Supplemental Digital Content is Available in the Text.
Individuals with fibromyalgia syndrome have impaired Hoffman reflex rate-dependent depression implicating spinal disinhibition as a pain-generating mechanism and novel treatment target.
Keywords: Fibromyalgia, Spinal disinhibition, H-reflex rate-dependent depression, Conditioned pain modulation
Abstract
Introduction:
Pain phenomenology in patients with fibromyalgia syndrome (FMS) shows considerable overlap with neuropathic pain. Altered neural processing leading to symptoms of neuropathic pain can occur at the level of the spinal cord, and 1 potential mechanism is spinal disinhibition. A biomarker of spinal disinhibition is impaired H-reflex rate-dependent depression (HRDD).
Objectives:
This study investigated whether patients with FMS exhibit evidence of spinal disinhibition.
Methods:
Thirty-one individuals with FMS and 20 healthy volunteers underwent testing of Hoffman reflex including HRDD, along with assessment of clinical signs and symptoms, pressure pain thresholds, temporal summation of pain (wind-up), and conditioned pain modulation (CPM). Small nerve fibre structure was quantified using intraepidermal nerve fibre density and corneal confocal microscopy.
Results:
Patients with FMS had significantly impaired HRDD at 1 Hz (P = 0.026) and 3 Hz (P = 0.011) and greater wind-up ratio (P = 0.008) compared with healthy controls. Patients with the most impaired HRDD also had the most inefficient CPM but HRDD was not associated with wind-up. Both HRDD and CPM were most impaired in patients with a shorter duration of disease.
Conclusion:
We demonstrate for the first time that people with FMS show evidence of spinal disinhibition, which is most dominant in shorter duration of disease and may represent a putative mechanism of pain generation in FMS. Identifying people with impairment of central pain processing at an early stage may provide opportunities for targeted mechanistically directed interventions. Longitudinal studies are warranted to tease out the precise contribution of these mechanisms.
1. Introduction
Fibromyalgia syndrome (FMS) is a common but poorly understood condition. It is a frequent cause of chronic widespread pain, often accompanied by fatigue, cognitive impairment, sleep, and mood disturbances.7 This complex and heterogenous syndrome presents challenges to both diagnosis and treatment,6 partly due to a lack of understanding of its pathoaetiology. It is debated whether the symptoms and signs of FMS reflect primarily peripheral or central sensitisation.8,10,15,40,44,65 People with FMS frequently report symptoms and signs seen in neuropathic pain including paraesthesia, hyperalgesia, and allodynia. Indeed, a subset of patients with FMS develops small fibre pathology,15,25,63 and microneurography studies in FMS demonstrate c-fibre nociceptor sensitisation.54
Whilst in some patients with pain syndromes, the underlying pathophysiology relates to peripheral nerve fibre degeneration and sensitisation, the functional significance of small fibre abnormalities and their relation to symptoms in FMS remains unclear.17,35,62 Furthermore, it is recognised that in FMS changes in the brain2,16,51 and spinal cord56,68 may generate, maintain, and modulate pain signalling. Both reduced pain modulation with inefficient descending pain inhibition,36,69 and/or increased temporal summation of pain,55 are reported in patients with FMS. However, recent studies in FMS using sensitivity-adjusted stimuli demonstrate effective processing of nociceptive input.55–58 Another potential mechanism of central sensitisation is spinal disinhibition, where disruption of intrinsic ligand-gated ionotropic inhibitory systems in the dorsal horn of the spinal cord leads to the amplification of incoming peripheral signals and increased ascending nociceptive drive.50 Spinal disinhibition has been extensively studied in preclinical neuropathic and inflammatory pain models50 and is proposed as a novel treatment target. However, the lack of a biomarker of spinal disinhibition as a pain-generating mechanism in humans has hindered the investigation of spinal inhibitory dysfunction in clinical pain conditions.
Noninvasive investigation of human spinal circuits using neurophysiological methods, for example, the Hoffman reflex (H-reflex), can provide valuable information about excitation and inhibition in the spinal cord.43 Hoffman reflex testing elicits a direct M wave and a longer latency trans-spinal H wave. The H-wave response is classically considered a monosynaptic circuit between type 1a muscle spindle afferents and motoneurons in the ventral spinal cord. However, it is subject to modification by intrinsic inhibitory oligo- and polysynaptic spinal circuits as well as supraspinal influences.4,34 These influences modulate the H-wave amplitude by altering the balance between excitatory and inhibitory inputs in the H-reflex pathway. An example, the diminution of the amplitude of the H wave with consecutive stimulations is termed H-reflex rate-dependent depression (HRDD).28
Translational studies indicate that impaired HRDD is a biomarker of spinal disinhibition, likely due to chloride dysregulation in the dorsal horn of the spinal cord, in animals and humans.33,34 We have previously demonstrated that HRDD is impaired in patients with painful diabetic neuropathy.38,68 Whether patients with FMS have evidence of spinal disinhibition is unknown. The primary aim of this study was to determine, using HRDD, whether patients with FMS display evidence of spinal disinhibition. We also aimed to determine whether HRDD is associated with presumed changes in central processing, wind-up, and conditioned pain modulation (CPM), as well as primary psychophysical characteristics of FMS.
2. Methods
Thirty-one patients with FMS were recruited to the DEFINE-FMS study (South West—Frenchay Research Ethics Committee—20/SW/0138) from physiotherapy-led musculoskeletal fibromyalgia services, pain clinics, as well as community-based fibromyalgia patient support groups. Patients underwent testing of HRDD, assessment of pressure pain thresholds (PPTs), temporal summation of pain and CPM, corneal confocal microscopy, quantification of intraepidermal nerve fibre density (IENFD) using skin biopsy, and completion of questionnaires to assess the presence of pain, neuropathic symptoms, and the impact of FMS on day-to-day life. To be included in this study, people with FMS aged ≥18 years satisfied the modified American College of Rheumatology 2016 diagnostic criteria.66 Twenty healthy volunteers (HV) were also recruited.
2.1. Assessment of H-reflex rate-dependent depression
For H-reflex studies, tibial nerve stimulation was performed using 1-ms square wave monophasic pulses delivered using surface silver–silver chloride electrodes, to the popliteal fossa. Surface silver–silver chloride recording electrodes with a diameter of 9 mm were placed on the long axis of the soleus muscle (Fig. 1A). Hoffman reflex recruitment curves were obtained to determine peak–peak H-wave and M-wave maximal amplitude, by incrementing stimulation current by 1 mA (1-ms duration). A random interstimulation interval with a minimum of 10 seconds was observed. For HRDD, a submaximal stimulus strength (to achieve a response of 75% of maximum H-reflex on the rising phase of the recruitment curve) was used. H-wave responses were recorded by assessing trains of 10 stimuli delivered at 1 and 3 Hz, to demonstrate that rate dependency is preserved in individuals with FMS. Traces were inspected to ensure they were free of volitional electromyographic activity. Hoffman reflex rate-dependent depression was calculated as the mean H-reflex amplitude of responses 2 to 5 of a stimulus train, expressed as a percentage of the amplitude of the first recorded H-reflex in the train67 (Fig. 1B, C). Therefore, a higher value of HRDD indicates a smaller degree of depression, which we will refer to as impairment of HRDD. Pain scores (NRS 0–10) were collected following each stimulus train.
Figure 1.
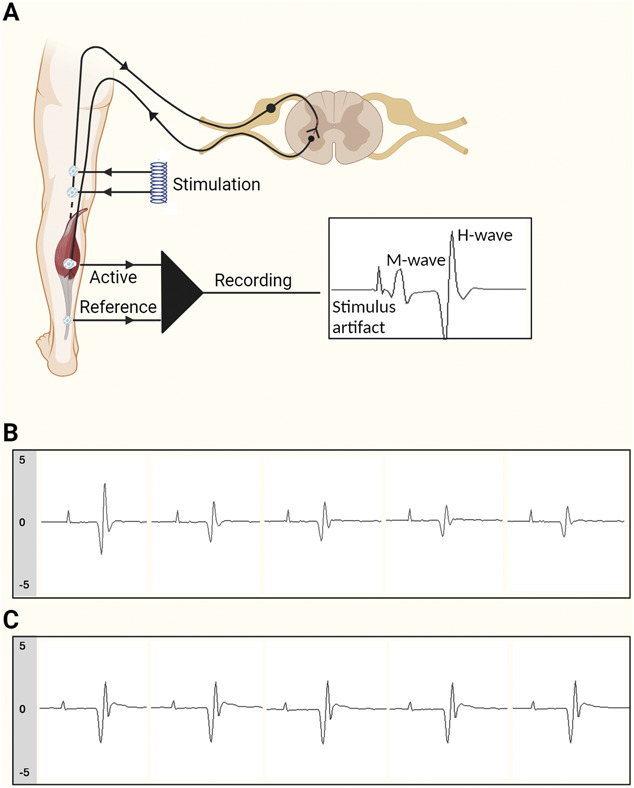
The H-reflex and HRDD. (A) Schematic representation for eliciting and recording the H-reflex (created with Biorender). (B) Representative trace of HRDD, using 1-Hz stimulation, in an individual with normal HRDD. (C) Representative trace of HRDD, using 1-Hz stimulation, in an individual with impaired HRDD. H-reflex, Hoffman reflex; HRDD, H-reflex rate-dependent depression.
2.2. Questionnaires
Participants completed 5 questionnaires to assess the presence of pain, neuropathic symptoms, and the impact of FMS on day-to-day life. The Revised Fibromyalgia Impact Questionnaire was administered to determine the severity of participants’ symptoms and functional impairment, including physical impairment, ability to work, restfulness, and mood.1 The Neuropathy Symptom Profile was used to assess sensory, autonomic, and motor neuropathy symptoms.14 The small Fibre Neuropathy Screenings List evaluated symptoms of purely small nerve fibre-related neuropathy.27 The PainDETECT screening tool evaluated neuropathic pain symptoms.18 An additional measure of current pain score was assessed using the McGill visual analogue scale (0–10).42
2.3. Corneal confocal microscopy
Participants underwent corneal examination with the Heidelberg Retina Tomograph 3 with Rostock Cornea Module (Heidelberg Eye Explorer; Heidelberg Engineering GmBH, Heidelberg, Germany), and images of the corneal subbasal nerve plexus were captured following an established protocol.59 Image selection was masked to the FMS/control group, and the average number of images for analysis per participant was 16. Automated analysis was conducted using ACCMetrics software (ACCMetrics: Malik Lab, Imaging Science, University of Manchester). Three corneal nerve parameters were quantified from each image: (1) corneal nerve fibre density (total number of main nerve fibres per square millimetre of corneal tissue [fibre no/mm2]); (2) corneal nerve branch density (number of branches of all main nerve fibres per square millimetre of corneal tissue [branch no./mm2]); and (3) corneal nerve fibre length (the total length of all main nerve fibres and branches [mm/mm2] within the images).59
2.4. Skin biopsy
Participants underwent punch biopsies to assess IENFD of the lateral proximal and distal thigh and distal leg, in accordance with a previously published protocol.3
2.5. Pressure pain threshold
Pressure pain threshold was evaluated using a pressure algometer (FDN200; Wagner Instruments, Riverside, CT) with a blunt contact area of 1 cm2 placed on the thenar eminence. The threshold was determined as the arithmetic mean of 3 recordings, and the raw data were log transformed and converted into a z-score to normalize the data for age, sex, and body site tested. Positive z-score values denote a gain in function and negative z-scores denote a loss of function.52 Values less than −1.96 (loss of function) or greater than 1.96 (gain of function) are considered abnormal.
2.6. Temporal summation of pain—wind-up
Wind-up ratio was assessed using a 256-mN pinprick stimulator. If the participant found testing too painful, a 128-mN pinprick stimulator was used. A train of 10 stimuli was applied to the dorsum of the right arm at a frequency of 1 per second. A pain score, using a 0 to 100 numerical rating scale, was recorded for the initial stimulus and the subsequent train of stimuli. The wind-up ratio was calculated as the arithmetic mean of the pain intensity rating for the series of stimuli divided by the arithmetic mean of the pain intensity rating for the single stimulus. The raw data were log transformed and converted into a z-score to normalize the data for age, sex, and body site tested. Positive z-score values denote a gain in function, and negative z-scores denote a loss of function.52 Values less than −1.96 (loss of function) or greater than 1.96 (gain of function) are considered abnormal.
2.7. Conditioned pain modulation
Conditioned pain modulation was used to assess the efficiency of diffuse noxious inhibitory control. The pressure pain threshold on the right abductor pollicis brevis was used as the test stimulus. A pressure algometer (FDN200; Wagner Instruments) with a blunt contact area of 1 cm2 was placed on the skin above the abductor hallucis muscle on the right hand. Pressure was applied with increasing intensity at a rate of 0.5 kg (50 kPa)/s. The participant indicated the moment the sensation of pressure changed to an additional painful “burning,” “stinging,” or “aching” sensation, and the respective value on the algometer was recorded. The test was repeated 3 times with a break of 10 seconds in between, and the mean value was recorded. The cold-pressor test was used as the conditioning stimulus, with the left hand of the patient immersed up to the wrist in a water bath of melting ice water for up to 180 seconds or as long as the participant could tolerate, with a minimum time of 45 seconds. Pain ratings, using a numerical rating scale of 0 to 100, were recorded every 15 seconds. Following the removal of the hand from the water bath, the test stimuli were repeated on the right hand (non-submerged) as detailed above. The CPM effect was calculated as the difference (preconditioning stimulus minus post) in raw PPTs. A negative value indicates efficient CPM.
2.8. Statistical methods
Statistical analyses were performed using GraphPad Prism statistical software (GraphPad Software, Inc, La Jolla, CA) and SPSS Version 29 (IBM). Continuous data (including HRDD, CPM, PPT, wind-up ratio, IENFD, and corneal confocal microscopy (CCM) parameters) were assessed for normality using quantile–quantile plots and Shapiro–Wilk test. Normal distributed data were reported as mean ± SD and analysed using an unpaired t test (FMS/HV) for between-group comparison. For nonnormally distributed data, the results were reported as median ± interquartile range (IQR) and analysed using Mann–Whitney test (FMS/HV) and Kruskal–Wallis test followed by a Dunn test (short-duration FMS/long-duration FMS/HV) for between-group comparison. Significance (P) was reported for values reaching the significance threshold set at <0.05. Correlations were performed using the Spearman rank test and expressed as a coefficient (r) with P values. Categorical data were analysed using χ2 Fisher exact test of association.
3. Results
Table 1 details the demographic, biochemical, clinical, and neuropathic characteristics of the study cohort. Data from 31 individuals with FMS and 20 healthy volunteers were included in the analysis. The details of current pain medication are documented in Supplementary Table 1 (http://links.lww.com/PR9/A279). Systematic analysis of medication was not completed due to the large degree of variability.
Table 1.
Demographic, biochemical, H-reflex rate-dependent depression, small nerve fibre parameters, and clinical characteristics in patients with fibromyalgia syndrome and healthy volunteers.
| FMS (n = 31) | HV (n = 20) | P | |
|---|---|---|---|
| Sex (F/M) | 29/2 | 15/5 | 0.060 |
| Age (y) | 49 (35–57) | 43 (31–58) | 0.105 |
| Duration of diagnosis (y) | 7 (3–10) | — | — |
| Duration of symptoms before diagnosis | 4 (2–8) | — | — |
| BMI (kg/m2) | 29.3 (22.9–34.3) | 24.7 (23.4–73.0) | 0.198 |
| BP (systolic) (mm Hg) | 129 ± 18 | 126 ± 12 | 0.760 |
| BP (diastolic) (mm Hg) | 81 12 | 76 6 | 0.058 |
| HbA1c (mmol/mol) | 36.1 ± 5.2 | 31.2 ± 7.8 | 0.309 |
| Cholesterol (mmol/L) | 5.0 ± 1.1 | 4.8 ± 0.8 | 0.498 |
| Triglycerides (mmol/L) | 1.5 ± 0.8 | 1.4 ± 0.7 | 0.731 |
| CNFD | 26.0 ± 6.1 | 27.1 ± 4.7 | 0.400 |
| CNBD | 34.9 ± 15.7 | 42.6 ± 22.9 | 0.196 |
| CNFL | 15.2 ± 3.2 | 16.8 ± 5.4 | 0.215 |
| IENFD proximal thigh | 8.7 ± 4.2 | 11.3 ± 5.5 | 0.502 |
| IENFD distal thigh | 9.0 ± 5.1 | 10.7 ± 8.2 | 0.441 |
| IENFD distal leg | 7.6 ± 3.6 | 8.3 ± 5.3 | 0.618 |
| Pressure pain threshold (z-score) | 3.79 ± 2.02 | 0.44 ± 1.68 | <0.001 |
| HRDD at 1Hz | 50.9 (42.6–68.7) | 41.8 (34.6–57.5) | 0.026 |
| HRDD at 3Hz | 46.8 (35.6–57.2) | 32.3 (25.2–40.9) | 0.011 |
| Depression (Y/N) | 28/3 | 1/19 | <0.001 |
| Depression (score out of 10) | 6 (2–8) | 0 (0–0) | <0.001 |
| Anxiety (Y/N) | 29/2 | 6/13 | <0.001 |
| Anxiety (score out of 10) | 7.5 (4–8) | 0 (0–2) | <0.001 |
| FIQR total (score out of 100) | 76 (50–81) | 0 (0–4) | <0.001 |
| SFNSL | 47 (37–60) | 2 (1–6) | <0.001 |
| PainDetect total (out of 38) | 23 (17–28) | 0 (0–1) | <0.001 |
| Neuropathy symptom profile | 20 (16–25) | 0 (0–1) | <0.001 |
| VAS current pain score | 80 (68–88) | 0 (0–17) | <0.001 |
Parametric data are shown as mean ± SD and analysed using an unpaired t test. Nonparametric data are shown as median (interquartile range) and analysed using the Mann–Whitney test. Values reaching the significance threshold set at <0.05 are in bold.
BMI, body mass index; BP, blood pressure; CNFD, corneal nerve fibre density; CNBD, corneal nerve branch density; CNFL, corneal nerve fibre length; FIQR, fibromyalgia impact questionnaire (revised); HbA1c, glycated haemoglobin; HV, healthy volunteers; HRDD, Hoffmann reflex rate-dependent depression; IENFD, intraepidermal nerve fibre density; SFNSL, small fibre neuropathy screening list; VAS, visual analogue scale.
3.1. Demographic, biochemical, and clinical characteristics
There was no difference in age, body mass index (BMI), or glycated haemoglobin (HbA1c) between FMS and healthy volunteers. In keeping with previous studies,19,29,46 approximately half (48.4%) of the individuals with FMS had IENFD below the normative range for age and sex in at least 1 location and 24% had an abnormality in corneal nerve fibre morphology.47,60 Whilst mean IENFDs and CCM parameters in patients with FMS were reduced compared with healthy volunteers, this did not reach a level of significance. As expected, PPT (FMS: mean 3.79, SD 2.02; HV: mean 0.44, SD 1.68; P < 0.001) was reduced (gain of function) in patients with FMS, and scores on pain and symptom questionnaires (Revised Fibromyalgia Impact Questionnaire; Small Fibre Neuropathy Screenings List; Neuropathy Symptom Profile; PainDetect; visual analogue scale, all P < 0.001) were higher in people with FMS compared with healthy volunteers. Frequency and severity of anxiety (FMS: median 7.5, IQR 4–8 [n = 29/31]; HV: median 0, IQR 0–2 [n = 6/20]; P < 0.001) and depression (FMS: median 6, IQR 2–8 [n = 28/31]; HV: median 0, IQR 0-0 [n = 1/20]; P < 0.001) were higher in patients with FMS compared with healthy volunteers.
3.2. Conditioned pain modulation and temporal summation of pain
The level of CPM did not differ between people with FMS and healthy volunteers. Temporal summation of pain was increased in people with FMS compared with healthy volunteers (Fig. 2).
Figure 2.
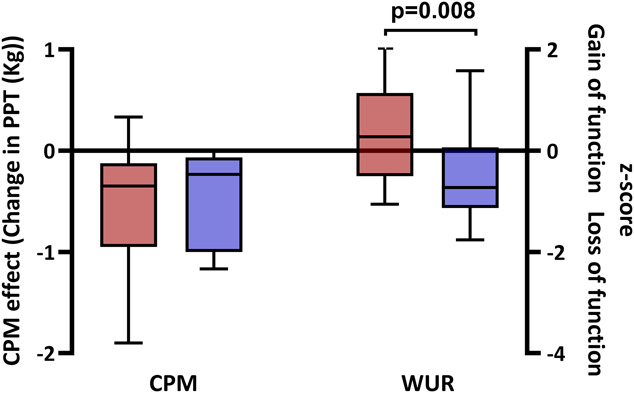
Conditioned pain modulation and temporal summation of pain in FMS. Box and whisker plot of CPM and temporal summation of pain (wind-up) in patients with FMS (red) and healthy volunteers (purple). Statistically significant P values are shown (Mann–Whitney). CPM, conditioned pain modulation; FMS, fibromyalgia syndrome; PPT, pressure pain threshold; WUR, wind-up ratio.
3.3. Hoffman reflex parameters
Table 2 details the H-reflex parameters in patients with FMS and healthy volunteers.
Table 2.
Hoffman reflex parameters in patients with fibromyalgia syndrome and healthy volunteers.
| FMS | HV | P | |
|---|---|---|---|
| Hmax | 3.70 (2.17–4.60) | 2.21 (1.96–4.41) | 0.534 |
| Mmax | 5.78 (4.11–8.20) | 7.86 (4.61–8.80) | 0.509 |
| Hmax/Mmax ratio | 0.59 (0.52–0.67) | 0.31 (0.11–0.62) | 0.094 |
Data are shown as median (interquartile range) and analysed using the Mann–Whitney test. Statistically significant P values are shown (Mann–Whitney).
FMS, fibromyalgia syndrome; Hmax, maximum amplitude of the H wave; Hmax/Mmax ratio, ratio of the maximum amplitudes of H and M waves; HV, healthy volunteers; Mmax, maximum amplitude of the M wave.
There was no significant difference in Hmax, Mmax, or Hmax/Mmax ratio between individuals with FMS and healthy volunteers.
3.4. Hoffman reflex rate-dependent depression
Individuals with FMS had impaired HRDD at 1 Hz (FMS: median 50.9, IQR 42.6–68.7; HV: median 41.8, IQR 34.6–57.5; P = 0.026) and 3 Hz (FMS: median 46.r8, IQR 35.6–57.2; HV: median 32.3, IQR 25.2–40.9; P = 0.011) compared with healthy volunteers (Fig. 3). Whilst a degree of overlap is observed, 13 patients with FMS (42%) had HRDD value (s) that fell outside the normal range of the healthy volunteers (>65.8 [45.73 + 20.06] at 1 Hz, >51.3 [35.20 + 16.08] at 3 Hz). Pain scores obtained during the HRDD trains were significantly higher in patients with FMS compared with healthy volunteers (1 Hz; FMS: median 6.0, IQR 4.0–6.23; HV: median 1.0, IQR 0–4.5; P = <0.001. Three Hertz; FMS: median 8.0, IQR 6.5–10; HV: median 4.0, IQR 1.25–6.25; P = <0.01). However, these pain scores were not associated with the degree of HRDD in either individuals with FMS (1 Hz: P = 0.180, rs = 0.277; 3 Hz: P = 0.387, rs = 0.199) or healthy volunteers (1 Hz: P = 0.471, rs = 0.192; 3 Hz: P = 0.408, rs = −0.213). No significant correlations were observed between HRDD and age, BMI, HbA1c, markers of small fibre pathology on skin biopsy and corneal confocal microscopy, or any pain and symptoms scores in individuals with FMS and healthy volunteers.
Figure 3.
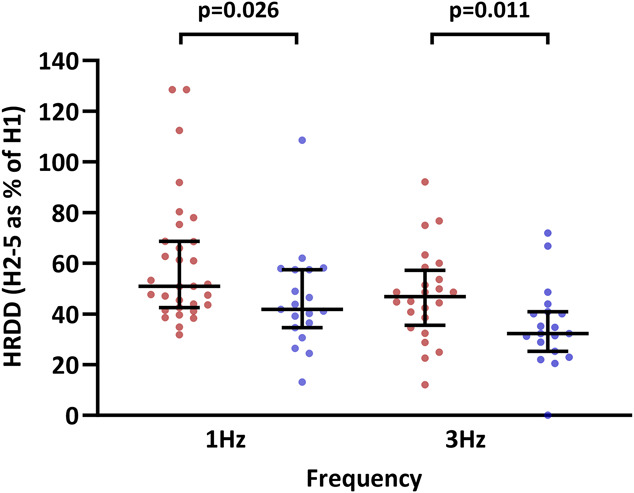
Hoffman reflex rate-dependent depression in FMS. Hoffman reflex rate-dependent depression at 1 and 3 Hz in patients with FMS (red circles) and healthy volunteers (purple circles). Statistically significant P values are shown (Mann–Whitney). FMS, fibromyalgia syndrome; HRDD, H-reflex rate-dependent depression.
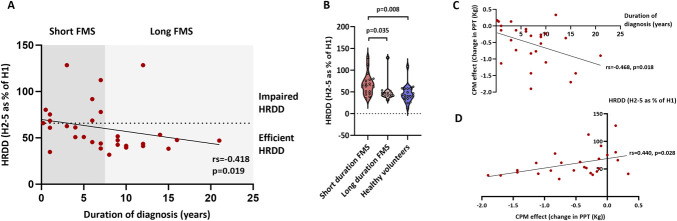
3.5. Hoffman reflex rate-dependent depression, conditioned pain modulation, and duration of disease diagnosis
Individuals with FMS with the most impaired HRDD also had the most inefficient CPM (rs = 0.468, P = 0.018), and both HRDD (rs = −0.418, P = 0.019) and CPM (rs = −0.440, P = 0.028) were most impaired in patients with the shortest disease duration. To further investigate these findings, patients with FMS were divided into 2 groups based on the median duration of FMS diagnosis.23 Patients with a short duration (7 years or less [n = 18]) had significantly more impaired HRDD compared with patients with a long duration (>7 years [n = 13]) (Fig. 4).
Figure 4.
Hoffman reflex rate-dependent depression during FMS disease progression. (A) Individual H-reflex rate-dependent depression (HRDD) data in patients with FMS with short (7 years or less) and long (greater than 7 years) disease duration. Spearman correlation (rs) and P value are reported. (B) Group HRDD data in patients with FMS with short (7 years or less) and long (greater than 7 years) disease duration and healthy volunteers. Kruskal–Wallis/Dunn test P values are reported. (C) Conditioned pain modulation and duration of disease. Spearman correlation (rs) and P values are reported. (D) Hoffman reflex rate-dependent depression and CPM. Spearman correlation (rs) and P values are reported. CPM, conditioned pain modulation; FMS, fibromyalgia syndrome; HRDD, H-reflex rate-dependent depression.
4. Discussion
This study reports for the first time evidence of spinal disinhibition in patients with FMS. The main finding is an impairment of HRDD, a biomarker of spinal inhibitory dysfunction, in ∼40% of patients with FMS. In addition, we provide evidence that this potential mechanism of pain generation is more pronounced in shorter duration of disease.
Multiple lines of evidence in preclinical studies indicate that spinal disinhibition is a prominent mechanism for the amplification of ascending nociceptive information in the context of both neuropathic and inflammatory pain.11,34 The effects of this disinhibition are to reduce the threshold for activation of ascending nociceptive pathways to peripheral stimulation.32 Whilst spinal disinhibition could potentially arise from several convergent pathways, a dominant mechanism appears to be the disruption of the chloride balance of postsynaptic neurons so that gamma-aminobutyric acid (GABA) ergic inputs into postsynaptic cells become depolarising (or less hyperpolarising).13 An important pathway leading to depolarising GABA in divergent preclinical models (nerve injury, diabetes, inflammation) is an increase in intracellular chloride due to a brain-derived neurotrophic factor (BDNF)-induced downregulation of the potassium/chloride cotransporter 2 (KCC2).9,11,13,34 In support of a similar mechanistic principle in humans, recordings from lamina I dorsal horn neurons in ex vivo human spinal cord slice preparations also indicate that BDNF can induce a depolarising switch in GABAergic transmission.11
This study used HRDD, a noninvasive biomarker of spinal inhibitory function, to investigate the presence of spinal disinhibition in FMS. Hoffman reflex rate-dependent depression is impaired in diabetic rodents that display both behavioural indices of pain and BDNF-dependent depolarising GABA.33,38 Importantly, in rodents, HRDD is normalised by interventions (eg, BDNF sequestration) that restore spinal inhibition and alleviate behavioural indices of pain; and it becomes impaired following interventions recapitulating the pathway to depolarising GABA (eg, KCC2 inhibition) that evoke behavioural indices of pain.30,33 Although the spinal circuits underlying the deficits in HRDD are not clear,34 these findings imply, at least in diabetic rodents, that the same mechanism underlying spinal disinhibition and indices of pain also results in impairment of HRDD. Underpinning the translational potential of these findings, impairment of HRDD is seen in individuals with painful diabetic neuropathy,38,68 most evidently in those with prominent mechanical pain hypersensitivity relative to mechanical pain detection.39 Unlike for painful diabetic neuropathy, currently, there is no parallel preclinical evidence in FMS to link impaired HRDD with chloride dysregulation in the dorsal horn of the spinal cord. Impairment of HRDD has been demonstrated in individuals with obesity/impaired glucose tolerance,53 for which there is an increased incidence in FMS.12,70 However, the current data show no correlation between obesity/impaired glucose tolerance and the degree of HRDD impairment. Furthermore, no significant group-level differences in BMI/HbA1c were seen between healthy control participants and individuals with FMS. Therefore, it seems unlikely that the abnormal HRDD in FMS is attributable to a prediabetic state or metabolic syndrome.
A previous investigation has demonstrated an increase in the Hmax:Mmax ratio, which also provides a measure of excitatory-inhibitory balance in the spinal cord,26 in individuals with FMS compared with controls.61 We did not show any significant alterations in the Hmax:Mmax ratio in individuals with FMS. Both our current findings and the previous study are from a relatively small cohort, potentially contributing to these differing findings. However, the Hmax:Mmax ratio is distinct from HRDD, has different underlying physiological mechanisms,26 and has not been associated with spinal disinhibition in clinical or preclinical studies in painful diabetic neuropathy.33,38 The nociceptive withdrawal reflex (NWR) has also been used to probe spinal excitability in chronic pain states37 with a reduction in threshold being taken as a measure of central hyperexcitability. Whilst in FMS, there is evidence of a reduced NWR threshold, the findings are weak and there is considerable heterogeneity.64 Furthermore, unlike impaired HRDD, alterations in the nociceptive withdrawal reflex have not been linked to a specific pain mechanism.
Further evidence of altered processing in the spinal cord comes from functional magnetic resonance imaging studies in individuals with FMS. For example, resting-state functional magnetic resonance imaging of the cervical cord in individuals with FMS has demonstrated a decrease and increase in the amplitude of low-frequency fluctuations in the dorsal and ventral cord, respectively.41 The significance of these alterations in the neural processes and whether they relate to spinal disinhibition or impaired HRDD are uncertain. However, they do potentially provide a link between dysfunction in predominant “sensory” and “motor” regions of the spinal cord that could be relevant to abnormalities in HRDD.
Psychophysical evidence of altered central processing—specifically, enhanced temporal summation of pain and inefficient diffuse noxious inhibitory control—has previously been reported in FMS studies.22,49,55 Compared with healthy volunteers, we found that temporal summation of pain to pinprick stimuli was enhanced in people with FMS. Temporal summation of pain is widely used as a proxy of processes causing wind-up in the spinal cord,24 and our findings could implicate enhanced wind-up in the spinal cord. However, although there is broad agreement that temporal summation is enhanced in FMS across studies,45 there is considerable interindividual heterogeneity and, like for HRDD in this study, a lack of correlation with clinical pain.48 Between study inconsistencies highlighted in a 2018 meta-analysis revealed that disparate measurement and phenotyping approaches relating to both wind-up and CPM, with the parameters type and site of stimulation, age, sample size, and medication control, may provide important sources of variability.45 Indeed, more recent studies using sensitivity-adjusted test stimuli suggest that individuals with fibromyalgia modulate nociceptive input as effectively as healthy volunteers.56–58 Whilst we did not use sensitivity-adjusted stimuli in our CPM paradigm, our findings suggest that, in FMS, central mechanisms may evolve over time; both HRDD and CPM were most impaired in the short duration of disease. Similar findings have been reported in patients with pDPN, whereby patients with shorter pain duration demonstrate less efficient CPM and higher temporal summation but with the same level of pain as those with longer duration.23 Longitudinal studies are required to investigate the potential time-dependency of central sensitisation mechanisms in FMS, but it is possible that spinal disinhibition and/or alterations in descending modulation could represent an initiating mechanism that subsequently becomes maintained by other processes. If, as these findings suggest, impaired central processing “normalises” with chronicity of pain, it may also explain in part the variance of findings in both research and clinical populations.22,49,57,58
Immune mechanisms have emerged as putative aetiopathological factors in FMS and have been linked to peripheral nervous system dysfunction. Passive transfer of IgG from individuals with FMS reproduces the sensory, motor, and pathological abnormalities in mice.21 The dominant hypothesis is that autoantibodies bind to satellite glial cells in the dorsal root ganglia (DRG) and sensitise nociceptor fibres.21 Whether this leads to secondary changes, including disinhibition, in the spinal cord is not known. Furthermore, as only approximately 50% of individuals display high levels of autoantibody binding to satellite glial cells,31 there may be other aetiological mechanisms. We did not measure antibody binding status in this study. Transfer of neutrophils from individuals with FMS to mice also results in reversible somatosensory hypersensitivity, neutrophil infiltration of the DRG, and, interestingly, sensitisation of deep dorsal horn neurons to noxious mechanical and thermal stimuli.5 This highlights the capacity of immune-related mechanisms involving the DRG to affect processing in neurons involved in central nociceptor processing and, potentially, segmental spinal reflexes. This is the likely case in painful diabetic neuropathy,34 where spinal disinhibition related to chloride dysregulation and impaired HRDD are likely secondary to alterations in peripheral nerves. Among healthy individuals, HRDD shows variation.68 An alternative reason for the impaired HRDD in a proportion of individuals with FMS is that spinal inhibitory function, and hence the degree of HRDD, reflects a trait that could prime the development of pain when triggered by an additional insult.
Limitations of this study include its cross-sectional design and relatively small number of participants. In addition, our findings relating to the duration of the disease were a representation of time since diagnosis. As the duration of symptoms before a confirmed FMS diagnosis is on average 5 years and is affected by multiple factors, the precise onset is unlikely to be determined to a single focal point.6,20 Furthermore, patients continued to take pain medication during the assessment period, and this was not systematically accounted for in the analysis. Medications would be expected to impact pain ratings, and treatments with antineuropathic pain drugs could potentially differentially alter HRDD.71 Larger scale prospective studies, determining longitudinal trends in HRDD, psychophysical parameters, and clinical symptoms and scores over the time course of FMS disease, are required. Larger studies are also needed to determine whether HRDD differs between individuals with FMS with and without small fibre pathology, although impairment of HRDD is not related to the severity of small fibre neuropathy in individuals with diabetic neuropathy.38,68 In addition, the response of HRDD to descending modulatory influences, eg, attention, and to antineuropathic therapies that modulate spinal dysfunction/inhibition is required.
In conclusion, we have demonstrated that patients with FMS have impairment of HRDD and therefore evidence of spinal disinhibition. Furthermore, our findings provide evidence that spinal inhibitory function is most impaired in patients with FMS in short-duration disease. Further investigations to expand these findings are needed, as identifying patients with impairment of central pain processing at an early stage may provide opportunities for time-dependent targeted mechanistic-based therapy.
Disclosures
The authors declare no direct competing interests.
Data sets are available from the corresponding author upon reasonable request, data transfer agreements and associated costs may be required.
Supplementary Material
Acknowledgments
The authors acknowledge funding by vs Arthritis (Grant number 22471) for this study (DEFINE-FMS).
With kind appreciation to the Fibromates patient support group for their input throughout this study.
Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A279.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
U. Alam and Andrew Marshall contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Jamie Burgess, Email: jamie.burgess@liverpool.ac.uk.
Andreas Goebel, Email: agoebel@liverpool.ac.uk.
Bernhard Frank, Email: bfrank@liverpool.ac.uk.
Uazman Alam, Email: ualam@liverpool.ac.uk.
Andrew Marshall, Email: andrew.marshall@liverpool.ac.uk.
References
- [1].Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther 2009;11:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bosma RL, Mojarad EA, Leung L, Pukall C, Staud R, Stroman PW. FMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum Brain Mapp 2016;37:1349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burgess J, Marshall A, Rapteas L, Hamill KJ, Marshall A, Malik RA, Frank B, Alam U. Automated immunohistochemistry of intra-epidermal nerve fibres in skin biopsies: a proof-of-concept study. J Peripher Nerv Syst 2024;29:329–38. [DOI] [PubMed] [Google Scholar]
- [4].Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 1984;52:435–48. [DOI] [PubMed] [Google Scholar]
- [5].Caxaria S, Bharde S, Fuller AM, Evans R, Thomas B, Celik P, Dell'Accio F, Yona S, Gilroy D, Voisin MB, Wood JN, Sikandar S. Neutrophils infiltrate sensory ganglia and mediate chronic widespread pain in fibromyalgia. Proc Natl Acad Sci U S A 2023;120:e2211631120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choy E, Perrot S, Leon T, Kaplan J, Petersel D, Ginovker A, Kramer E. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res 2010;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55. [DOI] [PubMed] [Google Scholar]
- [8].Costantini R, Affaitati G, Wesselmann U, Czakanski P, Giamberardino MA. Visceral pain as a triggering factor for fibromyalgia symptoms in comorbid patients. PAIN 2017;158:1925–37. [DOI] [PubMed] [Google Scholar]
- [9].Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005;438:1017–21. [DOI] [PubMed] [Google Scholar]
- [10].de Tommaso M, Vecchio E, Nolano M. The puzzle of fibromyalgia between central sensitization syndrome and small fiber neuropathy: a narrative review on neurophysiological and morphological evidence. Neurol Sci 2022;43:1667–84. [DOI] [PubMed] [Google Scholar]
- [11].Dedek A, Xu J, Kandegedara CM, Lorenzo L, Godin AG, De Koninck Y, Lombroso PJ, Tsai EC, Hildebrand ME. Loss of STEP61 couples disinhibition to N-methyl-d-aspartate receptor potentiation in rodent and human spinal pain processing. Brain 2019;142:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].D'Onghia M, Ciaffi J, Lisi L, Mancarella L, Ricci S, Stefanelli N, Meliconi R, Ursini F. Fibromyalgia and obesity: a comprehensive systematic review and meta-analysis. Semin Arthritis Rheum 2021;51:409–24. [DOI] [PubMed] [Google Scholar]
- [13].Doyon N, Vinay L, Prescott SA, De Koninck Y. Chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Neuron 2016;89:1157–72. [DOI] [PubMed] [Google Scholar]
- [14].Dyck PJ, Karnes J, O'Brien PC, Swanson CJ. Neuropathy Symptom Profile in health, motor neuron disease, diabetic neuropathy, and amyloidosis. Neurology 1986;36:1300–08. [DOI] [PubMed] [Google Scholar]
- [15].Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, Papagianni A, Saffer N, Meyer Zu Altenschildesche C, Kampik D, Malik RA, Sommer C, Uceyler N. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol 2019;86:504–16. [DOI] [PubMed] [Google Scholar]
- [16].Fallon N, Alghamdi J, Chiu Y, Sluming V, Nurmikko T, Stancak A. Structural alterations in brainstem of fibromyalgia syndrome patients correlate with sensitivity to mechanical pressure. Neuroimage Clin 2013;3:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fasolino A, Di Stefano G, Leone C, Galosi E, Gioia C, Lucchino B, Terracciano A, Di Franco M, Cruccu G, Truini A. Small-fibre pathology has no impact on somatosensory system function in patients with fibromyalgia. PAIN 2020;161:2385–93. [DOI] [PubMed] [Google Scholar]
- [18].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [19].Galosi E, Truini A, Di Stefano G. A systematic review and meta-analysis of the prevalence of small fibre impairment in patients with fibromyalgia. Diagnostics (Basel) 2022;12:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gendelman O, Amital H, Bar-On Y, Ben-Ami Shor D, Amital D, Tiosano S, Shalev V, Chodick G, Weitzman D. Time to diagnosis of fibromyalgia and factors associated with delayed diagnosis in primary care. Best Pract Res Clin Rheumatol 2018;32:489–99. [DOI] [PubMed] [Google Scholar]
- [21].Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, Sandor K, Vastani N, Maurer M, Cuhadar U, Sensi S, Nomura Y, Menezes J, Baharpoor A, Brieskorn L, Sandström A, Tour J, Kadetoff D, Haglund L, Kosek E, Bevan S, Svensson CI, Andersson DA. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest 2021;131:e144201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goubert D, Danneels L, Graven-Nielsen T, Descheemaeker F, Meeus M. Differences in pain processing between patients with chronic low back pain, recurrent low back pain, and fibromyalgia. Pain Physician 2017;20:307–18. [PubMed] [Google Scholar]
- [23].Granovsky Y, Nahman-Averbuch H, Khamaisi M, Granot M. Efficient conditioned pain modulation despite pain persistence in painful diabetic neuropathy. Pain Rep 2017;2:e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6:599–606. [DOI] [PubMed] [Google Scholar]
- [25].Grayston R, Czanner G, Elhadd K, Goebel A, Frank B, Üçeyler N, Malik RA, Alam U. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum 2019;48:933–40. [DOI] [PubMed] [Google Scholar]
- [26].Grosprêtre S, Martin A. H reflex and spinal excitability: methodological considerations. J Neurophysiol 2012;107:1649–54. [DOI] [PubMed] [Google Scholar]
- [27].Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: construction and cross-validation in sarcoidosis. Respir Med 2011;105:95–100. [DOI] [PubMed] [Google Scholar]
- [28].Ishikawa K, Ott K, Porter RW, Stuart D. Low frequency depression of the H wave in normal and spinal man. Exp Neurol 1966;15:140–56. [DOI] [PubMed] [Google Scholar]
- [29].Jänsch S, Evdokimov D, Egenolf N, Meyer Zu Altenschildesche C, Kreß L, Üçeyler N. Distinguishing fibromyalgia syndrome from small fiber neuropathy: a clinical guide. Pain Rep 2024;9:e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. PAIN 2008;140:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krock E, Morado-Urbina CE, Menezes J, Hunt MA, Sandström A, Kadetoff D, Tour J, Verma V, Kultima K, Haglund L, Meloto CB, Diatchenko L, Kosek E, Svensson CI. Fibromyalgia patients with elevated levels of anti-satellite glia cell immunoglobulin G antibodies present with more severe symptoms. PAIN 2023;164:1828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lavertu G, Côté SL, De Koninck Y. Enhancing K-Cl co-transport restores normal spinothalamic sensory coding in a neuropathic pain model. Brain 2014;137(pt 3):724–38. [DOI] [PubMed] [Google Scholar]
- [33].Lee-Kubli CA, Calcutt NA. Altered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic pain. PAIN 2014;155:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee-Kubli C, Marshall AG, Malik RA, Calcutt NA. The H-reflex as a biomarker for spinal disinhibition in painful diabetic neuropathy. Curr Diab Rep 2018;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Leone C, Galosi E, Esposito N, Falco P, Fasolino A, Di Pietro G, Di Stefano G, Camerota F, Vollert J, Truini A. Small-fibre damage is associated with distinct sensory phenotypes in patients with fibromyalgia and small-fibre neuropathy. Eur J Pain 2023;27:163–73. [DOI] [PubMed] [Google Scholar]
- [36].Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13:936–44. [DOI] [PubMed] [Google Scholar]
- [37].Linde LD, Duarte FC, Esmaeili H, Hamad A, Masani K, Kumbhare DA. The nociceptive flexion reflex: a scoping review and proposed standardized methodology for acquisition in those affected by chronic pain. Br J Pain 2021;15:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Marshall AG, Lee-Kubli C, Azmi S, Zhang M, Ferdousi M, Mixcoatl-Zecuatl T, Petropoulos IN, Ponirakis G, Fineman MS, Fadavi H, Frizzi K, Tavakoli M, Jeziorska M, Jolivalt CG, Boulton AJ, Efron N, Calcutt NA, Malik RA. Spinal disinhibition in experimental and clinical painful diabetic neuropathy. Diabetes 2017;66:1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marshall A, Kalteniece A, Ferdousi M, Azmi S, Jude EB, Adamson C, D'Onofrio L, Dhage S, Soran H, Campbell J, Lee-Kubli CA, Hamdy S, Malik RA, Calcutt NA, Marshall AG. Spinal disinhibition: evidence for a hyperpathia phenotype in painful diabetic neuropathy. Brain Commun 2023;5:fcad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Martínez-Lavín M. Fibromyalgia and small fiber neuropathy: the plot thickens. Clin Rheumatol 2018;37:3167–71. [DOI] [PubMed] [Google Scholar]
- [41].Martucci KT, Weber KA, Mackey SC. Altered cervical spinal cord resting-state activity in fibromyalgia. Arthritis Rheumatol 2019;71:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Melzack R. The short-form McGill Pain Questionnaire. PAIN 1987;30:191–7. [DOI] [PubMed] [Google Scholar]
- [43].Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 2003;28:144–60. [DOI] [PubMed] [Google Scholar]
- [44].Oaklander AL. What is the meaning of “small-fiber polyneuropathy” in fibromyalgia? An alternate answer. PAIN 2016;157:1366–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].O'Brien AT, Deitos A, Triñanes Pego Y, Fregni F, Carrillo-de-la-Peña MT. Defective endogenous pain modulation in fibromyalgia: a meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain 2018;19:819–36. [DOI] [PubMed] [Google Scholar]
- [46].Oudejans L, He X, Niesters M, Dahan A, Brines M, van Velzen M. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci Rep 2016;6:23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Perkins BA, Lovblom LE, Bril V, Scarr D, Ostrovski I, Orszag A, Edwards K, Pritchard N, Russell A, Dehghani C, Pacaud D, Romanchuk K, Mah JK, Jeziorska M, Marshall A, Shtein RM, Pop-Busui R, Lentz SI, Boulton AJM, Tavakoli M, Efron N, Malik RA. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia 2018;61:1856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Petersen KK, O'Neill S, Blichfeldt-Eckhardt MR, Nim C, Arendt-Nielsen L, Vægter HB. Pain profiles and variability in temporal summation of pain and conditioned pain modulation in pain-free individuals and patients with low back pain, osteoarthritis, and fibromyalgia. Eur J Pain 2024. [DOI] [PubMed] [Google Scholar]
- [49].Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. PAIN 2016;157:1704–10. [DOI] [PubMed] [Google Scholar]
- [50].Prescott SA. Synaptic inhibition and disinhibition in the spinal dorsal horn. Prog Mol Biol Transl Sci 2015;131:359–83. [DOI] [PubMed] [Google Scholar]
- [51].Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain 2011;12:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rolke R, Baron R, Maier C, Tolle TR, Treede DR, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [53].Salinas LF, Trujillo-Condes VE, Tecuatl C, Delgado-Lezama R, Cuellar CA. Impaired rate-dependent depression of the H-reflex in type-2 diabetes, prediabetes, overweight and obesity: a cross-sectional study. Medicine (Baltimore) 2022;101:e31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Serra J, Collado A, Solà R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 2014;75:196–208. [DOI] [PubMed] [Google Scholar]
- [55].Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. PAIN 2001;91:165–75. [DOI] [PubMed] [Google Scholar]
- [56].Staud R, Boissoneault J, Lai S, Mejia MS, Ramanlal R, Godfrey MM, Stroman PW. Spinal cord neural activity of patients with fibromyalgia and healthy controls during temporal summation of pain: an fMRI study. J Neurophysiol 2021;126:946–56. [DOI] [PubMed] [Google Scholar]
- [57].Staud R, Godfrey MM, Riley JL, Fillingim RB. Efficiency of pain inhibition and facilitation of fibromyalgia patients is not different from healthy controls: relevance of sensitivity-adjusted test stimuli. Br J Pain 2023;17:182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Staud R, Godfrey MM, Stroman PW. Fibromyalgia is associated with hypersensitivity but not with abnormal pain modulation: evidence from QST trials and spinal fMRI. Front Pain Res (Lausanne) 2023;4:1284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp 2011;47:2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, Ziegler D, Pacaud D, Romanchuk K, Perkins BA, Lovblom LE, Bril V, Singleton JR, Smith G, Boulton AJ, Efron N, Malik RA. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care 2015;38:838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Thabit MN, Ezat A, Ismael MA, Hadad S. Altered spinal excitability in patients with primary fibromyalgia: a case-control study. J Clin Neurol 2021;17:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Üçeyler N, Sommer C. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. PAIN 2013;154:2569. [DOI] [PubMed] [Google Scholar]
- [63].Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136(pt 6):1857–67. [DOI] [PubMed] [Google Scholar]
- [64].Van Oosterwijck S, Billens A, Cnockaert E, Danneels L, Mertens T, Dhondt E, Van Oosterwijck J. Spinal hyperexcitability in patients with chronic musculoskeletal pain or headache as evidenced by alterations in the nociceptive withdrawal reflex: a systematic review and meta-analysis. PAIN 2024. [DOI] [PubMed] [Google Scholar]
- [65].Vecchio E, Lombardi R, Paolini M, Libro G, Delussi M, Ricci K, Quitadamo SG, Gentile E, Girolamo F, Iannone F, Lauria G, de Tommaso M. Peripheral and central nervous system correlates in fibromyalgia. Eur J Pain 2020;24:1537–47. [DOI] [PubMed] [Google Scholar]
- [66].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- [67].Worthington A, Kalteniece A, Ferdousi M, D'Onofrio L, Dhage S, Azmi S, Adamson C, Hamdy S, Malik RA, Calcutt NA, Marshall AG. Optimal utility of H-reflex RDD as a biomarker of spinal disinhibition in painful and painless diabetic neuropathy. Diagnostics (Basel) 2021;11:1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Worthington A, Kalteniece A, Ferdousi M, Donofrio L, Dhage S, Azmi S, Adamson C, Hamdy S, Malik R, Calcutt N, Marshall A. Spinal inhibitory dysfunction in patients with painful or painless diabetic neuropathy. Diabetes Care 2021;44:1835–41. [DOI] [PubMed] [Google Scholar]
- [69].Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–15. [DOI] [PubMed] [Google Scholar]
- [70].Zetterman T, Markkula R, Kalso E. Glucose tolerance in fibromyalgia. Medicine (Baltimore) 2021;100:e27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhou X, Zhu Y, Wang Z, Lin Z, Zhu D, Xie C, Calcutt NA, Guan Y. Rate-dependent depression: a predictor of the therapeutic efficacy in treating painful diabetic peripheral neuropathy. Diabetes 2022;71:1272–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.