Abstract
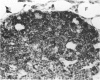
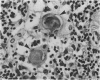
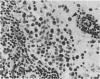
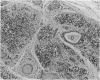
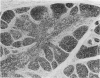
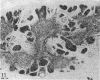
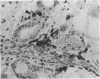
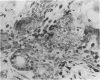
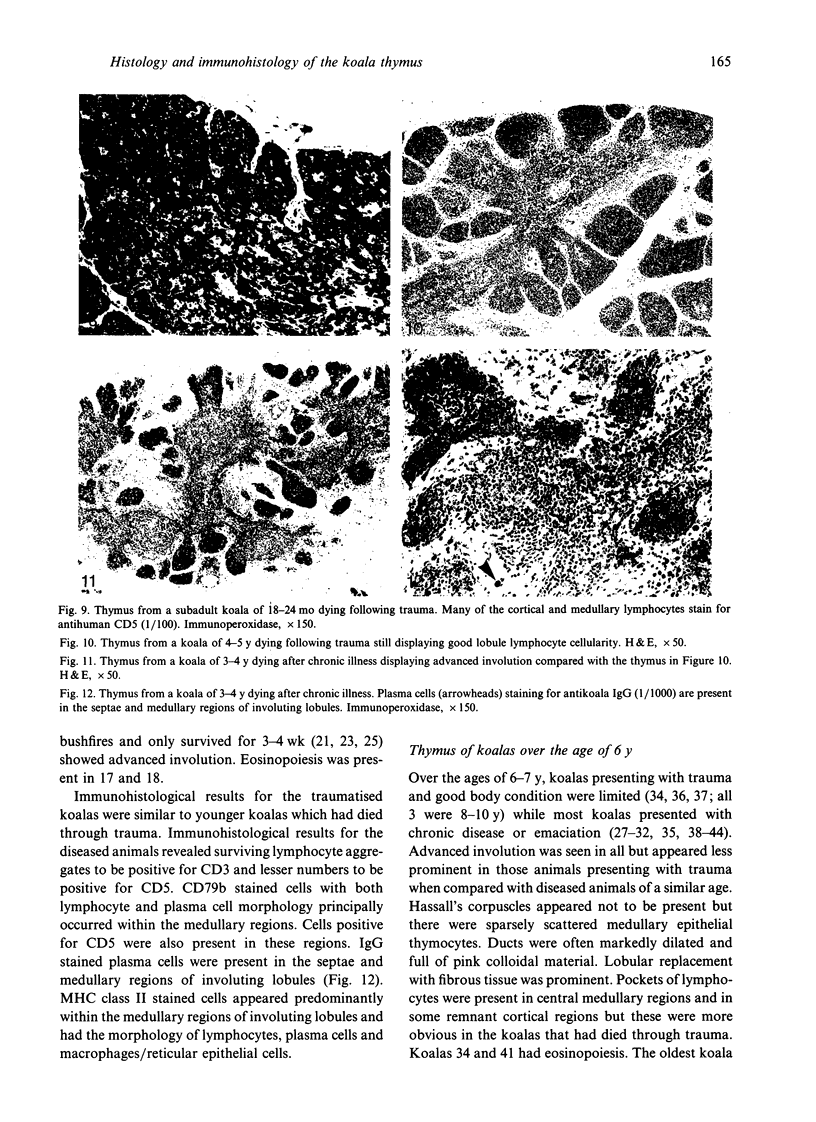
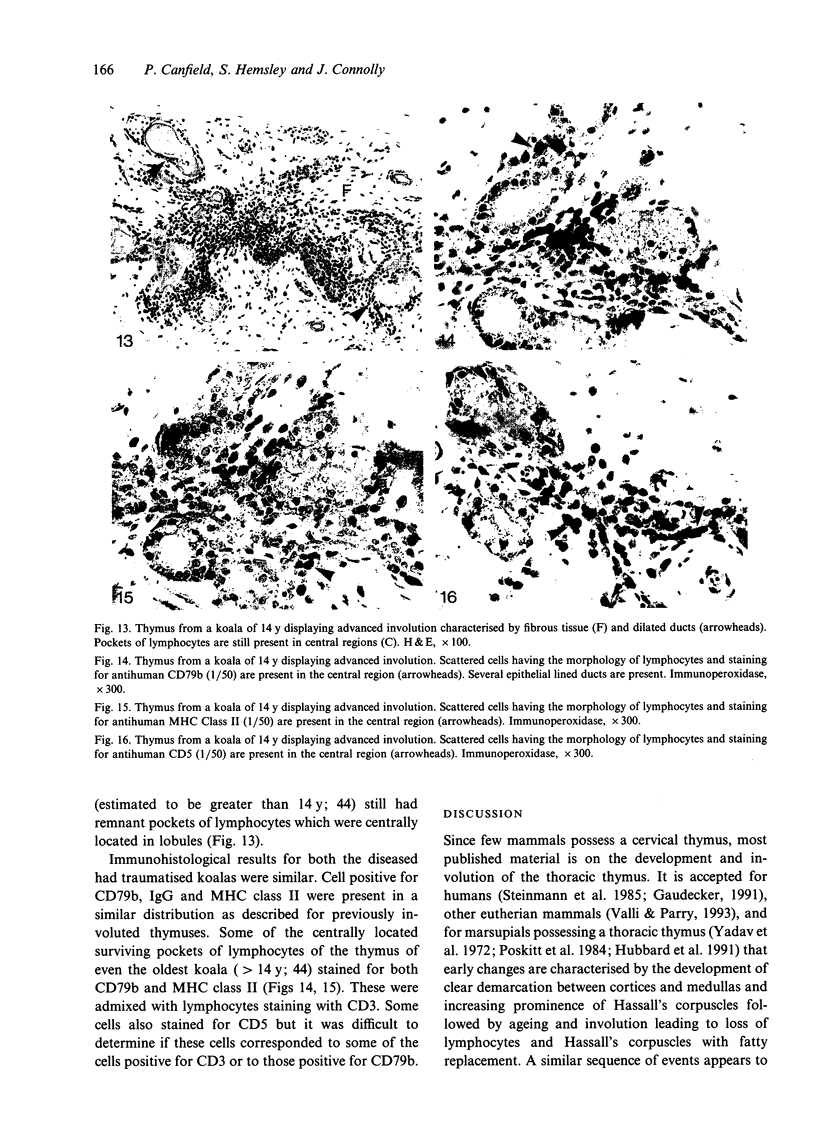
The thymuses of 44 koalas, ranging from less than 30 d to more than 14 y of age, were examined histologically and immunohistologically. The thymuses from 17 of these koalas dying acutely through trauma were regarded as not being significantly affected by disease and formed the basis for study of the normal thymus. Most other koalas had chronic illness and, consequently, disease affected (involuted) thymuses. Histologically, thymuses showed obvious corticomedullary differentiation with small Hassall's corpuscles visible in koalas more than 8 mo of age. Most cortical and medullary lymphocytes stained for CD3 and CD5 (T lymphocyte markers) while some cells (predominantly medullary) stained for CD79b (B lymphocytes and plasma cells), IgG (plasma cells) or MHC class II (reticular epithelium, macrophages and possibly lymphocytes). Adults of up to 5-6 y of age which had died through trauma had little evidence of involution and had prominent Hassall's corpuscles and medullary epithelial thymocytes. Thymic eosinopoiesis was an inconsistent finding. In traumatised animals over this age, involution was obvious with fibrous replacement of lobules, loss of Hassall's corpuscles and the development of dilated ducts lined by nonciliated epithelium. However, loss of lymphocytes was gradual and pockets of lymphocytes, centrally located in lobules, were still present in the oldest koala examined. In these involuted thymuses, remaining lymphocytes stained for CD3 and lesser numbers of CD5 and CD79b. Plasma cells were common and often stained both for IgG and MHC class II. Thymuses of chronically diseased koalas showed accelerated involution when age matched with thymuses from traumatised koalas. Chronically ill koalas as young as 18-24 mo showed advanced involution, but the morphological and immunohistological characteristics of involuted thymus from diseased koalas could not be distinguished from those of involuted thymuses derived from traumatised koalas. It was concluded that development of the koala thymus is completed at 8 mo of age and that for normal koalas involution is a gradual process which starts not at but after sexual maturity. Immunohistological characterisation of the thymus was comparable to that reported for a variety of eutherian mammals.
Full text
PDF

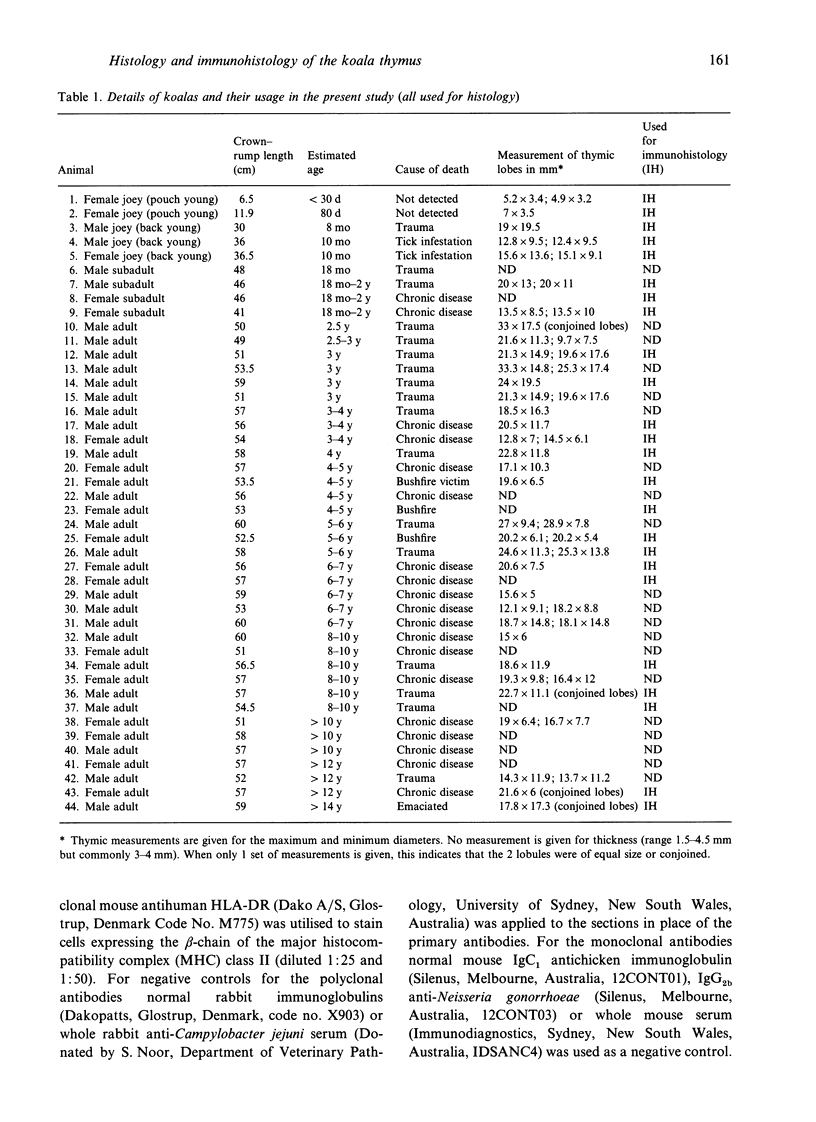




Images in this article
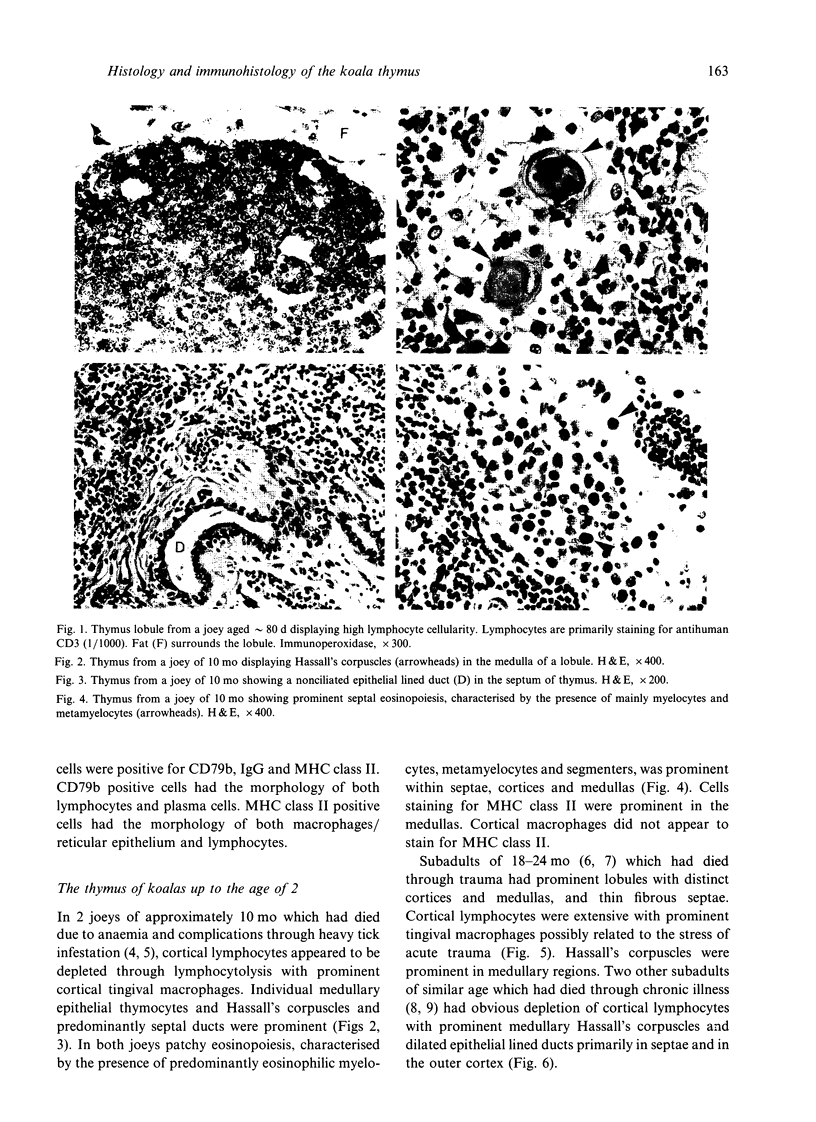
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B., Papadimitriou J. M. Development of lymphoid tissue in a marsupial, Setonix brachyurus (quokka). Acta Anat (Basel) 1975;91(4):594–611. [PubMed] [Google Scholar]
- Bodey B., Calvo W., Prummer O., Fliedner T. M., Borysenko M. Development and histogenesis of the thymus in dog. A light and electron microscopical study. Dev Comp Immunol. 1987 Winter;11(1):227–238. doi: 10.1016/0145-305x(87)90023-1. [DOI] [PubMed] [Google Scholar]
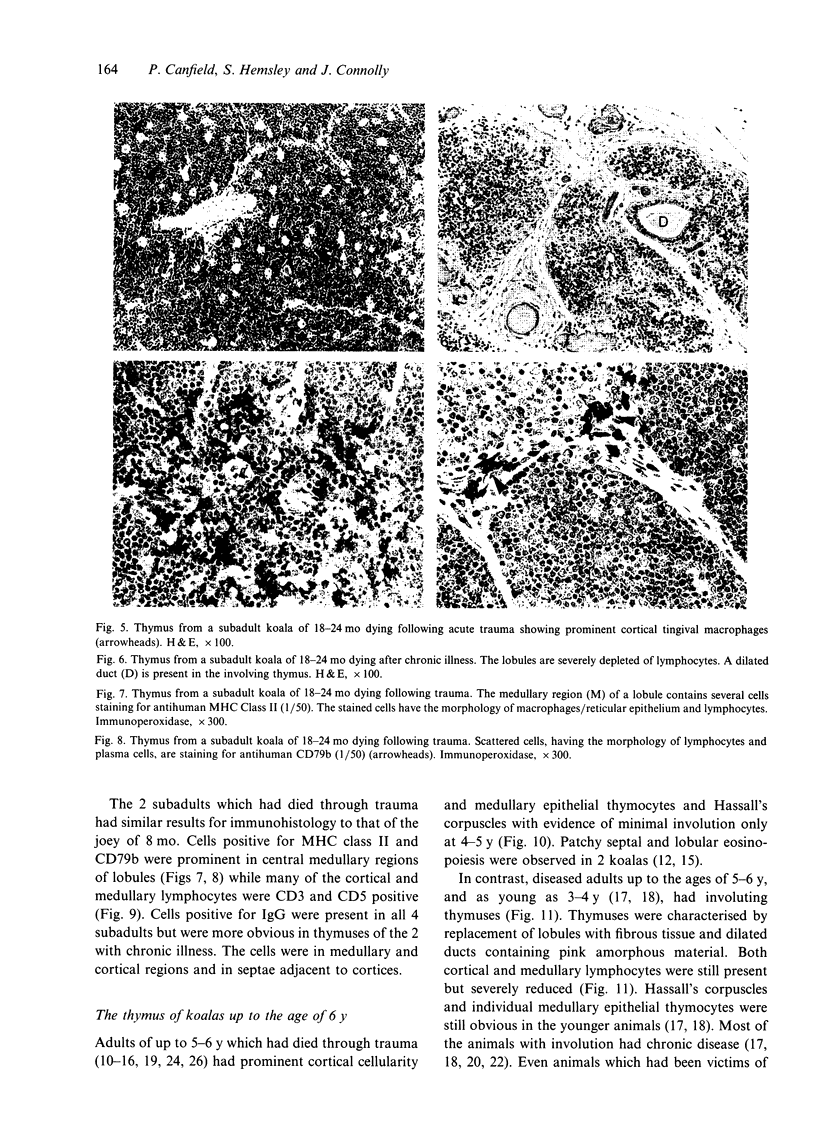
- Coutinho H. B., King G., Sewell H. F., Tighe P., Coutinho V. B., Robalinho T. I., Carvalho A. B. Immunocytochemical study of Peyer's patches follicular-associated epithelium in the marsupial, Didelphis albiventris. Dev Comp Immunol. 1993 Nov-Dec;17(6):537–548. doi: 10.1016/s0145-305x(05)80009-6. [DOI] [PubMed] [Google Scholar]
- Coutinho H. B., Nogueira J. C., King G., Coutinho V. B., Robalinho T. I., Amorim A. M., Cavalcanti V. M., Robins R. A., Sewell H. F. Immunocytochemical study of the ontogeny of Peyer's patches in the Brazilian marsupial Didelphis albiventris. J Anat. 1994 Oct;185(Pt 2):347–354. [PMC free article] [PubMed] [Google Scholar]
- Coutinho H. B., Sewell H. F., Tighe P., King G., Nogueira J. C., Robalinho T. I., Coutinho V. B., Cavalcanti V. M. Immunocytochemical study of the ontogeny of the marsupial Didelphis albiventris immune system. J Anat. 1995 Aug;187(Pt 1):37–46. [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Heinly C. S. Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med. 1995 Apr 1;181(4):1445–1458. doi: 10.1084/jem.181.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley S. W., Canfield P. J., Husband A. J. Immunohistological staining of lymphoid tissue in four Australian marsupial species using species cross-reactive antibodies. Immunol Cell Biol. 1995 Aug;73(4):321–325. doi: 10.1038/icb.1995.49. [DOI] [PubMed] [Google Scholar]
- Hubbard G. B., Saphire D. G., Hackleman S. M., Silva M. V., Vandeberg J. L., Stone W. H. Ontogeny of the thymus gland of a marsupial (Monodelphis domestica). Lab Anim Sci. 1991 Jun;41(3):227–232. [PubMed] [Google Scholar]
- Ishino S., Kadota K., Matsubara Y., Agawa H., Matsui N. Immunohistochemical studies on ontogeny of bovine lymphoid tissues. J Vet Med Sci. 1991 Oct;53(5):877–882. doi: 10.1292/jvms.53.877. [DOI] [PubMed] [Google Scholar]
- Jones M., Cordell J. L., Beyers A. D., Tse A. G., Mason D. Y. Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. J Immunol. 1993 Jun 15;150(12):5429–5435. [PubMed] [Google Scholar]
- McKenzie L. M., Cooper D. W. Low MHC class II variability in a marsupial. Reprod Fertil Dev. 1994;6(6):721–726. doi: 10.1071/rd9940721. [DOI] [PubMed] [Google Scholar]
- Nango K., Inaba M., Inaba K., Adachi Y., Than S., Ishida T., Kumamoto T., Uyama M., Ikehara S. Ontogeny of thymic B cells in normal mice. Cell Immunol. 1991 Mar;133(1):109–115. doi: 10.1016/0008-8749(91)90183-c. [DOI] [PubMed] [Google Scholar]
- Poskittt D. C., Barnett J., Duffey K., Kimpton W. G., Muller H. K. Involution of the thymus in marsupial mice. Dev Comp Immunol. 1984 Spring;8(2):483–488. doi: 10.1016/0145-305x(84)90056-9. [DOI] [PubMed] [Google Scholar]
- Simpson J. G., Gray E. S., Beck J. S. Age involution in the normal human adult thymus. Clin Exp Immunol. 1975 Feb;19(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Slade R. W., Hale P. T., Francis D. I., Graves J. A., Sturm R. A. The marsupial MHC: the tammar wallaby, Macropus eugenii, contains an expressed DNA-like gene on chromosome 1. J Mol Evol. 1994 May;38(5):496–505. doi: 10.1007/BF00178850. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Ossa-Gomez L. J. A quantitative histologic comparison of the thymus in 100 healthy and diseased adults. Am J Clin Pathol. 1981 Nov;76(5):657–665. doi: 10.1093/ajcp/76.5.657. [DOI] [PubMed] [Google Scholar]
- Stanley N. F., Yadav M., Waring H., Eadie M. The effect of thymectomy on response to various antigens of a marsupial Setonix brachyurus (Quokka). Aust J Exp Biol Med Sci. 1972 Dec;50(6):689–702. doi: 10.1038/icb.1972.62. [DOI] [PubMed] [Google Scholar]
- Steinmann G. G., Klaus B., Müller-Hermelink H. K. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985 Nov;22(5):563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Symington J. Note on the Thymus Gland in the Koala (Phascolarctus cinereus). J Anat Physiol. 1900 Jan;34(Pt 2):226–227. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R., Kotlarski I., Barton M. Further characterisation of the immune response of the koala. Vet Immunol Immunopathol. 1994 Apr;40(4):325–339. doi: 10.1016/0165-2427(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Yadav M., Papadimitriou J. M. The ultrastructure of the neonatal thymus of a marsupial, Setonix brachyurus. Aust J Exp Biol Med Sci. 1969 Dec;47(6):653–668. doi: 10.1038/icb.1969.163. [DOI] [PubMed] [Google Scholar]
- Yadav M., Stanley N. F., Waring H. The thymus glands of a marsupial, Setonix brachyurus (quokka), and their role in immune responses. Structure and growth of the thymus glands. Aust J Exp Biol Med Sci. 1972 Jun;50(3):347–356. doi: 10.1038/icb.1972.28. [DOI] [PubMed] [Google Scholar]
- von Gaudecker B. Functional histology of the human thymus. Anat Embryol (Berl) 1991;183(1):1–15. doi: 10.1007/BF00185830. [DOI] [PubMed] [Google Scholar]