Simple Summary
Ticks are one of the most problematic parasitic pests, world-wide. Infesting livestock, people, and their pets, ticks cause direct negative effects on their hosts through blood-feeding whilst also spreading significant diseases of veterinary and medical concern (e.g., Lyme disease). Controlling ticks through conventional chemical approaches is hampered by challenges associated with product performance, availability, and environmental safety, yet effective tick management is vital—particularly as populations of some species may be expanding because of climate change. To control several pest species in a more sustainable manner, researchers have increasingly been exploring the use of beneficial biological organisms as “biopesticides”, including entomopathogenic fungi. These fungi cause diseases in insects and other invertebrates in the natural environment, and many have shown promise for development as biopesticides against a range of pest species, ticks included. This review considers the potential of these beneficial fungi in controlling ticks, providing examples of their effective use against these parasitic pests from countries around the world. Details on the mode of action of entomopathogenic fungi against ticks, advantages and challenges to their use, and potential applications and prospects for their future practical development as biopesticides are also included.
Keywords: Beauveria bassiana, biological control, Entomopathogenic fungi, Metarhizium anisopliae, ticks
Abstract
Entomopathogenic fungi (EPFs) can infect and kill a diverse range of arthropods, including ticks (Acari: Ixodidae) that can transmit various diseases to animals and humans. Consequently, the use of EPFs as a biocontrol method for managing tick populations has been explored as an alternative to chemical acaricides, which may have harmful effects on the environment and non-target species. This review summarizes studies conducted on EPFs for tick control between 1998 and 2024, identifying 9 different EPF species that have been used against 15 different species of ticks. One of the most well-known and widely researched EPFs used against ticks is Metarhizium anisopliae, a fungus known for its ability to infect and kill various arthropods. When applied to tick-infested areas, M. anisopliae spores attach to the tick’s cuticle, germinate, and penetrate through the cuticle, leading to the eventual death of the tick due to the fungal infection. Whilst a number of studies support the potential of this and other EPF species against ticks, this review suggests that limitations to their effective use may include factors such as heat, humidity, and ultraviolet light (UV-A and UV-B). This comprehensive review aims to provide an overview of the literature on the potential of EPFs in tick control, focusing on their mode of action, previous field successes/failures, advantages, potential applications, and prospects for future practical developments.
1. Introduction
Ticks are obligatory blood feeders that transmit a diverse array of protozoan, bacterial, and viral pathogens of zoonotic importance. Ticks and mosquitoes have been reported as the main vectors of human and veterinary pathogens globally, although ticks are known to surpass all other arthropods in the variety of infectious agents they transmit [1,2]. Besides their role as biological and mechanical disease vectors, ticks have various additional direct impacts on the health and wellbeing of affected hosts, including blood loss, alopecia, fatal paralysis (where some ticks are also able to inject toxins), and exsanguination [3], with impacts affecting humans, livestock and wild animals alike. Infestations vary across this wide host range, dependent on host preference and agro-ecological location, with the availability of suitable habitats being affected by our changing climate.
Even though climate change has been reported as one of the main drivers of biodiversity loss, some species are able to respond by extending their geographical boundaries. Several tick species survive only in specific climatic conditions, for example, with changes in climate directly impacting their occurrence, while others are capable of adapting to a wide range of climatic conditions [4]. Additionally, generalist feeders and species known to utilize alternative hosts, such as the deer tick Ixodes scapularis, the cattle fever tick Rhipicephalus microplus, and the sheep tick Ixodes ricinus, are more likely to shift their expansion ranges as opposed to strictly host-specific ticks [5,6,7]. As in the tropics, many temperate regions are now characterized by hot and humid conditions that allow a more diverse tick population to establish, with the bulk of the literature on climate change implications on tick populations coming from North America or Europe [4].
The most studied ticks tend to be the most important from a medical and veterinary perspective [4], whereas most significant species globally in terms of abundance, distribution, and contribution to tick borne diseases (TBDs) include the brown dog tick Rhipicephalus sanguineus (the most prevalent tick globally), I. ricinus (dominating in Europe), Dermacentor reticulatus and Ixodes persulcatus (found in Siberia and more widely in Asia), the deer tick I. scapularis (occurring in most areas of North America and being a key vector of Lyme disease), Ixodes cookei (being present in most regions of the Quebec province in Canada and found throughout Northeastern North America), the Rocky Mountain wood tick Dermacentor andersoni (found in North, Central, and South America), Amblyomma ticks (occurring in South America, especially Amblyomma neumanni, Amblyomma cajennense, Amblyomma trieste, Amblyomma ovale and Amblyomma aureolatum) and the paralysis tick Ixodes holocyclus (known to occur in Queensland, Australia) [4,8,9].
Where ticks are present around the world, a major constraint for livestock animals and humans is the burden imposed by the TBDs they spread [10,11]. Both Ixodid and Argasid ticks are implicated in the transmission of dangerous pathogens, [12], where the Ixodid tick fauna consists of over 700 species worldwide, divided into two groups: the Prostriata (including the Ixodes genus) comprised of over 200 species, and the Metastriata (other whitemolecular markers have been studied and employed alongside conventional approaches, species identification is still problematic (especially for cryptic species) and is often challenged for many tick species, leading to controversy in nomenclature in the literature [13].
Tick-borne diseases are increasingly being identified as causes of human disease in many countries. The expansion of vector-borne diseases into diverse regions results from a complex interplay of multiple factors, including climate change, shifting host habitats, land use alterations, global trade, and animal movement [14,15,16]. Ticks also constitute a considerable hindrance to animal production, presenting significant risks that include morbidity and mortality through paralysis and anemia, welfare problems such as dermatitis, and associated economic impacts to livestock farming operations in countries where they occur [1,17]. The continued and sustainable production of milk and meat products from these animals is already a global concern when it comes to food security [18], with tick blood feeding from and pathogen transmission to these hosts adding to concerns. Ticks are responsible for the transmission of major destructive diseases in livestock, for example, Lyme disease and tick-borne encephalitis [1], and jeopardize food safety as a result [19]. These factors, coupled with the common occurrence of ticks developing resistance to acaricides presently used in their control, necessitate the need for novel control approaches coupled with integrated surveillance and pest management.
Synthetic acaricides have remained the gold standard for the control of ticks in recent decades despite delivering detrimental unintended consequences such as environmental pollution, human health risks, food contamination, and the development of resistance in the target pest population [20]. Numerous microorganisms also display pest control potential, however, including nematodes, fungi, and crystalliferous bacteria, all of which have been used to control a range of arthropod pests, including mosquitoes, and some of which have also been used against ticks. Biological control may also be delivered by larger species, where the parasitoid wasp Ixodiphagus hookeri was used for tick control in the 20th century [21], and predators such as birds, ants, rodents, shrews, and spiders have similarly been deployed to the same end. Notably, poultry can play a key role in removing ticks directly from infested animals, as well as from livestock housing [22].
From the above naturally derived solutions, the development of EPFs has demonstrated particularly promising potential as an “alternative” tick control method [23]. Working in this area, researchers have demonstrated that several factors play a critical role in the effectiveness of EPFs against these pests, including (1) tick innate responses to infection with EPFs; (2) mechanisms that potentiate the virulence of EPFs, including the impacts of toxic components and EPFs metabolites on target vectors; (3) propensity for mass production of EPFs strains adapted to broad spectrum climatic conditions across the wide geographic ranges that various tick vectors occur; and (4) identification and selection of rigorously virulent EPFs isolates [24].
Operator safety when deploying any pest control product is of utmost importance when implementing any biological control method, and EPFs have demonstrated a favorable safety profile, adding to their appeal as a reliable and sustainable solution for tick control [25]. Similar to other pest control agents, their use is subject to rigorous experimentation on target and non-target toxicity, risk assessments, and regulatory frameworks to ensure their safe and responsible application. These measures help minimize any potential risks associated with their deployment and guarantee that EPFs are applied in a manner that aligns with environmental and safety standards [26].
Previous reports have shown that treatments with EPFs exhibit anti-tick activity by inducing myco-acaricidal effects and inhibiting host reproduction [27]. According to White et al. [28], 17 fungal species have been isolated from ticks, with a few of these species being acari-specific and others being more generalist in their host range, infecting both insects and acari. Such species are especially interesting for their potential to target multiple pests, where two such generalist EPFs have been recently applied in tick biological control, namely M. anisoplae sensu lato and B. bassiana [28,29].
M. anisoplae is a prevalent entomopathogen that thrives on the cuticle of its host. It belongs to the Ascomycota phylum and the Clavicipitaceae family, with review and reclassification of the Metarhizium species complex recently undertaken [30]. Although this fungus has already undergone extensive examination and development as a biopesticide, studies suggest that further improvements could be realized through genetic engineering of M. anisopliae and formulation development to enhance application and effectiveness [31,32]. Beauveria bassiana is also widely used as a biopesticide, being an ascomycete arthropod pathogen belonging to the Cordycipitaceae family and the Hypocreales order [33]. Strains of B. bassiana have been effectively deployed against eggs, larvae, nymphs, and adults of the cayenne tick, A. cajennense s.l. and brown dog tick, and R. sanguineus s.l., among others [34,35,36,37]. A recent study has also compared the effectiveness of spray applications of M. anisopliae, Metarhizium brunneum, and B. bassiana against Dermacentor albipictus larvae, finding that M. anisopliae and M. brunneum isolates provided 74–99% control of unfed questing ticks and engorged larvae, while B. bassiana at similar concentrations delivered 30–64% control [38]. Authors elsewhere have further confirmed that M. brunneum is an effective anti-tick fungus under semi-field conditions [39], supporting its potential for wider commercial development and registration against ticks [40]. Similarly, Alonso-Díaz et al. [41] reported high mortality in various life stages of several tick species when exposed to M. anisopliae and M. anisopliae s. l. [18].
In addition to offering stand-alone solutions for tick control, Mesquita et al. [29] reported that EPFs disrupt the R. microplus gut microbiota, positively influencing tick susceptibility to acaricides. The tick gut microbiome is being increasingly targeted in medical and veterinary medicine following the realization that the composition of the microbiome could influence the vectorial capacity and biology of ticks [38,42,43,44]. Using 16S rRNA amplicon sequencing, researchers have gained deeper insights into the microbiome of different tick tissues [45,46] to support this area of research. Interactions between the tick and its microbiota regulate the tick peritrophic matrix and enhance tick epithelial integrity, vectorial capacity, and the pathogen transmission process, highlighting a potential target for anti-tick interventions [43,47]. Tick microbiota and bacterial symbionts that modulate the tick immune responses are, therefore, becoming new targets in tick control approaches [38].
Collectively, the body of work cited above provides strong evidence that ticks can succumb to EPF-mediated biological control methods, with the potential for combined use with other products. Further developing such integrated and sustainable tick control strategies could deliver significant gains for enhanced animal and human health.
2. The Significance of EPFs in Tick Control
The term “entomopathogen” encompasses a range of microorganisms that include parasites, bacteria, viruses, or fungi that are capable of infecting insects and other terrestrial arthropods, including mites, ticks, and spiders. These entomopathogens rely on a heterotrophic metabolism, which necessitates a life dependent on a host [48]. Bacillus thuringiensis, for example, is a rod-shaped and spore-forming bacterium that has been widely deployed to control defoliating insects in several sectors, particularly forestry [49]. The primary focus of the current review, however, is on EPFs, where there already exists a wide range of EPF-based biopesticides available in the market, most of which are derived from organisms belonging to the genera Beauveria, Metarhizium, Akanthomyces, and Cordyceps (all of which belong to the Ascomycota in the Order Hypocreales). These fungi have the ability to target a range of pests, thereby effectively combating various insect/acari-borne pathogens, including those carried by ticks [50]. To date, over a thousand EPF species have been identified as exhibiting a wide range of strategies and adaptations to successfully invade, reproduce within, and ultimately harm their arthropod hosts. This vast number of EPF species highlights the significant role they play in regulating insect populations in natural systems and their potential for application in pest management strategies [51]. Entomopathogenic Hypocreales are particularly well-specialized pathogens with a range of adaptions that have evolved to effectively infect insects and mites, including the ability to circumvent the host’s immune system defenses and the production of enzymes and degrading substances that can break down the host’s cuticle. As noted above, previous studies have already highlighted the potential of EPFs as a promising alternative to chemical pesticides for tick control, either in field or laboratory settings, with their use aligning with the growing demand for eco-friendly pest management practices [52].
One of the most notable advantages of EPFs is that they naturally exist in the environment and have a specific affinity for targeting arthropods such as ticks [18,53], thus minimizing impacts on higher non-target organisms and the environment [24]. Being naturally occurring, these fungi pose little discernable threat to water sources, soil quality, or air pollution, making them a sustainable and ecologically responsible solution to managing pests [54], ticks included [55]. Moreover, these fungi possess the ability to adapt and evolve with their host populations, reducing the likelihood of pests developing resistance to them [56].
In addition to implementing robust regulatory measures to approve EPF-based pest control products, efforts to raise public awareness and education on the benefits and safety of EPFs can play a crucial role in fostering acceptance and understanding of these sustainable solutions for tick control. By promoting knowledge and understanding, we can encourage the responsible use of EPFs as an effective and environmentally friendly method for managing tick populations [57]. Ultimately, the use of EPFs for tick control aligns with sustainable and eco-friendly practices, fostering a healthier environment for both humans and wildlife and aligning with the One Health principles.
3. The Use of EPFs in Tick Control
Entomopathogenic fungi are already widely utilized in some areas of pest management, with certain species such as B. bassiana and M. anisopliae emerging as “classic” examples of biopesticide candidates in particular geographic regions, offering alternatives to chemicals pesticides where these are neither economically nor ecologically sustainable [18]. Entomopathogenic fungi are nevertheless highly diverse, being heterotrophic, unicellular/multicellular, producing both sexual and asexual spores, and having wide global distributions [58]. They may also exert both direct and indirect effects on their hosts that can be leveraged in pest management, including in ticks. Metarhizium spp., for example, have been shown to deliver sub-lethal effects on ticks under field conditions by affecting their feeding behavior [59,60,61].
Whether through direct or indirect activity, EPFs show the potential to control ticks across all pest life stages, including eggs, larvae, nymphs, adults, and engorged females [62,63], where this control is perhaps unsurprisingly reported to also reduce the incidence of the diseases that these ticks spread [64]. To collate examples to support this potential, the current review adopted an unstructured approach, utilizing general search terms (e.g., “entomopathogenic fungus” and “biological control of ticks”) to identify the relevant literature. The resulting body of work identified confirms that studies to demonstrate this potential have been undertaken globally in a range of tick species, using a range of EPFs, in both laboratory and field settings, as summarized in Table 1 and Table 2.
In all, 39 laboratory and 29 field studies were identified that considered the effects of EPFs on a total of 15 different tick species between the years 1998 and 2024. Across these studies, nine species of fungi had been investigated, with the majority of studies focusing on M. anisopliae and B. bassiana, and most of this work being undertaken in Mexico, followed by Brazil and the USA. These studies reveal a wide range of outcomes regarding the use of numerous EPFs against varied tick species and life stages, which can be attributed to differences in study scope (e.g., target tick species, EPF strains), geographical locations, and variations in biotic and abiotic parameters. For instance, the choice of acaropathogen and its formulation can play a crucial role in tick control, where in work by Kirkland et al. [65], both M. anisopliae and B. bassiana were effective against R. microplus ticks, delivering a decrease in egg hatchability (EH), an increase in egg incubation period (EIP), and an increase in egg hatchability period (EHP). Similarly, [66] reported a reduction in egg hatchability of Rhipicephalus appendiculatus and Amblyomma variegatum after exposure to B. bassiana and M. anisopliae, but with both EPFs displaying variation in their efficacy dependent on their formulation (i.e., whether this was oil-based or aqueous). Differences may have also been attributed to variations in EPF strain virulence or environmental factors, where variations in tick species and their susceptibility to specific EPFs were evident [67,68,69]. Pirali-Kheirabadi et al. [67] reported a decrease in EH for A. cajennense when exposed to M. anisopliae, for example, whilst [69] did not find significant differences in EH for the same tick species when treated with B. bassiana. Onofre et al. [69] further showed varied results for Anocentor nitens ticks, with EH remaining largely unchanged when exposed to B. bassiana, but a decrease was seen in both EHP and egg hatchability index (EHI). It is important to consider such differences when designing tick control strategies, as the efficacy of EPFs may vary depending on any of these factors, as well as interactions between them.
Table 1.
Examples from the literature detailing results of studies where ticks were treated with conidial suspension of EPFs under laboratory conditions.
| Sr. No. | Entomopathogens (Strains) | Tick Species | Tick Stages | Result | Reference | Country |
|---|---|---|---|---|---|---|
| 1 | M. anisopliae (Ma 319, Ma 359, E9) | A. cajennense | Eggs and larvae | EH decreased, EIP and EHP were not different from control | [70] | Brazil |
| 2 | B. bassiana (Bb 986, Bb 747) | A. cajennense | ||||
| 3 |
B. bassiana (Bb 986) |
A. nitens | Eggs | EH and control group were same, decreased EHP and EHI | [71] | Brazil |
| 4 |
M. anisopliae (E6S1, E6S2 and CG491) |
B. microplus | Engorged females | 100% mortality of engorged females after 14 days | [72] | Brazil |
| 5 | M. anisopliae (ATCC 20500) and B. bassiana (ATCC 90517) |
Dermacentor variabilis Say, I. scapularis Say, and R. sanguineus Latrielle |
Nymph Adult |
90% nymph mortality, 50–70% adult mortality |
[65] | USA |
| 6 | M. anisopliae (IRAN 437 C, DEMI 001), B. bassiana (IRAN 403 C), L. psalliotae (IRAN 468 C, IRAN 518 C) | R. (B.) microplus | Engorged females | 90–100% adult mortality 35.5–88% EH reduced |
[67] | Iran |
| 7 |
B. bassiana (AM 09, CB 7, JAB 07) |
R. (B.) microplus | Inoculating eggs, larvae and engorged females | 1.36–65.58% EH decreased 0.8–70.49% larval mortality 96–100% adult mortality |
[68] | Brazil |
| 8 | M. anisopliae var. anisopliae | R. (B.) microplus | Engorged females | EH decreased 10.69–75.91% |
[69] | Brazil |
| 9 | M. anisopliae var. acridum | R. (B.) microplus | Engorged females | |||
| 10 |
B. bassiana (Bb28, Bb29 and Bb30) |
R. (B.) microplus | Egg and larvae | Decreased EH and EHP 30–80% |
[34] | Brazil |
| 11 |
M. anisopliae (Ma01, Ma02, Ma04) |
R. (B.) microplus | Eggs | EH decreased 24% to 83%, |
[73] | Brazil |
| 12 |
M. anisopliae (ARSEF3297) |
R. (B.) microplus | Engorged females | decreased EH, increased EHP | [74] | USA |
| 13 |
B. bassiana (JAB 07, CB 7, AM 9) |
R. sanguineus | Engorged females | EH decreased | [35] | Brazil |
| 14 | M. anisopliae (ESC1) | R. microplus | Engorged females | 100% mortality on 20th day | [75] | Mexico |
| 15 |
M. anisopliae (E9, 319) |
R. (B.) microplus | Eggs and larvae | EH decreased and EHP | [76] | Brazil |
| 16 | L. psalliotae (IRAN 468 C, IRAN 518 C), B. bassiana (IRAN 403 C), M. anisopliae (IRAN 437 C, DEMI 001) | B. annulatus | Engorged females | EH reduced up to 35.5%, 56.3%, and 89.1%, respectively | [67] | Iran |
| 17 | M. anisopliae Ma14, Ma34 | R. microplus | Adult and larvae | 100% adult mortality on 20th day | [77] | Mexico |
| 18 | M. anisopliae (M379) | R. microplus | Engorged females | 55.6% adult mortality on 15th day | [78] | Mexico |
| 19 | Nomuraea rileyi | R. microplus | Engorged females | 67.36% adult mortality | [79] | Brazil |
| 20 | B. bassiana (Bb986) | R. (B.) microplus | Engorged females and unfed larvae | 71% mortality of larvae on 30th day | [36] | Brazil |
| 21 | M. anisopliae (Ma 959) | R. (B.) microplus | 98.7% mortality of larvae on 30th day | |||
| 22 |
M. anisopliae (CG 37, CG 384 and IBCB 481) |
R. microplus | Larvae | 100% larval mortality after 14 days | [80] | Brazil |
| 23 |
M. anisopliae (strain 62) |
Hyalomma anatolicum | Larvae | 100% larval mortality after 5 days | [81] | Sudan |
| 24 | B. bassiana (Bb 112, Bb 113) | R. microplus | Larvae | 2.5–27% larval mortality on 20th day | [82] | Mexico |
| 25 | I. fumosorosea (Ifr22) | R. microplus | Larvae | 28.6% larval mortality on 16th day | ||
| 26 | B. bassiana (CD1123) | R. sanguineus | Eggs, larvae, nymph and adult | 100% reduction in EH, 100% reduction in larvae to nymph development, 95% adult mortality on 15th day | [37] | Italy |
| 27 | M. anisopliae (Ma136) and B. bassiana (Bb 115) | R. microplus | Engorged female | 99–100% adult mortality on 15th day | [83] | Mexico |
| 28 |
M. anisopliae (MaV 22, Ma 26, MaV55) |
R. microplus | Adult ticks | 3.3–100% adult mortality on 20th day | [84] | Mexico |
| 29 |
M. anisopliae (TIS-BR030) |
R. microplus | Larvae | 26.3–100% larval mortality in 15 days | [85] | Brazil |
| 30 |
M. anisopliae (MaV 01-54) B. bassiana (BbV01-06) |
R. microplus | Adult ticks | 3.3–86.7% adult mortality on 20th day | [86,87] | Mexico |
| 31 |
Purpureocillium lilacinum (PlV01) |
R. microplus | Adult ticks | 94.9% adult mortality on 20th day | [86] | Mexico |
| 32 | M. anisopliae and B. bassiana | R. sanguineus and H. longicornis | Adult ticks | 100% adult mortality in 3 days | [88] | Malaysia |
| 33 |
M. anisopliae (L04, L010, L047, L052, MET 32) B. bassiana (LO37) |
I. scapularis, D. variabilis, R. sanguineus | Engorged female | 100% adult mortality | [89] | Poland |
| 34 |
M. anisopliae (MaV25) |
A. mixtum | Larvae | 32.7% larval mortality on 20th day | [41] | Mexico |
| 35 | Aspergillus flavus (H-1), A. nitus (H-2) | Haemaphysalis longicornis | Unfed tick larvae | 100% larval mortality at 12th day | [90] | China |
| 36 | M. anisopliae (CG47) | R. microplus | Engorged females | LH reduced | [91] | Brazil |
| 37 | M. anisopliae sensu stricto (LCM S04) | R. microplus | Partially engorged | 100% adult mortality in 12 days | [29] | Brazil |
| 38 | M. anisopliae (LCM S01) | R. (B.) microplus | Engorged females | Reduced oviposition, EPI significantly decreased, LH remained same | [92] | Brazil |
| 39 | F. oxysporum | R. microplus | Adults | 100% adult mortality | [93] | Pakistan |
EIP = egg incubation period; EHP = egg hatchability period; EPI = egg production index; EH = egg hatchability; EHI = egg hatch inhibition; LH = larval hatchability.
Table 2.
Examples from the literature detailing results of studies where ticks were treated with conidial suspension of EPFs under field conditions.
| Sr. No. | Entomopathogens (Strains) | Tick Species | Tick Stages | Result | Reference | Country |
|---|---|---|---|---|---|---|
| 1 |
M. anisopliae (Ma01, Ma02) |
R. (B.) microplus | Engorged females | EPI reduced; LH decreased | [94] | Brazil |
| 2 | B. bassiana | R. microplus | Adults | 13–38% adult mortality | [95] | South Africa |
| 3 | M. robertsii (IP 146) | R. microplus | Larvae | 38.4% larval mortality | [23] | Brazil |
| 4 | M. anisopliae (LCM S01) | R. microplus | Larvae | 86% larval mortality | [96] | Brazil |
| 5 |
M. anisopliae (JEF-214, -279, and -290) |
H. longicornis | Nymphs | 80% nymph mortality in 7 days, rising to 100% in 14 days | [97] | Korea |
| 6 | B. bassiana (Baubassil ®) | R. (B.) microplus | Adults | 84.8% adult mortality | [98] | Colombia |
| 7 | M. anisopliae (ICIPE 7) and B. bassiana (ICIPE 718) | Rhipicephalus decoloratus | Larvae | 100% larval mortality on 20th day | [99] | Kenya |
| 8 |
M. anisopliae (ESALQ 1037, ESALQ E9) |
R. microplus | Engorged females | 90.53% adult mortality | [100] | Brazil |
| 9 |
B. bassiana (Balsamo, Vuillemin) |
Hyalomma lusitanicum | Adults | 78.63% reduction of ticks in spring till 30th day, with a 63.28% reduction till 60th day 35.7% reduction in summer till 30th day, with a 29.01% at 60th day |
[101] | Spain |
| 10 |
M. anisopliae sensu lato X-1c |
I. ricinus | Larvae and nymphs | 92% larval mortality 94% nymphs’ mortality |
[102] | Germany |
| 11 |
M. anisopliae (TIS-BR03) |
R. microplus | Engorged females | 97.0% adult mortality | [103] | Brazil |
| 12 |
M. brunneum (strain 7) |
R. (B) annulatus | Engorged Females |
93% adult mortality within 3–4 weeks during summer and 62.2% adult mortality within 6 weeks during summer | [39] | Israel |
| 13 |
M. brunneum (M-7) |
R. sanguineus | Larvae, nymphs, adults | larval mortality 49%; nymph mortality 79%; adult mortality 25.3% | [104] | Israel |
| 14 |
M. brunneum F52 |
I. scapularis | Nymphs | 50% nymph mortality in 7 days | [105] | USA |
| 15 |
M. anisopliae (NA1) |
Rhipicephalus evertsi evertsi and R. (B.) decoloratus | Adult | 83% control of tick populations | [106] | Namibia |
| 16 | M. anisopliae (Ma14, Ma34) | R. microplus | Adult and larvae | 67–100% adult mortality | [77] | Mexico |
| 17 |
M. anisopliae (Ma 14) I. fumosorosea |
R. microplus | Larvae | 94% larval mortality | [107] | Mexico |
| 18 | B. bassiana (ATCC 74040) | I. scapularis | Nymph | 38–58.7% control | [108] | USA |
| 19 | M. anisopliae (F52) | 55.6–84.6% control | ||||
| 20 |
M. anisopliae (Ma34) |
R. (B.) microplus | Engorged females | 45% control at day 1 and 5 | [109] | Mexico |
| 21 |
M. anisopliae (ARSEF3297 and IMI386697) |
R. (B.) microplus | Engorged females | Tick density was reduced (8.5 ± 0.6 and 19.1 ± 0.6 ticks/host) after 3 weeks | [110] | USA |
| 22 | M. anisopliae | R. (B.) microplus | Larvae | 86.9% to 94.08% control from day 35 to 48 post-infestation | [111] | Brazil |
| 23 |
M. anisopliae (ESALQ, 959) |
R. (B.) microplus | Larvae | 40.0% control | [112] | Brazil |
| 24 |
B. bassiana (Bb 986) |
A. nitens | Nymphs | 70.1% control | [113] | Brazil |
| 25 | B. bassiana | A. nitens | Adult | 50% on day 4–25 after treatment, | [114] | Brazil |
| 26 | M anisopliae and B. bassiana | A. variegatum and R. appendiculatus | Larvae, nymph, adult |
larvae 100%, nymph 40–50%, and adult 80–90% |
[66] | Namibia |
| 27 | M. anisopliae | R. (B.) microplus | Engorged females | 30% control on reproductive index | [115] | Brazil |
| 28 | M. anisopliae | R. (B.) microplus | Engorged female | 43.3% control | [116] | Brazil |
| 29 | M. anisopliae | R. (B.) microplus | Engorged females | 52% reduction in EPI | [117] | Brazil |
EPI = egg production index; LH = larval hatchability.
4. Mode of Action of EPFs Against Ticks
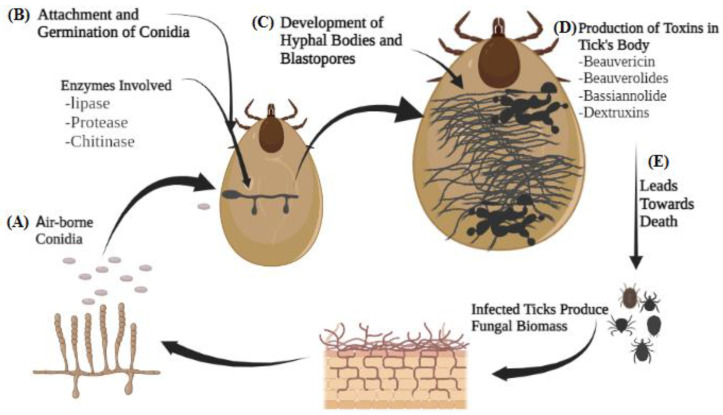
As shown in Table 1 and Table 2, EPFs often exhibit encouraging efficacy against ticks. Given this, it is perhaps surprising that there is a lack of research examining the defense mechanisms employed by ticks during fungal infections, or the mechanisms utilized by these fungi to infect ticks. EPF-based management of plant-feeding invertebrates is already widespread, with numerous examples of these fungi also controlling ectoparasitic species supporting similar potential impact in blood-feeding pests. However, the discussion below highlights the necessity for a more comprehensive understanding of these relationships to make best use of EPFs in tick control, framed against the following key steps in the tick EPFs infection process, as proposed by Beys-da-Silva et al. [118], and as also displayed in Figure 1.
Figure 1.
Schematic representation of the mode of action of EPFs against ticks. (A) Air-borne EPF conidia land on the cuticle of ticks attracted by hydrophobic mechanisms, (B) after adhesion and firm attachment germination and penetration into cuticle of the host takes place in the presence of cuticle degrading enzymes, viz. lipases, proteases, and chitinase. (C) After penetration, EPFs produce bunches of mycelia, fungal biomass, and blastospores, (D) which produce toxins in the tick’s body. (E) These toxins cause destruction of cellular processes, Malpighian tubes, muscular tissues, and middle intestine and insight flaccid paralysis in the tick’s body, which leads to death.
Recognition of the susceptible host by EPFs;
Adhesion of EPF conidia to the tick’s cuticle and subsequent germination;
Development of specialized EPFs structures, including germ tubes and appressoria;
Penetration of EPFs through the tick’s cuticle;
Vigorous EPF growth within the tick, leading to the death of the host;
Production of conidia once the EPF hyphae emerge through the tick’s cuticle.
4.1. EPFs Host Recognition, Conidial Adhesion, and Germination on the Host Cuticle
Air-borne conidia of EPFs land on the surface of the host’s cuticle, which is facilitated by hydrophobic mechanisms [119]. This adhesion process is primarily mediated by surface proteins known as adhesins [25] and hydrophobins [120], with the former having been identified in B. bassiana, also demonstrating the existence of homologous proteins [121]. The lipolytic activity observed in ticks enhances this process, specifically through enzymes including lipase and esterase, which have been identified as important in the adhesion and recognition of conidia during the process of infection in ticks by Metarhizium [122,123].
4.2. EPFS Penetration of the Host Cuticle
Once the conidia have firmly attached to a host, they enter a germination phase, facilitated by favorable environmental conditions. This germination process gives rise to a germination tube, which is followed by the formation of an appressorium or penetration peg that enables EPFs penetration into the tick’s cuticle [124]. Penetration is an active process that depends on the coordinated activity of hydrolytic cuticular enzymes like chitinases and lipases, as well as proteases, in addition to the physical force applied by the penetration peg or appressorium [124]. Various layers of cuticle contain different types of polymeric substrates which are degraded by the activity of the above enzymes, viz. proteases and chitinases [122]. Several proteases, including chymotrypsins, metallopeptidases, trypsins, exopeptidases, subtilisins, and aspartyl peptidases are involved in this process [125]. The expression of these proteases by EPFs is influenced by the specific composition of the host tick cuticle [126], where species of Metarhizium and Beauveria can produce as many as 11 different subtilisins, with the Pr1 subtilisin-like peptidases being particularly important for the pathogenicity of these EPFs against arthropods. These enzymes may also deliver further beneficial functions, playing a crucial role in both cuticle hydrolysis and nutrient acquisition for EPFs [121,125].
4.3. Development of EPFs and Release of Toxins in Tick Body
Upon entry into the host, the EPF develops to produce both mycelium and spores. These structures spread in the whole body of the tick by multiplication, utilizing its circulation system to invade various tissues. This colonization process serves as a pathway for nutrient absorption and establishment within the host [124,127], with virulence factors employed during this stage contributing to the spread of the EPFs within the arthropod’s body, eventually leading to the host’s death. Notably, mycotoxins produced by various EPF species during their growth, including Beauvericin, Beauverolides, Bassiannolide, and Destruxins, play a crucial role as toxic substances targeting ticks [127]. These toxins have the ability to disrupt various functions and structures within the tick’s body, including cellular processes, flaccid paralysis, Malpighian tubes, muscular tissues, and the middle intestine [58]. Beauvericin, a cyclic hexadepsipeptide belonging to the Enniatin (antibiotic) family, has been particularly well studied and is known to be present in various fungal species, including B. bassiana and Fusarium [128,129]. Apart from demonstrating insecticidal efficacy, this toxin also exhibits antiviral, antibacterial, and antifungal activity, being used alongside ketoconazole or miconazole for fungal control [130] and even showing promise against cancer or viral and bacterial infections in humans [131].
Once the host fully succumbs to the infection and the EPFs’ nutrient resources are depleted, the fungus breaks through the host’s outer covering, forms aerial mycelia, and begins the process of sporulation on the cadaver to aid in dispersion of the fungal biomass to infect a new host [25]. EPFs possess pathogenicity or virulence factors that confer them with the ability to target their hosts (e.g., ticks) during this phase, potentially allowing for effective pest control while minimizing harm to beneficial non-target organisms [65]. Nevertheless, EPFs are commonly regarded to lack specificity within certain host families, such as mites, and the extent of their specificity towards ticks has not been conclusively demonstrated [24]. Similarly, whilst specificity has been documented in numerous insect species, including Lymantria dispar, Diprion pini, Dendrolimus pini, Dendrolimus punctatus, Malacosoma disstria, and Fiorinia externa [132], several studies have suggested that even where EPFs specialize in a given species they may retain their capability to infect a wider range of hosts [118]. Within the Hypocreales order, for example, there are frequent observations of host range variations occurring at both species and strain levels, which have been attributed to the influence of environmental factors on EPF virulence characteristics [133]. Additionally, the pathogenic mechanisms of EPFs can also be affected by the host’s immune response and biological traits. A recent study provides evidence for the importance of EPF strain selection, by demonstrating significantly higher mortality rates in R. microplus and Amblyomma mixtum ticks after being exposed to a specific strain of EPFs. The experiment involved treating the ticks across four consecutive cycles, where EPF was used as a biological control agent [18]. Although the molecular and metabolic mechanisms responsible for the fungi’s increased effectiveness are not yet fully understood, the results clearly indicate that EPFs can effectively kill these tick species, especially when the most efficacious strains are used.
4.4. Ovicidal Effects of EPFs on Ticks
Entomopathogenic fungi are known for their ability to infect the eggs of various tick/insect species [134]. One such specific fungus is Purpureocillium lilacinum, which has been found to infect tick eggs and impede the emergence of larvae from them, with the fungus producing enzymes that break down the protective eggshell [135]. Notably, serine protease enzymes secreted by P. lilacinum play a key role in causing structural changes to the tick eggshell. Across several studies, the efficacy of certain isolates of P. lilacinum in significantly hindering the development of tick eggs has been demonstrated [28].
Aside from the enzymatic route, EPFs employ various other mechanisms to parasitize tick eggs. Fungal spores may attach to the surface of tick eggs and penetrate the eggshell, for example, leading to the death of the developing embryos directly [28,136], or disruption of egg development that interferes with the normal development of tick embryos. This can result in the emergence of abnormal or weakened larvae that are less likely to survive, reduced hatchability, and/or delayed hatching of larvae from eggs, thus impacting tick population growth [137]. Importantly, EPFs can persist in the environment and act as a reservoir, infecting ticks and their eggs over time.
Moreover, EPF may even offer the potential to reduce the burden of TBDs by directly targeting the pathogens that the ticks harbor, reducing the vector load within the ticks themselves to limit infection. Few studies seem to have explored this possibility in ticks, though work with mosquitoes suggests that treatment with B. bassiana did not have an influence on levels of malaria parasites within Anopheles stephensi [138]. This perhaps suggests that a more promising role for EPF is to target the vector directly.
4.5. Impact of EPF on Tick’s Immune System
It has been confirmed that the tick immune system is able to respond to infection by EPF. In work by Fiorotti et al. [139], for example, I. ricinus hemocytes were capable of phagocytosing Metarhizium robertsii conidia, protecting ticks against mild infection with this EPF. Studies exploring tick immune responses to EPF are nevertheless limited, and more work in this area could be recommended.
5. Factors Influencing the Effectiveness of EPFs in Tick Control
The effectiveness of EPFs is influenced by a wide range of factors. These factors include both biotic factors, like host range, latency, spore density, and dispersal, along with abiotic factors such as soil properties, temperature, humidity, rainfall, and sunlight. These factors dictate two distinct host ranges of EPFs, i.e., the ecological and physiological host ranges. The ecological host range refers to the infection and survival of EPFs on different host species under natural environmental conditions, whereas the physiological host range is defined by the EPFs’ infection and survival on various species under controlled conditions. Whilst the latter can be confirmed by experimentation under laboratory conditions, exploring the ecological host range poses substantial challenges and remains an area with limited research [133].
5.1. Ticks’ Counter Defenses
Several host-related factors, including the presence of a dark epidermal surface, high host density, and immunity, can influence the infection process of EPFs on ticks [140]. As would be expected, ticks have evolved defense mechanisms to counter fungal infections, which include increasing the production of antifungal compounds and activating innate immune responses, including reactive oxygen molecules, humoral melanization, and phagocytosis. As a result, EPFs intended for use as biocontrol agents must possess mechanisms to effectively overcome these host immune barriers and defense adaptations. Such mechanisms have, however, been observed in B. bassiana, which demonstrates increased levels of superoxide dismutase expression. This enzyme can play a crucial role in countering oxidative stress through detoxification of the extra hydrogen peroxide into water and molecular oxygen, thereby bolstering the fungus’ capacity to endure such conditions [141].
5.2. Environmental Factors
The EPF infection process, particularly the interactions between hosts and fungi, is significantly influenced by abiotic factors in the natural environment [142]. Environmental factors such as soil acidity, soil texture, and the abundance of organic matter, can all have a notable impact on the presence of EPFs, contributing significantly to colonization rates of these organisms on their hosts [140]. Temperature and humidity are also important environmental factors, with high RH levels typically needed to sustain the sporulation of EPFs [143].
Typically, EPFs display optimal spore germination and conidia viability at relative humidities as high as 95.5% and over. However, both Beauveria spp. and Metarhizium spp. have demonstrated the ability to infect their respective hosts even in conditions of low atmospheric humidity within microhabitats [140]. Studies have also consistently revealed that B. bassiana and M. anisopliae isolates exhibit strong growth across a broad temperature range, spanning from 8 to 37 °C. However, according to Abdul Qayyum et al. [140], the most suitable temperature for the germination and growth of EPFs consistently falls between 20 °C and 30 °C, with strains originating from warmer regions tending to perform better at higher temperatures, and vice versa [140].
Unlike B. bassiana, M. anisopliae demonstrates relatively strong tolerance to UV-B radiation. Furthermore, research has unveiled that natural isolates of both Beauveria spp. and Metarhizium spp. surpass non-native strains in terms of solar exposure tolerance and virulence, demonstrating adaption to local conditions from which they were originally isolated [37,144]. This may be an important consideration when deploying commercially formulated EPFs into environments that differ significantly from those found in the culturing/manufacturing facility for the product.
Soil acidity can also be important to EPF development and survival, where both B. bassiana and Metarhizium spp. display peak growth within a pH range of 3 to 9. In particular, mycelium growth thrives at pH 4.4, while pH 6 is ideal for optimal spore production [145]. Interestingly, while these fungi do not require a specific nitrogen source, urea has been found to be highly effective in promoting sporulation, particularly at higher concentrations.
Being living organisms, EPF will perform best within their preferred environmental ranges, with the risk that local climate change effects may render some EPF species unsuited to geographic areas where the limits of these ranges are exceeded. Whilst developments in product formulation technology may help to infer aspects of increased EPF climate resilience, a recent review emphasizes the importance of selection and use of “environmentally competent” strains to mitigate this risk.
6. Challenges and Limitations of Using EPFs Against Ticks
There are a number of issues and restrictions with using EPFs to control ticks that need to be resolved [18]. For one, current tick control methods, such as synthetic acaricides that affect the physiology, reproduction, or survival of ticks, might also exert negative effects on EPFs. Such incompatibility of conventional controls with EPFs has been demonstrated in other Acari and dictates that care must be taken when including EPFs within integrated tick management programs.
The effectiveness of EPFs can depend on the type of tick and its stage of development, where whilst some fungi show increased potency against specific species of ticks, others have little to no effect [24]. Interactions between culturing conditions and the genetic/phenotypic characteristics of the EPFs can also be important here, where Iwanicki et al. [146] concluded that two particular strains of Metarhizium (ESALQ1426 and ESALQ4676) delivered notably high efficacy against arthropods, likely due to their enhanced production of blastospores under the culturing conditions used (increased glucose and accelerated fermentation using pre-cultured, yeast-like cells). This further demonstrates a role for optimizing culturing techniques and EPF manufacturing protocols, where the authors also explored air-dried blastospores efficacy against R. microplus larvae and obtained good results. The importance of strain selection, as also noted earlier, has been confirmed in multiple works considering the worldwide management of ticks through the utilization of various disease-causing agents [62,63], where the value of identifying and culturing optimal fungal strains from the rich global diversity of EPF species is noted.
The environment in which EPFss are used is extremely important to their success [147] where, as previously noted, these fungi are extremely sensitive to heat, humidity, and ultraviolet light (UV-A and UV-B). Environmental parameters that are sub-optimal prevent fungal growth and reduce EPFs capacity to successfully infect and kill ticks [148]. It can, however, be difficult to ensure ideal conditions throughout the entire EPF application process, especially in outdoor settings where ticks are frequently found. In addition, arthropod hosts have also evolved mechanisms to resist infestation by EPFs, with many producing metabolites that lower the efficacy of EPFs [149,150].
The accessibility and availability of EPFs present another restriction to their use. Although there are many commercially available products containing EPF species, there are comparatively few fungal strains and formulations created specifically for controlling ticks [23,151], perhaps reflecting the difficulties in overcoming challenges regarding EPFs’ longevity and persistence in the outdoor environment in which EPFs would need to perform to target these pests. Conversely, relatively more EPF pesticides have been developed for use in controlled conditions (e.g., in glasshouse crops where environmental parameters are carefully managed), where efficacy against pests is also typically achieved by implementing a strategy that only needs to rely on the activity of the EPFs at the point of application, rather than relying on the continued development of successive fungal generations [152]. Within this framework, more than 170 EPF products have been created, drawing from a pool of at least 12 fungal species.
Despite there being an estimated 700 EPF species spanning around 90 genera, the commercially cultivated fungi primarily belong to Beauveria, Metarhizium, Lecanicillium, and Isaria species, likely due to their ease of large-scale production. Rather than expanding the EPF species used in products, the primary focus of many manufacturers has centered on technical aspects of biopesticide development, encompassing mass production, formulation, and the selection of fast-acting strains. Production prerequisites encompass cost-effectiveness, long-term stability, and, crucially, consistent field efficacy. Common methodologies to achieve these goals include generating dispersal units (diaspores) through inducing aerial conidiation on solid growth substrates, cultivating blastospores through yeast-like growth in liquid media, or cultivating hyphal biomass in either liquid or solid mediums [153,154,155]. Even with optimized manufacturing procedures, however, EPFs normally need to be applied repeatedly to achieve acceptable levels of pest control, which can increase the cost of employing these biocontrol agents, both in terms of buying the product and the extra labor needed for regular application.
Potential safety issues related to the use of EPFs must also be considered. Although these fungi are typically thought to be harmless for people and other non-target organisms, specific safety measures must still be taken to reduce any potential cross-species risks, especially for other invertebrates (see earlier). This entails using the right handling, storage, and application procedures to prevent unintentional exposure to people, animals, or beneficial insects [142,156,157,158].
A final challenge is that, depending on a particular situation and scope of use, the general efficacy of EPFs against ticks may vary. A given product may be efficient in restricted locations (e.g., a confined area), while its efficacy might decline when used in expansive landscapes with a variety of tick populations [159]. With this and the numerous other challenges above in mind, the integration of EPFs into existing tick control programs or strategies requires careful coordination and local adaptation to ensure synergistic effects and maximize overall tick population reduction.
In order to help overcome some of the above challenges, research into how to expand the range of biotic and abiotic parameters under which EPFs can perform well would be particularly welcome, seeking to make EPFs efficacy less dependent on temperature, humidity, and other environmental parameters. Similarly, identifying an EPF strain that could target multiple arthropod pest species, such as ticks, mites, mosquitoes, and flies, without affecting beneficial insects and other organisms would be groundbreaking. The identification of EPF strains displaying resistance to tick counter defenses, such as antifungal molecules, would also be worth pursuing to ensure optimum efficacy and impact. Finally, it would be useful to develop cost-effective EPF release devices that are able to sustain their populations and efficacy over a longer period, allowing single “applications” to target multiple stages and/or generations of the tick life cycle.
7. Regulatory Aspects and Guidelines for the Use of EPFs Against Ticks
Due to the growing need for sustainable and eco-friendly alternatives to conventional chemical pesticides, regulatory aspects and recommendations for the use of EPFs against ticks have attracted a lot of attention in recent years. In comparison to synthetic pesticides, utilizing EPFs reduces possible dangers to human health and the environment while providing a promising tick control method [160]. Nevertheless, regulatory frameworks and standard application procedures have been established to guarantee the safe and efficient use of these fungi [161]. Similarly to conventional products, numerous policymakers, governmental organizations, academic institutions, and business representatives are involved in the regulatory process controlling the use of EPFs against ticks [162], from efficacy testing and environmental toxicity testing to registration of products for field use [163], also evaluating consumer safety should fungal spores enter the food chain [164].
One of the key considerations in regulatory guidelines is the selection of appropriate fungal strains [165]. As repeatedly noted above, it is essential to identify and characterize the most effective and host-specific EPF strains for tick control [166], and regulatory bodies typically require extensive scientific data on the taxonomy, virulence, and host range at strain level. This information ensures that the fungi target ticks specifically, without posing a significant threat to non-target organisms [167].
Regulatory guidelines also place a strong emphasis on the creation of uniform formulations and application techniques, with standards for composition, stability, and quality control of fungal products provided [162,168,169], alongside details on application methods, doses, and timing to maximize effectiveness and reduce negative environmental effects [142]. For products to make it to market, consistent and repeatable outcomes in field tests are needed, with these regulations also specifying that handling, application, and disposal information must be included on product labels to support safe and effective use [170,171]. Clear and precise labeling has been noted as being essential to achieving this [172], and these recommendations should also stress any necessity for workers to receive the right training and certification prior to deploying a product [160].
Programs for long-term monitoring and surveillance are essential components of any regulatory framework. Here, the efficiency of EPFs is assessed through routine monitoring in treated locations, which also serves to identify any potential negative impacts on non-target organisms or the surrounding ecosystem [173]. Such monitoring programs also aid in identifying emerging resistance in tick populations and developing appropriate strategies to manage it accordingly [174], facilitating the choice of suitable strains, standardized formulations, and secure application techniques [152]. In short, regulatory processes and procedures already exist to support EPF development and deployment against ticks, where following these rules and recommendations encourages the responsible and sustainable use of EPFs, providing an efficient, sustainable, and safe tick control method.
8. Conclusions
EPFs represent a specialized group of micro-organisms with significant potential as biological control agents against ticks and other arthropods. As the limitations and negative environmental impacts of chemical acaricides become more evident, the demand for sustainable and eco-friendly tick control solutions has grown. EPFs, including species of the genera Beauveria, Metarhizium, Lecanicillium, and Isaria, have demonstrated efficacy in controlling a range of tick species, with numerous examples of their effective use in laboratory and field settings available in the literature, and presented within this review. Developing an improved understanding of the mode of action of EPF will allow researchers to optimize IPM programs for tick control, permitting the selection of strains best suited to deliver safe, high-efficacy control that complements local co-control methods being deployed and minimizes risks to non-target organisms and the wider environment. These fungi employ unique modes of action, infecting and ultimately killing ticks through various mechanisms, where their ability to colonize ticks and persist within their hosts offers a promising avenue for long-term tick management approaches. Research efforts have predominantly focused on understanding the ecological interactions between these fungi and their hosts, including the factors that influence successful colonization, such as inoculation methods and environmental sensitivity.
While commercial applications of EPF are currently limited to a few fungal species, ongoing research is paving the way for more efficient use of existing products as well as the development of new EPF species for commercial use. Ongoing development of mass production techniques and EPF formulations with extended shelf lives could be particularly important for ensuring the accessibility, cost-effectiveness, and residual activity of these products, the latter of which will be especially relevant to ensure efficacy against ticks when deployed in outdoor settings. Development of new EPF strains could also be supported through new and emerging technologies. Advances in molecular sequencing systems, for example, nanopore technologies, are allowing for improved and increasingly affordable/mobile genetic characterization pipelines, which could be used to identify novel EPF strains.
Author Contributions
Conceptulization, writing—original draft, review and editing, M.R.; conceptualization, supervision, writing—review and editing, M.S.S.; writing—review and editing, I.K.D., M.Z. and M.U.; writing original draft, review and editing, B.B.; investigation, writing—original draft and editing O.I.; supervision, writing—review and editing, N.A.R., H.M.R. and M.A.; investigation, conceptualization, supervision, writing—review and editing, investigation, O.A.S.; conceptualization, supervision, writing—review and editing, D.R.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This paper is a part of a Ph.D. research funded through the Higher Education Commission Indigenous Ph.D. fellowship Batch (II) phase (VI) Vide Pin # (520-146103-2AV6-119).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129:3–14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- 2.Colwell D.D., Dantas-Torres F., Otranto D. Vector-borne parasitic zoonoses: Emerging scenarios and new perspectives. Vet. Parasitol. 2011;182:14–21. doi: 10.1016/j.vetpar.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Van Hoy G., Marsh A.E., Carman M.K. Medicine and Surgery of Camelids. Wiley; Hoboken, NJ, USA: 2022. External parasites; pp. 174–223. [Google Scholar]
- 4.Gilbert L. The impacts of climate change on ticks and tick-borne disease risk. Annu. Rev. Entomol. 2021;66:373–388. doi: 10.1146/annurev-ento-052720-094533. [DOI] [PubMed] [Google Scholar]
- 5.Tsao J.I., Hamer S.A., Han S., Sidge J.L., Hickling G.J. The contribution of wildlife hosts to the rise of ticks and tick-borne diseases in North America. J. Med. Entomol. 2021;58:1565–1587. doi: 10.1093/jme/tjab047. [DOI] [PubMed] [Google Scholar]
- 6.Osbrink W.L., Thomas D.B., Lohmeyer K.H., Temeyer K.B. Climate change and alternative hosts complicate the eradication of cattle fever ticks (Acari: Ixodidae) in the southern United States, a review. Ann. Entomol. 2022;115:39–55. doi: 10.1093/aesa/saab034. [DOI] [Google Scholar]
- 7.Shad M., Usman M., Gardner Q.A. Structural-Functional Characterization of Cytochrome b in bc1 and b6 f Complexes along with Polymorphic Analysis. Pak. J. Zool. 2023;55:975. doi: 10.17582/journal.pjz/20220428110433. [DOI] [Google Scholar]
- 8.Raghavan R.K., Koestel Z., Ierardi R., Peterson A.T., Cobos M.E. Climatic suitability of the eastern paralysis tick, Ixodes holocyclus, and its likely geographic distribution in the year 2050. Sci. Rep. 2021;11:15330. doi: 10.1038/s41598-021-94793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunze S., Glock G., Kochmann J., Klimpel S. Ticks on the move climate change induced range shifts of three tick species in Europe: Current and future habitat suitability for Ixodes ricinus in comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol. Res. 2022;121:2241–2252. doi: 10.1007/s00436-022-07556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irshad N., Qayyum M., Hussain M., Khan M.Q. Prevalence of tick infestation and theileriosis in sheep and goats. Pak. Vet. J. 2010;30:178–180. [Google Scholar]
- 11.Nasreen N., Niaz S., Khan A., Ayaz S., Rashid M., Khattak I., Yu Z., Wang T., Al Sarraf M., Ali A. Molecular characterization of ticks infesting livestock in Khyber Pakhtunkhwa Province, Pakistan. Int. J. Acarol. 2020;46:165–170. doi: 10.1080/01647954.2020.1734082. [DOI] [Google Scholar]
- 12.Guglielmone A.A., Nava S., Robbins R.G. Geographic distribution of the hard ticks (Acari: Ixodidae) of the world by countries and territories. Zootaxa. 2023;5251:1–274. doi: 10.11646/zootaxa.5251.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Vanstreels R.E.T., Palma R.L., Mironov S.V. Arthropod parasites of Antarctic and Subantarctic birds and pinnipeds: A review of host-parasite associations. Int. J. Parasitol. Parasites Wildl. 2020;12:275–290. doi: 10.1016/j.ijppaw.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparagano O., George D., Giangaspero A., Špitalská E. Arthropods and associated arthropod-borne diseases transmitted by migrating birds. The case of ticks and tick-borne pathogens. Vet. Parasitol. 2015;213:61–66. doi: 10.1016/j.vetpar.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Sonenshine D.E. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health. 2018;15:478. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiffin H.S., Rajotte E.G., Sakamoto J.M., Machtinger E.T. Tick control in a connected world: Challenges, solutions, and public policy from a United States border perspective. Trop. Med. Infect. 2022;7:388. doi: 10.3390/tropicalmed7110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasirian H. Detailed new insights about tick infestations in domestic ruminant groups: A global systematic review and meta-analysis. J. Parasit. Dis. 2022;46:526–601. doi: 10.1007/s12639-021-01460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso-Díaz M.A., Fernández-Salas A. Entomopathogenic fungi for tick control in cattle livestock from Mexico. Front. Fungal Biol. 2021;2:657694. doi: 10.3389/ffunb.2021.657694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Vivas R.I., Grisi L., Pérez de León A.A., Villela H.S., Torres-Acosta J.F.d.J., Fragoso Sánchez H., Romero Salas D., Rosario Cruz R., Saldierna F., García Carrasco D. Potential economic impact assessment for cattle parasites in Mexico. Review. Rev. Mex. Cienc. Pecu. 2017;8:61–74. doi: 10.22319/rmcp.v8i1.4305. [DOI] [Google Scholar]
- 20.Waldman J., Klafke G.M., Vaz Junior I.d.S. Mechanisms of acaricide resistance in ticks. Acta Sci. Vet. 2023;51:14. doi: 10.22456/1679-9216.128913. [DOI] [Google Scholar]
- 21.Buczek A., Bartosik K., Buczek A.M., Buczek W., Kulina D. Abnormal development of Hyalomma marginatum ticks (Acari: Ixodidae) induced by plant cytotoxic substances. Toxins. 2019;11:445. doi: 10.3390/toxins11080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White A.L., Cumbie A., Brinkerhoff R.J., Hynes W.L., Gaff H.D. Release the hens: A study on the complexities of guinea fowl as tick control. J. Med. Entomol. 2024;61:410–417. doi: 10.1093/jme/tjad167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciano A.F., Mascarin G.M., Franco R.F.F., Golo P.S., Jaronski S.T., Fernandes É.K.K., Bittencourt V.R.E.P. Innovative granular formulation of Metarhizium robertsii microsclerotia and blastospores for cattle tick control. Sci. Rep. 2021;11:4972. doi: 10.1038/s41598-021-84142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes É.K., Bittencourt V.R., Roberts D.W. Perspectives on the potential of entomopathogenic fungi in biological control of ticks. Exp. Parasitol. 2012;130:300–305. doi: 10.1016/j.exppara.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Valero-Jiménez C.A., Wiegers H., Zwaan B.J., Koenraadt C.J., van Kan J.A. Genes involved in virulence of the entomopathogenic fungus Beauveria bassiana. Invertebr. Pathol. 2016;133:41–49. doi: 10.1016/j.jip.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Ostfeld R.S., Price A., Hornbostel V.L., Benjamin M.A., Keesing F. Controlling ticks and tick-borne zoonoses with biological and chemical agents. Bioscience. 2006;56:383–394. doi: 10.1641/0006-3568(2006)056[0383:CTATZW]2.0.CO;2. [DOI] [Google Scholar]
- 27.Fernandes E.K.K., Bittencourt V.R.E.P. Entomopathogenic fungi against South American tick species. Exp. Appl. Acarol. 2008;46:71–93. doi: 10.1007/s10493-008-9161-y. [DOI] [PubMed] [Google Scholar]
- 28.Ebani V.V., Mancianti F. Entomopathogenic fungi and bacteria in a veterinary perspective. Biology. 2021;10:479. doi: 10.3390/biology10060479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesquita E., da Costa D.P., Meirelles L.N., Camargo M.G., Corrêa T.A., Bittencourt V.R.E.P., da Silva Coelho I., Santos H.A., Humber R.A., Golo P.S. Entomopathogenic fungus treatment changes the gut bacterial diversity of Rhipicephalus microplus ticks. Parasite. Vec. 2023;16:185. doi: 10.1186/s13071-023-05790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehner S.A., Kepler R.M. Species limits, phylogeography and reproductive mode in the Metarhizium anisopliae complex. J. Invertebr. Pathol. 2017;148:60–66. doi: 10.1016/j.jip.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 31.St. Leger R. Biological Control: A Global Perspective: Case Studies from Around the World. CAB International; Wallingford, UK: 2007. Genetic modification for improvement of virulence of Metarhizium anisopliae as a microbial insecticide; pp. 328–335. [Google Scholar]
- 32.St. Leger R.J., Wang C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl. Microbiol. Biotechnol. 2010;85:901–907. doi: 10.1007/s00253-009-2306-z. [DOI] [PubMed] [Google Scholar]
- 33.Garrido-Jurado I., Fernández-Bravo M., Campos C., Quesada-Moraga E. Diversity of entomopathogenic Hypocreales in soil and phylloplanes of five Mediterranean cropping systems. J. Invertebr. Pathol. 2015;130:97–106. doi: 10.1016/j.jip.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes É.K.K., da Costa G.L., de Souza E.J., de Moraes A.M.L., Bittencourt V.R.E.P. Beauveria bassiana isolated from engorged females and tested against eggs and larvae of Boophilus microplus (Acari: Ixodidae) J. Basic Microbiol. 2003;43:393–398. doi: 10.1002/jobm.200310263. [DOI] [PubMed] [Google Scholar]
- 35.Prette N., Monteiro A.C., Garcia M.V., Soares V.E. Patogenicidade de isolados de Beauveria bassiana para ovos, larvas e ninfas ingurgitadas de Rhipicephalus sanguineus. Ciênc. Rural. 2005;35:855–861. doi: 10.1590/S0103-84782005000400017. [DOI] [Google Scholar]
- 36.Perinotto W., Angelo I., Golo P., Quinelato S., Camargo M., Sá F., Bittencourt V. Susceptibility of different populations of ticks to entomopathogenic fungi. Exp. Parasitol. 2012;130:257–260. doi: 10.1016/j.exppara.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Cafarchia C., Immediato D., Iatta R., Ramos R.A.N., Lia R.P., Porretta D., Figueredo L.A., Dantas-Torres F., Otranto D. Native strains of Beauveria bassiana for the control of Rhipicephalus sanguineus sensu lato. Parasit. Vec. 2015;8:80. doi: 10.1186/s13071-015-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narasimhan S., Fikrig E. Tick microbiome: The force within. Trends Parasitol. 2015;31:315–323. doi: 10.1016/j.pt.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samish M., Rot A., Ment D., Barel S., Glazer I., Gindin G. Efficacy of the entomopathogenic fungus Metarhizium brunneum in controlling the tick Rhipicephalus annulatus under field conditions. Vet. Parasitol. 2014;206:258–266. doi: 10.1016/j.vetpar.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan C.F., Parker B.L., Davari A., Lee M.R., Kim J.S., Skinner M. Evaluation of spray applications of Metarhizium anisopliae, Metarhizium brunneum and Beauveria bassiana against larval winter ticks, Dermacentor albipictus. Exp. Appl. Acarol. 2020;82:559–570. doi: 10.1007/s10493-020-00547-6. [DOI] [PubMed] [Google Scholar]
- 41.Alonso-Díaz M., Jiménez-Ruíz M., Fernández-Salas A. First Evidence of the Tickicide Effect of Entomopathogenic Fungi Isolated from Mexican Cattle Farms Against Amblyomma mixtum. J. Parasitol. 2022;108:539–544. doi: 10.1645/21-116. [DOI] [PubMed] [Google Scholar]
- 42.Narasimhan S., Rajeevan N., Liu L., Zhao Y.O., Heisig J., Pan J., Eppler-Epstein R., DePonte K., Fish D., Fikrig E. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe. 2014;15:58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aguilar-Díaz H., Quiroz-Castañeda R.E., Cobaxin-Cárdenas M., Salinas-Estrella E., Amaro-Estrada I. Advances in the study of the tick cattle microbiota and the influence on vectorial capacity. Front. Vet. Sci. 2021;8:710352. doi: 10.3389/fvets.2021.710352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet S.I., Pollet T. Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasit. Immunol. 2021;43:e12813. doi: 10.1111/pim.12813. [DOI] [PubMed] [Google Scholar]
- 45.Wu-Chuang A., Hodžić A., Mateos-Hernández L., Estrada-Peña A., Obregon D., Cabezas-Cruz A. Current debates and advances in tick microbiome research. CRPVBD. 2021;1:100036. doi: 10.1016/j.crpvbd.2021.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiesinger A., Wenderlein J., Ulrich S., Hiereth S., Chitimia-Dobler L., Straubinger R.K. Revealing the tick microbiome: Insights into midgut and salivary gland microbiota of female Ixodes ricinus Ticks. Int. J. Mol. Sci. 2023;24:1100. doi: 10.3390/ijms24021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghadamnan E. Characterization of Tick-Associated Microbiota in Spain Using a Nanopore-Based Metabarcoding Approach: Insights into Potential Zoonotic Pathogens. Università Ca’ Foscari Venezia; Venezia, Italy: 2023. [Google Scholar]
- 48.Bava R., Castagna F., Piras C., Musolino V., Lupia C., Palma E., Britti D., Musella V. Entomopathogenic fungi for pests and predators control in beekeeping. Vet. Sci. 2022;9:95. doi: 10.3390/vetsci9020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hajek A., Van Frankenhuyzen K. Microbial Control of Insect and Mite Pests. Elsevier; Amsterdam, The Netherlands: 2017. Use of entomopathogens against forest pests; pp. 313–330. [Google Scholar]
- 50.Quesada-Moraga E., Yousef-Naef M., Garrido-Jurado I. Biopesticides for Sustainable Agriculture. Burleigh Dodds Science Publishing; Sawston, UK: 2020. Advances in the use of entomopathogenic fungi as biopesticides in suppressing crop pests; pp. 63–98. [Google Scholar]
- 51.Ibrahim L., Hamieh A., Ghanem H., Ibrahim S. Pathogenicity of entomopathogenic fungi from Lebanese soils against aphids, whitefly and non-target beneficial insects. Int. J. Agric. Sci. 2011;3:156. [Google Scholar]
- 52.Rodríguez-Vivas R., Quiñones A., Fragoso S. Rodríguez-Vivas RI. Enfermedades de Importancia Económica en Producción Animal. McGraw-Hill; Mexico City, Mexico: 2005. Epidemiología y control de la garrapata Boophilus en México; pp. 571–592. [Google Scholar]
- 53.Weeks E.N., Machtinger E.T., Leemon D., Geden C.J. Pests and Vector-Borne Diseases in the Livestock Industry. Wageningen Academic Publishers; Wageningen, The Netherlands: 2018. Biological control of livestock pests: Entomopathogens; pp. 1142–1147. [DOI] [Google Scholar]
- 54.Barreto L.P., Luz C., Mascarin G.M., Roberts D.W., Arruda W., Fernandes É.K. Effect of heat stress and oil formulation on conidial germination of Metarhizium anisopliae ss on tick cuticle and artificial medium. J. Invertebr. Pathol. 2016;138:94–103. doi: 10.1016/j.jip.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007;17:553–596. doi: 10.1080/09583150701309006. [DOI] [Google Scholar]
- 56.Naranjo-Ortiz M.A., Gabaldón T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019;94:1443–1476. doi: 10.1111/brv.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Couceiro J.d.C., Fatoretto M.B., Demétrio C.G.B., Meyling N.V., Delalibera Jr Í. UV-B radiation tolerance and temperature-dependent activity within the entomopathogenic fungal genus Metarhizium in Brazil. Front. Fungal Biol. 2021;2:645737. doi: 10.3389/ffunb.2021.645737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mora M.A.E., Castilho A.M.C., Fraga M.E. Classification and infection mechanism of entomopathogenic fungi. Arq. Inst. Biol. 2017;84:e0552015. doi: 10.1590/1808-1657000552015. [DOI] [Google Scholar]
- 59.Gindin G., Samish M., Alekseev E., Glazer I. The susceptibility of Boophilus annulatus (Ixodidae) ticks to entomopathogenic fungi. Biocontrol Sci. Technol. 2001;11:111–118. doi: 10.1080/09583150020029790. [DOI] [Google Scholar]
- 60.Samish M., Gindin G., Alekseev E., Glazer I. Pathogenicity of entomopathogenic fungi to different developmental stages of Rhipicephalus sanguineus (Acari: Ixodidae) J. Parasitol. 2001;87:1355–1359. doi: 10.1645/0022-3395(2001)087[1355:POEFTD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 61.Hornbostel V.L., Ostfeld R.S., Zhioua E., Benjamin M.A. Sublethal effects of Metarhizium anisopliae (Deuteromycetes) on engorged larval, nymphal, and adult Ixodes scapularis (Acari: Ixodidae) J. Med. Entomol. 2004;41:922–929. doi: 10.1603/0022-2585-41.5.922. [DOI] [PubMed] [Google Scholar]
- 62.Samish M., Rehacek J. Pathogens and predators of ticks and their potential in biological control. Annu. Rev. Entomol. 1999;44:159–182. doi: 10.1146/annurev.ento.44.1.159. [DOI] [PubMed] [Google Scholar]
- 63.Samish M., Ginsberg H., Glazer I. Biological control of ticks. Parasitology. 2004;129:S389–S403. doi: 10.1017/S0031182004005219. [DOI] [PubMed] [Google Scholar]
- 64.Scholte E.-J., Knols B.G., Samson R.A., Takken W. Entomopathogenic fungi for mosquito control: A review. J. Insect Sci. 2004;4:19. doi: 10.1093/jis/4.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirkland B.H., Westwood G.S., Keyhani N.O. Pathogenicity of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae tick species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis. J. Med. Entomol. 2004;41:705–711. doi: 10.1603/0022-2585-41.4.705. [DOI] [PubMed] [Google Scholar]
- 66.Kaaya G.P., Hassan S. Entomogenous fungi as promising biopesticides for tick control. Exp. Appl. Acarol. 2000;24:913–926. doi: 10.1023/A:1010722914299. [DOI] [PubMed] [Google Scholar]
- 67.Pirali-Kheirabadi K., Haddadzadeh H., Razzaghi-Abyaneh M., Bokaie S., Zare R., Ghazavi M., Shams-Ghahfarokhi M. Biological control of Rhipicephalus (Boophilus) annulatus by different strains of Metarhizium anisopliae, Beauveria bassiana and Lecanicillium psalliotae fungi. Parasitol. Res. 2007;100:1297–1302. doi: 10.1007/s00436-006-0410-x. [DOI] [PubMed] [Google Scholar]
- 68.de Cássia Vieira Paião J., Monteiro A.C., do Nascimento Kronka S. Susceptibility of the cattle tick Boophilus microplus (Acari: Ixodidae) to isolates of the fungus Beauveria bassiana. World J. Microbiol. Biotechnol. 2001;17:245–251. doi: 10.1023/A:1016653700599. [DOI] [Google Scholar]
- 69.Onofre S.B., Miniuk C.M., de Barros N.M., Azevedo J.L. Pathogenicity of four strains of entomopathogenic fungi against the bovine tick Boophilus microplus. Am. J. Vet. Res. 2001;62:1478–1480. doi: 10.2460/ajvr.2001.62.1478. [DOI] [PubMed] [Google Scholar]
- 70.Souza E., Bittencourt V. Evaluation of in vitro effect of the fungi Beauveria bassiana and Metarhizium anisopliae on eggs and larvae of Amblyomma cajennense. Rev. Bras. Parasitai. Vet. 1999;8:127–131. [Google Scholar]
- 71.Carneiro M., Monteiro S., Daemon E., Bittencourt V. Effects of isolate 986 of the fungi Beauveria bassiana (Bals.) Vuill., on eggs of the tick Anocentor nitens (Neumann, 1897) (Acari: Ixodidae) Rev. Bras. Parasitol. Vet. 1999;8:59–62. [Google Scholar]
- 72.Frazzon A.P.G., Junior I.d.S.V., Masuda A., Schrank A., Vainstein M.H. In vitro assessment of Metarhizium anisopliae isolates to control the cattle tick Boophilus microplus. Vet. Parasitol. 2000;94:117–125. doi: 10.1016/S0304-4017(00)00368-X. [DOI] [PubMed] [Google Scholar]
- 73.Kamp Fernandes E.K., da Costa G.L., Lage de Moraes A.M., Pinheiro Bittencourt V.R.E. Entomopathogenic potential of Metarhizium anisopliae isolated from engorged females and tested in eggs and larvae of Boophilus microplus (Acari: Ixodidae) J. Basic Microbiol. Int. J. Biochem. Physiol. Genet. Morphol. Ecol. Microorg. 2004;44:270–274. doi: 10.1002/jobm.200410392. [DOI] [PubMed] [Google Scholar]
- 74.Polar P., Kairo M.T., Moore D., Pegram R., John S.-A. Comparison of water, oils and emulsifiable adjuvant oils as formulating agents for Metarhizium anisopliae for use in control of Boophilus microplus. Mycopathologia. 2005;160:151–157. doi: 10.1007/s11046-005-0120-4. [DOI] [PubMed] [Google Scholar]
- 75.Fernández-Ruvalcaba M., Zhioua E., García-Vázquez Z. Infectivity of Metarhizium anisopliae to susceptible and organophosphate-resistant Boophilus microplus strains. Tec. Pecu. Mex. 2005;43:433–440. [Google Scholar]
- 76.de Melo D.R., Reis R., Bittencourt V. In vitro patogenicity of the fungi Metarhizium anisopliae (Metschnikoff, 1879) Sorokin, 1883, on the tick Boophilus microplus (Canestrini, 1887) Rev. Bras. Parasitol. Vet. = Braz. J. Vet. Parasitol. Orgao Of. Col. Bras. Parasitol. Vet. 2006;15:157–162. [PubMed] [Google Scholar]
- 77.Ojeda-Chi M., Rodriguez-Vivas R., Galindo-Velasco E., Lezama-Gutiérrrez R. Laboratory and field evaluation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for the control of Rhipicephalus microplus (Acari: Ixodidae) in the Mexican tropics. Vet. Parasitol. 2010;170:348–354. doi: 10.1016/j.vetpar.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 78.Adames M., Fernández-Ruvalcaba M., Peña-Chora G., Hernández-Velázquez V.M. Effects of passages through a suitable host of the fungus, Metarhizium anisopliae, on the virulence of acaricide-susceptible and resistant strains of the tick, Rhipicephalus microplus. J. Insect Sci. 2011;11:21. doi: 10.1673/031.011.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perinotto W., Terra A., Angelo I., Fernandes E., Golo P., Camargo M., Bittencourt V. Nomuraea rileyi as biological control agents of Rhipicephalus microplus tick. Parasitol. Res. 2012;111:1743–1748. doi: 10.1007/s00436-012-3018-3. [DOI] [PubMed] [Google Scholar]
- 80.Quinelato S., Golo P.S., Perinotto W.M., Sá F.A., Camargo M.G., Angelo I.C., Moraes A.M., Bittencourt V.R. Virulence potential of Metarhizium anisopliae sl isolates on Rhipicephalus (Boophilus) microplus larvae. Vet. Parasitol. 2012;190:556–565. doi: 10.1016/j.vetpar.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 81.Suleiman E.A., Shigidi M., Hassan S. Metarhizium anisopliae as a biological control agent against Hyalomma anatolicum (Acari: Ixodidae) Pak. J. Biol. Sci. 2013;16:1943–1949. doi: 10.3923/pjbs.2013.1943.1949. [DOI] [PubMed] [Google Scholar]
- 82.Cruz-Avalos A.M., Cruz-Vazquez C., Lezama-Gutie R., Angel-Sahagun C., Vitela-Mendoza I. Selection of isolates of entomopathogenic fungus for control of Rhipicephalus microplus (Acari: Ixodidae) Trop. Subtrop. Agroecosyst. 2015;18:175–180. doi: 10.56369/tsaes.2050. [DOI] [Google Scholar]
- 83.Alcalá-Gómez J., Cruz-Vázquez C., Fernández-Ruvalcaba M., Ángel-Sahagún C., Vitela-Mendoza I., Ramos-Parra M. Virulence of Metarhizium anisopliae and Beauveria bassiana isolates and the effects of fungal infection on the reproduction potential of Rhiphicephalus microplus engorged females. Biocontrol Sci. Technol. 2017;27:931–939. doi: 10.1080/09583157.2017.1366422. [DOI] [Google Scholar]
- 84.Fernández-Salas A., Alonso-Díaz M., Alonso-Morales R., Lezama-Gutiérrez R., Rodríguez-Rodríguez J., Cervantes-Chávez J. Acaricidal activity of Metarhizium anisopliae isolated from paddocks in the Mexican tropics against two populations of the cattle tick Rhipicephalus microplus. Med. Vet. Entomol. 2017;31:36–43. doi: 10.1111/mve.12203. [DOI] [PubMed] [Google Scholar]
- 85.Webster A., Pradel E., Souza U.A., Martins J.R., Reck J., Schrank A., Klafke G. Does the effect of a Metarhizium anisopliae isolate on Rhipicephalus microplus depend on the tick population evaluated? Ticks. Tick. Borne. Dis. 2017;8:270–274. doi: 10.1016/j.ttbdis.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 86.Fernández-Salas A., Alonso-Díaz M.A., Alonso-Morales R.A. Effect of entomopathogenic native fungi from paddock soils against Rhipicephalus microplus larvae with different toxicological behaviors to acaricides. Exp. Parasitol. 2019;204:107729. doi: 10.1016/j.exppara.2019.107729. [DOI] [PubMed] [Google Scholar]
- 87.Fernández-Salas A., Alonso-Díaz M.Á., Morales R.A.A., Lezama-Gutiérrez R., Cervantes-Chávez J.A. Phylogenetic relationships and acaricidal effects of Beauveria bassiana obtained from cattle farm soils against Rhipicephalus microplus. J. Parasitol. 2018;104:275–282. doi: 10.1645/17-162. [DOI] [PubMed] [Google Scholar]
- 88.Dominic D.N., Siah J., Chan B., Nissom P.M. Evaluating the efficacy of oil-based entomopathogenic fungi conidial formulations on dog ticks. Trans. Sci. Technol. 2019;6:42–47. [Google Scholar]
- 89.Szczepańska A., Kiewra D., Plewa-Tutaj K., Dyczko D., Guz-Regner K. Sensitivity of Ixodes ricinus (L., 1758) and Dermacentor reticulatus (Fabr., 1794) ticks to entomopathogenic fungi isolates: Preliminary study. Parasitol. Res. 2020;119:3857–3861. doi: 10.1007/s00436-020-06805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y., Peng Z., He G., Xia Y., Zhao J., Wang H. Isolation and laboratory evaluation of two strains of Aspergillus for controlling Haemaphysalis longicornis (Acari: Ixodidae) Res. Squar. 2023:1–14. doi: 10.21203/rs.3.rs-3022560/v1. [DOI] [Google Scholar]
- 91.Da Paixão F.R.S., Muniz E.R., Catão A.M.L., Santos T.R., Luz C., Marreto R.N., Mascarin G.M., Fernandes É.K.K. Microsclerotial pellets of Metarhizium spp.: Thermotolerance and bioefficacy against the cattle tick. Appl. Microbiol. Biotechnol. 2023;107:2263–2275. doi: 10.1007/s00253-023-12467-7. [DOI] [PubMed] [Google Scholar]
- 92.Meirelles L.N., Mesquita E., Corrêa T.A., Bitencourt R.d.O.B., Oliveira J.L., Fraceto L.F., Camargo M.G., Bittencourt V.R.E.P. Encapsulation of entomopathogenic fungal conidia: Evaluation of stability and control potential of Rhipicephalus microplus. Ticks. Tick. Borne. Dis. 2023;14:102184. doi: 10.1016/j.ttbdis.2023.102184. [DOI] [PubMed] [Google Scholar]
- 93.Sumera N., Iqbal S., Khan S., Rehman Z.u. Fusarium oxysporum; its enhanced entomopathogenic activity with acidic silver nanoparticles against Rhipicephalus microplus ticks. Braz. J. Biol. 2023;84:e266741. doi: 10.1590/1519-6984.266741. [DOI] [PubMed] [Google Scholar]
- 94.Barbieri A., Rico I., Silveira C., Feltrin C., Dall B., Schrank A., Lozina L., Klafke G., Reck J. Field efficacy of Metarhizium anisopliae oil formulations against Rhipicephalus microplus ticks using a cattle spray race. Ticks. Tick. Borne. Dis. 2023;14:102147. doi: 10.1016/j.ttbdis.2023.102147. [DOI] [PubMed] [Google Scholar]
- 95.Zeina G., Ahmed M., Saeed M., Ziena L., Laing M. Field evaluation of Beauveria bassiana (Balsamo) Vuillemin isolates for the biocontrol of Rhipicephalus microplus (Canestrini) ticks on cattle. Exp. Parasitol. 2022;235:108215. doi: 10.1016/j.exppara.2022.108215. [DOI] [PubMed] [Google Scholar]
- 96.Mesquita E., Marciano A.F., Corval A.R., Fiorotti J., Corrêa T.A., Quinelato S., Bittencourt V.R., Golo P.S. Efficacy of a native isolate of the entomopathogenic fungus Metarhizium anisopliae against larval tick outbreaks under semifield conditions. BioControl. 2020;65:353–362. doi: 10.1007/s10526-020-10006-1. [DOI] [Google Scholar]
- 97.Lee M.R., Li D., Lee S.J., Kim J.C., Kim S., Park S.E., Baek S., Shin T.Y., Lee D.-H., Kim J.S. Use of Metarhizum aniopliae sl to control soil-dwelling longhorned tick, Haemaphysalis longicornis. J. Invertebr. Pathol. 2019;166:107230. doi: 10.1016/j.jip.2019.107230. [DOI] [PubMed] [Google Scholar]
- 98.Tofiño Rivera A.P., Ortega Cuadros M., Pedraza Claros B., Perdomo Ayola S.C., Moya Romero D.C. Efectividad de Beauveria bassiana (Baubassil®) sobre la garrapata común del ganado bovino Rhipicephalus microplus en el Departamento de la Guajira, Colombia. Rev. Argent. Microbiol. 2018;50:426–430. doi: 10.1016/j.ram.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 99.Murigu M.M., Nana P., Waruiru R.M., Nga’nga C.J., Ekesi S., Maniania N.K. Laboratory and field evaluation of entomopathogenic fungi for the control of amitraz-resistant and susceptible strains of Rhipicephalus decoloratus. Vet. Parasitol. 2016;225:12–18. doi: 10.1016/j.vetpar.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 100.Camargo M.G., Nogueira M.R., Marciano A.F., Perinotto W.M., Coutinho-Rodrigues C.J., Scott F.B., Angelo I.C., Prata M.C., Bittencourt V.R. Metarhizium anisopliae for controlling Rhipicephalus microplus ticks under field conditions. Vet. Parasitol. 2016;223:38–42. doi: 10.1016/j.vetpar.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 101.González J., Valcárcel F., Pérez-Sánchez J., Tercero-Jaime J., Cutuli M., Olmeda A. Control of Hyalomma lusitanicum (Acari: Ixodidade) ticks infesting Oryctolagus cuniculus (Lagomorpha: Leporidae) using the entomopathogenic fungus Beauveria bassiana (Hyocreales: Clavicipitaceae) in field conditions. J. Med. Entomol. 2016;53:1396–1402. doi: 10.1093/jme/tjw088. [DOI] [PubMed] [Google Scholar]
- 102.Wassermann M., Selzer P., Steidle J.L., Mackenstedt U. Biological control of Ixodes ricinus larvae and nymphs with Metarhizium anisopliae blastospores. Ticks. Tick. Borne. Dis. 2016;7:768–771. doi: 10.1016/j.ttbdis.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 103.Webster A., Reck J., Santi L., Souza U.A., Dall’Agnol B., Klafke G.M., Beys-da-Silva W.O., Martins J.R., Schrank A. Integrated control of an acaricide-resistant strain of the cattle tick Rhipicephalus microplus by applying Metarhizium anisopliae associated with cypermethrin and chlorpyriphos under field conditions. Vet. Parasitol. 2015;207:302–308. doi: 10.1016/j.vetpar.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 104.Rot A., Gindin G., Ment D., Mishoutchenko A., Glazer I., Samish M. On-host control of the brown dog tick Rhipicephalus sanguineus Latreille (Acari: Ixodidae) by Metarhizium brunneum (Hypocreales: Clavicipitaceae) Vet. Parasitol. 2013;193:229–237. doi: 10.1016/j.vetpar.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 105.Bharadwaj A., Stafford I., Kirby C., Behle R.W. Efficacy and environmental persistence of nootkatone for the control of the blacklegged tick (Acari: Ixodidae) in residential landscapes. J. Med. Entomol. 2014;49:1035–1044. doi: 10.1603/ME11251. [DOI] [PubMed] [Google Scholar]
- 106.Kaaya G., Samish M., Hedimbi M., Gindin G., Glazer I. Control of tick populations by spraying Metarhizium anisopliae conidia on cattle under field conditions. Exp. Appl. Acarol. 2011;55:273–281. doi: 10.1007/s10493-011-9471-3. [DOI] [PubMed] [Google Scholar]
- 107.Ángel-Sahagún C.A., Lezama-Gutiérrez R., Molina-Ochoa J., Pescador-Rubio A., Skoda S., Cruz-Vázquez C., Lorenzoni A., Galindo-Velasco E., Fragoso-Sánchez H., Foster J.E. Virulence of Mexican isolates of entomopathogenic fungi (Hypocreales: Clavicipitaceae) upon Rhipicephalus Boophilus microplus (Acari: Ixodidae) larvae and the efficacy of conidia formulations to reduce larval tick density under field conditions. Vet. Parasitol. 2010;170:278–286. doi: 10.1016/j.vetpar.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 108.Stafford K.C., Allan S.A. Field applications of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae F52 (Hypocreales: Clavicipitaceae) for the control of Ixodes scapularis (Acari: Ixodidae) J. Med. Entomol. 2014;47:1107–1115. doi: 10.1603/ME10019. [DOI] [PubMed] [Google Scholar]
- 109.Alonso-Díaz M., García L., Galindo-Velasco E., Lezama-Gutierrez R., Angel-Sahagún C., Rodríguez-Vivas R., Fragoso-Sánchez H. Evaluation of Metarhizium anisopliae (Hyphomycetes) for the control of Boophilus microplus (Acari: Ixodidae) on naturally infested cattle in the Mexican tropics. Vet. Parasitol. 2007;147:336–340. doi: 10.1016/j.vetpar.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 110.Polar P., de Muro M.A., Kairo M.T., Moore D., Pegram R., John S.-A., Roach-Benn C. Thermal characteristics of Metarhizium anisopliae isolates important for the development of biological pesticides for the control of cattle ticks. Vet. Parasitol. 2005;134:159–167. doi: 10.1016/j.vetpar.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 111.Basso L.M.d.S., Monteiro A.C., Belo M.A.d.A., Soares V.E., Garcia M.V., Mochi D.A. Controle de larvas de Boophilus microplus por Metarhizium anisopliae em pastagens infestadas artificialmente. Pesqui. Agropecu. Bras. 2005;40:595–600. doi: 10.1590/S0100-204X2005000600010. [DOI] [Google Scholar]
- 112.Bittencourt V., Bahiense T.C., Fernandes E.K., Souza E. Avaliação da ação in vivo de Metarhizium anisopliae (Metschnikoff, 1879) Sorokin, 1883 aplicado sobre Brachiaria decumbens infestada com larvas de Boophilus microplus (Canestrini, 1887)(Acari: Ixodidae) Rev. Bras. Parasitol. Vet. 2003;12:38–42. [Google Scholar]
- 113.Monteiro S.G., Bahiense T.C., Bittencourt V.R.E.P. Ação do fungo Beauveria bassiana (Balsamo) Vuillemin, 1912 sobre a fase parasitária do carrapato Anocentor nitens (Neumann, 1897) Schulze, 1937 (Acari: Ixodidae) Cienc. Rural. 2003;33:559–563. doi: 10.1590/S0103-84782003000300026. [DOI] [Google Scholar]
- 114.Bittencourt V., Souza E., Costa G., Fagundes A. Evaluation of a formulation of Beauveria bassiana for control of Anocentor nitens; Proceedings of the IV International Conference on Ticks and Tick-Born Pathogens; Banff, AB, Canada. 21–26 July 2002. [Google Scholar]
- 115.Bittencourt V.R.E.P., Mascarenhas A.G., Faccini J.L.H. Mecanismo de infecção do fungo Metarhizium anisopliae no carrapato Boophilus microplus em condições experimentais. Cienc. Rural. 1999;29:351–354. doi: 10.1590/S0103-84781999000200028. [DOI] [Google Scholar]
- 116.Castro A., Bittencourt V., Daemon E., Viegas E.d.C. Eficácia do fungo Metarhizium anisopliae sobre o carrapato Boophilus microplus em teste de estábulo. Rev. Univ. Rural Ser. Cienc. Vida. 1997;19:73–82. [Google Scholar]
- 117.Correia A.d.C., Fiorin A.C., Monteiro A.C., Veríssimo C.J. Effects of Metarhizium anisopliae on the tick Boophilus microplus (Acari: Ixodidae) in stabled cattle. J. Invertebr. Pathol. 1998;71:189–191. doi: 10.1006/jipa.1997.4719. [DOI] [PubMed] [Google Scholar]
- 118.Beys-da-Silva W.O., Rosa R.L., Berger M., Coutinho-Rodrigues C.J., Vainstein M.H., Schrank A., Bittencourt V.R., Santi L. Updating the application of Metarhizium anisopliae to control cattle tick Rhipicephalus microplus (Acari: Ixodidae) Exp. Parasitol. 2020;208:107812. doi: 10.1016/j.exppara.2019.107812. [DOI] [PubMed] [Google Scholar]
- 119.Ortiz-Urquiza A., Keyhani N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects. 2013;4:357–374. doi: 10.3390/insects4030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Skinner M., Parker B.L., Kim J.S. Integrated Pest Management. Academic Press; Cambridge, MA, USA: 2014. Role of entomopathogenic fungi in integrated pest management; pp. 169–191. [DOI] [Google Scholar]
- 121.Gao B.-J., Mou Y.-N., Tong S.-M., Ying S.-H., Feng M.-G. Subtilisin-like Pr1 proteases marking the evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence. 2020;11:365–380. doi: 10.1080/21505594.2020.1749487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santi L., Corrêa A.P.F., Silva L.A., Bresciani F.R., Schrank A., Vainstein M.H. The entomopathogen Metarhizium anisopliae can modulate the secretion of lipolytic enzymes in response to different substrates including components of arthropod cuticle. Fungal Biol. 2010;114:911–916. doi: 10.1016/j.funbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 123.Usman M., Atiq M., Rajput N.A., Sahi S.T., Shad M., Lili N., Iqbal S., Arif A.M., Ahmad U., Khan K.S. Efficacy of Green Synthesized Silver Based Nanomaterials Against Early Blight of Tomato Caused by Alternaria solani. J. Crop Health. 2024;76:105–115. doi: 10.1007/s10343-023-00957-7. [DOI] [Google Scholar]
- 124.Brunner-Mendoza C., Reyes-Montes M.d.R., Moonjely S., Bidochka M.J., Toriello C. A review on the genus Metarhizium as an entomopathogenic microbial biocontrol agent with emphasis on its use and utility in Mexico. Biocontrol Sci. Technol. 2019;29:83–102. doi: 10.1080/09583157.2018.1531111. [DOI] [Google Scholar]
- 125.Semenova T.A., Dunaevsky Y.E., Beljakova G.A., Belozersky M.A. Extracellular peptidases of insect-associated fungi and their possible use in biological control programs and as pathogenicity markers. Fungal Bio. 2020;124:65–72. doi: 10.1016/j.funbio.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 126.Freimoser F.M., Hu G., Leger R.J.S. Variation in gene expression patterns as the insect pathogen Metarhizium anisopliae adapts to different host cuticles or nutrient deprivation in vitro. Microbiology. 2005;151:361–371. doi: 10.1099/mic.0.27560-0. [DOI] [PubMed] [Google Scholar]
- 127.Maina U., Galadima I., Gambo F., Zakaria D. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018;6:27–32. [Google Scholar]
- 128.Hamill R.L., Higgens C., Boaz H., Gorman M. The structure op beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969;10:4255–4258. doi: 10.1016/S0040-4039(01)88668-8. [DOI] [Google Scholar]
- 129.Logrieco A., Moretti A., Castella G., Kostecki M., Golinski P., Ritieni A., Chelkowski J. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 1998;64:3084–3088. doi: 10.1128/AEM.64.8.3084-3088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L., Yan K., Zhang Y., Huang R., Bian J., Zheng C., Sun H., Chen Z., Sun N., An R. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc. Natl. Acad. Sci. USA. 2007;104:4606–4611. doi: 10.1073/pnas.0609370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Q., Xu L. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules. 2012;17:2367–2377. doi: 10.3390/molecules17032367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Augustyniuk-Kram A., Kram K.J. Forest Ecossistems-More Than Just Trees. IntechOpen; Rijeka, Croatia: 2012. Entomopathogenic fungi as an important natural regulator of insect outbreaks in forests; pp. 265–294. [Google Scholar]
- 133.Rohrlich C., Merle I., Mze Hassani I., Verger M., Zuin M., Besse S., Robene I., Nibouche S., Costet L. Variation in physiological host range in three strains of two species of the entomopathogenic fungus Beauveria. PLoS ONE. 2018;13:e0199199. doi: 10.1371/journal.pone.0199199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li M., Lin H., Li S., Xu A., Feng M. Efficiency of entomopathogenic fungi in the control of eggs of the brown planthopper Nilaparvata lugens Stål (Homopera: Delphacidae) Afr. J. Microbiol. Res. 2012;6:7162–7167. [Google Scholar]
- 135.Lin R., Qin F., Shen B., Shi Q., Liu C., Zhang X., Jiao Y., Lu J., Gao Y., Suarez-Fernandez M. Genome and secretome analysis of Pochonia chlamydosporia provide new insight into egg-parasitic mechanisms. Sci. Rep. 2018;8:1123. doi: 10.1038/s41598-018-19169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang L., Shi W.-B., Feng M.-G. Histopathological and molecular insights into the ovicidal activities of two entomopathogenic fungi against two-spotted spider mite. J. Invertebr. Pathol. 2014;117:73–78. doi: 10.1016/j.jip.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 137.Ali A.A.B. Effect of the entomopathogenic fungus Beauveria bassiana (Balsamo) on eggs and eclosing larvae of the tick Argas (Persicargas) persicus (Oken) Vet. Parasitol. 2022;306:109714. doi: 10.1016/j.vetpar.2022.109714. [DOI] [PubMed] [Google Scholar]
- 138.Heinig R.L., Thomas M.B. Interactions between a fungal entomopathogen and malaria parasites within a mosquito vector. Malar J. 2015;14:22. doi: 10.1186/s12936-014-0526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fiorotti J., Urbanová V., Gôlo P.S., Bittencourt V.R.E.P., Kopáček P. The role of complement in the tick cellular immune defense against the entomopathogenic fungus Metarhizium robertsii. Develop. Compa. Immunol. 2022;126:104234. doi: 10.1016/j.dci.2021.104234. [DOI] [PubMed] [Google Scholar]
- 140.Abdul Qayyum M., Bilal H., Ali H., Raza H., Wajid M. Factors affecting the epizootics of entomopathogenic fungi-A review. J. Bioresour. Manag. 2021;8:5. doi: 10.35691/JBM.1202.0204. [DOI] [Google Scholar]
- 141.Zibaee A., Malagoli D. Immune response of Chilo suppressalis Walker (Lepidoptera: Crambidae) larvae to different entomopathogenic fungi. Bull. Entom. Res. Lond. 2014;104:155–163. doi: 10.1017/S0007485313000588. [DOI] [PubMed] [Google Scholar]
- 142.Islam W., Adnan M., Shabbir A., Naveed H., Abubakar Y.S., Qasim M., Tayyab M., Noman A., Nisar M.S., Khan K.A. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021;159:105122. doi: 10.1016/j.micpath.2021.105122. [DOI] [PubMed] [Google Scholar]
- 143.Steenberg T., Kilpinen O. Synergistic interaction between the fungus Beauveria bassiana and desiccant dusts applied against poultry red mites (Dermanyssus gallinae) Exp. Appl. Acarol. 2014;62:511–524. doi: 10.1007/s10493-013-9757-8. [DOI] [PubMed] [Google Scholar]
- 144.Figueroa L.B.P., Ferreira J.M., Mamani R.C.C., de Freitas Soares F.E. Entomopathogenic Fungi: Prospects and Challenges. Springer Nature; Singapore: 2024. Biochemistry, Pathogenesis, and Parasitism of Beauveria; pp. 227–245. [Google Scholar]
- 145.Teng C. Studies on the biology of Beauveria bassiana (Bals.) Vuill. with reference to microbial control of insect pests. Acta Bot. Sin. 1962;10:69–72. [Google Scholar]
- 146.Iwanicki N.S.A., de Oliveira Ferreira B., Mascarin G.M., Júnior Í.D. Modified Adamek’s medium renders high yields of Metarhizium robertsii blastospores that are desiccation tolerant and infective to cattle-tick larvae. Fungal Biol. 2018;122:883–890. doi: 10.1016/j.funbio.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 147.Ekesi S., Dimbi S., Maniania N. Use of Entomopathogenic Fungi in Biological Pest Management. CAB International; Wallingford, UK: 2007. The role of entomopathogenic fungi in the integrated management of fruit flies (Diptera: Tephritidae) with emphasis on species occurring in Africa; pp. 239–274. [Google Scholar]
- 148.Fernandes É.K., Rangel D.E., Braga G.U., Roberts D.W. Tolerance of entomopathogenic fungi to ultraviolet radiation: A review on screening of strains and their formulation. Curr. Genet. 2015;61:427–440. doi: 10.1007/s00294-015-0492-z. [DOI] [PubMed] [Google Scholar]
- 149.Ignoffo C. Environmental factors affecting persistence of entomopathogens. Fla. Entomol. 1992;75:516–525. doi: 10.2307/3496133. [DOI] [Google Scholar]
- 150.Moore D., Prior C. The potential of mycoinsecticides. Biocon. News. Inform. 1993;14:31–40. [Google Scholar]
- 151.Eisen L., Stafford III K.C. Barriers to effective tick management and tick-bite prevention in the United States (Acari: Ixodidae) J. Med. Entomol. 2021;58:1588–1600. doi: 10.1093/jme/tjaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Faria M.R., Wraight S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007;43:237–256. doi: 10.1016/j.biocontrol.2007.08.001. [DOI] [Google Scholar]
- 153.Vega F.E., Goettel M.S., Blackwell M., Chandler D., Jackson M.A., Keller S., Koike M., Maniania N.K., Monzón A., Ownley B.H. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009;2:149–159. doi: 10.1016/j.funeco.2009.05.001. [DOI] [Google Scholar]
- 154.Rego R.O., Trentelman J.J., Anguita J., Nijhof A.M., Sprong H., Klempa B., Hajdusek O., Tomás-Cortázar J., Azagi T., Strnad M. Counterattacking the tick bite: Towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit. Vec. 2019;12:229. doi: 10.1186/s13071-019-3468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mitton G.A., Meroi Arcerito F., Cooley H., Fernandez de Landa G., Eguaras M.J., Ruffinengo S.R., Maggi M.D. More than sixty years living with Varroa destructor: A review of acaricide resistance. Int. J. Pest Manag. 2022:1–18. doi: 10.1080/09670874.2022.2094489. [DOI] [Google Scholar]
- 156.Srivastava C.N., Maurya P., Sharma P., Mohan L. Prospective role of insecticides of fungal origin. Entomol. Res. 2009;39:341–355. doi: 10.1111/j.1748-5967.2009.00244.x. [DOI] [Google Scholar]
- 157.Vukicevich E., Lowery T., Bowen P., Úrbez-Torres J.R., Hart M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016;36:48. doi: 10.1007/s13593-016-0385-7. [DOI] [Google Scholar]
- 158.Fischhoff I.R., Keesing F., Ostfeld R.S. The tick biocontrol agent Metarhizium brunneum (M. anisopliae)(strain F52) does not reduce non-target arthropods. PLoS ONE. 2017;12:e0187675. doi: 10.1371/journal.pone.0187675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goettel M., Glare T. Insect Control: Biological and Synthetic Agents. CAB International; Wallingford, UK: 2010. 11 Entomopathogenic fungi and their role in regulation of insect populations. [Google Scholar]
- 160.Tuininga A.R., Miller J.L., Morath S.U., Daniels T.J., Falco R.C., Marchese M., Sahabi S., Rosa D., Stafford K.C. Isolation of entomopathogenic fungi from soils and Ixodes scapularis (Acari: Ixodidae) ticks: Prevalence and methods. J. Med. Entomol. 2014;46:557–565. doi: 10.1603/033.046.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hadi J., Brightwell G. Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods. 2021;10:1226. doi: 10.3390/foods10061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lamichhane J.R., Bischoff-Schaefer M., Bluemel S., Dachbrodt-Saaydeh S., Dreux L., Jansen J.P., Kiss J., Köhl J., Kudsk P., Malausa T. Identifying obstacles and ranking common biological control research priorities for Europe to manage most economically important pests in arable, vegetable and perennial crops. Pest Manag. Sci. 2017;73:14–21. doi: 10.1002/ps.4423. [DOI] [PubMed] [Google Scholar]
- 163.Kalia A., Gosal S. Effect of pesticide application on soil microorganisms. Arch. Agron. Soil Sci. 2011;57:569–596. doi: 10.1080/03650341003787582. [DOI] [Google Scholar]
- 164.Akutse K.S., Subramanian S., Maniania N., Dubois T., Ekesi S. Biopesticide research and product development in Africa for sustainable agriculture and food security–Experiences from the International Centre of Insect Physiology and Ecology (icipe) Front. Sustain. Food Syst. 2020;4:563016. doi: 10.3389/fsufs.2020.563016. [DOI] [Google Scholar]
- 165.Marchiondo A., Holdsworth P., Fourie L., Rugg D., Hellmann K., Snyder D., Dryden M. World Association for the Advancement of Veterinary Parasitology (WAAVP): Guidelines for evaluating the efficacy of parasiticides for the treatment, prevention and control of flea and tick infestations on dogs and cats. Vet. Parasitol. 2013;194:84–97. doi: 10.1016/j.vetpar.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 166.Brooks C.S., Hefty P.S., Jolliff S.E., Akins D.R. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 2003;71:3371–3383. doi: 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lorenz S.-C. Ph.D. Thesis. University of Bielefeld; Bielefeld, Germany: 2019. Development of a Biological Tick Control Agent Based on an Innovative Attract–and–Kill Strategy. [Google Scholar]
- 168.Horowitz R., Richards A., DuLaney M., Ericson M., Green C., Lubelczyk C., Munderloh U., Nicolson G., Paddock C., Perdue S. Report of the Other Tick-Borne Diseases and Co-Infections Subcommittee to the Tick-Borne Disease Working Group. US Department of Health and Human Services, Tick-Borne Disease Working Group; Washington, DC, USA: 2020. [Google Scholar]
- 169.Sullivan C.F., Parker B.L., Skinner M. A review of commercial Metarhizium and Beauveria based biopesticides for the biological control of ticks in the USA. Insects. 2022;13:260. doi: 10.3390/insects13030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pasupathi P., Sindhu S., Ponnusha B.S., Ambika A. Biomedical waste management for health care industry. Int. J. Biol. Med. Res. 2011;2:472–486. [Google Scholar]
- 171.Stull J.W., Bjorvik E., Bub J., Dvorak G., Petersen C., Troyer H.L. 2018 AAHA infection control, prevention, and biosecurity guidelines. J. Am. Anim. Hosp. Assoc. 2018;54:297–326. doi: 10.5326/JAAHA-MS-6903. [DOI] [PubMed] [Google Scholar]
- 172.Marucheck A., Greis N., Mena C., Cai L. Product safety and security in the global supply chain: Issues, challenges and research opportunities. J. Oper. Manag. 2011;29:707–720. doi: 10.1016/j.jom.2011.06.007. [DOI] [Google Scholar]
- 173.Cranford P.J., Kamermans P., Krause G., Mazurié J., Buck B.H., Dolmer P., Fraser D., Van Nieuwenhove K., Francis X., Sanchez-Mata A. An ecosystem-based approach and management framework for the integrated evaluation of bivalve aquaculture impacts. Aquac. Environ. Interact. 2012;2:193–213. doi: 10.3354/aei00040. [DOI] [Google Scholar]
- 174.Langwig K.E., Voyles J., Wilber M.Q., Frick W.F., Murray K.A., Bolker B.M., Collins J.P., Cheng T.L., Fisher M.C., Hoyt J.R. Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ. 2015;13:195–202. doi: 10.1890/140241. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.