Abstract
Tert-butyl phenolic antioxidants (TBP-AOs) are employed to inhibit oxidation and function as stabilizers and protectants in a broad spectrum of consumer products, such as food packaging, adhesives, lubricants, plastics, and cosmetics. The extensive utilization of TBP-AOs results in human exposure through various pathways. Furthermore, some TBP-AOs have been identified as potential endocrine disruptors and may cause liver and lung damage, as well as allergic reactions. Considering their varied applications and potential toxicity, a detailed evaluation of their safety profiles is imperative. However, existing research is often segmented and tends to focus narrowly on specific compounds. Consequently, this review collates recent data on TBP-AOs regarding their production, exposure, and toxicity, incorporating different databases and prior studies, as well as predictions of toxicity using ADMET. Our review strives to offer a comprehensive overview of the characteristics and health effects of TBP-AOs to guide future research and inform policy decisions.
Keywords: tert-butyl phenolic antioxidants, consumer products, exposure, toxicity
1. Introduction
Antioxidants are agents that can delay or prevent autoxidation by inhibiting the formation of free radicals or by interrupting their propagation. Among these, phenolic antioxidants constitute a significant class of compounds that impede oxidation processes crucial to both commercial and biological systems [1]. These antioxidants are regularly incorporated into consumer products to curb oxidation and enhance shelf life, thereby playing an essential role in preserving the quality and stability of products vulnerable to oxidative degradation.
Within the group of synthetic phenolic antioxidants, numerous compounds resemble tert-butyl phenol. These compounds consist of one or more tert-butyl phenol units, with each comprising at least one tert-butyl group attached to a phenol ring [2]. They have gained widespread use in various consumer goods, including food, cosmetics, and personal care products [2]. Referred to as tert-butyl phenol antioxidants (TBP-AOs), these substances belong to a subclass of synthetic phenolic antioxidants and share common characteristics with other phenolic compounds, such as stabilizing and protecting materials from oxidative degradation. However, TBP-AOs may demonstrate distinctive properties due to their tert-butyl phenol functional groups. Tert-butyl phenol (TBP), with its basic structure of a tert-butyl group linked to a phenol ring, is extensively utilized as an antioxidant, UV stabilizer, and precursor in the manufacture of plastics, rubber, petroleum, paints, coatings, and pharmaceuticals, especially in industrial environments, owing to its high stability [3].
Like most synthetic phenolic antioxidants, TBP-AOs are primarily used in consumer products to protect against environmental oxidative effects. However, the antioxidant properties of TBP-AOs may extend beyond simple preservation due to their capability to combat oxidative stress. The antioxidative properties of TBP-AOs have attracted attention for their potential therapeutic applications in various health conditions. For example, 2,6-di-tert-butylphenol (AO701) derivatives have shown potential neuroprotective effects by reducing glutamate-induced oxidative toxicity in neuronal cells and demonstrating beneficial outcomes in an in vivo rodent model of ischemic stroke, indicating low toxicity and promising therapeutic possibilities [4]. These compounds also inhibit lipid peroxidation induced by mersalyl in rat kidney cortical mitochondria, suggesting a potential protective role against free-radical-mediated nephrotoxicity [5]. Tert-butyl hydroquinone (TBHQ) mitigates oxidative stress and apoptosis in various cell types by enhancing antioxidant enzyme activities and activating the Nrf2 pathway, while also offering protective effects against arsenic toxicity, colorectal cancer metastasis, retinal oxidative damage, cartilage destruction in osteoarthritis, and metabolic issues [6,7,8,9,10,11,12]. 2,4-di-tert-butylphenol (AO33) exhibits a range of protective and therapeutic effects, including antioxidant properties, improved cognitive function in mice, inhibition of viral and bacterial activities, and anti-inflammatory and anticancer effects [13,14,15,16,17,18,19]. These findings indicate that TBP-AOs play a significant role not only in product preservation but also in potential therapeutic strategies for various diseases and conditions associated with oxidative stress.
The widespread use of TBP-AOs in consumer goods underscores their importance, yet it also necessitates a thorough understanding of their potential health and environmental impacts. Although TBP-AOs offer significant benefits in various applications, some of these compounds may induce toxicity in the human body, raising concerns about their safety and the necessity for regulatory scrutiny [2]. Many other TBP-AOs have insufficient safety information or limited research available. This lack of knowledge poses challenges to assessing their potential health risks and environmental impacts. Our review aims to provide a comprehensive examination of the chemical structure, characteristics, uses, current research, and ADMET predictions concerning the health impacts of TBP-AOs. It highlights their critical role in modern industry and daily life while also guiding future research efforts.
2. Chemical Structure and Properties
TBP-AOs are characterized by one or more tert-butyl phenol units linked together, with each unit featuring one or more tert-butyl groups, −(C(CH₃)₃), attached to the phenol ring. The phenol group, identified by its hydroxyl (-OH) group bonded to an aromatic ring, is essential in the antioxidant properties of TBP-AOs due to its capacity to donate hydrogen atoms. This donation neutralizes free radicals and reduces oxidative stress [20]. The large tert-butyl group increases the phenol’s stability by providing steric hindrance, protecting it from rapid oxidation [21]. Consequently, these functional groups collaborate to enhance the effectiveness of TBP-AOs in preventing oxidation, making them extremely efficient in various industrial applications where oxidative stability is crucial.
2.1. Phenol Group
Phenolic compounds, consisting of one or more phenol groups with different substituents on the aromatic ring, are categorized based on their functional groups. When carboxylic acids are either directly attached or separated by a C=C bond, they are classified as hydroxybenzoic acids or hydroxycinnamic acids, respectively. Compounds with multiple phenol units are termed polyphenols [22]. These compounds exhibit antimicrobial, antioxidant, and anti-inflammatory properties, making them useful in pharmaceuticals for treating cardiovascular disease, diabetes, cancer, and hypertension. They also serve as food preservatives and additives, with additional roles in the cosmetic and packaging industries [22]. In particular, polyphenols, found naturally in fruits and vegetables, are recognized for their therapeutic benefits and technological applications [23]. In the fields of cosmetics and dermatology, phenolic compounds are primarily used for their antioxidant properties, which help prevent premature aging, provide photoprotection, and treat sensitive or sun-damaged skin [24]. In medical and pharmaceutical research, their significant health benefits, including their role as natural chemopreventives for age-related metabolic disorders and their ability to scavenge free radicals, have made phenolic compounds highly esteemed [25].
2.2. Tert-Butyl Group
The tert-butyl group, represented by the formula (CH₃)₃C-, is a large alkyl substituent in organic chemistry that significantly influences the structure and properties of the molecules it attaches to [21]. Its considerable size can induce steric hindrance, potentially slowing or inhibiting reactions at adjacent sites. This group is crucial in organic chemistry and is extensively used in both research and industrial settings, particularly in drug synthesis, to alter the chemical and physical properties of various compounds. In commercial products and pharmaceuticals, tert-butyl groups can enhance biological activity by increasing the solubility of compounds in organic solvents, thanks to the hydrophobic nature of its methyl groups, and by stabilizing compounds through the protection of functional groups [26]. In organic synthesis, it frequently serves as a protective group for other functional groups, such as hydroxyl groups, during complex synthesis procedures by providing steric hindrance, protecting sensitive areas in molecules, and minimizing unwanted reactions [27].
2.3. Tert-Butyl Phenol
When attached to the aromatic ring, the tert-butyl group profoundly affects the structure and properties of phenol by introducing significant steric hindrance. This hindrance can reduce the reactivity of phenol, particularly in reactions requiring close proximity of reagents to the aromatic ring, such as electrophilic aromatic substitution [28]. The tert-butyl group acts as an electron-donating group through the inductive effect, enhancing the electron density on both the aromatic ring and the hydroxyl group [29,30]. It stabilizes phenolic compounds by slowing oxidation rates, stabilizing phenoxy radicals during antioxidant activity, and increasing effectiveness in preventing oxidation. Additionally, it lowers the acidity of cation radicals, making tert-butylated phenols effective antioxidants [31]. The hydrophobic nature of the tert-butyl group augments the overall hydrophobic character of the molecule, influencing its solubility in various solvents and making it suitable for non-polar environments, such as plastics and oils. Overall, the tert-butyl group markedly enhances the stability, reactivity, and physical properties of phenol derivatives, making tert-butyl phenolic compounds valuable in various industrial applications, particularly as antioxidants and stabilizers.
2.4. Classifications
Based on the number of tert-butyl phenol groups present, TBP-AOs are classified into three categories: mono-TBP, di-TBP, and poly-TBP. This classification underscores the diversity of TBP-AOs, allowing for tailored applications based on their antioxidative properties and structural complexity. Table 1 displays the chemical names, structures, and common names of some frequently used TBP-AOs in each category.
Table 1.
Classification and common TBP-AOs.
| Group | No. | Chemicals | Structure | Common Name | Abbreviation | CAS. No |
|---|---|---|---|---|---|---|
| Mono-TBP | 1 | 4-Tert-butylphenol |

|
PTBP | PTBP | 98-54-4 |
| 2 | 2-Tert-Butyl-4,6-dimethylphenol |

|
Antioxidant AO30 | AO30 | 1879-09-0 | |
| 3 | 2,6-Di-tert-butylphenol |

|
Ethanox 701 | AO701 | 128-39-2 | |
| 4 | Butylated Hydroxytoluene |

|
BHT | BHT | 128-37-0 | |
| 5 | 2,6-Di-tert-Butyl-4-(dimethylaminomethyl)phenol |

|
Antioxidant 703 | AO703 | 88-27-7 | |
| 6 | Diethyl 3,5-di-tert-butyl-4-hydroxybenzylphosphonate |

|
Antioxidant 1222 | AO1222 | 976-56-7 | |
| 7 | Octadecyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate |
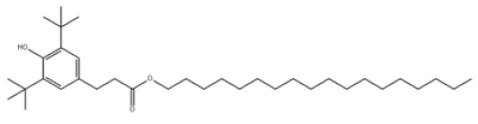
|
Antioxidant 1076 | AO1076 | 2082-79-3 | |
| 8 | 4-((4,6-Bis(octylthio)-1,3,5-triazin-2-yl)amino)-2,6-di-tert-butylphenol |

|
Antioxidant 565 | AO565 | 991-84-4 | |
| 9 | Calcium bis(ethyl 3,5-di-tert-butyl-4-hydroxybenzylphosphonate) |
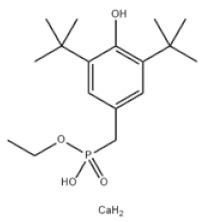
|
Antioxidant 1425 | AO1425 | 65140-91-2 | |
| 10 | 2,4,6-Tri-tert-butylphenol |
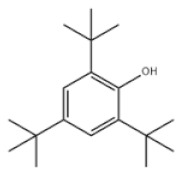
|
Antioxidant 246 | AO246 | 732-26-3 | |
| 11 | 2-Tert-Butyl-4-methoxyphenol |

|
3-BHA | BHA | 121-00-6 | |
| 12 | 2,4-Di-tert-butylphenol |

|
Antioxidant 33 | AO33 | 96-76-4 | |
| 13 | Tert-Butylhydroquinone |

|
TBHQ | TBHQ | 1948-33-0 | |
| 14 | 3,5-Di-tert-butyl-4-hydroxybenzyl alcohol |
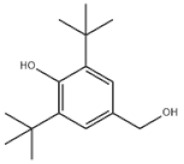
|
Antioxidant 754 | AO754 | 88-26-6 | |
| 15 | Octyl-3,5-di-tert-butyl-4-hydroxy-hydrocinnamate |
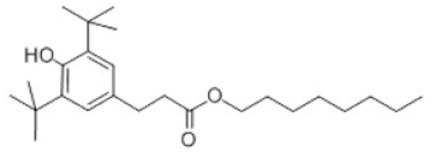
|
Antioxidant 1135 | AO1135 | 125643-61-0 | |
| 16 | 11-Methyldodecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate |
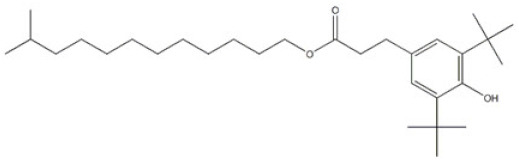
|
Antioxidant 1077 | AO1077 | 847488-62-4 | |
| 17 | 4-Sec-Butyl-2,6-di-tert-butylphenol |

|
ISONOX 132 | AO132 | 17540-75-9 | |
| 18 | Methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate |

|
Ralox 35 | AO35 | 6386-38-5 | |
| 19 | 6-Tert-Butyl-m-cresol |

|
TBMC | 88-60-8 | ||
| Di-TBP | 20 | 2,2′-Methylenebis(4-methyl-6-tert-butylphenol) |

|
Antioxidant 2246 | AO2246 | 119-47-1 |
| 21 | 2,2′-Thiobis(6-tert-butyl-p-cresol) |

|
Antioxidant 1081 | AO1081 | 90-66-4 | |
| 22 | 2,2′-Methylenebis(4-ethyl-6-tert-butylphenol) |
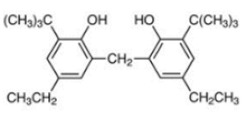
|
Antioxidant 425 | AO425 | 88-24-4 | |
| 23 | Santowhite |

|
Santowhite | STW | 85-60-9 | |
| 24 | 2-Tert-Butyl-6-(3-tert-butyl-2-hydroxy-5-methylbenzyl)-4-methylphenyl acrylate |
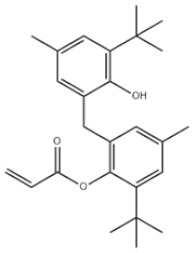
|
Antioxidant 3052 | AO3052 | 61167-58-6 | |
| 25 | 4,4′-Methylenebis(2,6-Di-tert-butylphenol) |

|
Antioxidant 702 | AO702 | 118-82-1 | |
| 26 | 1,2-Bis(3,5-di-tert-butyl-4-hydroxyhydrocinnamoyl)hydrazine |

|
Antioxidant 1024 | AO1024 | 32687-78-8 | |
| 27 | Triethylene glycol bis(3-tert-butyl-4-hydroxy-5-methylphenyl)propionate |

|
Antioxidant 245 | AO245 | 36443-68-2 | |
| 28 | N,N’-Propane-1,3-diylbis[3-(3,5-DI-tert-butyl-4-hydroxyphenyl)propionamide] |

|
Antioxidant 1019 | AO1019 | 69851-61-2 | |
| 29 | 3,9-Bis(2,4-di-tert-butylphenoxy)-2,4,8,10-tetraoxa-3,9-diphosphaspiro[5.5]undecane |

|
Ultranox 626 | AO626 | 26741-53-7 | |
| 30 | Bis(2,6-di-tert-butyl-4-methylphenyl)pentaerythritol diphosphite |

|
Antioxidant PEP-36 | AO36 | 80693-00-1 | |
| 31 | Hexamethylene bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate] |

|
Irganox 259 | AO259 | 35074-77-2 | |
| 32 | Irganox 1035 |
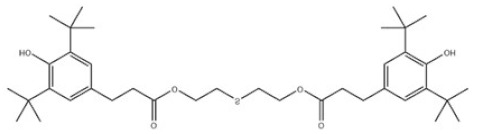
|
Antioxidant 1035 | AO1035 | 41484-35-9 | |
| 33 | Sumilizer AG 80 |

|
Antioxidant AO80 | AO80 | 90498-90-1 | |
| 34 | 2,2′-Ethylidenebis(4,6-di-tert-butylphenol) |
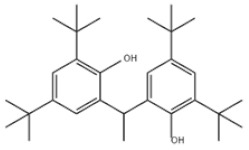
|
Antioxidant 1290 | AO1290 | 35958-30-6 | |
| 35 | Benzenepropanamide, N,N’-1,6-hexanediylbis(3,5-bis(1,1-dimethylethyl)-4-hydroxy- |

|
Antioxidant 1098 | AO1098 | 23128-74-7 | |
| 36 | Naugard XL-1 |

|
Antioxidant MD-697 | AO697 | 70331-94-1 | |
| 37 | 4,4′-Thiobis(6-tert-butyl-m-cresol) |

|
Antioxidant 300 | AO300 | 96-69-5 | |
| Poly-TBP | 38 | 1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylphenyl)butane |

|
Antioxidant CA | AOCA | 1843-03-4 |
| 39 | Tris(2,4-di-tert-butylphenyl) phosphite |

|
Antioxidant 168 | AO168 | 31570-04-4 | |
| 40 | 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris[[4-(1,1-dimethylethyl)-3-hydroxy-2,6-dimethylphenyl]methyl]- |
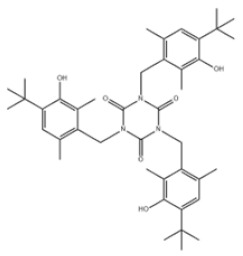
|
Antioxidant 1790 | AO1790 | 40601-76-1 | |
| 41 | 1,3,5-Trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)benzene |

|
Antioxidant 1330 | AO1330 | 1709-70-2 | |
| 42 | 1,3,5-Tris[(3,5-ditert-butyl-4-hydroxyphenyl)methyl]-1,3,5-triazinane-2,4,6-trione |

|
Antioxidant 3114 | AO3114 | 27676-62-6 | |
| 43 | Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) |
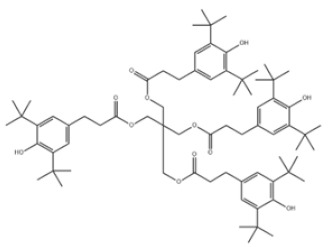
|
Antioxidant 1010 | AO1010 | 6683-19-8 |
2.5. Physical Properties
TBP-AOs display a variety of physical properties that vary significantly due to differences in their molecular structures, such as the number of tert-butyl groups and the nature of the attached R-groups. These variations affect important characteristics, including molecular weight, melting point, boiling point, and their physical state at room temperature, which can be either solid or liquid. Table 2 provides a summary of some of these physical properties, as taken from the Chemicalbook database. As shown in Table 2, most TBP-AOs are typically solid and appear white to light yellow, although some can exist as liquids at room temperature. The phenolic group in TBP-AOs imparts weak acidic properties, with pKa values ranging from around 10 to 12. Additionally, most TBP-AOs have a LogP greater than 1, indicating a tendency to dissolve in lipids, which raises concerns about their absorption in the human body. Therefore, caution is advised regarding exposure to TBP-AOs, as their physical properties suggest that they can easily enter biological systems.
Table 2.
Physical properties of some TBP-AOs.
| No. | Compounds | MW (g/mol) |
Melting Point | Boiling Point | Form | Color | LogP | pKa |
|---|---|---|---|---|---|---|---|---|
| 1 | PTBP | 150.22 | 96–101 °C | 236–238 °C | Flakes or pastilles | White to light beige | 3 at 23 °C | 10.23 at 25 °C |
| 2 | AO30 | 178.27 | 22 °C | 249 °C | Powder to lump to clear liquid | White or colorless to light yellow | 3.64 at 35 °C | 12.00 ± 0.23 |
| 3 | AO701 | 206.32 | 34–37 °C | 253 °C | Crystalline solid | White to light yellow | 4.5 at 24 °C | 12.16 ± 0.40 |
| 4 | BHT | 220.35 | 69–73 °C | 265 °C | Crystals | White | 5.2 | 14 (uncertain) |
| 5 | AO703 | 263.42 | 93–94 °C | 172 °C at 30 mm Hg | Powder to crystal | White to yellow to orange | 4.24 | 11.17 ± 0.70 |
| 6 | AO1222 | 356.44 | 122 °C | 417.0 ± 33.0 °C | Powder to crystal | White to almost white | 2.9 at 23 °C | 12.04 ± 0.40 |
| 7 | AO1076 | 530.86 | 50–52 °C | 568.1 ± 45.0 °C | - | White to off-white | 13.930 | 12.33 ± 0.40 |
| 8 | AO565 | 588.95 | 92–95 °C | 670.7 ± 65.0 °C | Solid | White to off-white | 13.2 at 25 °C | 12.27 ± 0.70 |
| 9 | AO1425 | 370.48 | - | - | - | - | −0.1 at 23 °C | - |
| 10 | AO246 | 262.43 | 125–130 °C | 277 °C | Buffered aqueous glycerol solution | Off-white to pale yellow | 7.1 at 35 °C | 12.61 ± 0.40 |
| 11 | BHA | 180.24 | 58–64 °C | 269 °C | Powder/waxy solid | White to off-white | 1–2.82 at 25–27 °C | 11.83 ± 0.18 |
| 12 | AO33 | 206.32 | 53–56 °C | 265 °C | Crystalline solid | White to yellow | 4.8 at 23 °C | 11.56 ± 0.18 |
| 13 | TBHQ | 166.22 | 127–129 °C | 295 °C | Crystalline powder | White to light tan; may contain black specks | 1.521 at 25 °C | 10.80 ± 0.18 |
| 14 | AO754 | 236.35 | 139–141 °C | 214 °C at 40 mm Hg | Solid: particulate/powder | White to yellow to orange | 3.16 at 20 °C | 12.01 ± 0.40 |
| 15 | AO1135 | 390.6 | - | 343–370.65 °C at 101 325 Pa | Oil | Colorless | 7.18–9.2 at 0–30 °C | - |
| 16 | AO1077 | 460.73 | - | 220–245 °C at 101.3 kPa | Viscous | - | 3.56 at 25 °C | - |
| 17 | AO132 | 262.43 | 25 °C | 141–142 °C at 10 mm Hg | Liquid | White or colorless to yellow | 7.2 at 20 °C | 11.85 ± 0.70 |
| 18 | AO35 | 292.41 | 60–67 °C | 125–130 °C (Press: 0.1 Torr) | Powder to crystal | White to almost white | 4.866 | 12.17 ± 0.40 |
| 19 | TBMC | 164.24 | 20 °C | 117–118 °C at 12 mm Hg | - | Colorless to red to green | 3.97 | 11.45 ± 0.10 |
| 20 | AO2246 | 340.5 | 123–127 °C | 187 °C | Solid | White to off-white | 6.25 at 20 °C | 11.32 ± 0.48 |
| 21 | AO1081 | 358.54 | 84–85 °C | 431.1 ± 45.0 °C | - | - | 6.5 at 25 °C | 10.30 ± 0.50 |
| 22 | AO425 | 368.55 | 119–122 °C | 458.86 °C | Solid | White to off-white | 8.95 at 20 °C | 11.37 ± 0.48 |
| 23 | STW | 382.58 | 211 °C | 469.7 °C | Powder to crystal | White to almost white | 6.4 at 20 °C | 10.44 ± 0.20 |
| 24 | AO3052 | 394.55 | 128–133 °C | 491 °C | Solid | White to off-white | - | 11.66 ± 0.48 |
| 25 | AO702 | 424.66 | 155–159 °C | 289 °C at 40 mm Hg | solid | White | 8.9 at 25 °C | 12.03 ± 0.40 |
| 26 | AO1024 | 552.79 | 60–67 °C | 652.6 ± 55.0 °C | Solid | White to off-white | 4.8 at 23 °C | 11.10 ± 0.50 |
| 27 | AO245 | 586.77 | 79–81 °C | 602.88 °C | Solid | White to off-white | 4.7 at 23 °C | 11.44 ± 0.25 |
| 28 | AO1019 | 594.87 | - | 711.0 ± 60.0 °C | - | - | 8.13 | 12.08 ± 0.40 |
| 29 | AO626 | 604.69 | 160–178 °C | 555.8 ± 50.0 °C | Solid | White to off-white | 10.9 at 25 °C | - |
| 30 | AO36 | 632.75 | 235–240 °C | 577.0 ± 50.0 °C | - | - | 6 at 25 °C | - |
| 31 | AO259 | 638.92 | 102–105 °C | 648.1 ± 55.0 °C | Solid | Off-white | - | 12.03 ± 0.40 |
| 32 | AO1035 | 642.94 | 78 °C | 659.4 ± 55.0 °C | Solid | White to off-white | - | 12.02 ± 0.40 |
| 33 | AO80 | 740.98 | 125 °C | 755.7 ± 55.0 °C | Solid | White to off-white | - | 11.44 ± 0.25 |
| 34 | AO1290 | 438.68 | 162–164 °C | 464.8 ± 40.0 °C | - | - | 9.840 | 11.28 ± 0.50 |
| 35 | AO1098 | 636.95 | 156–161 °C | 740.1 ± 60.0 °C | Solid | White to off-white | 9.6 at 25 °C | 12.08 ± 0.40 |
| 36 | AO697 | 696.91 | 178 °C | - | Solid | White to off-white | 8.1 at 35 °C | 11.48 ± 0.46 |
| 37 | AO300 | 358.54 | 160–165 °C | 460.94 °C | Powder | White to off-white | 5.24 at 25 °C | 10.76 ± 0.36 |
| 38 | AOCA | 544.81 | 183–190 °C | 578.54 °C | - | - | 12.7 at 25 °C | 10.38 ± 0.20 |
| 39 | AO168 | 646.94 | 181–184 °C | 594.2 ± 50.0 °C | Powder | White | 18 at 25 °C | - |
| 40 | AO1790 | 699.92 | 163–165 °C | 793.8 ± 60.0 °C | Solid | White to off-white | 15.281 at 20 °C | 11.36 ± 0.28 |
| 41 | AO1330 | 775.2 | 248–250 °C | 739.54 °C | Solid | White to off-white | 17.17 | 11.91 ± 0.40 |
| 42 | AO3114 | 784.08 | 218–220 °C | 757.9 ± 60.0 °C | - | White to off-white | 15.18 | 11.45 ± 0.40 |
| 43 | AO1010 | 1177.66 | 115–118 °C | 779.1 °C | Solid | White to off-white | 18.832 | 11.71 ± 0.40 |
All data were taken from Chemicalbook (https://www.chemicalbook.com/) on 24 November 2024. MW: molecular weight; pKa: negative logarithm of the acid dissociation constant; LogP: logarithm of the partition coefficient.
3. Applications in Consumer Products
TBP-AOs serve as stabilizers and protectants across a broad spectrum of consumer products. They appear in adhesives, sealants, lubricants, greases, plastics, polymers, and rubber used in both industrial and household settings. Moreover, TBP-AOs are found in everyday items, such as detergents, cosmetics, and personal care products, as well as in food coatings. Table 3 presents the applications of some widely used TBP-AOs according to different databases, including the European Chemicals Agency (ECHA), Chemicalbook, and ChemBK.
Table 3.
Consumer uses of some common TBP-AOs.
| No. | Compounds | Consumer Products and Materials Using TBP-AOs | ||
|---|---|---|---|---|
| ECHA | Chemicalbook | ChemBK | ||
| 1 | PTBP | Sealants, adhesives, and coatings. | Sealants, adhesives, coating products, polymers, and other materials. | |
| 2 | AO30 | Fuels, hydraulic fluids, metal working fluids, lubricants, and greases. | Jet and rocket fuels. | Fuel, acrylic acid polymerization, and pharmaceuticals. |
| 3 | AO701 | Fuels, lubricants, and greases. | Fuels, lubricants, plastics, rubber, and polymers. | Fuel, rubber and plastic, UV absorber, and pesticide and dye applications. |
| 4 | BHT | Cleaning products, plant protection, lubricants, greases, adhesives, sealants, polishes, waxes, coating, and fertilizers. | Oils and fat-containing foods. | Rubber, plastic, gasoline, oil, and food. |
| 5 | AO703 | Rubber, synthetic resin, gasoline, and oil. | ||
| 6 | AO1222 | Polyester, polycondensation, dimethyl terephthalate, polyamide, and UV absorber applications. | ||
| 7 | AO1076 | Coatings, lubricants, greases, adhesives, sealants, polishes, waxes, air care, and cleaning products. | Plastics, synthetic fibers, elastomers, adhesives, waxes, oils, fats, polyolefin, and other polymers. | Resin, rubber, petroleum, polyolefin, and polyvinyl chloride. |
| 8 | AO565 | Adhesives and sealants. | Resin, elastomers, adhesives, polystyrene, polyamide, and polyolefin. | Resins, elastomers, rubber, adhesives, and ABS plastic. |
| 9 | AO1425 | Rosin, resin, and polymers (*). | ||
| 10 | AO246 | Electromagnetic bushing. | Rubber. | |
| 11 | BHA | Cosmetics and personal care products. | Cosmetics, topical medications, foods, fuels, rubber, plastics, paints, and glues. | Organic raw materials, biochemical reagents, etc. |
| 12 | AO33 | Fuels. | Polyolefins, UV stabilizers, pharmaceuticals, and fragrances. | Used as a chemical intermediate, light stabilizer, UV absorber, and plasticizer. |
| 13 | TBHQ | Cosmetics, edible fats and vegetable oils, potato chips, and dry cereal. | Cosmetics, edible oils and fats, lard, frying foods, rubber, resin, plastics, pharmaceuticals, etc. | |
| 14 | AO754 | Gasoline and other hydrocarbons. | ||
| 15 | AO1135 | Polymers, food packaging, and textile staining. | Rubber, elastomer, metal deactivators, polyether, polyurethane, oil. | |
| 16 | AO1077 | Plastics, synthetic fiber, waxes, greases, elastomers, and rubber. | ||
| 17 | AO132 | Polyols, PVC, adhesives, and functional fluids. | ||
| 18 | AO35 | Cosmetics, biocides, fragrances, polishes, waxes, cleaning, air care, and personal care products. | Polyethylene. | |
| 19 | TBMC | Lubricating oil and others. | Organic synthesis intermediate. | |
| 20 | AO2246 | Fuels, adhesives, sealants, lubricants, greases, and hydraulic and metal working fluids. | Distilled biodiesel. | Rubber, latex, other materials, and petroleum products. |
| 21 | AO1081 | Rubber and polymer materials. | ||
| 22 | AO425 | Rubber and synthetic resin. | ||
| 23 | STW | Coatings, adhesives, and sealants. | Polyolefin and rubber. | |
| 24 | AO3052 | Adhesive, plastic, and elastomer materials. | Cosmetics, food processing, plastics, rubber, paint, drugs. | |
| 25 | AO702 | Coatings, lubricants and greases, and washing and cleaning products. | Polymers and resin. | |
| 26 | AO1024 | Packaging polymers, resins, adhesives, and food contact. | Polyolefin materials, wires, cables, and insulating materials. | |
| 27 | AO245 | Adhesives and polymers for food contact. | Rubber, latex, resins, and polymers. | |
| 28 | AO1019 | Wires and cables and metal-contact materials (*). | ||
| 29 | AO626 | Coatings, adhesives, sealants, inks, and toners. | Food contact, rubber, elastomers, coatings, adhesives, polymers, and plastics. | Polymer materials. |
| 30 | AO36 | Plastics. | ||
| 31 | AO259 | Lubricants and greases. | ||
| 32 | AO1035 | Wire, cable, polymers, resins, and adhesives. | Coatings, plastics, and rubber. | |
| 33 | AO80 | Polymers, plastics, rubber, adhesives, sealants, etc. | ||
| 34 | AO1290 | Act as antioxidant. | ||
| 35 | AO1098 | Plastics, adhesives, elastomers, polymers, and fibers. | Polymers, resin, and rubber. | |
| 36 | AO697 | Cables, polymers, resin, and other materials. | ||
| 37 | AO300 | Rubber, plastics, and food contact polymers. | Polyethylene packaging film, rubber, resin, etc. | |
| 38 | AOCA | Adhesives and sealants. | Polymers, resins, and light-colored rubber products. | |
| 39 | AO168 | Coatings, adhesives and sealants, inks, and toners. | Polymers, resin, plastics, binding agent, rubber, and petroleum. | Polymers, fiber, resin, and other plastics. |
| 40 | AO1790 | Polystyrene and rubber-modified polystyrene in food contact. | Nylon, pipes, agricultural films, household appliances, polymers, resin, and plastics. | |
| 41 | AO1330 | Adhesives, sealants, lubricants, and greases. | Polymers, elastomers, fibers, adhesives, waxes, oils, and fats | Polymers, plastics, resin, and rubber. |
| 42 | AO3114 | Adhesives and sealants. | Nonfatty food packaging and propylene copolymers. | Polymers. |
| 43 | AO1010 | Coatings, adhesives, sealants, lubricants, greases, polishes, waxes, and cleaning and air care products. | Plastics, fibers, elastomers, adhesives, waxes, oils, and fats. | Polymers, resin, and plastic products. |
(*) Intended use, but not mentioned in databases. ECHA: “https://www.epa.gov/ (accessed on 23 September 2024)”. Chemicalbook: “https://www.chemicalbook.com/ (accessed on 23 September 2024)”. ChemBK: https://www.chembk.com/ (accessed on 23 September 2024)”.
3.1. Adhesives and Sealants
Adhesives and sealants are essential in various industries, notably in non-woven fabrics (such as baby diapers, feminine hygiene products, and medical supplies), tapes, furniture adhesives, and industrial and construction applications. Antioxidants are crucial in preventing the thermal degradation of polymers in adhesives, sealants, and coatings. Hindered phenolic antioxidants, such as Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (AO1010) and Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate (AO1076), are preferred for their enhanced thermal stability that boosts processing and durability [32]. Butylated hydroxytoluene (BHT) is commonly used in shoe adhesives and has been linked to allergic contact dermatitis in users [33,34]. By incorporating these antioxidants, manufacturers can enhance the durability and longevity of their products, thus fulfilling the rigorous performance standards of contemporary applications.
3.2. Lubricants and Greases
Lubricants and greases are employed to reduce friction, wear, and heat in mechanical components. They are widely used in household items like appliances, vehicles, and tools, as well as in industrial machinery. Lubricants consist of base oils and additives, such as antioxidants and anti-wear agents, to enhance performance. In contrast, greases are semi-solid or solid lubricants [35]. TBP-AOs play a pivotal role in lubricants and greases, enhancing the oxidative resistance of base oils, which improves thermal stability and extends the service life of the lubricant [35,36]. The efficacy of TBP-AOs is attributed to their hindered phenol structure, which protects by donating hydrogen from their phenolic hydroxyl group. This reaction with RO· and ROO· radicals mirrors the mechanism seen in aromatic amine molecules, effectively inhibiting oxidative degradation in lubricating oils [36].
3.3. Plastics, Polymers, and Rubber
Polymers, plastics, and rubbers are vital materials in everyday life, offering versatility and functionality across a wide range of applications. These materials are found in common items, such as packaging, containers, household appliances, toys, and vehicle components. TBP-AOs significantly enhance the durability and performance of these items. For example, Tris(2,4-di-tert-butylphenyl) phosphite (AO168), a widely used antioxidant in plastics, has been found to contaminate various laboratory reagents, potentially biasing ecotoxicological and toxicological studies. Additionally, oxidized AO168 has been detected in reverse osmosis and deionized water containers [37]. AO701 and 4,4′-bis(2,6-di-tert-butylphenol) are commonly employed as antioxidants in the manufacturing of rubber to prevent degradation from oxygen and heat exposure, thus enhancing product durability and performance [38,39]. Other TBP-AOs, like 1,3,5-trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl) benzene (AO1330), AO1010, and AO1076, are used to stabilize crosslinked and non-crosslinked polyethylene materials [40,41]. Like other phenolic antioxidants, TBP-AOs protect these materials through various mechanisms. They serve as thermal stabilizers, safeguarding against degradation due to high temperatures, and they protect against UV damage by neutralizing free radicals [42,43]. Additionally, they mitigate oxidative degradation by neutralizing oxygen radicals, helping to maintain the polymers’ flexibility and elasticity, which is essential for applications involving repeated flexing, thus extending product lifespan [44]. Moreover, they delay aging processes, such as embrittlement and cracking, particularly in outdoor environments, while preserving mechanical strength and elongation properties to ensure consistent performance under demanding conditions [45].
3.4. Cosmetics and Personal Care Products
The increasing interest in both natural and synthetic antioxidants for use in cosmetics and personal care products is fueled by their ability to shield the skin from oxidative stress and prevent age-related damage. TBP-AOs, known for their antioxidant properties, have become essential components in skincare and beauty formulations. Compounds like 2-tert-butyl-4-methoxyphenol (BHA), BHT, and TBHQ are integrated into a diverse range of cosmetics and personal care products, such as foam stabilizers [46], hair dyes [47], lipsticks, eye shadows, blushers, and skin creams [48,49]. In addition to their antioxidative capabilities, certain TBP-AOs also inhibit tyrosinase, an enzyme critical for melanin production, and boost glutathione reductase activity, making them effective for use in skin-lightening and anti-pigmentation products [50,51,52,53,54].
3.5. Food and Coatings
Foods are prone to various oxidative reactions initiated by specific enzymes or molecular oxygen, which generate reactive free radicals. These reactions can lead to undesirable flavors and odors, reduced nutritional value, and diminished shelf life of products. Antioxidants play a crucial role as additives that impede these oxidative processes, thus prolonging the shelf life of foods without altering their intrinsic properties. They are employed in food packaging and coatings that are in direct contact with food items. Additives in these materials may stay within the polymer, degrade due to environmental influences, or migrate into the food, potentially reducing the effectiveness of antioxidants in polymers and posing safety risks to humans via food consumption. Therefore, rigorous standards for the stability and safety of these food additives are essential. Natural phenolic compounds are extensively used in food packaging, as well as in edible films and coatings [55]. TBP-AOs, including AO1076, AO1010 [56,57], and AO168 [58,59], are frequently utilized as antioxidant additives in food packaging polymers to counteract polymer degradation caused by oxygen, light, and heat due to their durability and safety.
3.6. Other Applications
TBP-AOs are utilized in various sectors beyond food packaging, where they perform critical functions as antioxidants and preservatives. For example, AO1076 is a commonly used phenolic antioxidant in several polymer-based medical devices, such as catheters, to protect against microbial contamination [60,61]. Santowhite (STW) serves in cage implant systems to prevent surface cracking and flaking [62]. In pharmaceutical containers, the use of BHT, AO168, and AO1010 may introduce risks to patients or affect the quality of the product [63]. Moreover, compounds like AO1010, Benzenepropanamide, N,N’-1,6-hexanediylbis(3,5-bis(1,1-dimethylethyl)-4-hydroxy- (AO1098), and 1,3,5-tris[(3,5-ditert-butyl-4-hydroxyphenyl)methyl]-1,3,5-triazinane-2,4,6-trione (AO3114) aid in reducing the formation of malodor molecules in fabrics during the use of laundry detergents [56].
4. Human Exposure
TBP-AOs are found in various daily use items. Consequently, human exposure to these compounds can occur through multiple pathways, each with distinct risks based on the nature of contact, including dermal contact, inhalation, oral intake, and environmental exposure. The extent of human exposure to TBP-AOs varies considerably due to factors like geographic location, age, personal characteristics, consumption patterns, and the degree to which these chemicals penetrate products, the body, and the environment. Despite the importance of these variations, existing data on TBP-AO exposure remain insufficient. Some exposure routes for TBP-AOs include the following.
4.1. Dermal Contact
TBP-AOs are found in personal care products, cosmetics, and plastics. Direct application or dermal contact during use can lead to absorption of TBP-AOs. The extent to which these substances are absorbed, retained in human skin, and penetrate the circulation has not been thoroughly investigated. However, research using a consumer-like dermal exposure model showed that ten different polymer additives, including 4,4′-Thiobis(6-tert-butyl-m-cresol) (AO300), 2,2′-Methylenebis(4-methyl-6-tert-butylphenol) (AO2246), 2,2′-Thiobis(6-tert-butyl-p-cresol) (AO1081), and Diethyl 3,5-di-tert-butyl-4-hydroxybenzylphosphonate (AO1222), can penetrate and distribute within the intact porcine and human skin barriers depending on their lipophilicity [64]. A study on the dermal absorption of AO300 found that approximately 20% of the applied dose was absorbed in mice, with rats showing significantly lower absorption rates, under 2% [65]. The dermal absorption rate of BHT in animal skin models varies, ranging from 0.07% in pig skin to 11.1% in fuzzy rat skin [66]. Higher absorption rates may contribute to increased systemic exposure and potential toxicity, making it crucial to understand these rates when evaluating the safety and potential health risks of these compounds in consumer products. In humans, daily dermal intake of TBP-AOs varies with different daily contact with items, such as personal care products and paperboard food packages [67,68]. Additionally, TBP-AOs may induce allergic contact dermatitis upon exposure to items containing these compounds, such as cosmetics [69], gloves [70], wound bandages [71], medical devices [72,73], and other contact materials.
4.2. Inhalation Exposure
TBP-AOs are widely used in industrial settings, particularly in the production of paints, adhesives, plastics, and rubber. The use of TBP-AOs in these applications can lead to inhalation exposure for workers and individuals near manufacturing environments. During the production and application processes, TBP-AOs may be released into the air as vapors or particulates, posing potential respiratory risks. Workers who handle these materials may be particularly susceptible to exposure, potentially experiencing a range of health effects, including respiratory irritation and other systemic impacts. A 49-year-old non-atopic male factory worker developed asthma while cleaning mixing drums containing TBHQ, a common additive in food and cosmetics that can cause allergic contact dermatitis, suggesting that TBHQ may be the cause of his asthma [74]. TBP-AOs, such as AO1076, AO168, and their oxidation products, were detected in the atmosphere where plastic waste was incinerated, contributing to air pollution and increasing the risk of inhalation exposure to TBP-AOs [75].
4.3. Oral Exposure
For many years, researchers have been studying the oral exposure and potential toxic effects of additives that leach from consumer products into food or body fluids. TBP-AOs, commonly found in materials that contact food, can transfer from packaging or storage containers into the food itself. Gao et al. discovered high migration levels of TBP-AOs, including BHT, BHA, AO2246, Irganox 1035 (AO1035), AO1010, and AO1330, with BHT and AO2246 notably exceeding specific migration levels in certain food simulants [76]. These chemicals exhibit substantial migration levels, raising concerns about potential TBP-AO exposure through food intake. This poses possible health risks and suggests that glass containers might be a safer alternative to plastic for storing oil-based foods. Monitoring and determining the specific migration levels of these additives is crucial for ensuring food quality. Additionally, handling materials containing TBP-AOs and then consuming food without washing hands could also lead to ingestion.
The estimated daily intake (EDI) of specific TBP-AOs from food sources has been examined in numerous studies, revealing considerable variability influenced by various factors, such as the region, the subjects, and the types of foods containing these compounds. This variability underscores the significance of context in evaluating exposure levels. As summarized in Table 4, various studies report differing EDI values for certain TBP-AOs, reflecting regional exposure differences. Further research is essential to provide more comprehensive data on the EDI of other TBP-AOs across diverse populations and food sources.
Table 4.
Summary of estimated daily intake (EDI) of certain TBP-AOs from foods.
| Compounds | Mean EDI (mg/kg bw/day) |
Subjects | Country | Year | Ref. |
|---|---|---|---|---|---|
| BHA | 0.14–0.17 | 16,014 households | Brazil | 1997–1999, 2003 | [77] |
| 5.49–12.12 (mg/person/day) |
13,000 individuals of all ages | Canada | 1973 | [78] | |
| 0–0.04 | 11,525 individuals of all ages | Korea | 1998 | [79] | |
| 0.001–0.017 | 3003 individuals of all ages | France | 1998–1999 | [80] | |
| 0–0.72 | 706 children (aged 1–36 months) | France | 2005 | [81] | |
| 5.1 × 10−4–0.3 | 134 samples | Korea | 2005 | [82] | |
| 0.15 | 230 children (aged 9–18 years) | Lebanon | 2002–2003 | [83] | |
| BHA and/or BHT | 0.075 | 5898 individuals of all ages | The Netherlands | 1987/1988 | [84] |
| BHT | 0.09–0.11 | 17,014 households | Brazil | 1997–1999, 2003 | [77] |
| 0.13–0.39 | 13,000 individuals of all ages | Canada | 1973 | [78] | |
| 1.56 × 10−5–0.04 | 11,525 individuals of all ages | Korea | 1998 | [79] | |
| 0–0.013 | 3003 individuals of all ages | France | 1998–1999 | [80] | |
| 0–0.267 | 441–4079 individuals of all ages | France, Italy, the UK, Ireland | 1992–2007 | [85] | |
| 0.018–0.025 | 230 children (age 9–18 years) | Lebanon | 2002–2003 | [83] | |
| 7.5 × 10−4–0.29 | 131 samples | Korea | 2005 | [82] | |
| 0.003–0.087 | 32 different dietary surveys on peoples of all ages | Europe (22 countries) | 2010 | [86] | |
| 1.4 × 10−03 | Snack and cookie samples | China | 2019 | [67] | |
| 2.51 × 10−5–4.71 × 10−5 | 1780 individuals (aged 6 months–17 years) | Spain | 2012–2014 | [87] | |
| 6.61 × 10−3 | 952 individuals of all ages | China | 2021 | [88] | |
| TBHQ | 0.11–0.14 | 18 014 households | Brazil | 1997–1999, 2003 | [77] |
| 1.2 × 10−6–0.04 | 11,525 individuals of all ages | Korea | 1998 | [79] | |
| 2.5 × 10−4–0.28 | 104 samples | Korea | 2005 | [82] | |
| AO1076 | 1.07 × 10−3 | Snack and cookie samples | China | 2019 | [67] |
| AO2246 | 7.81 × 10−5 | Snack and cookie samples | China | 2019 | [67] |
| AO245 | 1.40 × 10−5 | Snack and cookie samples | China | 2019 | [67] |
4.4. Gestational Exposure
Gestational exposure to environmental chemicals, including TBP-AOs, poses significant health risks to both pregnant women and their developing fetuses. TBP-AOs can cross the placental barrier and potentially impact fetal development. A recent study investigated prenatal exposure to 46 plasticizers and antioxidants in 109 pairs of maternal and cord serum samples to assess the ability of these chemicals to cross the placenta [89]. The results indicated that TBP-AOs, such as AO33 and BHT, along with their primary transformation products, demonstrated relatively high transplacental transfer efficiencies [89]. The transformation products of BHT exhibit greater potential for maternal transfer compared to BHT itself, whereas AO2246 and AO33 were found in significant levels in cord plasma and placenta, rather than in maternal blood [90]. These findings emphasize that TBP-AOs, including AO33, can permeate the placental barrier, suggesting potential adverse effects on fetal development. This underscores the need for further research and regulatory measures to mitigate prenatal exposure to harmful environmental chemicals.
4.5. Environmental Exposure
TBP-AOs persist in the environment and can contaminate the air, soil, and water through industrial waste or degradation of consumer products. Research on antioxidants in household dust from various locations has revealed the widespread presence of TBP-AOs, such as Triethylene glycol bis(3-tert-butyl-4-hydroxy-5-methylphenyl)propionate (AO245), 2,6-di-tert-butyl-4-(dimethylaminomethyl)phenol (AO703), AO33, AO701, BHA, and BHT, as well as their derivatives, across different regions [91,92]. Individuals residing in contaminated areas might be exposed to TBP-AOs through dust, drinking water, or the food chain, leading to an increased risk of health issues. A study exploring the potential endocrine-disrupting effects of the migrating compound AO33 suggested that migration from plastic pipes could lead to prolonged exposure, with significant variations in migration levels depending on the type of plastic pipe and the manufacturer [93]. Additionally, 4-tert-butylphenol (PTBP), used as a hardener in epoxy resins, can leach from steel coatings upon contact with water and induce acute estrogenic effects [94].
Certain TBP-AOs, although initially considered safe, can undergo environmental transformations resulting in highly toxic byproducts. For instance, AO701, deemed non-toxic by European Union standards, forms a degradation product through photodegradation under natural sunlight, 2,5-di-tert-butylphenol, that exhibits significant toxicity to marine bacteria [95]. Consequently, the environmental impact of these compounds may be more severe than initially assessed, necessitating a comprehensive evaluation of their behavior and transformation in natural waters.
4.6. Occupational Exposure
In addition to daily exposure, occupational exposure to TBP-AOs and their derivatives significantly increases the risk of toxicity. Workers in industries that manufacture or use these compounds may encounter higher concentrations, thereby elevating the risk of acute and chronic health effects. Table 5 presents the production volume and the total number of workers exposed to specific TBP-AOs in 2019, as reported to the U.S. Environmental Protection Agency (EPA) by various companies, along with the annual volume noted by the European Chemicals Agency (ECHA). These data underscore the potential extent of exposure and associated health risks, emphasizing the need for rigorous monitoring and regulation of occupational exposure to TBP-AOs to safeguard workers’ health. Implementing safety measures is critical to minimize the risks these chemicals pose in the workplace.
Table 5.
Production summary of TBP-AOs.
| No. | Chemicals | EPA | ECHA | |
|---|---|---|---|---|
| Total Exposed Workers * | 2019 National Aggregated Production (lbs.) | Annual Manufactured and Import Volume (tons) | ||
| 1 | PTBP | 235–515 | 20,000,000–100,000,000 | ≥10,000 |
| 2 | AO30 | 25–60 | 1,000,000–20,000,000 | 100–1000 |
| 3 | AO701 | 75–220 | 100,000,000–1,000,000,000 | 1000–10,000 |
| 4 | BHT | 905–2265 | 1,000,000–10,000,000 | 10,000–100,000 |
| 5 | AO703 | 10–35 | <1,000,000 | 100–1000 |
| 6 | AO1222 | - | - | 10–100 |
| 7 | AO1076 | 970–2700 | 20,000,000–100,000,000 | ≥10,000 |
| 8 | AO565 | 25–80 | 100,000–<500,000 | 100–1000 |
| 9 | AO1425 | 10–45 | <1,000,000 | 100–1000 |
| 10 | AO246 | 75–160 | 20,000,000–100,000,000 | 100–1000 |
| 11 | BHA | - | - | ≥10 |
| 12 | AO33 | 125–260 | 20,000,000–100,000,000 | ≥1000 |
| 13 | TBHQ | - | <1,000,000 | 100–1000 |
| 14 | AO754 | - | - | 1–10 |
| 15 | AO1135 | 620–1390 | 10,000,000–50,000,000 | - |
| 16 | AO1077 | - | - | 10–100 |
| 17 | AO1320 | 25–49 | 1,000,000–20,000,000 | 10–100 |
| 18 | AO35 | 3000–5996 | 20,000,000–100,000,000 | 1–100 |
| 19 | TBMC | 25–49 | 1,000,000–20,000,000 | - |
| 20 | AO2246 | 700–1750 | 1,000,000–10,000,000 | 1000–10,000 |
| 21 | AO1081 | - | - | 10–100 |
| 22 | AO425 | <10 | <1,000,000 | 1–10 |
| 23 | STW | - | 100,000–500,000 | 100–1000 |
| 24 | AO3052 | - | - | 10–100 |
| 25 | AO702 | 50–110 | 1,000,000–20,000,000 | 100–1000 |
| 26 | AO1024 | 50–130 | 1,000,000–10,000,000 | 100–1000 |
| 27 | AO245 | 200–1040 | 1,000,000–10,000,000 | 1000–10,000 |
| 28 | AO1019 | - | - | 10–100 |
| 29 | AO626 | 50–120 | 1,000,000–20,000,000 | 1000–10,000 |
| 30 | AO36 | <10 | 138713 | 10 |
| 31 | AO259 | 500–1020 | <1,000,000 | 100–1000 |
| 32 | AO1035 | 500–1020 | 1,000,000–20,000,000 | 100–1000 |
| 33 | AO80 | <10 | <1,000,000 | - |
| 34 | AO1290 | 60–125 | <1,000,000 | - |
| 35 | AO1098 | 50–110 | <1,000,000 | 1000–10,000 |
| 36 | AO697 | 50–100 | <1,000,000 | 100–1000 |
| 37 | AO300 | 50–110 | 100,000–500,000 | 100–1000 |
| 38 | AOCA | 1050–10130 | 100,000–500,000 | 100–1000 |
| 39 | AO168 | 1985–4995 | 10,000,000–50,000,000 | 10,000–100,000 |
| 40 | AO1790 | 75–160 | 1,000,000–20,000,000 | 100–1000 |
| 41 | AO1330 | 100–240 | 1,000,000–20,000,000 | 1000–10,000 |
| 42 | AO3114 | 75–180 | 1,000,000–10,000,000 | 1000–10,000 |
| 43 | AO1010 | 2070–5490 | 50,000,000–100,000,000 | ≥10,000 |
*: The categorization of workers reasonably likely to be exposed to chemicals was reported as a range with 9 options: 0–10, 10–25, 25–50, 50–100, 100–500, 500–1000, 1000–10,000, more than 10,000, and NKRA (Other and Not Known or Reasonably Ascertainable). The total number of workers exposed to a specific chemical is calculated by summing the upper and lower limits of the worker ranges reported by all companies producing that chemical. EPA: Environmental Protection Agency. ECHA: European Chemicals Agency.
In summary, human exposure to TBP-AOs can occur through dermal contact, inhalation, oral ingestion, and environmental exposure, with each posing specific health risks. To mitigate these risks, it is critical to regulate TBP-AO usage, develop safer alternatives, and ensure safe working environments.
5. Human Health Effects
The toxicity of TBP-AOs is a significant concern due to their widespread use in various applications and the risk of migration from materials to food, pharmaceuticals, and other consumer products. Studies have indicated that some TBP-AOs may cause adverse effects, including skin toxicity, liver toxicity, lung toxicity, and endocrine disruption. In the skin, TBP-AOs can lead to atopic dermatitis and depigmentation in the skin. In the liver, these compounds may cause hepatotoxic effects, including changes in liver enzyme activity, disruption of metabolic functions, and induction of cell death. In the lung, certain TBP-AOs are linked to asthma, pneumotoxicity, tumor development, and lung damage. Furthermore, in the endocrine system, TBP-AOs might disrupt normal hormone functions and potentially lead to reproductive toxicity.
While research on the toxic effects of TBP-AOs in humans is still sparse, some in vitro and in vivo studies have been undertaken to assess their toxicological profiles and potential mechanisms of action. However, the complete range of these toxicities and their mechanisms is still not fully understood, with only a few TBP-AOs having been thoroughly investigated regarding their toxicity and safety profiles. Below is a summary of current insights into the impact of TBP-AOs on human health.
5.1. Skin Toxicity
Atopic dermatitis and depigmentation are significant skin toxicities associated with TBP-AOs. Numerous studies have demonstrated that TBP-AOs can cause allergic contact dermatitis when individuals come into contact with consumer products containing these compounds, such as shoes [96], cosmetics [69], and medical devices [70,71,72,73]. While the precise mechanism remains unclear, several patients have developed allergic contact dermatitis following exposure to latex medical devices that contain AO300 [70,72], BHA [70], and 2-tert-Butyl-6-(3-tert-butyl-2-hydroxy-5-methylbenzyl)-4-methylphenyl acrylate (AO3052) [73]. Furthermore, PTBP can exacerbate atopic-dermatitis-like skin lesions in mice by enhancing T-helper 2-type immune responses and increasing inflammatory cytokines, which suggests its potential to aggravate allergic skin conditions [97]. The presence of TBP-AOs in various products raises concerns about their potential to trigger or exacerbate atopic dermatitis in susceptible individuals, underscoring the importance of carefully evaluating these compounds in consumer products.
Depigmentation is another skin toxicity linked to TBP-AOs, particularly PTBP. This compound has been found to induce oxidative stress and apoptosis in melanocytes, leading to depigmentation disorders, such as vitiligo and leukoderma [98,99]. The oxidative damage and endoplasmic reticulum stress in melanocytes highlight the severe effects PTBP can have on skin health, contributing to significant issues like cutaneous depigmentation and exacerbation of atopic dermatitis. Additionally, PTBP derivatives, including TBHQ, 4-hydroxyanisole, and 4-tert-butyl catechol, which are commonly used in consumer applications, have caused depigmentation in animal models [50,100]. Notably, 4-tert-butyl catechol has been shown to stimulate the formation of pheomelanosomes in skin melanocytes by activating glutathione-metabolizing enzymes and inhibiting the oxidation of eumelanin intermediates [101]. Furthermore, cases of cutaneous depigmentation have also been reported in occupational exposures to AO33 in the rubber industry [102].
5.2. Liver Toxicity
TBP-AOs can induce various hepatotoxic effects, including modulation of liver enzymes, disruption of hepatic metabolism, apoptosis, and necrosis. For example, PTBP has been shown to impair liver development and function in zebrafish, resulting in liver damage, increased lipid accumulation, altered activities of metabolic enzymes, and changes in the expression of key metabolic and inflammatory genes, ultimately leading to tissue dysfunction [103]. In addition, PTBP can induce apoptosis, necroptosis [104], and ferroptosis [105], highlighting its potential to cause significant liver damage.
In contrast, BHT presents a complex profile regarding liver toxicity. BHT is renowned for its capacity to prevent chemically induced tumors and reduce the acute toxic effects of various chemicals through multiple mechanisms [106,107,108,109,110]. Owing to its antioxidant capabilities, BHT has been used as a reference compound in studies assessing the efficacy of antioxidants in defending against oxidative stress and associated damage [108]. Additionally, BHT offers protective effects against liver damage induced by specific carcinogens, such as diethylnitrosamine [107] and aflatoxin B1 [109,110]. However, prolonged exposure to BHT can result in hepatotoxic effects, characterized by elevated liver enzymes and histopathological alterations [111]. It also influences the expression of genes associated with both phase I and phase II metabolism in the liver, highlighting its potential effects on hepatic function and the significance of gene expression profiling in comprehending the toxicity of food additives [112]. Moreover, BHT exacerbates liver injury and oxidative stress when administered in conjunction with LPS [113].
Some TBP-AOs also exhibit hepatotoxic effects. For example, BHA and AO2246 disrupt hepatic lipid homeostasis and fatty acid levels [114,115]. 2,2′-Methylenebis(4-ethyl-6-tert-butylphenol) (AO425) adversely affects mitochondrial function and enzyme activity in the liver [116]. Meanwhile, 2,4,6-Tri-tert-butylphenol (AO246) causes liver injury characterized by focal necrosis and microcytic anemia, especially in female rats, and it does not induce neoplastic responses [117]. The complex and often multifaceted nature of liver toxicity associated with various TBP-AOs underscores the need for a comprehensive understanding of their mechanisms of action and effects.
5.3. Lung Toxicity
Exposure to TBP-AOs and their derivatives in daily life, particularly through inhalation, raises concerns about their toxicity to the respiratory system. Pneumotoxicity has been widely observed with BHT, especially in relation to tumor development and lung damage.
BHT has exhibited dose-dependent pneumotoxicity in CD-1 mice, resulting in respiratory distress, lung congestion, alveolar collapse, dilated alveolar ducts, and epithelial cell degeneration or necrosis, while no similar effects were observed in BHA-treated mice or other tested species [118]. However, BHA intensifies BHT-induced lung toxicity in mice by increasing hydrogen peroxide formation and promoting the peroxidase-dependent conversion of BHT into a toxic metabolite [119]. Furthermore, BHT serves as a non-genotoxic tumor promoter, notably enhancing tumor development when combined with a carcinogen, although it does not independently induce tumors [120]. It can foster lung tumor growth through the induction of chronic inflammation [121], an increase in reactive oxygen species, the deactivation of key antioxidant enzymes [122], and the activation of functional TLR4 [123]. This resulting inflammation is instrumental in tumor promotion and increases lung tumor multiplicity when paired with carcinogen treatment.
Conversely, TBHQ demonstrates dose- and time-dependent cytotoxic and genotoxic effects on lung cancer cells and endothelial cells, leading to apoptosis and DNA fragmentation [124] or causing cytotoxicity through the electrophilic reactions of its metabolites [125]. A recent study has associated TBHQ with respiratory issues, such as asthma, highlighted by a case where a factory worker developed asthma symptoms following exposure [74]. While numerous studies have examined the respiratory system impacts of certain TBP-AOs, the safety of other compounds in this group remains unverified. Considering the frequent use of TBP-AOs in industrial applications, workers and individuals near these areas might be at risk of inhalation exposure. Further research is imperative to assess the risk of respiratory toxicity of other TBP-AOs and their potential health implications.
5.4. Endocrine Disruption
Endocrine disruption refers to the interference of chemicals with the body’s hormonal systems. Several TBP-AOs, particularly PTBP, BHT, and BHA, have been identified as endocrine disruptors. TBP-AOs can act as either agonists or antagonists to key hormones, such as testosterone, androgens, estrogen, and progesterone, thereby impacting normal endocrine function. These compounds may mimic hormonal action, activate receptors, or block natural hormones from exerting their effects, particularly when influencing aromatase activity and altering estrogen levels. However, the effects of some TBP-AOs on hormone levels are inconsistent among studies.
For instance, PTBP binds to estrogen receptors, promoting cell proliferation and enhancing the expression of estrogen-regulated proteins, such as the progesterone receptor and pS273, albeit with minimal estrogen antagonism [126]. PTBP reduces estradiol and testosterone secretion in isolated ovarian follicles from rats without affecting aromatase activity, highlighting its potential impact on steroidogenesis in follicles [127]. However, other studies have shown that PTBP markedly increases testosterone and progesterone levels by up to sevenfold in fetal rat testes [128] and inhibits aromatase in JEG-3 cells [129].
Additionally, BHT and BHA are well-documented for their potential to disrupt endocrine systems, significantly affecting hormonal balance and reproductive health. In T47D-Kbluc and MCF-7 breast cancer cells, BHT and BHA exhibited both estrogenic and anti-estrogenic activities [130]. Another study on pregnant mice demonstrated that BHT increases estrogen and progesterone levels at 200 mg/kg/day [131]. Conversely, another study found that BHA, BHT, and AO2246 did not bind directly to ERα or exhibit estrogenic effects; instead, they notably enhanced E2 secretion by disrupting steroidogenic processes in the human adenocarcinoma cell line, H295R [132]. Moreover, BHA and BHT displayed anti-androgenic properties by inhibiting DHT-induced luciferase activity in a concentration-dependent manner in the androgen receptor of MDA-kb2 human breast cancer cells [133].
Finally, the modulation of the endocrine system by TBP-AOs can lead to reproductive toxicity. In males, BHT disrupts calcium homeostasis and induces endoplasmic reticulum stress in Leydig cells, resulting in testicular toxicity and adversely affecting male reproductive health [134]. In females, studies involving immature and ovariectomized rodents have indicated that TBP-AOs, such as BHA, AO300, and STW, can induce uterotrophic activity following repeated treatment and dietary exposure [135,136]. Additionally, exposure to BHT during pregnancy significantly decreased maternal body weight, the number of implantation sites, and uterine weight, while increasing serum levels of estrogen and progesterone, indicative of potential reproductive toxicity [131].
The endocrine disruption effects of TBP-AOs, as summarized in Table 6, have been recognized in various studies. Although some TBP-AOs are identified as potential endocrine disruptors, research into their endocrine-disrupting effects and underlying mechanisms remains sparse. Existing studies offer some insight into how TBP-AOs may influence key hormones, such as testosterone, androgens, estrogen, and progesterone; however, the specific pathways and mechanisms are still largely undefined. This lack of a comprehensive understanding underscores the urgent need for further research to elucidate the effects of TBP-AOs on hormonal systems and their role in endocrine disruption. Detailed investigations are crucial to determine the mechanisms of action of TBP-AOs and their potential health risks, particularly concerning reproductive and metabolic disorders. The impacts of TBP-AOs on the endocrine system show considerable variation across studies, influenced by various factors such as cell types and experimental models, highlighting the need for standardized methods to evaluate their endocrine-disrupting potential.
Table 6.
Summary of endocrine disruption by TBP-AOs.
| Compounds | Model | Treatment | Testosterone (LOEL) | Androgen | Aromatase | Progesterone (LOEL) | Estrogen (LOEL) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Period | AGO | ANT | AGO | ANT (IC50) | AGO | ANT (IC50) | AGO | ANT | AGO | ANT | |||
| PTBP | Isolated fetuses’ testes | 10–500 mg/L | 24 h | 100 mg/L | - | - | - | - | - | 100 mg/L | - | - | - | [128] |
| Isolated immature rat ovarian follicles | 0.01–1 μM | 3 days | 0.01 μM | 0.1 μM | - | - | - | - | - | - | - | 0.1 μM | [127] | |
| 5 days | - | 0.1 μM | - | - | N | - | - | - | - | 0.01 μM | ||||
| MCF-7 cells | 10 nM–10 μM | 24 h | - | - | - | - | - | - | - | - | - | 10 μM | [126] | |
| JEG-3 cells | 5–500 μM | 24 h | - | - | - | - | - | 283 μM | - | - | - | - | [129] | |
| BHT | T47D-Kbluc cells | 0.3–200 μM | 24 h | - | - | - | - | - | - | - | - | N | IC25 = 15,734 μM | [130] |
| MCF-7 cells | 0.3–200 μM | 24 h | - | - | - | - | - | - | - | - | 10 μM | N | ||
| MDA-kb2 cells | 0.3–300 μM | 24 h | - | - | N | 43.2 μM | - | - | - | - | - | - | [133] | |
| Pregnant CD1 mice | 200,400 mg/kg/d | 6 days | - | - | - | - | - | - | 200 mg/kg/d | - | 200 mg/kg/d | - | [131] | |
| H295R cells | 1–100 μM | 48 h | N | - | N | - | - | - | - | N | 100 μM | - | [132] | |
| BHA | T47D-Kbluc cells | 0.3–100 μM | 24 h | - | - | - | - | - | - | - | - | 100 μM | IC50 = 100.22 μM | [130] |
| MCF-7 cells | 0.3–200 μM | 24 h | - | - | - | - | - | - | - | - | EC25 = 5.53 μM, EC40 = 8.96 μM |
IC50 = 116.83 μM | ||
| H295R cells | 1–100 μM | 48 h | N | - | N | - | - | - | - | N | 1 μM | - | [132] | |
| Zebrafish gonads | 1–5 μM | 21 days | 1 μM | - | - | - | - | - | - | - | 1 μM | - | ||
| MDA-kb2 cells | 0.3–300 μM | 24 h | - | - | N | 172.5 μM | - | - | - | - | - | - | [133] | |
| TBHQ | H295R cells | 0.01–1 μM | 48 h | N | - | N | - | - | - | - | N | N | - | [132] |
| AO2246 | H295R cells | 0.01–1 μM | 48 h | N | - | N | - | - | - | - | N | 0.1 μM | - | |
| AO701 | JEG-3 cells | 50–200 μM | 24 h | - | - | - | - | - | N | - | - | - | - | [129] |
| AO246 | JEG-3 cells | 3–320 μM | 24 h | - | - | - | - | - | 58 μM | - | - | - | - | |
N: No significant difference. LOEL: Lowest Observed Effect Level. IC50: Inhibitory Concentration 50%. EC50: Effective Concentration 50%.
6. Prediction of Toxicities Using ADMET
Many TBP-AOs are currently in use and are anticipated to be employed in various applications in the future. Nevertheless, research on their toxicity remains inadequate, raising concerns about potential health hazards. To address this issue, we utilize ADMETlab 2.0 “https://admetmesh.scbdd.com/ (accessed on 27 September 2024)”. as a crucial tool for evaluating the toxicological characteristics of these compounds. The method for using ADMETlab 2.0 has been described in a prior study [137]. In this review, predicted probability values are represented by three symbols indicating the likelihood of a compound being active: (_) for low probability (probability score: 0–0.3), (+) for medium probability (probability score: 0.3–0.7), and (+++) for high probability (probability score: 0.7–1). This classification allows for a rapid assessment of the compounds’ toxicological profiles. Employing ADMET models enables us to understand their potential harmful effects, subsequently guiding future research to ensure safer applications and inform regulatory decisions. The predicted toxicity of TBP-AOs from ADMET analysis is presented in Table 7. According to these predictions, most TBP-AOs are likely to induce skin and eye irritation and respiratory toxicity and impact endocrine regulation and cellular stress responses. However, there is a notable absence of comprehensive studies on these toxicities of TBP-AOs. This research gap underscores the urgent need for further investigation to fully understand their potential health implications. Specifically, ADMET predictions indicate that many TBP-AOs might affect estrogen regulation, as confirmed by several studies. Moreover, the antioxidative properties of TBP-AOs could modulate key pathways involved in oxidative stress, inflammation, and cellular protection. Understanding these interactions is vital for determining the overall impact of TBP-AOs on health and disease, as well as their potential risks and benefits. As predicted, TBP-AOs may influence peroxisome proliferator-activated receptors (PPARs), which are crucial in lipid metabolism and inflammatory responses [138,139], as well as cellular responses to stress and protection mechanisms, such as the antioxidant response element (ARE), the heat shock factor response element (HSE), and mitochondrial membrane potential (MMP). Given these potential impacts, investigating how TBP-AOs affect these pathways and their implications for human health is essential. Consequently, additional research is needed to explore the effects of TBP-AOs on these processes.
Table 7.
Potential for toxicity induction predicted using ADMET.
| No. | Compounds | Skin Sensitivity |
Eye Corrosion/ Eye Irritation |
Respiratory | Endocrine Disruption | PPARs | ARE | HSE | MMP | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen Receptor |
Aromatase | Estrogen Receptor |
||||||||||||
| EC | EI | AR | AR-LBD | ER | ER-LBD | |||||||||
| 1 | PTBP | +++ | +++ | +++ | + | +++ | +++ | + | +++ | |||||
| 2 | AO30 | +++ | +++ | +++ | + | + | + | |||||||
| 3 | AO701 | +++ | +++ | +++ | + | + | +++ | +++ | ||||||
| 4 | BHT | +++ | +++ | +++ | + | +++ | +++ | |||||||
| 5 | AO703 | +++ | +++ | +++ | + | +++ | ||||||||
| 6 | AO1222 | + | + | +++ | +++ | +++ | + | +++ | ||||||
| 7 | AO1076 | +++ | +++ | + | + | + | + | + | ||||||
| 8 | AO565 | +++ | +++ | +++ | +++ | + | +++ | +++ | +++ | +++ | ||||
| 9 | AO1425 | +++ | +++ | + | +++ | +++ | +++ | |||||||
| 10 | AO246 | + | +++ | +++ | + | + | + | +++ | + | +++ | ||||
| 11 | BHA | +++ | +++ | +++ | +++ | + | + | +++ | ||||||
| 12 | AO33 | +++ | +++ | +++ | + | + | +++ | + | + | +++ | ||||
| 13 | TBHQ | +++ | +++ | +++ | + | +++ | +++ | +++ | +++ | +++ | ||||
| 14 | AO754 | +++ | +++ | +++ | + | + | +++ | |||||||
| 15 | AO1135 | +++ | +++ | +++ | + | + | +++ | + | +++ | |||||
| 16 | AO1077 | +++ | +++ | +++ | + | + | + | +++ | ||||||
| 17 | AO132 | + | +++ | + | + | +++ | +++ | |||||||
| 18 | AO35 | + | + | +++ | +++ | |||||||||
| 19 | TBMC | + | +++ | +++ | + | + | +++ | |||||||
| 20 | AO2246 | +++ | +++ | + | + | +++ | + | +++ | +++ | |||||
| 21 | AO1081 | +++ | +++ | +++ | + | + | +++ | + | +++ | +++ | ||||
| 22 | AO425 | +++ | +++ | + | +++ | +++ | +++ | +++ | +++ | |||||
| 23 | STW | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |||
| 24 | AO3052 | +++ | +++ | +++ | + | + | + | +++ | +++ | +++ | +++ | |||
| 25 | AO702 | +++ | +++ | + | + | +++ | + | +++ | + | + | +++ | |||
| 26 | AO1024 | +++ | + | +++ | +++ | +++ | +++ | |||||||
| 27 | AO245 | +++ | + | + | + | +++ | +++ | +++ | ||||||
| 28 | AO1019 | +++ | + | +++ | +++ | + | +++ | |||||||
| 29 | AO626 | + | +++ | +++ | + | + | +++ | |||||||
| 30 | AO36 | +++ | +++ | + | + | |||||||||
| 31 | AO259 | +++ | +++ | + | + | + | +++ | + | + | +++ | ||||
| 32 | AO1035 | +++ | +++ | + | + | + | +++ | + | + | +++ | ||||
| 33 | AO80 | + | + | + | + | + | +++ | |||||||
| 34 | AO1290 | +++ | +++ | + | + | +++ | +++ | +++ | + | + | +++ | |||
| 35 | AO1098 | +++ | + | +++ | +++ | + | +++ | |||||||
| 36 | AO697 | +++ | +++ | + | +++ | +++ | + | +++ | ||||||
| 37 | AO300 | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ||||
| 38 | AOCA | +++ | +++ | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |||
| 39 | AO168 | +++ | +++ | + | + | + | +++ | |||||||
| 40 | AO1790 | +++ | +++ | + | +++ | +++ | +++ | |||||||
| 41 | AO1330 | +++ | +++ | + | +++ | + | + | + | +++ | +++ | ||||
| 42 | AO3114 | +++ | +++ | +++ | +++ | +++ | +++ | |||||||
| 43 | AO1010 | +++ | + | +++ | + | + | +++ | +++ | ||||||
+++: high probability of being active (probability score: 0.7–1). +: medium probability of being active (probability score: 0.3–0.7). (blank): low probability of being active (probability score: 0–0.3). EC/EI: eye corrosion/irritation; AR/ER: androgen/estrogen receptor; AR/ER-LBD: Androgen/Estrogen Receptor Ligand Binding Domain; PPARs: peroxisome proliferator-activated receptors; ARE: antioxidant response element; HSE: heat shock factor response element; MMP: mitochondrial membrane potential.
7. Future Perspective
Aside from TBP-AOs, many other antioxidants are being explored for their similar protective properties while presenting lower health risks. These alternatives aim to maintain product stability and safety without compromising consumer health. Natural antioxidants, such as vitamin C, vitamin E, phenolic acids, phenolic diterpenes, and flavonoids extracted from fruits and plants, provide excellent protection against oxidation [140]. These substances are generally recognized as safe and have demonstrated effectiveness in various applications, including food and cosmetics. Additionally, novel antioxidants, like peptides, proteins, and enzymes, show promise for use in food preservation, cosmetics, and therapeutics [141,142]. However, these compounds have some limitations, such as low antioxidant activity, instability under temperature, moisture, and oxygen, and high costs associated with extraction and purification processes. Therefore, synthetic antioxidants, including TBP-AOs, are still widely used in consumer products. Research is ongoing to develop modified versions of these compounds that retain their effectiveness while reducing potential health risks.
As TBP-AOs are widely used in consumer products and the demand for effective stabilizers grows, TBP-AOs are likely to see expanded use in food packaging, cosmetics, and industrial formulations owing to their antioxidant, physical, and biological properties. This increased usage will necessitate greater regulatory scrutiny and comprehensive risk assessments. However, the safety profiles of these compounds are not well-understood, raising concerns about potential health impacts. There is an urgent need for comprehensive research on the toxicological effects of TBP-AOs and standardized testing methods to elucidate their biological effects.
Continued research into their toxicity is essential for shaping industry policies and practices, ultimately ensuring enhanced protection for public health and the environment. Comprehensive information gathering is required, including extensive literature reviews and meta-analyses on their safety and efficacy, development of standardized testing protocols to assess their toxicological profiles, and establishment of clear guidelines for their safe use in consumer products. By implementing these strategies, we can deepen our understanding of the implications of TBP-AOs and ensure their use is consistent with public health and environmental safety priorities.
Author Contributions
N.M.H.H.: data collection and analysis, writing the manuscript. K.P.: data collation, review, and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the Korea Ministry of Environment (MOE) Project No. RS-2023-00215856.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yehye W.A., Rahman N.A., Ariffin A., Abd Hamid S.B., Alhadi A.A., Kadir F.A., Yaeghoobi M. Understanding the Chemistry behind the Antioxidant Activities of Butylated Hydroxytoluene (BHT): A Review. Eur. J. Med. Chem. 2015;101:295–312. doi: 10.1016/j.ejmech.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Liu R., Mabury S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020;54:11706–11719. doi: 10.1021/acs.est.0c05077. [DOI] [PubMed] [Google Scholar]
- 3.Fiege H., Voges H.-W., Hamamoto T., Umemura S., Iwata T., Miki H., Fujita Y., Buysch H.-J., Garbe D., Paulus W. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley; Hoboken, NJ, USA: 2000. Phenol Derivatives. [Google Scholar]
- 4.Jacques M.T., de Souza V., Barbosa F.A.R., Faria Santos Canto R., Lopes S.C., Prediger R.D., Braga A.L., Aschner M., Farina M. Novel Probucol Analogue, 4,4′-Diselanediylbis (2,6-Di-Tert-Butylphenol), Prevents Oxidative Glutamate Neurotoxicity In Vitro and Confers Neuroprotection in a Rodent Model of Ischemic Stroke. ACS Chem. Neurosci. 2023;14:2857–2867. doi: 10.1021/acschemneuro.3c00138. [DOI] [PubMed] [Google Scholar]
- 5.FUJITA T., FUJIMOTO Y. Effect of various diuretics on lipid peroxidation in rat renal cortical mitochondria and in the supernatant. Jpn. J. Pharmacol. 1981;31:795–800. doi: 10.1016/S0021-5198(19)52801-3. [DOI] [PubMed] [Google Scholar]
- 6.Eleazu C.O., Obeten U.N., Ozor G., Njemanze C.C., Eleazu K.C., Egedigwe-Ekeleme A.C., Okorie U.C., Ogunwa S.C., Adeolu A.I., Okoh P.-F.N., et al. Tert-Butylhydroquinone Abrogates Fructose-Induced Insulin Resistance in Rats via Mitigation of Oxidant Stress, NFkB-Mediated Inflammation in the Liver but Not the Skeletal Muscle of High Fructose Drinking Rats. J. Food Biochem. 2022;46:e14473. doi: 10.1111/jfbc.14473. [DOI] [PubMed] [Google Scholar]
- 7.Yang B., Huang H., He Q., Lu W., Zheng L., Cui L. Tert-Butylhydroquinone Prevents Oxidative Stress-Mediated Apoptosis and Extracellular Matrix Degradation in Rat Chondrocytes. Evidence-Based Complement. Altern. Med. 2021;2021:1905995. doi: 10.1155/2021/1905995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Li J., Xiang X., Zhou B., Zhao C., Wei Q., Sun Y., Chen J., Lai B., Luo Z., et al. Tert-Butylhydroquinone Attenuates Osteoarthritis by Protecting Chondrocytes and Inhibiting Macrophage Polarization. Bone Joint Res. 2021;10:704–713. doi: 10.1302/2046-3758.1011.BJR-2020-0242.R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y., Wang J., Wei F., Gu Q., Tian M., Lv H.-B. Tert-Butylhydroquinone Protects the Retina from Oxidative Stress in STZ-Induced Diabetic Rats via the PI3K/Akt/ENOS Pathway. Eur. J. Pharmacol. 2022;935:175297. doi: 10.1016/j.ejphar.2022.175297. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Cao H., He W., Zhang X., Xu R. Tert-Butylhydroquinone-Induced Formation of High-Molecular-Weight P62: A Novel Mechanism in the Activation of Nrf2-Keap1. Cell Biol. Int. 2022;46:1345–1354. doi: 10.1002/cbin.11849. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Hu S., Yang J., Yuan L., Han L., Liang F., Zhang F., Zhao H., Liu Y., Gao N. Arenobufagin Inhibits Lung Metastasis of Colorectal Cancer by Targeting C-MYC/Nrf2 Axis. Phytomedicine. 2024;127:155391. doi: 10.1016/j.phymed.2024.155391. [DOI] [PubMed] [Google Scholar]
- 12.Duan X., Li J., Li W., Xing X., Zhang Y., Li W., Zhao L., Sun G., Gao X., Li B. Antioxidant Tert-Butylhydroquinone Ameliorates Arsenic-Induced Intracellular Damages and Apoptosis through Induction of Nrf2-Dependent Antioxidant Responses as Well as Stabilization of Anti-Apoptotic Factor Bcl-2 in Human Keratinocytes. Free Radic. Biol. Med. 2016;94:74–87. doi: 10.1016/j.freeradbiomed.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Saha P., Hegde M., Chakraborty K., Singha A., Mukerjee N., Ghosh D., Kunnumakkara A.B., Khan M.S., Ahmad M.I., Ghosh A., et al. Targeted Inhibition of Colorectal Cancer Proliferation: The Dual-Modulatory Role of 2,4-DTBP on Anti-Apoptotic Bcl-2 and Survivin Proteins. J. Cell Mol. Med. 2024;28:e18150. doi: 10.1111/jcmm.18150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra R., Kushveer J.S., Khan M.I.K., Pagal S., Meena C.K., Murali A., Dhayalan A., Venkateswara Sarma V. 2,4-Di-Tert-Butylphenol Isolated from an Endophytic Fungus, Daldinia Eschscholtzii, Reduces Virulence and Quorum Sensing in Pseudomonas Aeruginosa. Front. Microbiol. 2020;11:1668. doi: 10.3389/fmicb.2020.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y.W., Lim Y., Cho S.K. 2,4-Di-tert-butylphenol, a Potential HDAC6 Inhibitor, Induces Senescence and Mitotic Catastrophe in Human Gastric Adenocarcinoma AGS Cells. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:675–683. doi: 10.1016/j.bbamcr.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Reshma V.R., Nair Devi Velayudhan Jayasree P.G.B., Baby S. Anti-Inflammatory and Anticancer Activities of Erythrodiol-3-Acetate and 2,4-Di-Tert-Butylphenol Isolated from Humboldtia Unijuga. Nat. Prod. Res. 2020;34:2319–2322. doi: 10.1080/14786419.2018.1531406. [DOI] [PubMed] [Google Scholar]
- 17.Yang L., Wang R., Lin W., Li B., Jin T., Weng Q., Zhang M., Liu P. Efficacy of 2,4-Di-Tert-Butylphenol in Reducing Ralstonia Solanacearum Virulence: Insights into the Underlying Mechanisms. ACS Omega. 2024;9:4647–4655. doi: 10.1021/acsomega.3c07887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S.J., Kim J.K., Kim H.K., Harris K., Kim C.-J., Park G.G., Park C.-S., Shin D.-H. 2,4-Di-Tert-Butylphenol from Sweet Potato Protects Against Oxidative Stress in PC12 Cells and in Mice. J. Med. Food. 2013;16:977–983. doi: 10.1089/jmf.2012.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo M., Peng J., Guo P., Wang Q., Zhang L., Shen H., Chen F., Zhang P., Lin S., Gao H., et al. Inhalation of 2,4-Di-Tert-Butylphenol-Loaded Micelles Suppresses Respiratory Syncytial Virus Infection in Mice. Antiviral Res. 2024;226:105880. doi: 10.1016/j.antiviral.2024.105880. [DOI] [PubMed] [Google Scholar]
- 20.Kruk J., Aboul-Enein B.H., Duchnik E., Marchlewicz M. Antioxidative Properties of Phenolic Compounds and Their Effect on Oxidative Stress Induced by Severe Physical Exercise. J. Physiol. Sci. 2022;72:19. doi: 10.1186/s12576-022-00845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisel P., Al-Momani L., Müller M. The Tert-Butyl Group in Chemistry and Biology. Org. Biomol. Chem. 2008;6:2655–2665. doi: 10.1039/b800083b. [DOI] [PubMed] [Google Scholar]
- 22.Mamari H.H. Al Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis. In: Badria F.A., editor. Phenolic Compounds. IntechOpen; Rijeka, Croatia: 2021. p. 4. [Google Scholar]
- 23.de Araújo F.F., de Paulo Farias D., Neri-Numa I.A., Pastore G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021;338:127535. doi: 10.1016/j.foodchem.2020.127535. [DOI] [PubMed] [Google Scholar]
- 24.de Lima Cherubim D.J., Buzanello Martins C.V., Oliveira Fariña L., da Silva de Lucca R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020;19:33–37. doi: 10.1111/jocd.13093. [DOI] [PubMed] [Google Scholar]
- 25.Gasmi A., Mujawdiya P.K., Noor S., Lysiuk R., Darmohray R., Piscopo S., Lenchyk L., Antonyak H., Dehtiarova K., Shanaida M., et al. Polyphenols in Metabolic Diseases. Molecules. 2022;27:6280. doi: 10.3390/molecules27196280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westphal M.V., Wolfstädter B.T., Plancher J.-M., Gatfield J., Carreira E.M. Evaluation of Tert-Butyl Isosteres: Case Studies of Physicochemical and Pharmacokinetic Properties, Efficacies, and Activities. ChemMedChem. 2015;10:461–469. doi: 10.1002/cmdc.201402502. [DOI] [PubMed] [Google Scholar]
- 27.Chan S.-C., Palone A., Bietti M., Costas M. Tert-Butyl as a Functional Group: Non-Directed Catalytic Hydroxylation of Sterically Congested Primary C−H Bonds. Angew. Chem. Int. Ed. 2024;63:e202402858. doi: 10.1002/anie.202402858. [DOI] [PubMed] [Google Scholar]
- 28.Charlton N.C., Mastyugin M., Török B., Török M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules. 2023;28:1057. doi: 10.3390/molecules28031057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouellette R.J., Rawn J.D. 5—Aromatic Compounds. In: Ouellette R.J., Rawn J.D., editors. Principles of Organic Chemistry. Elsevier; Boston, MA, USA: 2015. pp. 133–162. [Google Scholar]
- 30.Mtashobya L. Insights on Tert-Butyl Alkylation Effects on Fluorobenzene. Sci. Afr. 2021;11:e00659. doi: 10.1016/j.sciaf.2020.e00659. [DOI] [Google Scholar]
- 31.Nemeth T., de Wild T., Gubler L., Nauser T. Impact of Substitution on Reactions and Stability of One-Electron Oxidised Phenyl Sulfonates in Aqueous Solution. Phys. Chem. Chem. Phys. 2022;24:895–901. doi: 10.1039/D1CP04518K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollande L., Domenek S., Allais F. Chemo-Enzymatic Synthesis of Renewable Sterically-Hindered Phenolic Antioxidants with Tunable Polarity from Lignocellulose and Vegetal Oil Components. Int. J. Mol. Sci. 2018;19:3358. doi: 10.3390/ijms19113358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liszewski W., Owen B., Fournier E., Kerchinsky L., Wei J., Scheman A. Assessment of Contact Allergens in “Hypoallergenic” Athletic Shoes by Mass Spectrometry. Dermatitis. 2023;34:532–535. doi: 10.1089/derm.2022.0102. [DOI] [PubMed] [Google Scholar]
- 34.Kaniwa M.-A., Isama K., Nakamura A., Kantoh H., Itoh M., Miyoshi K., Saito S., Shono M. Identification of Causative Chemicals of Allergic Contact Dermatitis Using a Combination of Patch Testing in Patients and Chemical Analysis. Contact Dermat. 1994;30:26–34. doi: 10.1111/j.1600-0536.1994.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 35.Murru C., Badía-Laíño R., Díaz-García M.E. Oxidative Stability of Vegetal Oil-Based Lubricants. ACS Sustain. Chem. Eng. 2021;9:1459–1476. doi: 10.1021/acssuschemeng.0c06988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia D., Wang Y., Liu H., Yan J., Lin H., Han S. Research Progress of Antioxidant Additives for Lubricating Oils. Lubricants. 2024;12:115. doi: 10.3390/lubricants12040115. [DOI] [Google Scholar]
- 37.Hermabessiere L., Receveur J., Himber C., Mazurais D., Huvet A., Lagarde F., Lambert C., Paul-Pont I., Dehaut A., Jezequel R., et al. An Irgafos® 168 Story: When the Ubiquity of an Additive Prevents Studying Its Leaching from Plastics. Sci. Total Environ. 2020;749:141651. doi: 10.1016/j.scitotenv.2020.141651. [DOI] [PubMed] [Google Scholar]
- 38.Cui S., Yu Y., Zhan T., Zhang C., Zhuang S. 2,6-Di-Tert-Butylphenol and Its Quinone Metabolite Trigger Aberrant Transcriptional Responses in C57BL/6 Mice Liver. Sci. Total Environ. 2021;778:146322. doi: 10.1016/j.scitotenv.2021.146322. [DOI] [PubMed] [Google Scholar]
- 39.Akhmadullin R.M., Gatiyatullin D.R., Vasil’ev L.A., Akhmadullina A.G., Mukmeneva N.A., Cherezova E.N., Yang M. Performance of 4,4’-Bis(2,6-Di-Tert-Butylphenol) in Stabilization of Isoprene Rubber and Polypropylene. Russ. J. Appl. Chem. 2015;88:833–838. doi: 10.1134/S1070427215050171. [DOI] [Google Scholar]
- 40.Thörnblom K., Palmlöf M., Hjertberg T. The Extractability of Phenolic Antioxidants into Water and Organic Solvents from Polyethylene Pipe Materials—Part I. Polym. Degrad. Stab. 2011;96:1751–1760. doi: 10.1016/j.polymdegradstab.2011.07.023. [DOI] [Google Scholar]
- 41.Elena Dreassi M.B., Corti P. Evaluation of Release of Antioxidant from High Density Polyethylene by Planar Chromatography. Food Addit. Contam. 1998;15:466–472. doi: 10.1080/02652039809374667. [DOI] [PubMed] [Google Scholar]
- 42.Xu J., Hao Y., Yang Z., Li W., Xie W., Huang Y., Wang D., He Y., Liang Y., Matsiko J., et al. Rubber Antioxidants and Their Transformation Products: Environmental Occurrence and Potential Impact. Int. J. Environ. Res. Public Health. 2022;19:14595. doi: 10.3390/ijerph192114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manteghi A., Ahmadi S., Arabi H. Enhanced Thermo-Oxidative Stability through Covalent Attachment of Hindered Phenolic Antioxidant on Surface Functionalized Polypropylene. Polymer. 2018;138:41–48. doi: 10.1016/j.polymer.2018.01.048. [DOI] [Google Scholar]
- 44.Almeida S., Ozkan S., Gonçalves D., Paulo I., Queirós C.S.G.P., Ferreira O., Bordado J., dos Santos R. A Brief Evaluation of Antioxidants, Antistatics, and Plasticizers Additives from Natural Sources for Polymers Formulation. Polymers. 2023;15:6. doi: 10.3390/polym15010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barret J., Gijsman P., Swagten J., Lange R.F.M. The Interaction of a Phenolic Anti-Oxidant and an Aromatic Amine in a Thermo-Oxidative Ageing Process. Polym. Degrad. Stab. 2002;75:367–374. doi: 10.1016/S0141-3910(01)00241-5. [DOI] [Google Scholar]
- 46.Siritham C., Thammakhet-Buranachai C., Thavarungkul P., Kanatharana P. A Stir Foam Composed of Graphene Oxide, Poly(Ethylene Glycol) and Natural Latex for the Extraction of Preservatives and Antioxidant. Microchimica Acta. 2018;185:148. doi: 10.1007/s00604-017-2643-z. [DOI] [PubMed] [Google Scholar]
- 47.Le Coz C.J., Schneider G.-A. Contact Dermatitis from Tertiary-Butylhydroquinone in a Hair Dye, with Cross-Sensitivity to BHA and BHT. Contact Dermat. 1998;39:39–40. doi: 10.1111/j.1600-0536.1998.tb05819.x. [DOI] [PubMed] [Google Scholar]
- 48.White I.R., Lovell C.R., Cronin E. Antioxidants in Cosmetics. Contact Dermat. 1984;11:265–267. doi: 10.1111/j.1600-0536.1984.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 49.Guan Y., Chu Q., Fu L., Ye J. Determination of Antioxidants in Cosmetics by Micellar Electrokinetic Capillary Chromatography with Electrochemical Detection. J. Chromatogr. A. 2005;1074:201–204. doi: 10.1016/j.chroma.2005.03.063. [DOI] [PubMed] [Google Scholar]
- 50.Patrick E., Juberg D.R., O’Donoghue J., Maibach H.I. Depigmentation with Tert-Butyl Hydroquinone Using Black Guinea Pigs. Food Chem. Toxicol. 1999;37:169–175. doi: 10.1016/S0278-6915(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 51.Thörneby-Andersson K., Sterner O., Hansson C. Tyrosinase-Mediated Formation of a Reactive Quinone from the Depigmenting Agents, 4-Tert-Butylphenol and 4-Tert-Butylcatechol. Pigment. Cell Res. 2000;13:33–38. doi: 10.1034/j.1600-0749.2000.130107.x. [DOI] [PubMed] [Google Scholar]
- 52.Yonemoto K., Gellin G.A., Epstein W.L., Fukuyama K. Glutathione Reductase Activity in Skin Exposed to 4-Tertiary Butyl Catechol. Int. Arch. Occup. Environ. Health. 1983;51:341–345. doi: 10.1007/BF00378347. [DOI] [PubMed] [Google Scholar]
- 53.Mansur J.D., Fukuyama K., Gellin G.A., Epstein W.L. Effects of 4-Tertiary Butyl Catechol on Tissue Cultured Melanocytes. J. Investig. Dermatol. 1978;70:275–279. doi: 10.1111/1523-1747.ep12541510. [DOI] [PubMed] [Google Scholar]
- 54.Yonemoto K., Gellin G.A., Epstein W.L., Fukuyama K. Reduction in Eumelanin by the Activation of Glutathione Reductase and γ-Glutamyl Transpeptidase after Exposure to a Depigmenting Chemical. Biochem. Pharmacol. 1983;32:1379–1382. doi: 10.1016/0006-2952(83)90450-1. [DOI] [PubMed] [Google Scholar]
- 55.Singh A.K., Kim J.Y., Lee Y.S. Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules. 2022;27:7513. doi: 10.3390/molecules27217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heiserman W.M., Can S.Z., Walker R.A., Begley T.H., Limm W. Interfacial Behavior of Common Food Contact Polymer Additives. J. Colloid. Interface Sci. 2007;311:587–594. doi: 10.1016/j.jcis.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 57.Rani M., Shim W.J., Han G.M., Jang M., Song Y.K., Hong S.H. Benzotriazole-Type Ultraviolet Stabilizers and Antioxidants in Plastic Marine Debris and Their New Products. Sci. Total Environ. 2017;579:745–754. doi: 10.1016/j.scitotenv.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 58.Markley L.C., González Bonet A.M., Ogungbesan A., Bandele O.J., Bailey A.B., Patton G.W. Safety Assessment for Tris(2,4-Di-Tert-Butylphenyl) Phosphite (Irgafos 168) Used as an Antioxidant and Stabilizer in Food Contact Applications. Food Chem. Toxicol. 2023;178:113877. doi: 10.1016/j.fct.2023.113877. [DOI] [PubMed] [Google Scholar]
- 59.Wu X., Liu P., Shi H., Wang H., Huang H., Shi Y., Gao S. Photo Aging and Fragmentation of Polypropylene Food Packaging Materials in Artificial Seawater. Water Res. 2021;188:116456. doi: 10.1016/j.watres.2020.116456. [DOI] [PubMed] [Google Scholar]
- 60.Saunier J., Mazel V., Paris C., Yagoubi N. Polymorphism of Irganox 1076®: Discovery of New Forms and Direct Characterization of the Polymorphs on a Medical Device by Raman Microspectroscopy. Eur. J. Pharm. Biopharm. 2010;75:443–450. doi: 10.1016/j.ejpb.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Saunier J., Herry J.-M., Marlière C., Renault M., Bellon-Fontaine M.-N., Yagoubi N. Modification of the Bacterial Adhesion of Staphylococcus Aureus by Antioxidant Blooming on Polyurethane Films. Mater. Sci. Eng. C. 2015;56:522–531. doi: 10.1016/j.msec.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y., Zhao Q., Anderson J.M., Hiltner A., Lodoen G.A., Payet C.R. Effect of Some Additives on the Biostability of a Poly(Etherurethane) Elastomer. J. Biomed. Mater. Res. 1991;25:725–739. doi: 10.1002/jbm.820250604. [DOI] [PubMed] [Google Scholar]
- 63.Parris P., Martin E.A., Stanard B., Glowienke S., Dolan D.G., Li K., Binazon O., Giddings A., Whelan G., Masuda-Herrera M., et al. Considerations When Deriving Compound-Specific Limits for Extractables and Leachables from Pharmaceutical Products: Four Case Studies. Regul. Toxicol. Pharmacol. 2020;118:104802. doi: 10.1016/j.yrtph.2020.104802. [DOI] [PubMed] [Google Scholar]
- 64.Bartsch N., Girard M., Schneider L., Van De Weijgert V., Wilde A., Kappenstein O., Vieth B., Hutzler C., Luch A. Chemical Stabilization of Polymers: Implications for Dermal Exposure to Additives. J. Environ. Sci. Health Part. A. 2018;53:405–420. doi: 10.1080/10934529.2017.1412192. [DOI] [PubMed] [Google Scholar]
- 65.Birnbaum L.S., Heaney S.M. Dermal Absorption of the Antioxidant 4,4′-Thiobis(6-Tert-Butyl-m-Cresol) in Sencar Mice and Fischer Rats. Toxicol. Lett. 1987;37:13–19. doi: 10.1016/0378-4274(87)90161-5. [DOI] [PubMed] [Google Scholar]
- 66.Final Report on the Safety Assessment of BHT. Int. J. Toxicol. 2002;21:19–94. doi: 10.1080/10915810290096513. [DOI] [PubMed] [Google Scholar]
- 67.Ji X., Liu J., Liang J., Feng X., Liu X., Wang Y., Chen X., Qu G., Yan B., Liu R. The Hidden Diet: Synthetic Antioxidants in Packaged Food and Their Impact on Human Exposure and Health. Environ. Int. 2024;186:108613. doi: 10.1016/j.envint.2024.108613. [DOI] [PubMed] [Google Scholar]
- 68.Liu R., Mabury S.A. Synthetic Phenolic Antioxidants in Personal Care Products in Toronto, Canada: Occurrence, Human Exposure, and Discharge via Greywater. Environ. Sci. Technol. 2019;53:13440–13448. doi: 10.1021/acs.est.9b04120. [DOI] [PubMed] [Google Scholar]
- 69.Angelini E., Marinaro C., Carrozzo A.M., Bianchi L., Delogu A., Giannelo G., Nini G. Allergic Contact Dermatitis of the Lip Margins from Para-Tertiary-Butylphenol in a Lip Liner. Contact Dermat. 1993;28:146–148. doi: 10.1111/j.1600-0536.1993.tb03375.x. [DOI] [PubMed] [Google Scholar]
- 70.Rich P., Belozer M.L., Norris P., Storrs F.J. Allergic Contact Dermatitis to Two Antioxidants in Latex Gloves: 4,4′-Thiobis(6-Tert-Butyl-Meta-Cresol) (Lowinox 44S36) and Butylhydroxyanisole: Allergen Alternatives for Glove-Allergic Patients. J. Am. Acad. Dermatol. 1991;24:37–43. doi: 10.1016/0190-9622(91)70006-N. [DOI] [PubMed] [Google Scholar]
- 71.Myers L.P., Law B.F., Fedorowicz A., Siegel P.D., Butterworth L.F., Anderson S.E., Sussman G., Shapiro M., Meade B.J., Beezhold D. Identification of Phenolic Dermal Sensitizers in a Wound Closure Tape. J. Immunotoxicol. 2007;4:303–310. doi: 10.1080/15476910701680236. [DOI] [PubMed] [Google Scholar]
- 72.Antelmi A., Lejding T., Bruze M., Mowitz M., Dahlin J. 4,4′-Thiobis(2-Tert-Butyl-5-Methylphenol), an Antioxidant in Medical Devices That May Cause Allergic Contact Dermatitis. Contact Dermatitis. 2023;89:103–106. doi: 10.1111/cod.14335. [DOI] [PubMed] [Google Scholar]
- 73.Mowitz M., Lejding T., Ulriksdotter J., Antelmi A., Bruze M., Svedman C. Further Evidence of Allergic Contact Dermatitis Caused by 2,2′-Methylenebis(6-Tert-Butyl-4-Methylphenol) Monoacrylate, a New Sensitizer in the Dexcom G6 Glucose Sensor. Dermatitis. 2022;33:287–292. doi: 10.1097/DER.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 74.Sherson D.L., Jacobsen I.B., Thomsen G.F. The Antioxidant, Tert-Butylhydroquinone: A New Cause of Asthma. Occup. Med. 2023;73:109–111. doi: 10.1093/occmed/kqac093. [DOI] [PubMed] [Google Scholar]
- 75.Simoneit B.R.T., Medeiros P.M., Didyk B.M. Combustion Products of Plastics as Indicators for Refuse Burning in the Atmosphere. Environ. Sci. Technol. 2005;39:6961–6970. doi: 10.1021/es050767x. [DOI] [PubMed] [Google Scholar]
- 76.Gao Y., Gu Y., Wei Y. Determination of Polymer Additives–Antioxidants and Ultraviolet (UV) Absorbers by High-Performance Liquid Chromatography Coupled with UV Photodiode Array Detection in Food Simulants. J. Agric. Food Chem. 2011;59:12982–12989. doi: 10.1021/jf203257b. [DOI] [PubMed] [Google Scholar]
- 77.Gisele C., Maziero C.B., Toledo M.C.F. Estimates of the Theoretical Maximum Daily Intake of Phenolic Antioxidants BHA, BHT and TBHQ in Brazil. Food Addit. Contam. 2001;18:365–373. doi: 10.1080/02652030120645. [DOI] [PubMed] [Google Scholar]
- 78.Kirkpatrick D.C., Lauer B.H. Intake of Phenolic Antioxidants from Foods in Canada. Food Chem. Toxicol. 1986;24:1035–1037. doi: 10.1016/0278-6915(86)90285-1. [DOI] [PubMed] [Google Scholar]
- 79.Suh H.-J., Chung M.-S., Cho Y.-H., Kim J.-W., Kim D.-H., Han K.-W., Kim C.-J. Estimated Daily Intakes of Butylated Hydroxyanisole (BHA), Butylated Hydroxytoluene (BHT) and Tert-Butyl Hydroquinone (TBHQ) Antioxidants in Korea. Food Addit. Contam. 2005;22:1176–1188. doi: 10.1080/02652030500195288. [DOI] [PubMed] [Google Scholar]
- 80.Nawel Bemrah J.-C.L., Volatier J.-L. Assessment of Dietary Exposure in the French Population to 13 Selected Food Colours, Preservatives, Antioxidants, Stabilizers, Emulsifiers and Sweeteners. Food Addit. Contam. Part. B. 2008;1:2–14. doi: 10.1080/19393210802236943. [DOI] [PubMed] [Google Scholar]
- 81.Mancini F.R., Paul D., Gauvreau J., Volatier J.L., Vin K., Hulin M. Dietary Exposure to Benzoates (E210–E213), Parabens (E214–E219), Nitrites (E249–E250), Nitrates (E251–E252), BHA (E320), BHT (E321) and Aspartame (E951) in Children Less than 3 Years Old in France. Food Addit. Contam. Part. A. 2015;32:293–306. doi: 10.1080/19440049.2015.1007535. [DOI] [PubMed] [Google Scholar]
- 82.Suh H.-J., Choi S.-H. Safety Assessment of Estimated Daily Intakes of Antioxidants in Korean Using Dietary Survey Approach and Food Supply Survey Approach. Korean J. Food Sci. Technol. 2010;42:762–767. [Google Scholar]
- 83.Soubra L., Sarkis D., Hilan C., Verger P. Dietary Exposure of Children and Teenagers to Benzoates, Sulphites, Butylhydroxyanisol (BHA) and Butylhydroxytoluen (BHT) in Beirut (Lebanon) Regul. Toxicol. Pharmacol. 2007;47:68–77. doi: 10.1016/j.yrtph.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Verhagen H., Deerenberg I., Marx A., ten Hoor F., Henderson P.T., Kleinjans J.C.S. Estimate of the Maximal Daily Dietary Intake of Butylated Hydroxyanisole and Butylated Hydroxytoluene in The Netherlands. Food Chem. Toxicol. 1990;28:215–220. doi: 10.1016/0278-6915(90)90033-J. [DOI] [PubMed] [Google Scholar]
- 85.Vin K., Connolly A., McCaffrey T., McKevitt A., O’Mahony C., Prieto M., Tennant D., Hearty A., Volatier J.L. Estimation of the Dietary Intake of 13 Priority Additives in France, Italy, the UK and Ireland as Part of the FACET Project. Food Addit. Contam. Part. A. 2013;30:2050–2080. doi: 10.1080/19440049.2013.851417. [DOI] [PubMed] [Google Scholar]
- 86.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the Re-Evaluation of Butylated Hydroxytoluene BHT (E 321) as a Food Additive. EFSA J. 2012;10:2588. doi: 10.2903/j.efsa.2012.2588. [DOI] [Google Scholar]
- 87.Lestido-Cardama A., de Quirós A., Bustos J., Lomo M.L., Paseiro Losada P., Sendón R. Estimation of Dietary Exposure to Contaminants Transferred from the Packaging in Fatty Dry Foods Based on Cereals. Foods. 2020;9:1038. doi: 10.3390/foods9081038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X., Xu M., Yang A., Wang Y., Hou S., Zheng N., Liang D., Hua X., Dong D. Health Risks of Population Exposure to Phthalic Acid Esters through the Use of Plastic Containers for Takeaway Food in China. Sci. Total Environ. 2021;785:147347. doi: 10.1016/j.scitotenv.2021.147347. [DOI] [Google Scholar]
- 89.Tang S., Sun X., Qiao X., Cui W., Yu F., Zeng X., Covaci A., Chen D. Prenatal Exposure to Emerging Plasticizers and Synthetic Antioxidants and Their Potency to Cross Human Placenta. Environ. Sci. Technol. 2022;56:8507–8517. doi: 10.1021/acs.est.2c01141. [DOI] [PubMed] [Google Scholar]
- 90.Du B., Zhang Y., Lam J.C.W., Pan S., Huang Y., Chen B., Lan S., Li J., Luo D., Zeng L. Prevalence, Biotransformation, and Maternal Transfer of Synthetic Phenolic Antioxidants in Pregnant Women from South China. Environ. Sci. Technol. 2019;53:13959–13969. doi: 10.1021/acs.est.9b04709. [DOI] [PubMed] [Google Scholar]
- 91.Tan H., Yang L., Huang Y., Tao L., Chen D. “Novel” Synthetic Antioxidants in House Dust from Multiple Locations in the Asia-Pacific Region and the United States. Environ. Sci. Technol. 2021;55:8675–8682. doi: 10.1021/acs.est.1c00195. [DOI] [PubMed] [Google Scholar]
- 92.Wang W., Asimakopoulos A.G., Abualnaja K.O., Covaci A., Gevao B., Johnson-Restrepo B., Kumosani T.A., Malarvannan G., Minh T.B., Moon H.-B., et al. Synthetic Phenolic Antioxidants and Their Metabolites in Indoor Dust from Homes and Microenvironments. Environ. Sci. Technol. 2016;50:428–434. doi: 10.1021/acs.est.5b04826. [DOI] [PubMed] [Google Scholar]
- 93.Liu Z., Yin H., Dang Z. Do Estrogenic Compounds in Drinking Water Migrating from Plastic Pipe Distribution System Pose Adverse Effects to Human? An Analysis of Scientific Literature. Environ. Sci. Pollut. Res. 2017;24:2126–2134. doi: 10.1007/s11356-016-8032-z. [DOI] [PubMed] [Google Scholar]
- 94.Bell A.M., Baier R., Kocher B., Reifferscheid G., Buchinger S., Ternes T. Ecotoxicological Characterization of Emissions from Steel Coatings in Contact with Water. Water Res. 2020;173:115525. doi: 10.1016/j.watres.2020.115525. [DOI] [PubMed] [Google Scholar]
- 95.Cui S., Zhang X., Liu J., Zhou L., Shang Y., Zhang C., Liu W., Zhuang S. Natural Sunlight-Driven Aquatic Toxicity Enhancement of 2,6-Di-Tert-Butylphenol toward Photobacterium Phosphoreum. Environ. Pollut. 2019;251:66–71. doi: 10.1016/j.envpol.2019.04.123. [DOI] [PubMed] [Google Scholar]
- 96.Oztas P., Polat M., Cinar L., Alli N. Shoe Dermatitis from Para-Tertiary Butylphenol Formaldehyde. Contact Dermat. 2007;56:294–295. doi: 10.1111/j.1600-0536.2006.01014.x. [DOI] [PubMed] [Google Scholar]
- 97.Sadakane K., Ichinose T., Takano H., Yanagisawa R., Koike E., Inoue K. The Alkylphenols 4-Nonylphenol, 4-Tert-Octylphenol and 4-Tert-Butylphenol Aggravate Atopic Dermatitis-like Skin Lesions in NC/Nga Mice. J. Appl. Toxicol. 2014;34:893–902. doi: 10.1002/jat.2911. [DOI] [PubMed] [Google Scholar]
- 98.Yang F., Sarangarajan R., Caroline Le Poole I., Boissy R.E., Medrano E.E. The Cytotoxicity and Apoptosis Induced by 4-Tertiary Butylphenol in Human Melanocytes Are Independent of Tyrosinase Activity. J. Investig. Dermatol. 2000;114:157–164. doi: 10.1046/j.1523-1747.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 99.Manga P., Sheyn D., Yang F., Sarangarajan R., Boissy R.E. A Role for Tyrosinase-Related Protein 1 in 4-Tert-Butylphenol-Induced Toxicity in Melanocytes: Implications for Vitiligo. Am. J. Pathol. 2006;169:1652–1662. doi: 10.2353/ajpath.2006.050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nair X., Tramposch K.M. The Yucatan Miniature Swine as an in Vivo Model for Screening Skin Depigmentation. J. Dermatol. Sci. 1991;2:428–433. doi: 10.1016/0923-1811(91)90007-K. [DOI] [PubMed] [Google Scholar]
- 101.Kawashima T., Yonemoto K., Gellin G.A., Epstein W.L., Fukuyama K. Effects of 4-Tertiary Butyl Catechol on Glutathione-Metabolizing Enzymes In Vivo and In Vitro. J. Investig. Dermatol. 1984;82:53–56. doi: 10.1111/1523-1747.ep12259115. [DOI] [PubMed] [Google Scholar]
- 102.Harris J.E. Chemical-Induced Vitiligo. Dermatol. Clin. 2017;35:151–161. doi: 10.1016/j.det.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H., Liu J., Qiang S., Che Y., Hu T. 4-Tert-Butylphenol Impairs the Liver by Inducing Excess Liver Lipid Accumulation via Disrupting the Lipid Metabolism Pathway in Zebrafish. Environ. Pollut. 2024;356:124385. doi: 10.1016/j.envpol.2024.124385. [DOI] [PubMed] [Google Scholar]
- 104.Cui J., Qiu M., Liu Y., Liu Y., Tang Y., Teng X., Li S. Nano-Selenium Protects Grass Carp Hepatocytes against 4-Tert-Butylphenol-Induced Mitochondrial Apoptosis and Necroptosis via Suppressing ROS-PARP1 Axis. Fish. Shellfish. Immunol. 2023;135:108682. doi: 10.1016/j.fsi.2023.108682. [DOI] [PubMed] [Google Scholar]
- 105.Cui J., Zhou Q., Yu M., Liu Y., Teng X., Gu X. 4-Tert-Butylphenol Triggers Common Carp Hepatocytes Ferroptosis via Oxidative Stress, Iron Overload, SLC7A11/GSH/GPX4 Axis, and ATF4/HSPA5/GPX4 Axis. Ecotoxicol. Environ. Saf. 2022;242:113944. doi: 10.1016/j.ecoenv.2022.113944. [DOI] [PubMed] [Google Scholar]
- 106.Ansar S., Tabassum H., Jameil N. Al Protective Effect of Butylated Hydroxytoluene on Ferric Nitrilotriacetate Induced Hepatotoxicity and Oxidative Stress in Mice. Hum. Exp. Toxicol. 2013;32:513–521. doi: 10.1177/0960327113477876. [DOI] [PubMed] [Google Scholar]
- 107.Fahim S.A., Ibrahim S., Tadros S.A., Badary O.A. Protective Effects of Butylated Hydroxytoluene on the Initiation of N-Nitrosodiethylamine-Induced Hepatocellular Carcinoma in Albino Rats. Hum. Exp. Toxicol. 2023;42:09603271231165664. doi: 10.1177/09603271231165664. [DOI] [PubMed] [Google Scholar]
- 108.Al-Qahtani W.H., Binobead M.A. Anti-Inflammatory, Antioxidant and Antihepatotoxic Effects of Spirulina Platensis against d-Galactosamine Induced Hepatotoxicity in Rats. Saudi J. Biol. Sci. 2019;26:647–652. doi: 10.1016/j.sjbs.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guarisco J.A., Hall J.O., Coulombe R.A. Mechanisms of Butylated Hydroxytoluene Chemoprevention of Aflatoxicosis—Inhibition of Aflatoxin B1 Metabolism. Toxicol. Appl. Pharmacol. 2008;227:339–346. doi: 10.1016/j.taap.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 110.Guarisco J.A., Hall J.O., Coulombe R.A. Butylated Hydroxytoluene Chemoprevention of Aflatoxicosis—Effects on Aflatoxin B1 Bioavailability, Hepatic DNA Adduct Formation, and Biliary Excretion. Food Chem. Toxicol. 2008;46:3727–3731. doi: 10.1016/j.fct.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 111.Abou-Hadeed A.H., Mohamed A.T., Hegab D.Y., Ghoneim M.H. Ethoxyquin and Butylated Hydroxy Toluene Distrub the Hematological Parameters and Induce Structural and Functional Alterations in Liver of Rats. Arch. Razi Inst. 2021;76:1765–1776. doi: 10.22092/ari.2021.356439.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stierum R., Conesa A., Heijne W., van Ommen B., Junker K., Scott M.P., Price R.J., Meredith C., Lake B.G., Groten J. Transcriptome Analysis Provides New Insights into Liver Changes Induced in the Rat upon Dietary Administration of the Food Additives Butylated Hydroxytoluene, Curcumin, Propyl Gallate and Thiabendazole. Food Chem. Toxicol. 2008;46:2616–2628. doi: 10.1016/j.fct.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 113.Engin A.B., Bukan N., Kurukahvecioglu O., Memis L., Engin A. Effect of Butylated Hydroxytoluene (E321) Pretreatment versus l-Arginine on Liver Injury after Sub-Lethal Dose of Endotoxin Administration. Environ. Toxicol. Pharmacol. 2011;32:457–464. doi: 10.1016/j.etap.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 114.Sun Z., Tang Z., Yang X., Liu Q.S., Zhang J., Zhou Q., Jiang G. 3-Tert-Butyl-4-Hydroxyanisole Impairs Hepatic Lipid Metabolism in Male Mice Fed with a High-Fat Diet. Environ. Sci. Technol. 2022;56:3204–3213. doi: 10.1021/acs.est.1c07182. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi O., Hiraga K. Effects of Four Bisphenolic Antioxidants on Lipid Contents of Rat Liver. Toxicol. Lett. 1981;8:77–86. doi: 10.1016/0378-4274(81)90142-9. [DOI] [PubMed] [Google Scholar]
- 116.Takagi A., Kawasaki N., Momma J., Aida Y., Ohno Y., Hasegawa R., Kurokawa Y. Toxicity Studies of a Synthetic Antioxidant, 2, 2′-Methylenebis (4-ethyl-6-tert-butylphenol) in Rats 2. Uncoupling Effect on Oxidative Phosphorylation of Liver Mitochondria. J. Toxicol. Sci. 1993;18:49–55. doi: 10.2131/jts.18.49. [DOI] [PubMed] [Google Scholar]
- 117.Matsumoto K., Ochiai T., Sekita K., Uchida O., Furuya T., Kurokawa Y. Chronic Toxicity of 2, 4, 6-Tri-tert-butylphenol in Rats. J. Toxicol. Sci. 1991;16:167–179. doi: 10.2131/jts.16.167. [DOI] [PubMed] [Google Scholar]
- 118.Yoshifumi M., Michihito T., Fumio F., Kazuhiro T., Hidetaka S., Yuzo H. Pneumotoxicity of Butylated Hydroxytoluene Applied Dermally to CD-1 Mice. Toxicol. Lett. 1986;34:99–105. doi: 10.1016/0378-4274(86)90151-7. [DOI] [PubMed] [Google Scholar]
- 119.Thompson D.C., Trush M.A. Studies on the Mechanism of Enhancement of Butylated Hydroxytoluene-Induced Mouse Lung Toxicity by Butylated Hydroxyanisole. Toxicol. Appl. Pharmacol. 1988;96:122–131. doi: 10.1016/0041-008X(88)90254-2. [DOI] [PubMed] [Google Scholar]
- 120.Bauer A.K., Dwyer-Nield L.D. Chapter 10—Two-Stage 3-Methylcholanthrene and Butylated Hydroxytoluene-Induced Lung Carcinogenesis in Mice. In: Galluzzi L., Buqué A., editors. Carcinogen-Driven Mouse Models of Oncogenesis. Volume 163. Academic Press; Cambridge, MA, USA: 2021. pp. 153–173. Methods in Cell Biology. [DOI] [PubMed] [Google Scholar]
- 121.Vikis H.G., Gelman A.E., Franklin A., Stein L., Rymaszewski A., Zhu J., Liu P., Tichelaar J.W., Krupnick A.S., You M. Neutrophils Are Required for 3-Methylcholanthrene-Initiated, Butylated Hydroxytoluene-Promoted Lung Carcinogenesis. Mol. Carcinog. 2012;51:993–1002. doi: 10.1002/mc.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shearn C.T., Fritz K.S., Thompson J.A. Protein Damage from Electrophiles and Oxidants in Lungs of Mice Chronically Exposed to the Tumor Promoter Butylated Hydroxytoluene. Chem. Biol. Interact. 2011;192:278–286. doi: 10.1016/j.cbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 123.Bauer A.K., Dixon D., DeGraff L.M., Cho H.-Y., Walker C.R., Malkinson A.M., Kleeberger S.R. Toll-Like Receptor 4 in Butylated Hydroxytoluene–Induced Mouse Pulmonary Inflammation and Tumorigenesis. JNCI J. Natl. Cancer Inst. 2005;97:1778–1781. doi: 10.1093/jnci/dji403. [DOI] [PubMed] [Google Scholar]
- 124.Eskandani M., Hamishehkar H., Ezzati Nazhad Dolatabadi J. Cytotoxicity and DNA Damage Properties of Tert-Butylhydroquinone (TBHQ) Food Additive. Food Chem. 2014;153:315–320. doi: 10.1016/j.foodchem.2013.12.087. [DOI] [PubMed] [Google Scholar]
- 125.Endo S., Nishiyama A., Suyama M., Takemura M., Soda M., Chen H., Tajima K., El-Kabbani O., Bunai Y., Hara A., et al. Protective Roles of Aldo-Keto Reductase 1B10 and Autophagy against Toxicity Induced by p-Quinone Metabolites of Tert-Butylhydroquinone in Lung Cancer A549 Cells. Chem. Biol. Interact. 2015;234:282–289. doi: 10.1016/j.cbi.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 126.Olsen C.M., Meussen-Elholm E.T.M., Holme J.A., Hongslo J.K. Brominated Phenols: Characterization of Estrogen-like Activity in the Human Breast Cancer Cell-Line MCF-7. Toxicol. Lett. 2002;129:55–63. doi: 10.1016/S0378-4274(01)00469-6. [DOI] [PubMed] [Google Scholar]
- 127.Myllymäki S., Haavisto T., Vainio M., Toppari J., Paranko J. In Vitro Effects of Diethylstilbestrol, Genistein, 4-Tert-Butylphenol, and 4-Tert-Octylphenol on Steroidogenic Activity of Isolated Immature Rat Ovarian Follicles. Toxicol. Appl. Pharmacol. 2005;204:69–80. doi: 10.1016/j.taap.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Haavisto T.E., Adamsson N.A., Myllymäki S.A., Toppari J., Paranko J. Effects of 4-Tert-Octylphenol, 4-Tert-Butylphenol, and Diethylstilbestrol on Prenatal Testosterone Surge in the Rat. Reprod. Toxicol. 2003;17:593–605. doi: 10.1016/S0890-6238(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 129.Pérez-Albaladejo E., Lacorte S., Porte C. Differential Toxicity of Alkylphenols in JEG-3 Human Placental Cells: Alteration of P450 Aromatase and Cell Lipid Composition. Toxicol. Sci. 2019;167:336–346. doi: 10.1093/toxsci/kfy243. [DOI] [PubMed] [Google Scholar]
- 130.Pop A., Drugan T., Gutleb A.C., Lupu D., Cherfan J., Loghin F., Kiss B. Estrogenic and Anti-Estrogenic Activity of Butylparaben, Butylated Hydroxyanisole, Butylated Hydroxytoluene and Propyl Gallate and Their Binary Mixtures on Two Estrogen Responsive Cell Lines (T47D-Kbluc, MCF-7) J. Appl. Toxicol. 2018;38:944–957. doi: 10.1002/jat.3601. [DOI] [PubMed] [Google Scholar]
- 131.Sun Z., Gao R., Chen X., Liu X., Ding Y., Geng Y., Mu X., Liu T., Li F., Wang Y., et al. Exposure to Butylated Hydroxytoluene Compromises Endometrial Decidualization during Early Pregnancy. Environ. Sci. Pollut. Res. 2021;28:42024–42036. doi: 10.1007/s11356-021-13720-0. [DOI] [PubMed] [Google Scholar]
- 132.Yang X., Song W., Liu N., Sun Z., Liu R., Liu Q.S., Zhou Q., Jiang G. Synthetic Phenolic Antioxidants Cause Perturbation in Steroidogenesis in Vitro and in Vivo. Environ. Sci. Technol. 2018;52:850–858. doi: 10.1021/acs.est.7b05057. [DOI] [PubMed] [Google Scholar]
- 133.Pop A., Drugan T., Gutleb A.C., Lupu D., Cherfan J., Loghin F., Kiss B. Individual and Combined in Vitro (Anti)Androgenic Effects of Certain Food Additives and Cosmetic Preservatives. Toxicol. Vitr. 2016;32:269–277. doi: 10.1016/j.tiv.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 134.Ham J., Lim W., Whang K.-Y., Song G. Butylated Hydroxytoluene Induces Dysregulation of Calcium Homeostasis and Endoplasmic Reticulum Stress Resulting in Mouse Leydig Cell Death. Environ. Pollut. 2020;256:113421. doi: 10.1016/j.envpol.2019.113421. [DOI] [PubMed] [Google Scholar]
- 135.Takahashi O., Oishi S. Male Reproductive Toxicity of Four Bisphenol Antioxidants in Mice and Rats and Their Estrogenic Effect. Arch. Toxicol. 2006;80:225–241. doi: 10.1007/s00204-005-0033-5. [DOI] [PubMed] [Google Scholar]
- 136.Kang H.G., Jeong S.H., Cho J.H., Kim D.G., Park J.M., Cho M.H. Evaluation of Estrogenic and Androgenic Activity of Butylated Hydroxyanisole in Immature Female and Castrated Rats. Toxicology. 2005;213:147–156. doi: 10.1016/j.tox.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 137.Xiong G., Wu Z., Yi J., Fu L., Yang Z., Hsieh C., Yin M., Zeng X., Wu C., Lu A., et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021;49:W5–W14. doi: 10.1093/nar/gkab255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lamas Bervejillo M., Ferreira A.M. Understanding Peroxisome Proliferator-Activated Receptors: From the Structure to the Regulatory Actions on Metabolism. In: Trostchansky A., Rubbo H., editors. Bioactive Lipids in Health and Disease. Springer International Publishing; Cham, Switzerland: 2019. pp. 39–57. [DOI] [PubMed] [Google Scholar]
- 139.Christofides A., Konstantinidou E., Jani C., Boussiotis V.A. The Role of Peroxisome Proliferator-Activated Receptors (PPAR) in Immune Responses. Metabolism. 2021;114:154338. doi: 10.1016/j.metabol.2020.154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brewer M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- 141.Vilchis-Landeros M.M., Vázquez-Meza H., Vázquez-Carrada M., Uribe-Ramírez D., Matuz-Mares D. Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. Int. J. Mol. Sci. 2024;25:5675. doi: 10.3390/ijms25115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shahidi F., Zhong Y. Novel Antioxidants in Food Quality Preservation and Health Promotion. Eur. J. Lipid Sci. Technol. 2010;112:930–940. doi: 10.1002/ejlt.201000044. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable.


