Abstract
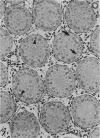
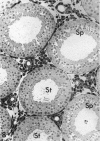
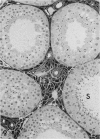
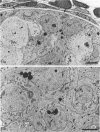
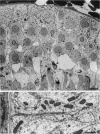
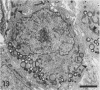
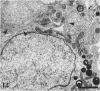
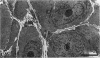
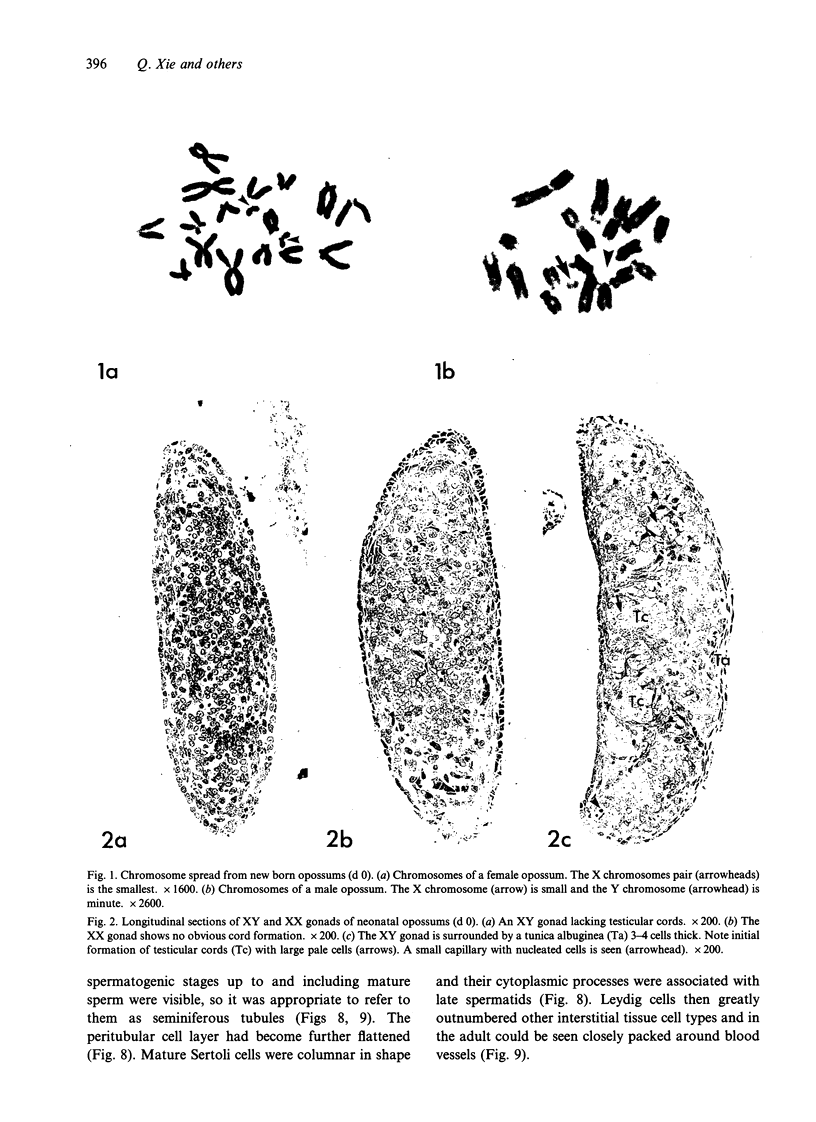
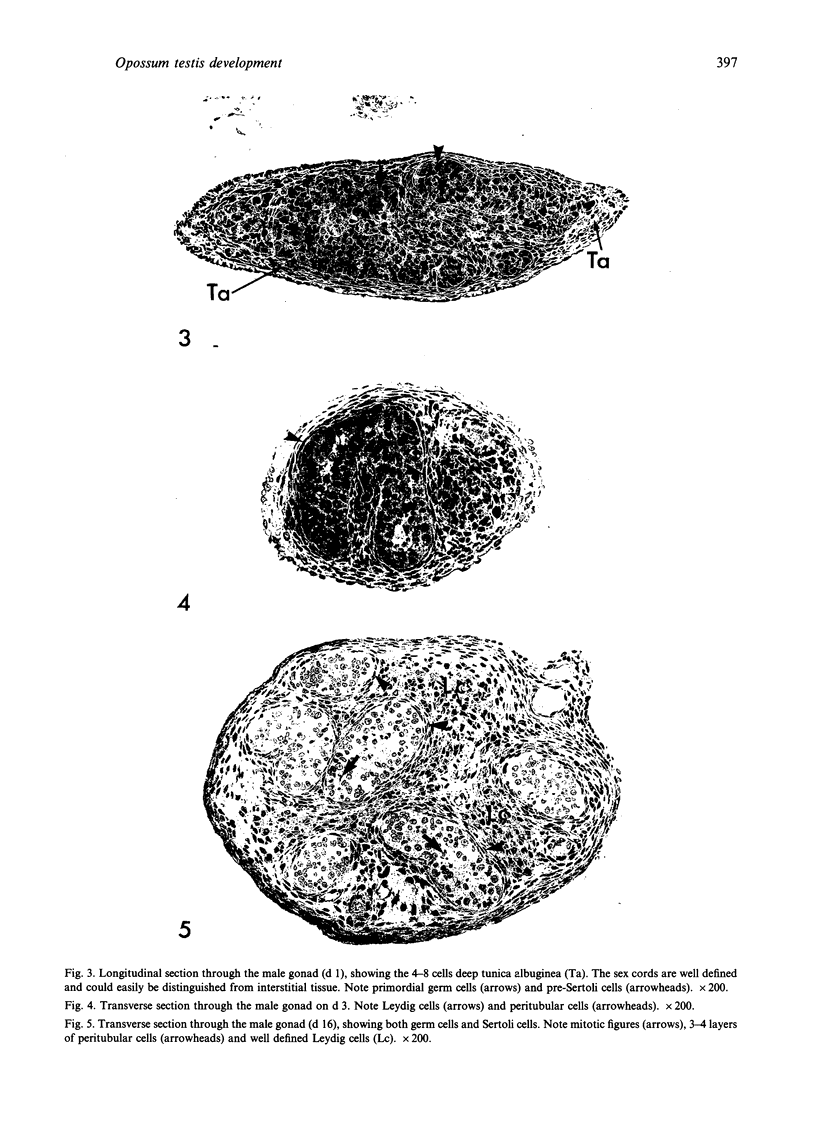
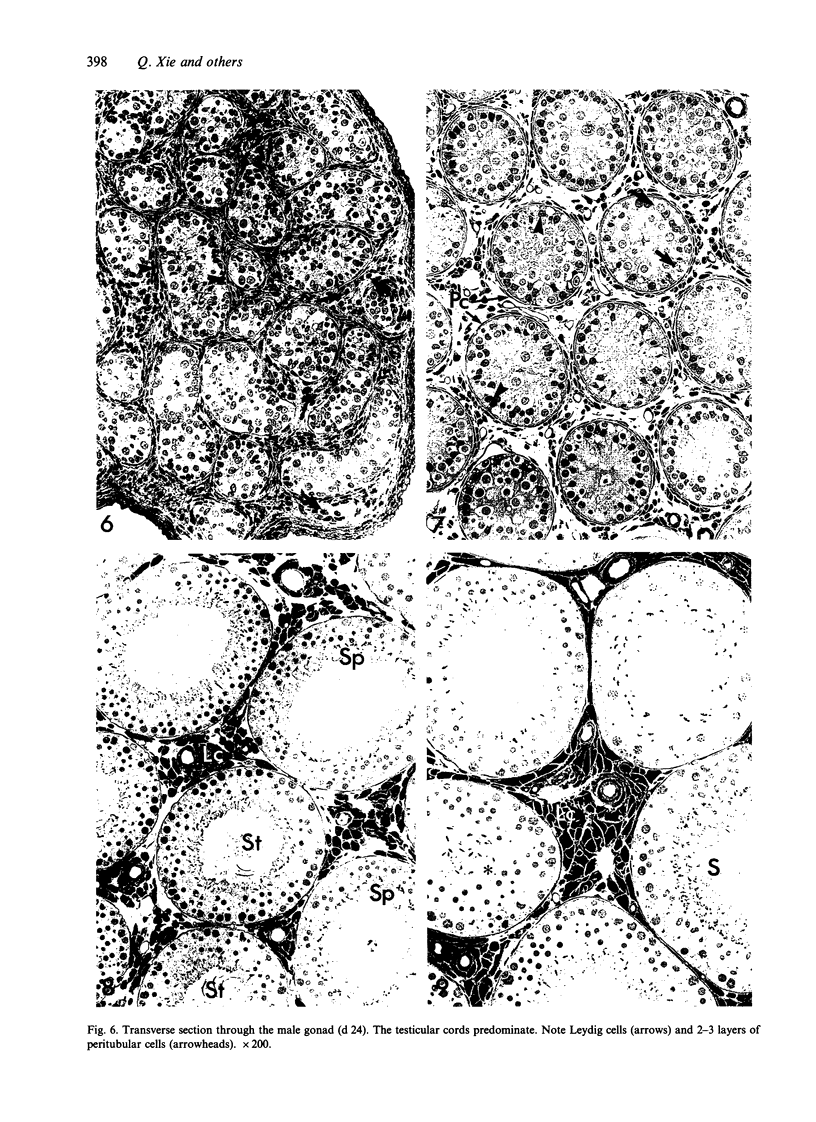
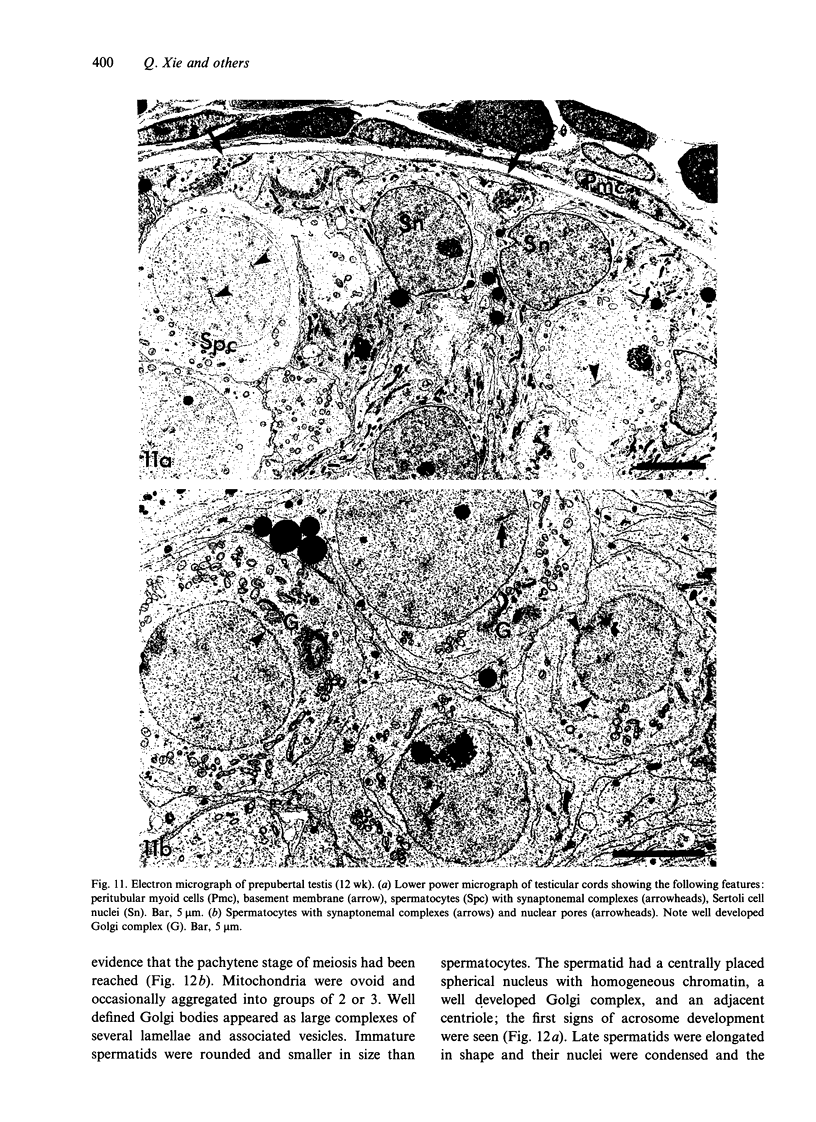
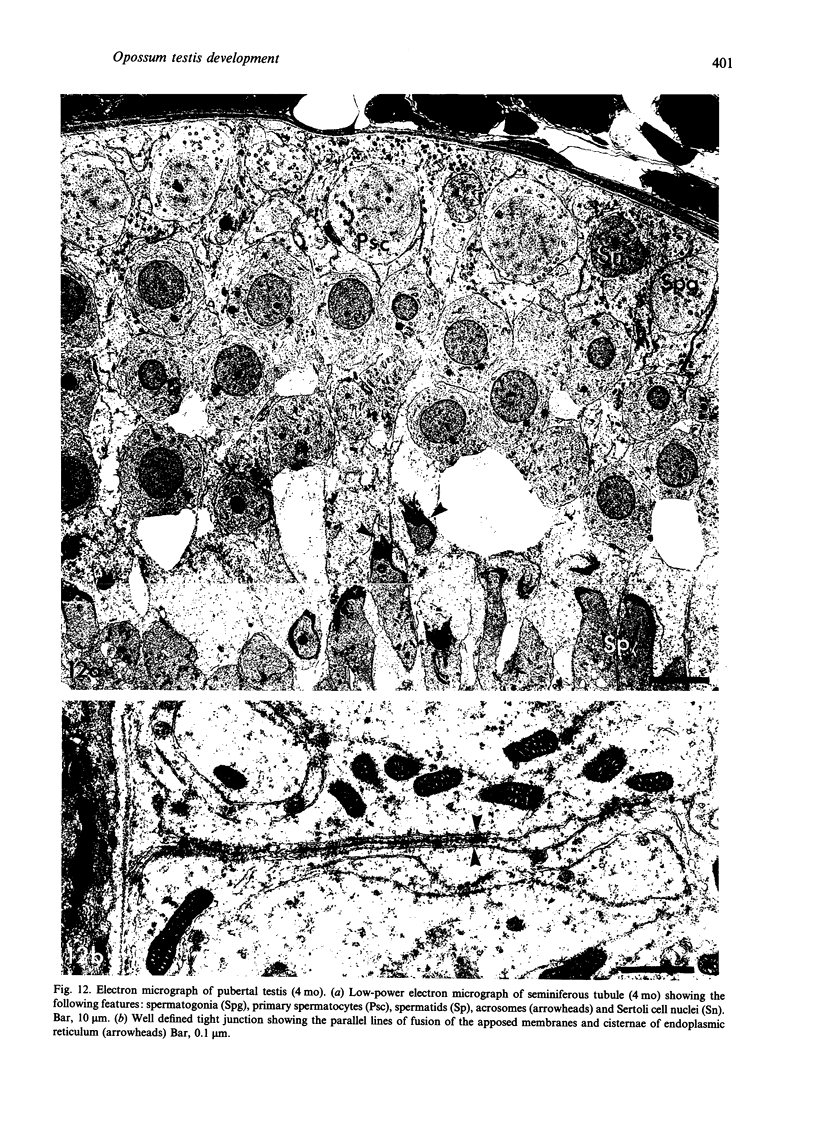
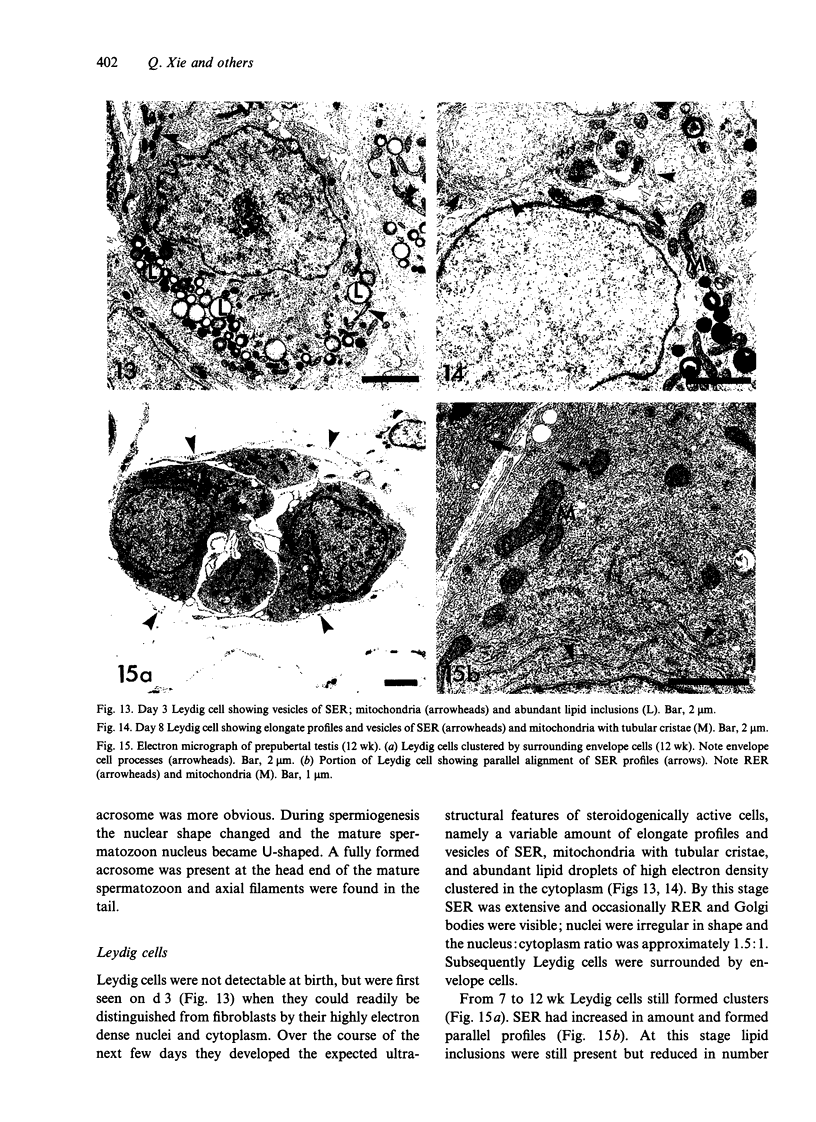
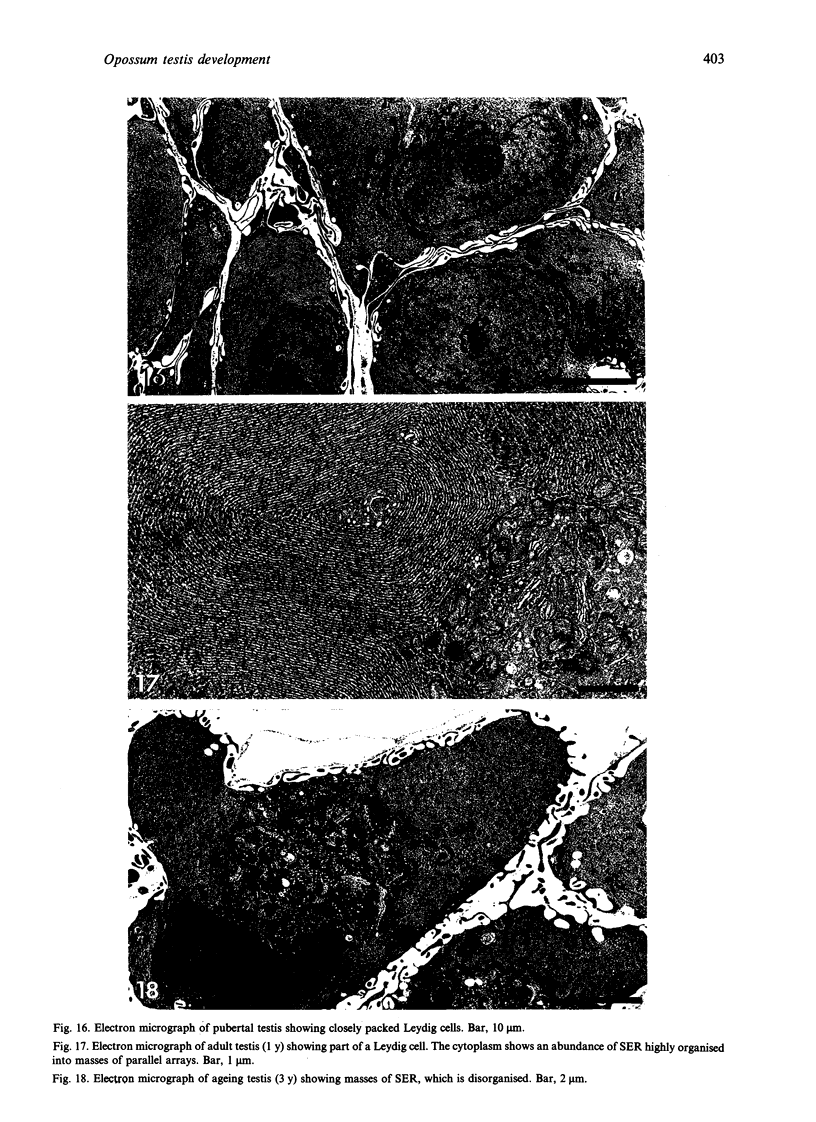
Testis development in the grey short-tailed opossum, Monodelphis domestica, was investigated by light and electron microscopy in 180 animals. On the day of birth, half the karyotyped males were found to have histologically differentiated testes. By day (d) 1 testicular cords were clearly distinguished in all XY gonads and the tunica albuginea was fully developed. At this stage the large and pale primordial germ cells could be differentiated from dark pre-Sertoli cells. From d 3 the testis became progressively rounded and testicular cords were surrounded by peritubular cells. Leydig cells were then distinguishable by the expected ultrastructural features of steroidogenically active cells, showing abundant vesicles of SER, extensive mitochondria with tubular cristae and numerous lipid inclusions. Subsequently these cells formed clusters and were surrounded by envelope cells until wk 12. Testes were located in the abdomen, attached to the large mesonephroi, until d 24 after birth when they began their descent to the scrotal sac. From 7 wk the interstitial tissue became less cellular. At the prepubertal stage (12 wk), the seminiferous tubules lacked lumina. Leydig cell cytoplasm was electron-dense with increased amounts of SER forming parallel profiles. By 4 mo (pubertal stage), seminiferous tubules were patent and various spermatogenic stages, including spermatozoa, were seen for the first time. Leydig cells then greatly outnumbered other interstitial tissue cells and were closely-packed around blood vessels but no longer clustered by envelope cells; their SER was very highly organised into masses of parallel arrays and lipid inclusions were reduced. In the adult (1 y) Leydig cells reached their greatest size; their morphological features resembled those seen at 4 mo except that lipid inclusions were sparse. In ageing Leydig cells (2-3 y), large amounts of SER were present but disorganised.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Moore H. D., Burgess A. M., Mittwoch U. Gonadal sex differentiation in embryos and neonates of the marsupial, Monodelphis domestica: arrest of testis development in postterm embryos. J Anat. 1993 Apr;182(Pt 2):267–273. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Moore H. D., Penfold L. M., Burgess A. M., Mittwoch U. Gonadal sex differentiation in the neonatal marsupial, Monodelphis domestica. Development. 1990 Jul;109(3):699–703. doi: 10.1242/dev.109.3.699. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN A. K., FAWCETT D. W. The normal fine structure of opossum testicular interstitial cells. J Biophys Biochem Cytol. 1961 Mar;9:653–670. doi: 10.1083/jcb.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavicchia J. C., Sacerdote F. L. Correlation between blood-testis barrier development and onset of the first spermatogenic wave in normal and in busulfan-treated rats: a lanthanum and freeze-fracture study. Anat Rec. 1991 Jul;230(3):361–368. doi: 10.1002/ar.1092300309. [DOI] [PubMed] [Google Scholar]
- Fadem B. H. Evidence for the activation of female reproduction by males in a marsupial, the gray short-tailed opossum (Monodelphis domestica). Biol Reprod. 1985 Aug;33(1):112–116. doi: 10.1095/biolreprod33.1.112. [DOI] [PubMed] [Google Scholar]
- Fadem B. H., Rayve R. S. Characteristics of the oestrous cycle and influence of social factors in grey short-tailed opossums (Monodelphis domestica). J Reprod Fertil. 1985 Mar;73(2):337–342. doi: 10.1530/jrf.0.0730337. [DOI] [PubMed] [Google Scholar]
- Fadem B. H., Tesoriero J. V., Whang M. Early differentiation of the gonads in the gray short-tailed opossum (Monodelphis domestica). Biol Neonate. 1992;61(2):131–136. doi: 10.1159/000243542. [DOI] [PubMed] [Google Scholar]
- Gondos B., Paup D. C., Ross J., Gorski R. A. Ultrastructural differentiation of Leydig cells in the fetal and postnatal hamster testis. Anat Rec. 1974 Mar;178(3):551–565. doi: 10.1002/ar.1091780304. [DOI] [PubMed] [Google Scholar]
- Harding H. R., Carrick F. N., Shorey C. D. Crystalloid inclusions in the Sertoli cell of the koala, Phascolarctos cinereus (Marsupialia). Cell Tissue Res. 1982;221(3):633–642. doi: 10.1007/BF00215707. [DOI] [PubMed] [Google Scholar]
- Kerr J. B., Knell C. M. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development. 1988 Jul;103(3):535–544. doi: 10.1242/dev.103.3.535. [DOI] [PubMed] [Google Scholar]
- Kuopio T., Paranko J., Pelliniemi L. J. Basement membrane and epithelial features of fetal-type Leydig cells in rat and human testis. Differentiation. 1989 Jun;40(3):198–206. doi: 10.1111/j.1432-0436.1989.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Kuopio T., Tapanainen J., Pelliniemi L. J., Huhtaniemi I. Developmental stages of fetal-type Leydig cells in prepubertal rats. Development. 1989 Oct;107(2):213–220. doi: 10.1242/dev.107.2.213. [DOI] [PubMed] [Google Scholar]
- Mackay S., Smith R. A. Mouse gonadal differentiation in vitro in the presence of fetal calf serum. Cell Differ Dev. 1989 Jun;27(1):19–28. doi: 10.1016/0922-3371(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Maitland P., Ullmann S. L. Gonadal development in the opossum, Monodelphis domestica: the rete ovarii does not contribute to the steroidogenic tissues. J Anat. 1993 Aug;183(Pt 1):43–56. [PMC free article] [PubMed] [Google Scholar]
- O W. S., Short R. V., Renfree M. B., Shaw G. Primary genetic control of somatic sexual differentiation in a mammal. Nature. 1988 Feb 25;331(6158):716–717. doi: 10.1038/331716a0. [DOI] [PubMed] [Google Scholar]
- Olson G. E., Winfrey V. P. Changes in actin distribution during sperm development in the opossum, Monodelphis domestica. Anat Rec. 1991 Jun;230(2):209–217. doi: 10.1002/ar.1092300208. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner J. B. Nuclear shaping in marsupial spermatids. J Ultrastruct Res. 1972 Sep;40(5):498–512. doi: 10.1016/s0022-5320(72)80038-8. [DOI] [PubMed] [Google Scholar]
- Renfree M. B., Short R. V. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Philos Trans R Soc Lond B Biol Sci. 1988 Dec 1;322(1208):41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- Rodger J. C. The testis and its excurrent ducts in American caenolestid and didelphid marsupials. Am J Anat. 1982 Mar;163(3):269–282. doi: 10.1002/aja.1001630307. [DOI] [PubMed] [Google Scholar]
- Russell L. D., Bartke A., Goh J. C. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat. 1989 Mar;184(3):179–189. doi: 10.1002/aja.1001840302. [DOI] [PubMed] [Google Scholar]
- Taggart D. A., Johnson J. L., O'Brien H. P., Moore H. D. Why do spermatozoa of American marsupials form pairs? A clue from the analysis of sperm-pairing in the epididymis of the grey short-tailed opossum, Monodelphis domestica. Anat Rec. 1993 Jul;236(3):465–478. doi: 10.1002/ar.1092360307. [DOI] [PubMed] [Google Scholar]
- Taggart D. A., Johnson J., Temple-Smith P. D. Testicular and epididymal development in the brown marsupial mouse, Antechinus stuartii (Dasyuridae, Marsupialia). Anat Embryol (Berl) 1993 Jul;188(1):87–100. doi: 10.1007/BF00191454. [DOI] [PubMed] [Google Scholar]
- Ullmann S. L. Differentiation of the gonads and initiation of mammary gland and scrotum development in the brushtail possum Trichosurus vulpecula (Marsupialia). Anat Embryol (Berl) 1993 May;187(5):475–484. doi: 10.1007/BF00174423. [DOI] [PubMed] [Google Scholar]
- Ullmann S. L. Early differentiation of the testis in the native cat, Dasyurus viverrinus (Marsupialia). J Anat. 1984 Jun;138(Pt 4):675–688. [PMC free article] [PubMed] [Google Scholar]
- Zirkin B. R., Ewing L. L. Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat Rec. 1987 Oct;219(2):157–163. doi: 10.1002/ar.1092190208. [DOI] [PubMed] [Google Scholar]