Abstract
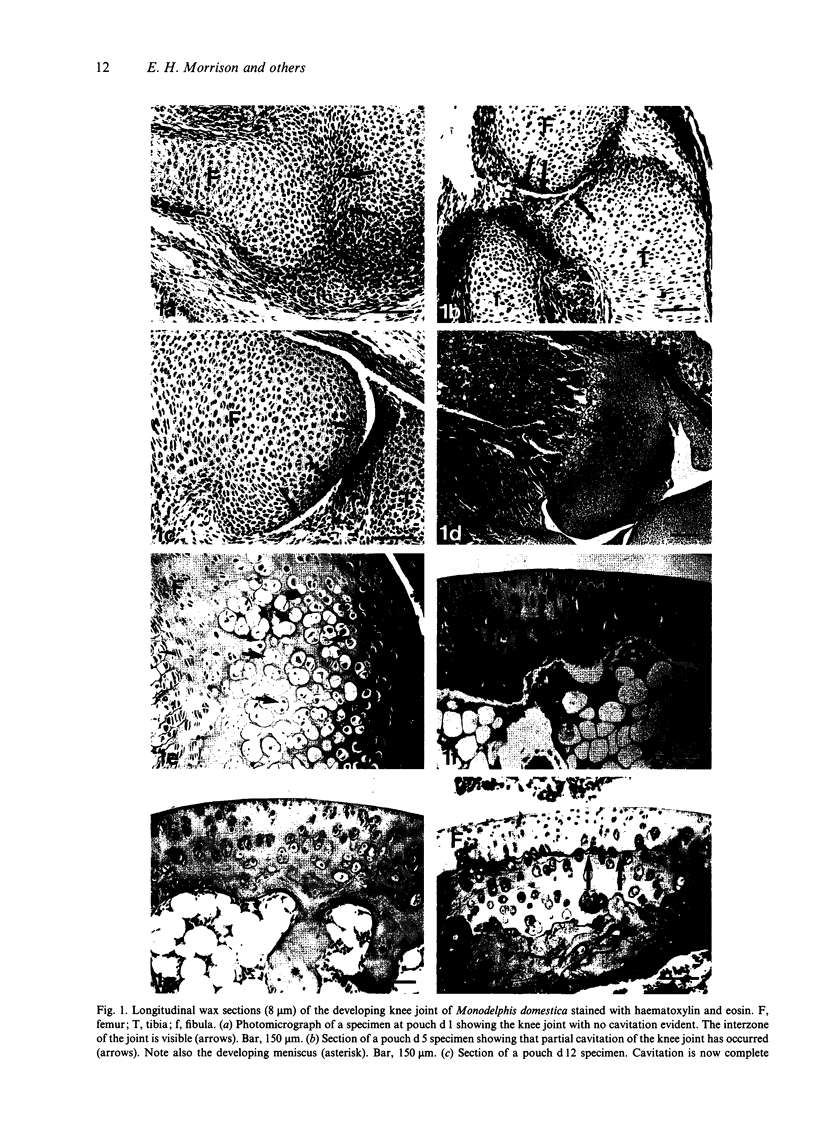
Articular cartilage is both morphologically and biochemically heterogeneous. Its susceptibility to degenerative diseases such as arthritis and its limited repair capacity have made cartilage the focus of intense study; surprisingly, little is known of its development. Using a panel of specific antibodies, we have documented the temporal and spatial patterns of collagen types I, II, III, VI and X in the developing knee cartilage of the marsupial Monodelphis domestica from parturition to adulthood. Type I collagen was initially detected in the presumptive articular cartilage of the epiphyses in addition to the perichondrium. By 14 d postparturition, type I collagen was not detectable in the epiphyseal cartilage apart from insertion sites of ligaments and tendons of the joint. Similarly, type III collagen was detected at insertion sites of the major ligaments and tendons and within the perichondrium/periosteum but was never detected in the cartilage per se. Type II collagen was predictably distributed throughout the cartilage matrix and was also detected in the perichondrium. Type VI collagen was widely distributed throughout the cartilage matrix at parturition, but during development became restricted to a pericellular location particularly towards the presumptive articular cartilage, i.e. the epiphysis. Interestingly, generalised matrix immunopositivity was only retained in the hypertrophic cartilage of the secondary centre of ossification. After the formation of the secondary centre, type VI collagen became localised pericellularly in the deeper regions of the articular cartilage but was absent in the cartilage of the growth plate. Type X collagen showed a novel distribution pattern. In addition to being synthesised by hypertrophic chondrocytes, this collagen type was also expressed transiently by some cells at the presumptive articular surface. Furthermore, these surface chondrocytes also stained histochemically for alkaline phosphatase, suggesting that they were terminally differentiated. The fate of these terminally differentiated cells is unknown.
Full text
PDF



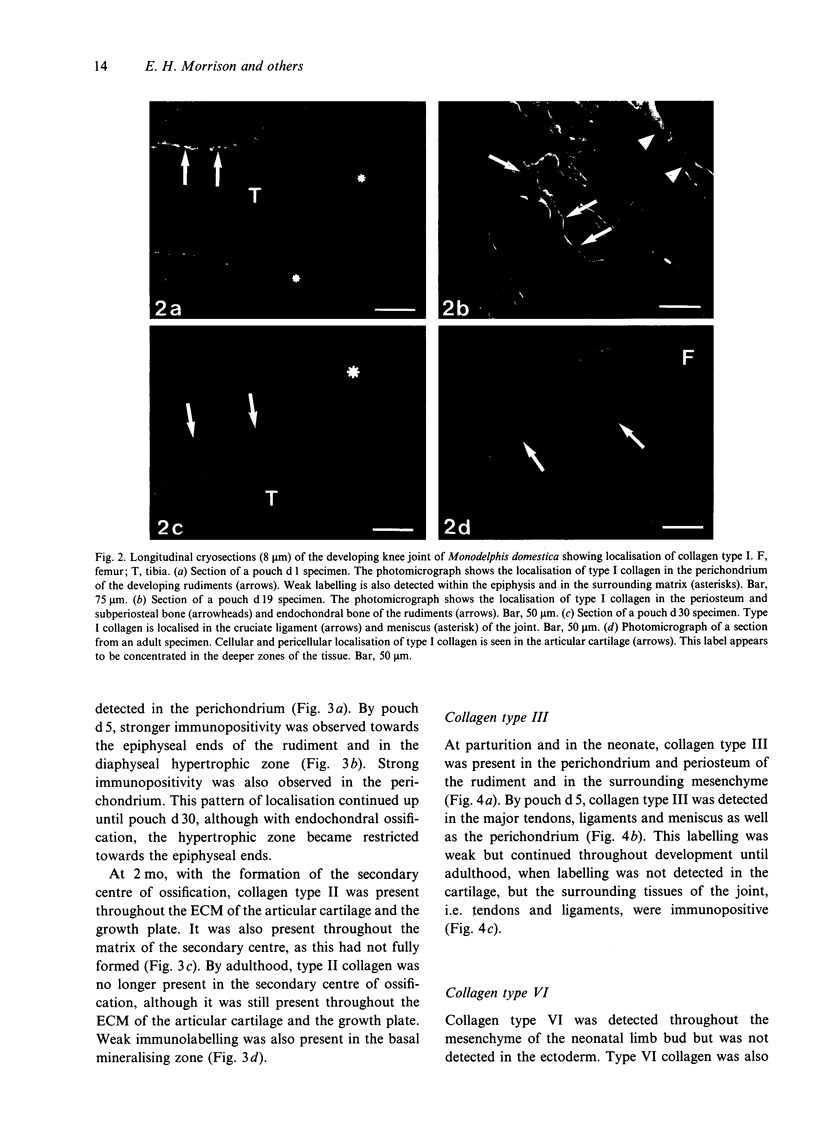
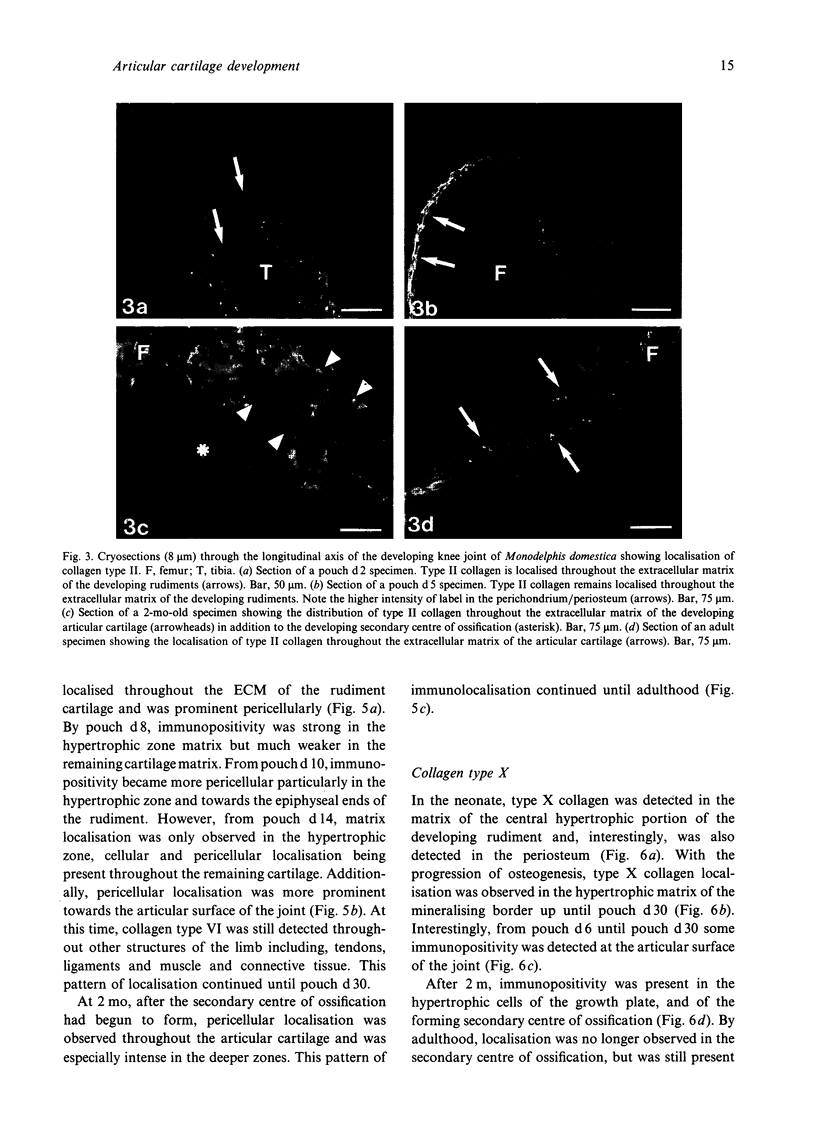
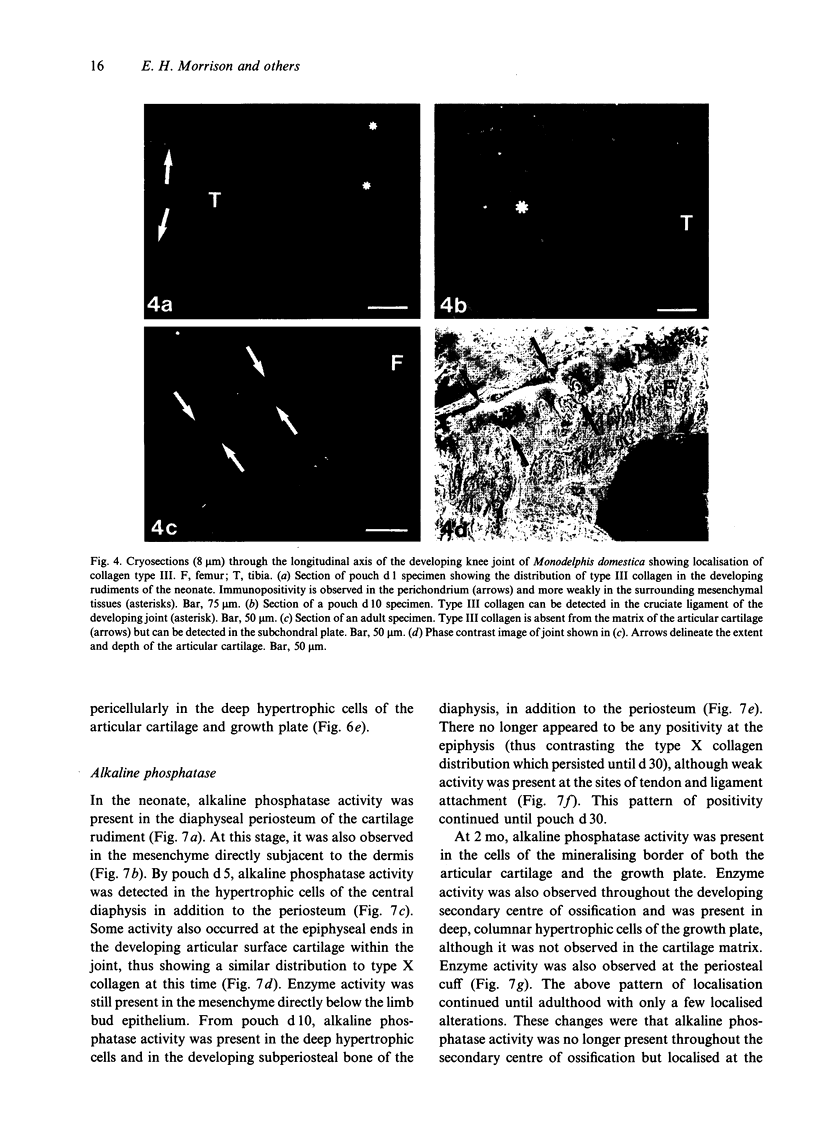

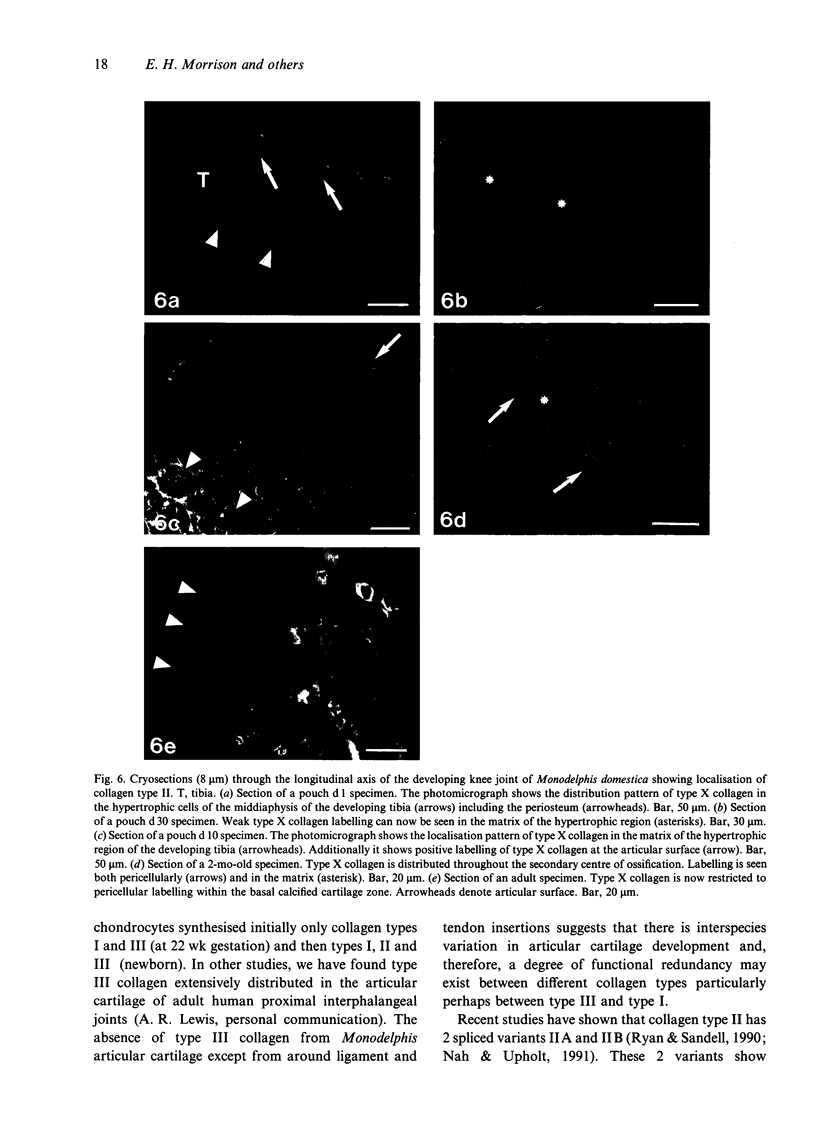



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron J. E., Carter D. H. Rapid preparation of fresh-frozen undecalcified bone for histological and histochemical analysis. J Histochem Cytochem. 1987 Mar;35(3):361–369. doi: 10.1177/35.3.2434557. [DOI] [PubMed] [Google Scholar]
- Amenta P. S., Gil J., Martinez-Hernandez A. Connective tissue of rat lung. II: Ultrastructural localization of collagen types III, IV, and VI. J Histochem Cytochem. 1988 Sep;36(9):1167–1173. doi: 10.1177/36.9.3403967. [DOI] [PubMed] [Google Scholar]
- Archer C. W., Morrison H., Pitsillides A. A. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat. 1994 Jun;184(Pt 3):447–456. [PMC free article] [PubMed] [Google Scholar]
- Archer C. W. Skeletal development and osteoarthritis. Ann Rheum Dis. 1994 Oct;53(10):624–630. doi: 10.1136/ard.53.10.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J Natl Cancer Inst. 1958 Mar;20(3):601–615. [PubMed] [Google Scholar]
- Beertsen W., van den Bos T. Alkaline phosphatase induces the mineralization of sheets of collagen implanted subcutaneously in the rat. J Clin Invest. 1992 Jun;89(6):1974–1980. doi: 10.1172/JCI115805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner H. G., van Beersum S. E., Warman M. L., Olsen B. R., Ropers H. H., Mariman E. C. A Stickler syndrome gene is linked to chromosome 6 near the COL11A2 gene. Hum Mol Genet. 1994 Sep;3(9):1561–1564. doi: 10.1093/hmg/3.9.1561. [DOI] [PubMed] [Google Scholar]
- Carter D. H., Sloan P., Aaron J. E. Trabecular generation de novo. A morphological and immunohistochemical study of primary ossification in the human femoral anlagen. Anat Embryol (Berl) 1992 Aug;186(3):229–239. doi: 10.1007/BF00174144. [DOI] [PubMed] [Google Scholar]
- Chan D., Cole W. G., Chow C. W., Mundlos S., Bateman J. F. A COL2A1 mutation in achondrogenesis type II results in the replacement of type II collagen by type I and III collagens in cartilage. J Biol Chem. 1995 Jan 27;270(4):1747–1753. [PubMed] [Google Scholar]
- Cheah K. S., Lau E. T., Au P. K., Tam P. P. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development. 1991 Apr;111(4):945–953. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- Craig F. M., Bentley G., Archer C. W. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987 Mar;99(3):383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Diab M., Wu J. J., Shapiro F., Eyre D. Abnormality of type IX collagen in a patient with diastrophic dysplasia. Am J Med Genet. 1994 Feb 15;49(4):402–409. doi: 10.1002/ajmg.1320490411. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Apon S., Wu J. J., Ericsson L. H., Walsh K. A. Collagen type IX: evidence for covalent linkages to type II collagen in cartilage. FEBS Lett. 1987 Aug 17;220(2):337–341. doi: 10.1016/0014-5793(87)80842-6. [DOI] [PubMed] [Google Scholar]
- Galotto M., Campanile G., Robino G., Cancedda F. D., Bianco P., Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. J Bone Miner Res. 1994 Aug;9(8):1239–1249. doi: 10.1002/jbmr.5650090814. [DOI] [PubMed] [Google Scholar]
- Gardner E., O'Rahilly R. The early development of the knee joint in staged human embryos. J Anat. 1968 Jan;102(Pt 2):289–299. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H., Schröter-Kermani C., Merker H. J. Localization of collagen type VI in articular cartilage of young and adult mice. Cell Tissue Res. 1993 Apr;272(1):155–160. doi: 10.1007/BF00323581. [DOI] [PubMed] [Google Scholar]
- Haines R. W. The development of joints. J Anat. 1947 Jan;81(Pt 1):33–55. [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Rubin K., Klareskog L., Larsson E., Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986 Mar;29(3):400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Keene D. R., Engvall E., Glanville R. W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988 Nov;107(5):1995–2006. doi: 10.1083/jcb.107.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty C. M., Cummings C., Whittaker S. P., Shuttleworth C. A., Grant M. E. Isolation and ultrastructural analysis of microfibrillar structures from foetal bovine elastic tissues. Relative abundance and supramolecular architecture of type VI collagen assemblies and fibrillin. J Cell Sci. 1991 Aug;99(Pt 4):797–807. doi: 10.1242/jcs.99.4.797. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Kwan A. P., Holmes D. F., Schor S. L., Grant M. E. Type X collagen, a product of hypertrophic chondrocytes. Biochem J. 1985 Apr 15;227(2):545–554. doi: 10.1042/bj2270545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A. P., Cummings C. E., Chapman J. A., Grant M. E. Macromolecular organization of chicken type X collagen in vitro. J Cell Biol. 1991 Aug;114(3):597–604. doi: 10.1083/jcb.114.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A. P., Dickson I. R., Freemont A. J., Grant M. E. Comparative studies of type X collagen expression in normal and rachitic chicken epiphyseal cartilage. J Cell Biol. 1989 Oct;109(4 Pt 1):1849–1856. doi: 10.1083/jcb.109.4.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboy P. S., Shapiro I. M., Uschmann B. D., Oshima O., Lin D. Gene expression in mineralizing chick epiphyseal cartilage. J Biol Chem. 1988 Jun 15;263(17):8515–8520. [PubMed] [Google Scholar]
- Maddox P. H., Jenkins D. 3-Aminopropyltriethoxysilane (APES): a new advance in section adhesion. J Clin Pathol. 1987 Oct;40(10):1256–1257. doi: 10.1136/jcp.40.10.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Nah H. D., Upholt W. B. Type II collagen mRNA containing an alternatively spliced exon predominates in the chick limb prior to chondrogenesis. J Biol Chem. 1991 Dec 5;266(34):23446–23452. [PubMed] [Google Scholar]
- Oxford J. T., Doege K. J., Horton W. E., Jr, Morris N. P. Characterization of type II and type XI collagen synthesis by an immortalized rat chondrocyte cell line (IRC) having a low level of type II collagen mRNA expression. Exp Cell Res. 1994 Jul;213(1):28–36. doi: 10.1006/excr.1994.1169. [DOI] [PubMed] [Google Scholar]
- Petit B., Ronzière M. C., Hartmann D. J., Herbage D. Ultrastructural organization of type XI collagen in fetal bovine epiphyseal cartilage. Histochemistry. 1993 Sep;100(3):231–239. doi: 10.1007/BF00269096. [DOI] [PubMed] [Google Scholar]
- Poole C. A., Ayad S., Schofield J. R. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988 Aug;90(Pt 4):635–643. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- Quarto R., Dozin B., Bonaldo P., Cancedda R., Colombatti A. Type VI collagen expression is upregulated in the early events of chondrocyte differentiation. Development. 1993 Jan;117(1):245–251. doi: 10.1242/dev.117.1.245. [DOI] [PubMed] [Google Scholar]
- Ralphs J. R., Benjamin M., Thornett A. Cell and matrix biology of the suprapatella in the rat: a structural and immunocytochemical study of fibrocartilage in a tendon subject to compression. Anat Rec. 1991 Oct;231(2):167–177. doi: 10.1002/ar.1092310204. [DOI] [PubMed] [Google Scholar]
- Rooney P., Archer C. W. The development of the perichondrium in the avian ulna. J Anat. 1992 Dec;181(Pt 3):393–401. [PMC free article] [PubMed] [Google Scholar]
- Ryan M. C., Sandell L. J. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990 Jun 25;265(18):10334–10339. [PubMed] [Google Scholar]
- Sandell L. J., Morris N., Robbins J. R., Goldring M. B. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991 Sep;114(6):1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. J., Nalin A. M., Reife R. A. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dyn. 1994 Feb;199(2):129–140. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. Developmental acquisition of type X collagen in the embryonic chick tibiotarsus. Dev Biol. 1985 Feb;107(2):373–381. doi: 10.1016/0012-1606(85)90319-7. [DOI] [PubMed] [Google Scholar]
- Sloan P., Carter D. H., Kielty C. M., Shuttleworth C. A. An immunohistochemical study examining the role of collagen type VI in the rodent periodontal ligament. Histochem J. 1993 Jul;25(7):523–530. doi: 10.1007/BF00159289. [DOI] [PubMed] [Google Scholar]
- Stephens M., Kwan A. P., Bayliss M. T., Archer C. W. Human articular surface chondrocytes initiate alkaline phosphatase and type X collagen synthesis in suspension culture. J Cell Sci. 1992 Dec;103(Pt 4):1111–1116. doi: 10.1242/jcs.103.4.1111. [DOI] [PubMed] [Google Scholar]
- Swiderski R. E., Daniels K. J., Jensen K. L., Solursh M. Type II collagen is transiently expressed during avian cardiac valve morphogenesis. Dev Dyn. 1994 Aug;200(4):294–304. doi: 10.1002/aja.1002000404. [DOI] [PubMed] [Google Scholar]
- Swiderski R. E., Solursh M. Localization of type II collagen, long form alpha 1(IX) collagen, and short form alpha 1(IX) collagen transcripts in the developing chick notochord and axial skeleton. Dev Dyn. 1992 Jun;194(2):118–127. doi: 10.1002/aja.1001940205. [DOI] [PubMed] [Google Scholar]
- Treilleux I., Mallein-Gerin F., le Guellec D., Herbage D. Localization of the expression of type I, II, III collagen, and aggrecan core protein genes in developing human articular cartilage. Matrix. 1992 Jun;12(3):221–232. doi: 10.1016/s0934-8832(11)80065-x. [DOI] [PubMed] [Google Scholar]
- Vandenberg P. Molecular basis of heritable connective tissue disease. Biochem Med Metab Biol. 1993 Feb;49(1):1–12. doi: 10.1006/bmmb.1993.1001. [DOI] [PubMed] [Google Scholar]
- Vialle-Presles M. J., Hartmann D. J., Franc S., Herbage D. Immunohistochemical study of the biological fate of a subcutaneous bovine collagen implant in rat. Histochemistry. 1989;91(3):177–184. doi: 10.1007/BF00490129. [DOI] [PubMed] [Google Scholar]
- Wardale R. J., Duance V. C. Quantification and immunolocalisation of porcine articular and growth plate cartilage collagens. J Cell Sci. 1993 Aug;105(Pt 4):975–984. doi: 10.1242/jcs.105.4.975. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Aubert-Foucher E., Dublet B., Eichenberger D., Font B., Goldschmidt D. Structure and function of the fibril-associated collagens. Biochem Soc Trans. 1991 Nov;19(4):820–824. doi: 10.1042/bst0190820. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Mayne R. Type IX collagen proteoglycan from cartilage is covalently cross-linked to type II collagen. J Biol Chem. 1988 Feb 5;263(4):1615–1618. [PubMed] [Google Scholar]
- von der Mark K. Localization of collagen types in tissues. Int Rev Connect Tissue Res. 1981;9:265–324. doi: 10.1016/b978-0-12-363709-3.50012-7. [DOI] [PubMed] [Google Scholar]
- von der Mark K., von der Mark H., Gay S. Study of differential collagen synthesis during development of the chick embryo by immunofluroescence. II. Localization of type I and type II collagen during long bone development. Dev Biol. 1976 Oct 15;53(2):153–170. doi: 10.1016/0012-1606(76)90220-7. [DOI] [PubMed] [Google Scholar]