbons R., Pelini S. The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae) Myrmecol. News. 2012;17:133–146. doi: 10.25849/myrmecol.news_017:133. [DOI] [Google Scholar]
- 6.Blüthgen N., Feldhaar H. 2009: Chapter 7 Food and shelter: How resources influence ant ecology. In: Lach L., Parr C., Abbott K., editors. Ant Ecology. Oxford University Press; Oxford, UK: 2009. pp. 115–136. [Google Scholar]
- 7.Chanam J., Sheshshayee M.S., Kasinathan S., Jagdeesh A., Joshi K.A., Borges R.M. Nutritional benefits from domatia inhabitants in an ant–plant interaction: Interlopers do pay the rent. Funct. Ecol. 2014;28:1107–1116. doi: 10.1111/1365-2435.12251. [DOI] [Google Scholar]
- 8.Delabie J., Ospina M., Zabala G. Relaciones entre hormigas y plantas: Una introducción. In: Fernández F., editor. Introducción a Las Hormigas de la Región Neotropical. Biota Colomb.; Bogotá, Colombia: 2003. pp. 167–180. [DOI] [Google Scholar]
- 9.Carvajal V.d.l.A., Ospina F., Estévez J., Llano C.A. Invertebrados Asociados a Bromelias: Una Diversidad Escondida. Editorial Universidad de Caldas; Caldas, Colombia: 2008. p. 35. [Google Scholar]
- 10.Ojembarrena J.A., Chanampa M., Vidal P., Guerra R., Olivieri F., Neila F.J., Bedoya C. Sistemas vegetales que mejoran la calidad ambiental de las ciudades. Boletín CF+S. 2010;42/43:211–223. [Google Scholar]
- 11.Hernández S.A. Thesis. Universidad Autónoma del Estado de Morelos; Morelos, Mexico: 2020. Distribución altitudinal de hormigas en una vertiente de la Sierra Montenegro, Morelos, México. [Google Scholar]
- 12.Areche J.C., Mallma B.M. Thesis. Universidad Nacional de Huancavelica; Pampas, Peru: 2014. [(accessed on 11 August 2024)]. Sistema de control de temperatura y humedad para prevenir el ataque de hongos y bacterias en los cultivos de orquídeas en el Instituto Nacional de Investigación Agraría Junín. Available online: http://repositorio.unh.edu.pe/handle/UNH/782. [Google Scholar]
- 13.Urcuqui A.M. Thesis. Universidad Autónoma de Occidente; Santiago de Cali, Colombia: 2005. Caracterización de los hábitos alimentarios de las hormigas arrieras en el bosque seco tropical del Jardín Botánico de la ciudad de Santiago de Cali. [DOI] [Google Scholar]
- 14.Gallego-Ropero M.C., Salguero B. Ensamblaje de hormigas del bosque seco tropical, jardín botánico de Cali. Coulomb Force. 2015;18:139–150. doi: 10.14483/udistrital.jour.colomb.for.2015.1.a08. [DOI] [Google Scholar]
- 15.Guzmán-Mendoza R., Castaño-Meneses G., Herrera-Fuentes M.D.C. Variación espacial y temporal de la diversidad de hormigas en el Jardín Botánico del valle de Zapotitlán de las Salinas, Puebla. Rev. Mex. Biodivers. 2010;81:427–435. doi: 10.22201/ib.20078706e.2010.002.233. [DOI] [Google Scholar]
- 16.Martínez C.L., Riquelme M.B., Santadino M.V., de Haro A.M., Barañao J.J. Estudios sobre el comportamiento de forrajeo de Acromyrmex lundi Guering (Himenoptera, Formicidae) y su efecto sobre el crecimiento de procedencias de Eucalyptus globulus Labill. (Myrtaceae) Rev. Árvore. 2015;39:189–198. doi: 10.1590/0100-67622015000100018. [DOI] [Google Scholar]
- 17.Carvajal S., Peña-Pinela C. Familia Cecropiaceae. Flora del Bajío y de Regiones Adyacentes. Instituto de Ecología A.C.; Michoacán, Mexico: 1997. pp. 1–10. Fascículo 53. [DOI] [Google Scholar]
- 18.Mora-Pineda G. Thesis. Universidad de Costa Rica; San Pedro, Costa Rica: 2014. [(accessed on 10 September 2024)]. Interaccione de los nematodos Sclerorhabditis (Rhabditidae) encontrados en troncos de Cecropia (Urticaceae) junto a las hormigas Azteca (Formicidae: Dolichoderinae) Available online: http://biologia.ucr.ac.cr/TesisLic/GeovannyMoraPineda.pdf. [Google Scholar]
- 19.Barrera-Bello Á.M., Torres-González A.M. Basic ecology of ant gardens in a dry-premontane transitional forest. Rev. Biol. Trop. 2022;70:526–540. doi: 10.15517/rev.biol.trop..v70i1.41703. [DOI] [Google Scholar]
- 20.Morales-Linares J., Flores-Palacios A., Ramos-Robles M.I., Vásquez-Bolaños M. Listado de angiospermas epífitas que conforman jardines de hormigas de Azteca gnava (Formicidae) en el sureste de México. Acta Bot. Mex. 2023;130:e2129. doi: 10.21829/abm130.2023.2129. [DOI] [Google Scholar]
- 21.Longino J.T. A taxonomic review of the genus Azteca (Hymenoptera: Formicidae) in Costa Rica and a global revision of the aurita group. Zootaxa. 2007;1491:1–63. doi: 10.11646/zootaxa.1491.1.1. [DOI] [Google Scholar]
- 22.Hölldobler B., Wilson E.O. The Ants. Springer; Berlin/Heidelberg, Germany: 1990. p. 752. [Google Scholar]
- 23.Edwards D., Hassall M., Sutherland W.J., Yu D.W. Selection for protection in an ant–plant mutualism: Host sanctions, host modularity, and the principal–agent game. Proc. Biol. Sci. 2006;273:595–602. doi: 10.1098/rspb.2005.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales-Linares J., Corona-López A.M., Toledo-Hernández V.H., Flores-Palacios A. Ant-gardens: A specialized ant-epiphyte mutualism capable of facing the effects of climate change. Biodivers. Conserv. 2021;30:1165–1187. doi: 10.1007/s10531-021-02138-2. [DOI] [Google Scholar]
- 25.Morales-Linares J., García-Franco J.G., Flores-Palacios A., Valenzuela-González J.E., Mata-Rosas M., Díaz-Castelazo C. Vascular epiphytes and host trees of ant-gardens in an anthropic landscape in southeastern Mexico. Sci. Nat. 2016;103:96. doi: 10.1007/s00114-016-1421-9. [DOI] [PubMed] [Google Scholar]
- 26.Orivel J., Dejan A. Selection of epiphyte seeds by ant-garden ants. Ecoscience. 1999;6:51–55. doi: 10.1080/11956860.1999.11952205. [DOI] [Google Scholar]
- 27.Quinteros-Gómez Y., Millán B., Gómez-Ticerán D., Angeles-Alvarez F., Salinas-Inga A., Macedo-Bedoya J., Olórtegui S., Balbuena-Serrano Á. Diversity and species of vascular epiphytes in Tingana, the highest flooded forest in Peru. Mires Peat. 2024;31:1–22. doi: 10.19189/MaP.2023.OMB.Sc.2321074. [DOI] [Google Scholar]
- 28.Quinteros-Gómez Y., Monroy-Vilchis O., Zarco-González M.M., Endara-Agramont Á.R., Pacheco X.P. Composición florística, estructura y estatus de conservación de los aguajales de la palma Mauritia flexuosa en el piedemonte amazónico en el departamento de San Martín, Perú. Rev. Mex. Biodivers. 2021;92:e923186. doi: 10.22201/ib.20078706e.2021.92.3186. [DOI] [Google Scholar]
- 29.PEAM . Boletín Meteorológico e Hidrológico del Alto Mayo, 1996–2004. El Proyecto Especial Alto Mayo (PEAM) Departamento de San Martín, SENAMHI; Moyobamba, Peru: 2004. p. 57. [Google Scholar]
- 30.Alva J.E., Meneses J., Chang L.A., Lara J.L., Nishimura T. Efectos en el Terreno Ocasionados Por Los Sismos de Alto Mayo en Perú. IX Congreso Nacional de Ingeniería Civil; Ica, Peru: 1992. [Google Scholar]
- 31.Quinteros-Gómez Y.M., Cabrera D., Macedo-Bedoya J., Santos-Linares V., Salinas-Inga A. Propagación vegetativa de Vanilla pompona subsp. grandiflora (Orchidaceae) en territorios inundables del Valle del Alto Mayo, Perú. Acta Bot. Mex. 2024;131:e2309. doi: 10.21829/abm131.2024.2309. [DOI] [Google Scholar]
- 32.Quinteros-Gómez Y., Zarco-González M., Ticerán D., Agramont A., Monroy-Vilchis O. Effects of human disturbance on above-ground carbon stocks in north-west Amazonian Mauritia flexuosa peat swamp forests. Mires Peat. 2023;29:1–19. doi: 10.19189/MaP.2021.OMB.StA.2300. [DOI] [Google Scholar]
- 33.Longino J.T. The Crematogaster (Hymenoptera, Formicidae, Myrmicinae) of Costa Rica. Zootaxa. 2003;151:1–150. doi: 10.11646/zootaxa.151.1.1. [DOI] [Google Scholar]
- 34.Mackay W. Volume 1 Introduction, Keys to the Sub-Genera and Species Complexes and the Subgenus Camponotus. Lambert Academic Publishing; Saarbrücken, Germany: 2019. New world carpenter ants of the hyperdiverse genus Camponotus; p. 420. [Google Scholar]
- 35.Feitosa R.M., Dias A.M. An illustrated guide for the identification of ant subfamilies and genera in Brazil. Insect Syst. Evol. 2024;55:451–571. doi: 10.1163/1876312X-bja10062. [DOI] [Google Scholar]
- 36.Sjoberg D. ggsankey: Sankey. Alluvial and Sankey Bump Plots, 564. 2022. Stockholm, Sweden. [(accessed on 25 August 2024)]. Available online: https://github.com/davidsjoberg/ggsankey.
- 37.Oksanen F., Blanchet F.G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P., O’Hara R., Simpson G., Solymos P. vegan: Community Ecology Package, R package version 2.4-4; Helsinki, Finland, 2024. [(accessed on 15 September 2024)]. Available online: https://cran.r-project.org/package=vegan.
- 38.Davidson D.W. Ecological studies of neotropical ant gardens. Ecology. 1988;69:1138–1152. doi: 10.2307/1941268. [DOI] [Google Scholar]
- 39.Dubuisson J.Y., Bary S., Ebihara A., Carnero-Diaz E., Boucheron-Dubuisson E., Hennequin S. Epiphytism, anatomy and regressive evolution in trichomanoid filmy ferns (Hymenophyllaceae) Bot. J. Linn. Soc. 2013;173:573–593. doi: 10.1111/boj.12106. [DOI] [Google Scholar]
- 40.Blüthgen N., Schmit-Neuerburg V., Engwald S., Barthlott W. Ants as epiphyte gardeners: Comparing the nutrient quality of ant and termite canopy substrates in a Venezuelan lowland rain forest. J. Trop. Ecol. 2001;17:887–894. doi: 10.1017/S0266467401001651. [DOI] [Google Scholar]
- 41.Laviski B.F.d.S., Mahyé-Nunes A.J., Nunes-Freitas A.F. Structure of ant-diaspore networks and their functional outcomes in a Brazilian Atlantic Forest. Sociobiology. 2021;68:e7104. doi: 10.13102/sociobiology.v68i3.7104. [DOI] [Google Scholar]
- 42.Goodale U.M., Ashton M.S., Berlyn G.P., Gregoire T.G., Singhakumara B.M.P., Tennakoon K.U. Disturbance and tropical pioneer species: Patterns of association across life history stages. For. Ecol. Manag. 2012;277:54–66. doi: 10.1016/j.foreco.2012.04.020. [DOI] [Google Scholar]
- 43.Campos R.I., Soares J.P., Martins R.P., Ribeiro S.P. Effect of habitat structure on ant assemblages (Hymenoptera: Formicidae) associated to two pioneer tree species. Sociobiology. 2006;47:721–737. [Google Scholar]
- 44.Stokes K.E., Ward K.A., Colloff M.J. Alterations in flood frequency increase exotic and native species richness of understory vegetation in a temperate floodplain eucalypt forest. Plant Ecol. 2010;211:219–233. doi: 10.1007/s11258-010-9833-7. [DOI] [Google Scholar]
- 45.López-Acosta J.C., Dirzo R. Aspectos relevantes sobre la historia natural de las plantas hemiepífitas estranguladoras. Interciencia. 2015;40:190–197. [Google Scholar]
- 46.Dejean A., Orivel J., Rossi V., Roux O., Lauth J., Malé P.J.G., Céréghino R., Leroy C. Predation success by a plant-ant indirectly favours the growth and fitness of its host Myrmecophyte. PLoS ONE. 2013;8:e59405. doi: 10.1371/journal.pone.0059405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-Teuber M., Heil M. Comparative anatomy and physiology of myrmecophytes: Ecological and evolutionary perspectives. Res. Rep. Biodivers. Stud. 2015;4:21–32. doi: 10.2147/RRBS.S60420. [DOI] [Google Scholar]
- 48.Orivel J., Lambs L., Malé P.J.G., Leroy C., Grangier J., Otto T., Quilichini A., Dejean A. Dynamics of the association between a long-lived understory myrmecophyte and its specific associated ants. Oecologia. 2011;165:369–376. doi: 10.1007/s00442-010-1739-5. [DOI] [PubMed] [Google Scholar]
- 49.Tie S., Wang J., He N., Zhao Z., Liu Y. Biodiversity and ecological network of epiphytic bryophytes and their host trees in the forests of the southeastern Qinghai-Tibet Plateau. Ecol. Indic. 2023;146:109781. doi: 10.1016/j.ecolind.2022.109781. [DOI] [Google Scholar]
- 50.Vital M., Castro M.M., Zeringóta V., Prezoto F. Myrmecofauna of urban gardens in southeast region of Brazil = Mirmecofauna de jardins urbanos na região sudeste do Brasil. Biosci. J. 2015;31:1205–1212. doi: 10.14393/BJ-v31n4a2015-26130. [DOI] [Google Scholar]
- 51.Bizerril M., Vieira E. Azteca ants as antiherbivore agents of Tococa formicaria (Melastomataceae) in Brazilian cerrado. Stud. Neotrop. Fauna Environ. 2002;37:145–149. doi: 10.1076/snfe.37.2.145.8585. [DOI] [Google Scholar]
- 52.Michelangeli F.A. Ant Protection against herbivory in three species of Tococa (Melastomataceae) occupying different environments. Biotropica. 2003;35:181–188. doi: 10.1111/j.1744-7429.2003.tb00277.x. [DOI] [Google Scholar]
- 53.Silva B.M.d.S., Lima J.D., Dantas V.A.V., Moraes W.d.S., Sabonaro D.Z. Efeito da luz no crescimento de mudas de Hymenaea parvifolia Huber. Rev. Árvore. 2007;31:1019–1026. doi: 10.1590/S0100-67622007000600006. [DOI] [Google Scholar]
- 54.Cipriano J., Martins L., Deus M.d.S.M.d., Peron A.P. O gênero Hymenaea e Euas espécies mais importantes do. Cad. Pesqui. 2014;26:41–51. doi: 10.17058/CP.V26I2.5248. [DOI] [Google Scholar]
- 55.Ribeiro R.D., Cardoso D.B.O.S., Cardoso D., de Lima H.C. A new species of Hymenaea (Leguminosae: Caesalpinioideae) with a revised identification key to the genus in the Brazilian Atlantic forest. Syst. Bot. 2015;40:151–156. doi: 10.1600/036364415X686440. [DOI] [Google Scholar]
- 56.Raine C.A., Farrar D.R., Sheffield E. A new Hymenophyllum species in the Appalachians represented by independent gametophyte colonies. Am. Fern J. 1991;81:109–118. doi: 10.2307/1547542. [DOI] [Google Scholar]
- 57.Torres-Torres J.J., Medina-Arroyo H.H., Martinez-Guardia M. Germinación y crecimiento inicial de Hymenaea oblongifolia Huber en el municipio de Istmina, Chocó, Colombia. Entramado. 2018;14:230–242. doi: 10.18041/1900-3803/entramado.2.4760. [DOI] [Google Scholar]
- 58.Corbara B., Dejean A., Orivel J. Les « jardins de fourmis, une association plantes-fourmis originale. Année Biol. 1999;38:73–89. doi: 10.1016/S0003-5017(99)80027-0. [DOI] [Google Scholar]
- 59.Dejean A., Corbara B., Orivel J., Snelling R.R., Delabie J., Belin-Depoux M. The importance of ant gardens in the pioneer vegetal formations of French Guiana (Hymenoptera: Formicidae) Sociobiology. 2000;35:425–439. [Google Scholar]
- 60.Cedeño A., Mérida T., Zegarra J. Ant Gardens of Surumoni, Venezuela. [(accessed on 25 August 2024)];Selbyana. 1999 20:125–132. Available online: https://www.jstor.org/stable/41760015. [Google Scholar]
- 61.Leal L.C., Jacovak C.C., Bobrowiec P.E.D., Camargo J.L.C., Peixoto P.E.C. The role of parabiotic ants and environment on epiphyte composition and protection in ant gardens. Sociobiology. 2017;64:276–283. doi: 10.13102/sociobiology.v64i3.1219. [DOI] [Google Scholar]
- 62.Jiménez I.V., Miranda A.F. Epiphyte orchid diversity in a Yungas montane forest in the Cotapata National Park and Integrated Management Natural Area, La Paz—Bolivia. Lankesteriana. 2007;7:49–52. doi: 10.15517/lank.v7i1-2.18434. [DOI] [Google Scholar]
- 63.Bianchi J.S., Kersten R.d.A. Edge effect on vascular epiphytes in a subtropical Atlantic Forest. Acta Bot. Bras. 2014;28:120–126. doi: 10.1590/S0102-33062014000100012. [DOI] [Google Scholar]
- 64.Farrell A.D., Evelyn S., Lennon A.M., Umaharan P. Genotypic variation in senescence and water relations in cut flowers of Anthurium andraeanum (Hort.) Hortscience. 2012;47:133–1337. doi: 10.21273/HORTSCI.47.9.1333. [DOI] [Google Scholar]
- 65.Rajeevan P.K., Kumari P.K.V., Prasada Rao G.S.L.H.V., Liji P.V., Mohan S. Performance evaluation of cut flower varieties of anthurium under two agroclimatic conditions. J. Ornam. Hortic. 2007;10:177–180. [Google Scholar]
- 66.Sonnleitner M., Dullinger S., Wanek W., Zechmeister H.G. Microclimatic patterns correlate with the distribution of epiphyllous bryophytes in a tropical lowland rain forest in Costa Rica. J. Trop. Ecol. 2009;25:321–330. doi: 10.1017/S0266467409006002. [DOI] [Google Scholar]
- 67.Kleinfeldt S.E. Ant-Gardens: The interaction of Codonanthe crassifolia (Gesneriaceae) and Crematogaster longispina (Formicidae) Ecology. 1978;59:449–456. doi: 10.2307/1936574. [DOI] [Google Scholar]
- 68.Beattie A.J. The Evolutionary Ecology of Ant–Plant Mutualisms. Cambridge University Press; Cambridge, UK: 1985. [DOI] [Google Scholar]
- 69.Said C. Les nectaires floraux des Crassulacées Etude morphologique, histologique et anatomique. Lett. Bot. 1982;129:231–240. doi: 10.1080/01811797.1982.10824548. [DOI] [Google Scholar]
- 70.Gontcharova S.B., Gontcharov A.A. Molecular phylogeny and systematics of flowering plants of the family Crassulaceae DC. Mol. Biol. 2009;43:794–803. doi: 10.1134/S0026893309050112. [DOI] [PubMed] [Google Scholar]
- 71.Symmank L., Samain M.-S., Smith J.F., Pino G., Stoll A., Goetghebeur P., Neinhuis C., Wanke S. The extraordinary journey of Peperomia subgenus Tildenia (Piperaceae): Insights into diversification and colonization patterns from its cradle in Peru to the Trans-Mexican Volcanic Belt. J. Biogeogr. 2011;38:2337–2349. doi: 10.1111/j.1365-2699.2011.02586.x. [DOI] [Google Scholar]
- 72.Mathieu G., Symmank L., Callejas R., Wanke S., Neinhuis C., Goetghebeur P., Samain M.-S. New geophytic Peperomia (Piperaceae) species from Mexico, Belize and Costa Rica. Rev. Mex. Biodivers. 2011;82:357–382. doi: 10.22201/IB.20078706E.2011.2.1199. [DOI] [Google Scholar]
- 73.Takemori N.K., Bona C., Alquini Y. Anatomia comparada das folhas de espécies de Peperomia (Piperaceae): I. Ontogênese do tecido aqüífero e dos estômatos. Acta Bot. Bras. 2003;17:387–394. doi: 10.1590/S0102-33062003000300006. [DOI] [Google Scholar]
- 74.Horner H.T. Peperomia leaf cell wall interface between the multiple hypodermis and crystal-containing photosynthetic layer displays unusual pit fields. AoB Plants. 2012;109:1307–1316. doi: 10.1093/aob/mcs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinheiro F., Cozzolino S. Epidendrum (Orchidaceae) as a model system for ecological and evolutionary studies in the Neotropics. Taxon. 2013;62:77–88. doi: 10.1002/tax.621007. [DOI] [Google Scholar]
- 76.Rocha C.F.D., Bergallo H.G. Bigger ant colonies reduce herbivory and herbivore residence time on leaves of an ant-plant: Azteca muelleri vs. Coelomera ruficornis on Cecropia pachystachya. Oecologia. 1992;91:249–252. doi: 10.1007/BF00317792. [DOI] [PubMed] [Google Scholar]
- 77.Dejean A., Grangier J., Leroy C., Orivel J. Predation and aggressiveness in host plant protection: A generalization using ants from the genus Azteca. Sci. Nat. 2009;96:57–63. doi: 10.1007/s00114-008-0448-y. [DOI] [PubMed] [Google Scholar]
- 78.Quinteros-Gómez Y.M., Macedo-Bedoya J. Primer reporte de jardines de hormigas en renacales de Piedemonte Andino-Amazónico en Perú. Lilloa. 2024:149–158. doi: 10.30550/j.lil/1927. [DOI] [Google Scholar]
- 79.Grasso D.A., Pandolfi C., Bazihizina N., Nocentini D., Nepi M., Mancuso S. Extrafloral-nectar-based partner manipulation in plant–ant relationships. AoB Plants. 2015;7:lv002. doi: 10.1093/aobpla/plv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alencar C., Nogueira A., Vicente R., Coutinho Í. Plant species with larger extrafloral nectaries produce better quality nectar when needed and interact with the best ant partners. J. Exp. Bot. 2023;74:4613–4627. doi: 10.1093/jxb/erad160. [DOI] [PubMed] [Google Scholar]
- 81.Oliveira P.S., Freitas A.V. Ant-plant-herbivore interactions in the neotropical cerrado savanna. Sci. Nat. 2004;91:557–570. doi: 10.1007/s00114-004-0585-x. [DOI] [PubMed] [Google Scholar]
- 82.Cupul-Magaña F.G. Hymenoptera: Mirmecofauna (Hymenoptera: Formicidae) común del estero “El Salado” y Puerto Vallarta, Jalisco, México. Dugesiana. 2004;11:13–20. doi: 10.32870/dugesiana.v11i1.3801. [DOI] [Google Scholar]
- 83.Czaczkes T.J., Heinze J., Ruther J. Nest Etiquette—Where ants go when nature calls. PLoS ONE. 2015;10:e0118376. doi: 10.1371/journal.pone.0118376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrone O., Vega A.S., Maier M. Elaiosomes in Urochloa paucispicata (Poaceae: Panicoideae: Paniceae): Anatomy and chemical composition. Flora. 2000;195:303–310. doi: 10.1016/S0367-2530(17)30989-1. [DOI] [Google Scholar]
- 85.Sheridan S.L., Iversen K.A., Itagaki H. The role of chemical senses in seed-carrying behavior by ants: A behavioral, physiological, and morphological study. J. Insect Physiol. 1996;42:149–159. doi: 10.1016/0022-1910(95)00087-9. [DOI] [Google Scholar]
- 86.Youngsteadt E., Nojima S., Häberlein C., Schulz S., Schal C. Seed odor mediates an obligate ant–plant mutualism in Amazonian rainforests. Proc. Natl. Acad. Sci. USA. 2008;105:4571–4575. doi: 10.1073/pnas.0708643105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kattan G.H., Murcia C., Aldana R.C., Usma S. Relaciones entre hormigas y Melastomataceas en un bosque lluvioso del Pacífico colombiano. [(accessed on 10 June 2024)];BMEUV. 2011 9:1–10. Available online: https://hdl.handle.net/10893/765. [Google Scholar]
- 88.Orivel J., Dejean A., Errard C. Active role of two Ponerine ants in the elaboration of ant gardens. [(accessed on 5 May 2024)];Biotropica. 1998 30:487–491. doi: 10.1111/j.1744-7429.1998.tb00085.x. Available online: http://www.jstor.org/stable/2389135. [DOI] [Google Scholar]
- 89.Céréghino R., Leroy C., Dejean A., Corbara B. Ants mediate the structure of phytotelm communities in an ant-garden bromeliad. Ecology. 2010;91:1549–1556. doi: 10.1890/09-1534.1. [DOI] [PubMed] [Google Scholar]
- 90.Benzing D.H. Bromeliaceae: Profile of an Adaptive Radiation. Cambridge University Press; Cambridge, UK: 2000. [DOI] [Google Scholar]
- 91.Dejean A., Azémar F., Naskrecki P., Tindo M., Rossi V., Faucher C., Gryta H. Mutualistic interactions between ants and fungi: A review. Ecol. Evol. 2023;13:e10386. doi: 10.1002/ece3.10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farji-Brener A.G.F. Modificaciones al suelo realizadas por hormigas cortadoras de hojas (Formicidae, Attini): Una revisión de sus efectos sobre la vegetación. Ecol. Austral. 1992;2:87–94. [Google Scholar]
- 93.Risch S.J., Carroll C.R. The ecological role of ants in two Mexican agroecosystems. Oecologia. 1982;55:114–119. doi: 10.1007/BF00386726. [DOI] [PubMed] [Google Scholar]
- 94.Weissflog A., Kaufmann E., Maschwitz U. Ant gardens of Camponotus (Myrmotarsus) irritabilis (Hymenoptera: Formicidae: Formicinae) and Hoya elliptica (Apocynaceae) in Southeast Asia. Asian Myrmecol. 2017;9:e009001. doi: 10.20362/am.009001. [DOI] [Google Scholar]
- 95.Montoya-Lerma J., Giraldo-Echeverri C., Armbrecht I., Farji-Brener A.G., Calle Z. Leaf-cutting ants revisited: Towards rational management and control. Int. J. Pest Manag. 2012;58:225–247. doi: 10.1080/09670874.2012.663946. [DOI] [Google Scholar]
- 96.Souza A.F., Martins F.R. Spatial variation and dynamics of flooding, canopy openness, and structure in a neotropical swamp forest. Plant Ecol. 2005;180:161–173. doi: 10.1007/s11258-004-7811-7. [DOI] [Google Scholar]
- 97.Queiroz A.C.M., Ribas C.R. Canopy cover negatively affects arboreal ant species richness in a tropical open habitat. Braz. J. Biol. 2016;76:864–870. doi: 10.1590/1519-6984.02015. [DOI] [PubMed] [Google Scholar]
- 98.Mera Y.A., Gallego M.C., Armbrecht I. Interacciónes entre hormigas e insectos en follaje de cafetales de sol y sombra, Cauca-Colombia. Rev. Col. Entomol. 2010;36:116–126. doi: 10.25100/socolen.v36i1.9131. [DOI] [Google Scholar]
- 99.Gutiérrez-Martínez P.R., Acuña-Sánchez D. Patrones diarios de actividad de la hormiga Azteca constructor (Hymenoptera: Formicidae) y su relación con la presencia de alimento. UNED Res. J. 2013;5:219–225. doi: 10.22458/urj.v5i2.269. [DOI] [Google Scholar]
- 100.Mueller U.G., Scott J.J., Ishak H.D., Cooper M., Rodrigues A. Monoculture of leafcutter ant gardens. PLoS ONE. 2010;5:e12668. doi: 10.1371/journal.pone.0012668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Álvarez C., Jiménez-Ríos L., Iniesta-Pallarés M., Jurado-Flores A., Molina-Heredia F., Ng C.K.Y., Mariscal V. Symbiosis between cyanobacteria and plants: From molecular studies to agronomic applications. J. Exp. Bot. 2023;74:6145–6157. doi: 10.1093/jxb/erad261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lobry de Bruyn L.A. Ants as bioindicators of soil function in rural environments. Agric. Ecosyst. Environ. 1999;74:425–441. doi: 10.1016/S0167-8809(99)00047-X. [DOI] [Google Scholar]
- 103.Sanabria C., Lavelle P., Fonte S.J. Ants as indicators of soil-based ecosystem services in agroecosystems of the Colombian Llanos. Appl. Soil Ecol. 2014;84:24–30. doi: 10.1016/j.apsoil.2014.07.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study can be requested from the corresponding author via email: tavomonroyvilchis@gmail.com.