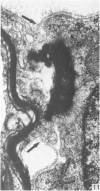
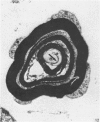
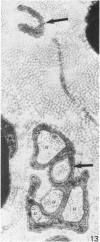
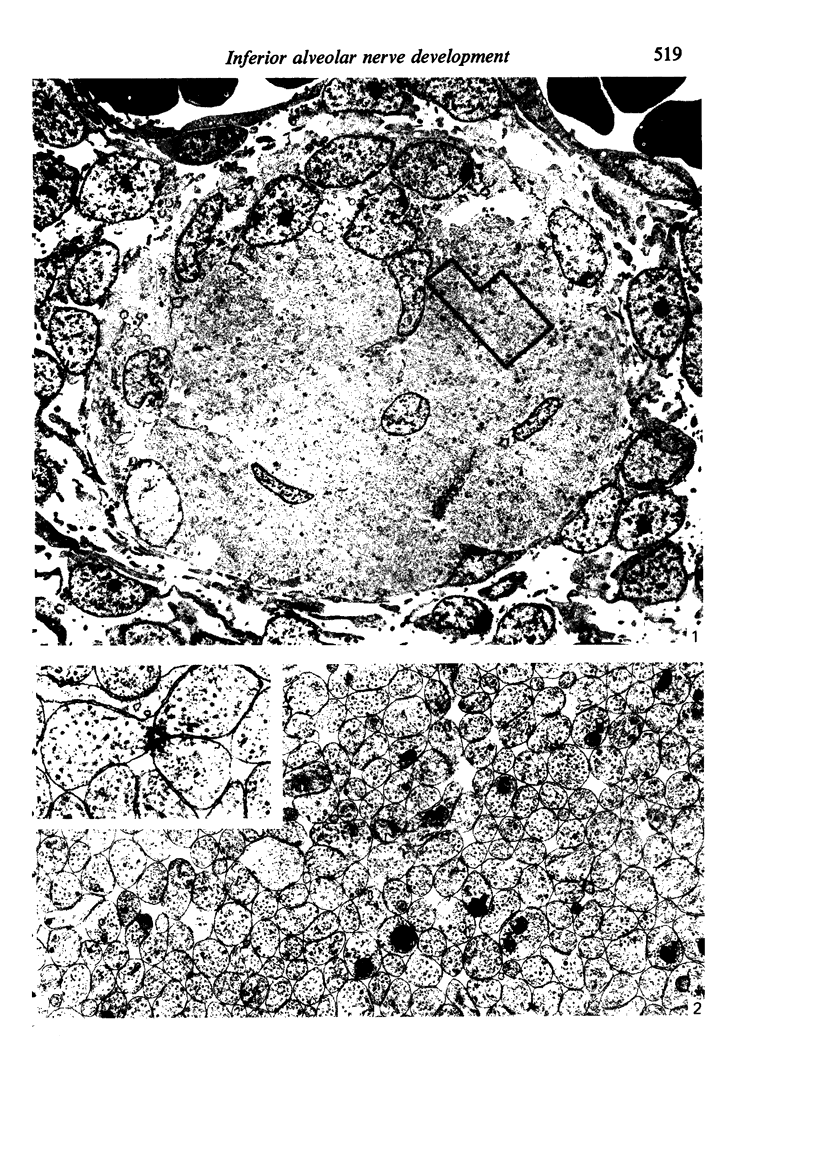
Abstract
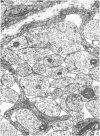
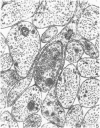
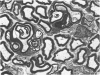
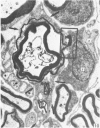
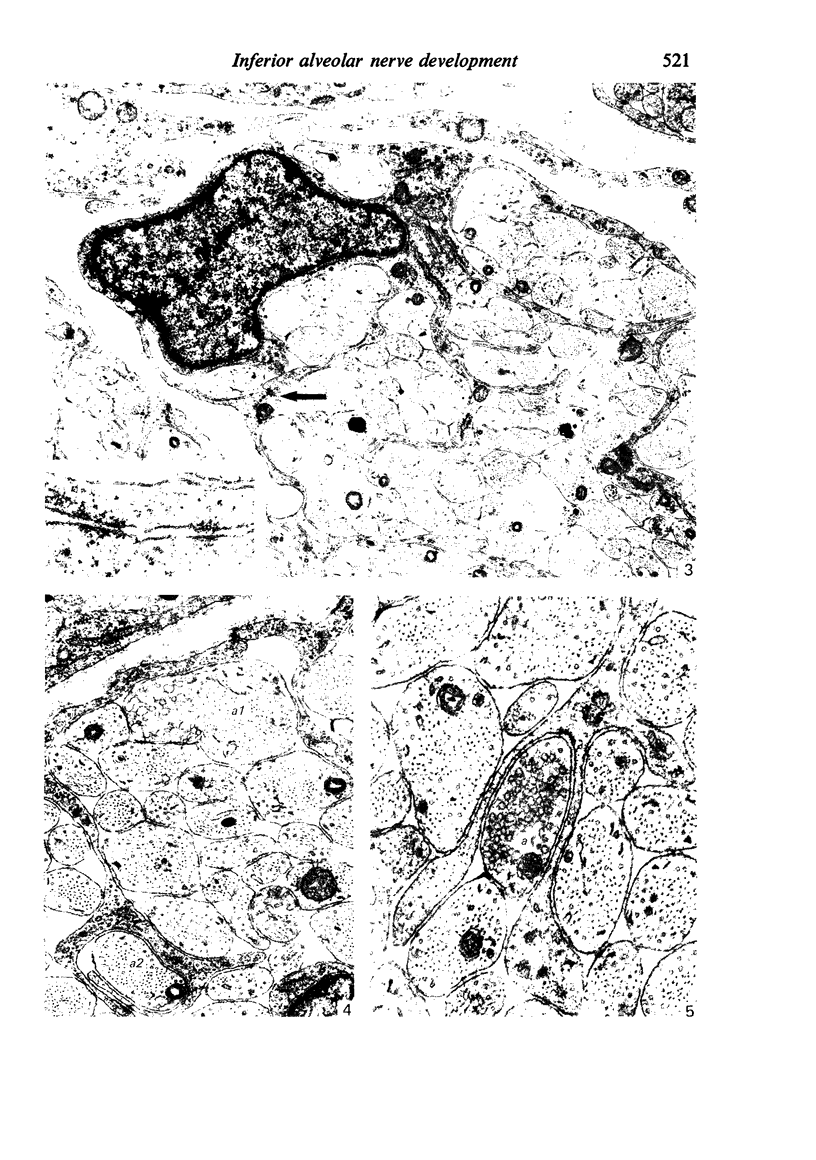
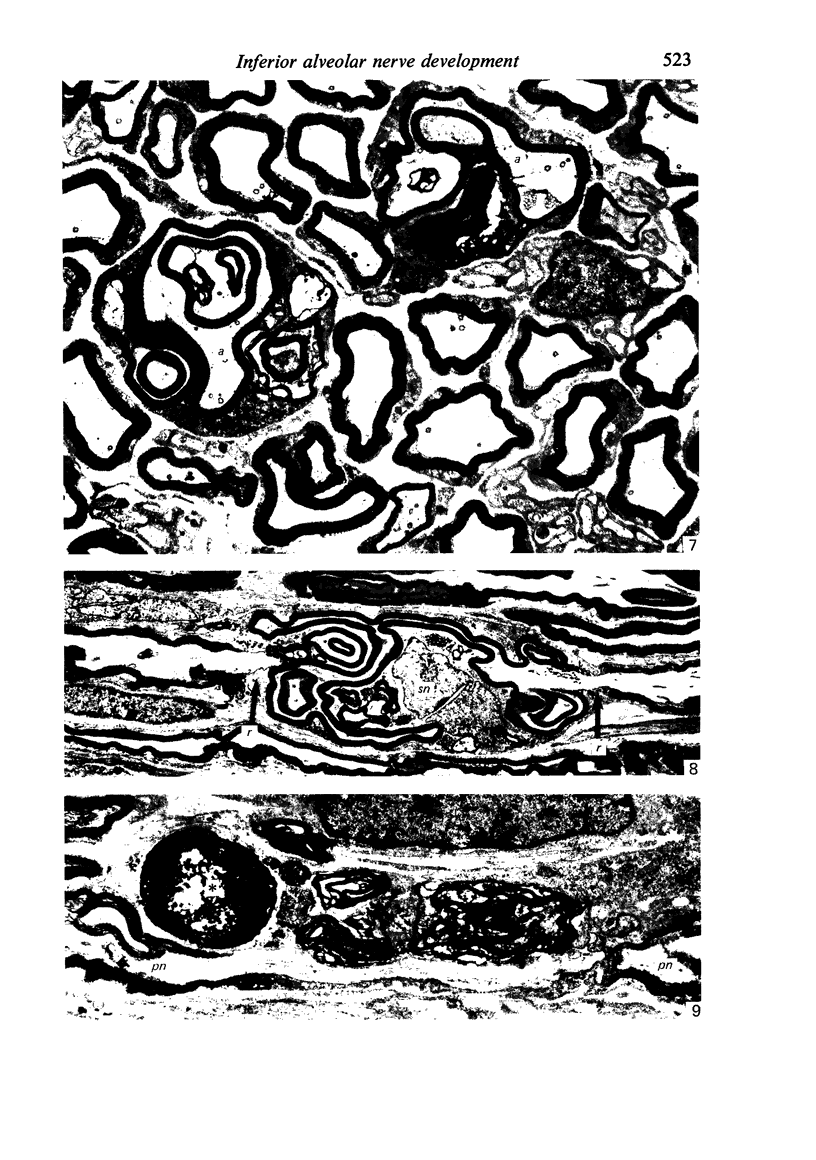
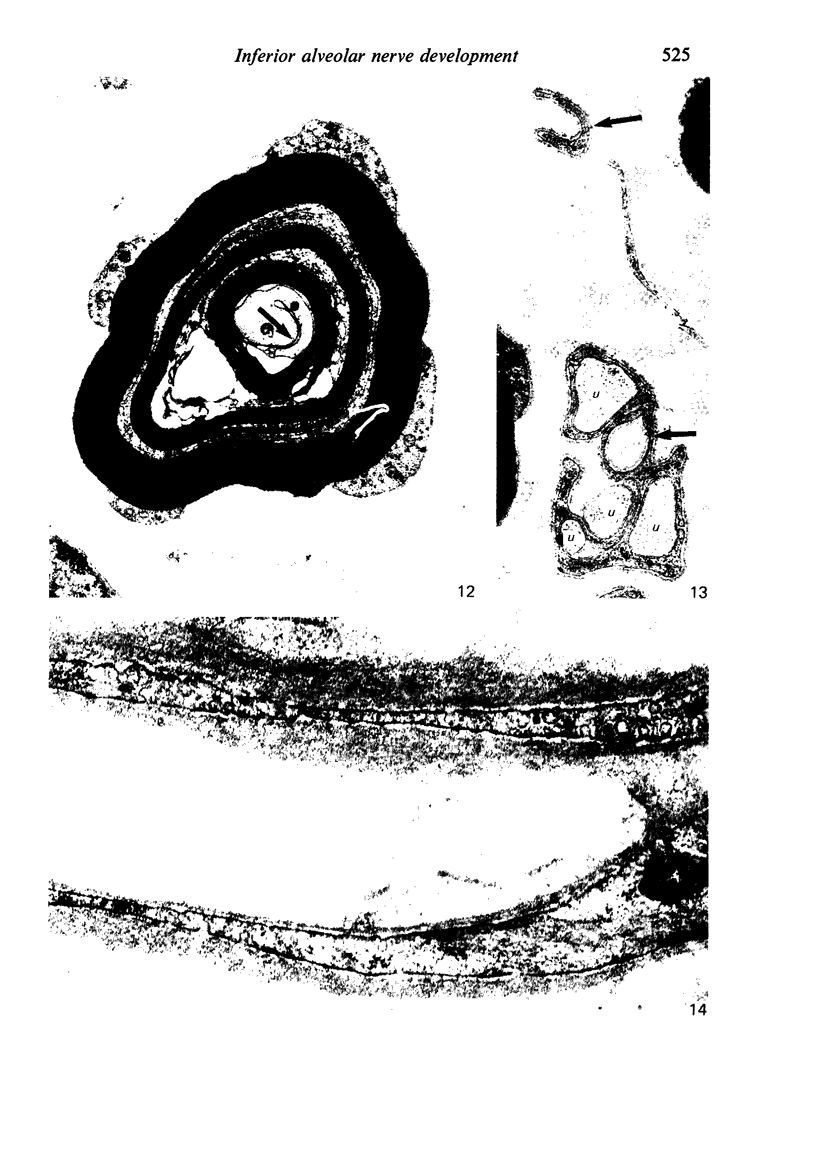
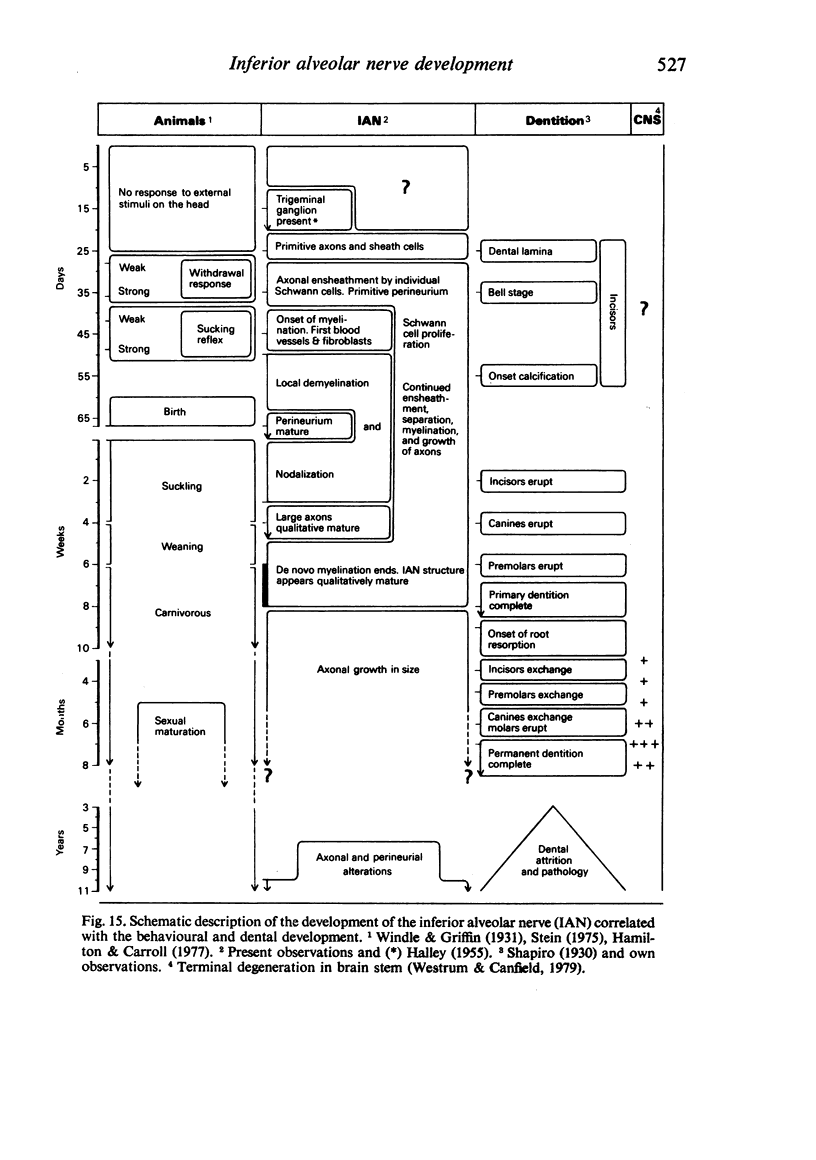
The qualitative structural development of the inferior alveolar nerve was studied by electron microscopy in 56 pre- and postnatal kittens and 21 young and old adult cats. At 25 days post conception the nerve was composed of a bundle of small axons enclosed by primitive sheath cells. Three weeks later myelination had been initiated. Axons measuring 2-3 micrometers underwent local demyelination from 2 weeks before to 3 weeks after birth.This was accompanied and followed by nodalization of larger axons. A typical perineurium was first apparent in the newborn kitten. Six to eight weeks postnatally, the nerve appeared qualitatively mature, although axonal growth was far from completed. This coincides with achievement of a fully mature primary dentition shortly after the weanling period. Apart from a continued size growth, no changes were observed in the nerve during the transition from the primary to the permanent dentition. In the inferior alveolar nerve of old cats, axonal and perineurial changes co-existed with signs of dental attrition and pathology.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN J. T., THOMAS P. K. Retrograde changes in fibre size following nerve section. J Anat. 1962 Jan;96:121–129. [PMC free article] [PubMed] [Google Scholar]
- Aguayo A. J., Terry L. C., Bray G. M. Spontaneous loss of axons in sympathetic unmyelinated nerve fibers of the rat during development. Brain Res. 1973 May 17;54:360–364. doi: 10.1016/0006-8993(73)90061-9. [DOI] [PubMed] [Google Scholar]
- Billings-Gagliardi S., Webster H. F., O'Connell M. F. In vivo and electron microscopic observations on Schwann cells in developing tadpole nerve fibers. Am J Anat. 1974 Nov;141(3):375–391. doi: 10.1002/aja.1001410308. [DOI] [PubMed] [Google Scholar]
- Bradlaw R. The Histology and Histopathology of the Dental Innervation: (Section of Odontology). Proc R Soc Med. 1939 Jul;32(9):1040–1053. [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B., Williams A. K., Wood P. M., Uitto J., Jeffrey J. J. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980 Jan;84(1):184–202. doi: 10.1083/jcb.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cerro M. P., Snider R. S. Studies on the developing cerebellum. Ultrastructure of the growth cones. J Comp Neurol. 1968 Jul;133(3):341–362. doi: 10.1002/cne.901330305. [DOI] [PubMed] [Google Scholar]
- Ekholm J. Postnatal changes in cutaneous reflexes and in the discharge pattern of cutaneous and articular sense organs. A morphological and physiological study in the cat. Acta Physiol Scand Suppl. 1967;297:1–130. [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968 Oct;27(4):546–570. [PubMed] [Google Scholar]
- GAMBLE H. J., EAMES R. A. AN ELECTRON MICROSCOPE STUDY OF THE CONNECTIVE TISSUES OF HUMAN PERIPHERAL NERVE. J Anat. 1964 Oct;98:655–663. [PMC free article] [PubMed] [Google Scholar]
- Gamble H. J., Breathnach A. S. An electron-microscope study of human foetal peripheral nerves. J Anat. 1965 Jul;99(Pt 3):573–584. [PMC free article] [PubMed] [Google Scholar]
- Gamble H. J. Further electron microscope studies of human foetal peripheral nerves. J Anat. 1966 Jul;100(Pt 3):487–502. [PMC free article] [PubMed] [Google Scholar]
- Gibson J. D. The origin of the neural macrophage: a quantitative ultrastructural study of cell population changes during Wallerian degeneration. J Anat. 1979 Aug;129(Pt 1):1–19. [PMC free article] [PubMed] [Google Scholar]
- HALLEY G. The placodal relations of the neural crest in the domestic cat. J Anat. 1955 Apr;89(2):133–152. [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. M., Carroll K. K. Plasma cholesterol levels in suckling and weaned kittens, puppies, and guinea pigs. Lipids. 1977 Feb;12(2):145–148. doi: 10.1007/BF02533284. [DOI] [PubMed] [Google Scholar]
- KARLSSON U., SCHULTZ R. L. FIXATION OF THE CENTRAL NERVOUS SYSTEM FROM ELECTRON MICROSCOPY BY ALDEHYDE PERFUSION. I. PRESERVATION WITH ALDEHYDE PERFUSATES VERSUS DIRECT PERFUSION WITH OSMIUM TETROXIDE WITH SPECIAL REFERENCE TO MEMBRANES AND THE EXTRACELLULAR SPACE. J Ultrastruct Res. 1965 Feb;12:160–186. doi: 10.1016/s0022-5320(65)80014-4. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. The perineurium as a diffusion barrier to protein tracers. Differences between mature and immature animals. Acta Neuropathol. 1971;17(2):127–138. doi: 10.1007/BF00687488. [DOI] [PubMed] [Google Scholar]
- MOHIUDDIN A. The fate of the nerves of the deciduous teeth. J Anat. 1950 Jul;84(3):319–323. [PMC free article] [PubMed] [Google Scholar]
- MOHIUDDIN A. The post-natal development of the inferior dental nerve of the cat. J Anat. 1951 Jan;85(1):24–35. [PMC free article] [PubMed] [Google Scholar]
- Matthews B., Robinson P. P. The course of post-ganglionic sympathetic fibres distributed with the trigeminal nerve in the cat. J Physiol. 1980 Jun;303:391–401. doi: 10.1113/jphysiol.1980.sp013294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. H., Hudson A. R., Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. 3. Changes in the axons of the proximal stump. Z Zellforsch Mikrosk Anat. 1972;124(2):131–164. doi: 10.1007/BF00335677. [DOI] [PubMed] [Google Scholar]
- Mustafa G. Y., Gamble H. J. Changes in axonal numbers in developing human trochlear nerve. J Anat. 1979 Mar;128(Pt 2):323–330. [PMC free article] [PubMed] [Google Scholar]
- Mustafa G. Y., Gamble H. J. Observations on the development of the connective tissues of developing human nerve. J Anat. 1978 Sep;127(Pt 1):141–155. [PMC free article] [PubMed] [Google Scholar]
- Ochoa J., Mair W. G. The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol. 1969;13(3):217–239. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- Ochoa J. The sural nerve of the human foetus: electron microscope observations and counts of axons. J Anat. 1971 Feb;108(Pt 2):231–245. [PMC free article] [PubMed] [Google Scholar]
- Oldfors A. Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol. 1980;49(1):43–49. doi: 10.1007/BF00692218. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. The course, relations and distribution of the inferior alveolar nerve and its branches in the cat. Anat Rec. 1979 Oct;195(2):265–271. doi: 10.1002/ar.1091950203. [DOI] [PubMed] [Google Scholar]
- Samorajski T. Age differences in the morphology of posterior tibial nerves of mice. J Comp Neurol. 1974 Oct 15;157(4):439–445. doi: 10.1002/cne.901570406. [DOI] [PubMed] [Google Scholar]
- Schwieler G. H. Respiratory regulation during postnatal development in cats and rabbits and some of its morphological substrate. Acta Physiol Scand Suppl. 1968;304:1–123. [PubMed] [Google Scholar]
- Skoglund S. Growth and differentiation, with special emphasis on the central nervous system. Annu Rev Physiol. 1969;31:19–42. doi: 10.1146/annurev.ph.31.030169.000315. [DOI] [PubMed] [Google Scholar]
- Sohal G. S., Weidman T. A. Development of the trochlear nerve: loss of axons during normal ontogen. Brain Res. 1978 Mar 10;142(3):455–465. doi: 10.1016/0006-8993(78)90908-3. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Thomas P. K. Ultrastructural studies of the dying-back process. II. The sequestration and removal by Schwann cells and oligodendrocytes of organelles from normal and diseases axons. J Neurocytol. 1974 Dec;3(6):763–783. doi: 10.1007/BF01097197. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol. 1970 Jan;44(1):62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrum L. E., Canfield R. C. Normal loss of milk teeth causes degeneration in brain stem. Exp Neurol. 1979 Jul;65(1):169–177. doi: 10.1016/0014-4886(79)90257-7. [DOI] [PubMed] [Google Scholar]