Abstract
The perennial species Hypericum perforatum, commonly known as St. John’s Wort, is well regarded for its medicinal attributes, particularly its strong anti-inflammatory and antidepressant effects. Hypericum perforatum L., commonly known as balsam, is extensively employed in both traditional and contemporary medicine due to its biological properties, although the plant’s medicine distribution is limited to Europe and Asia. This study pioneers the investigation of Hypericum perforatum cultivation in a Mediterranean country, specifically Greece, focusing on the effects of irrigation and biostimulants of two distinct genotypes on quantitative (height, drug yield, essential oil yield) and qualitative (essential oil content and composition) characteristics. A field trial was conducted at the experimental farm of the Agrotechnology Department at the University of Thessaly, located in the Larissa region. This study investigated various testing varieties under different irrigation levels and biostimulant applications. The results underscore the importance of customized irrigation and biostimulant strategies in improving yield and quality during the second growing season, establishing a foundation for sustainable agricultural progress. Notably, irrigated treatments significantly increased plant height, dry biomass yield, and essential oil production per hectare. Specifically, the essential oil yields for irrigated treatments were nearly double those of rainfed treatments, with 219 kg/ha for rainfed and 407 kg/ha for irrigated. The genotype played a crucial role in influencing production potential, height, flowering, and essential oil composition, with one variety demonstrating biennial blooming and modified essential oil compounds. While irrigation positively impacted yield, it also reduced certain essential oil compounds while increasing β-pinene content. The effects of biostimulants varied based on their composition, with some enhancing and others diminishing essential oil content. Notably, the biostimulant containing algae with auxin and cytokinin (B2) proved to be the most effective in improving the therapeutic profile. This study offers valuable insights into the cultivation of H. perforatum in a Mediterranean climate, highlighting the necessity for ongoing research into native populations, irrigation levels, biostimulants, fertilization, and other factors that affect crop yield and quality characteristics.
Keywords: Hypericum perforatum, yield, essential oil, aromatic–medicinal plants, biostimulants, irrigation
1. Introduction
Hypericum perforatum L., widely recognized as St. John’s Wort, stands out as the most prominent perennial species within the Hypericaceae family, found across Central and Eastern Europe, Asia, North Africa, and North America [1,2]. This plant has a long history of medicinal use, spanning over two millennia [3]. Driven by the demand for natural therapeutic agents and the beneficial biological properties of Hypericum perforatum L., it is frequently incorporated into medical practices [4,5,6,7,8]. Its extracts are noted for various applications, including antiviral, antimicrobial, anti-inflammatory, and antitumor properties [9], as well as their effectiveness in alleviating symptoms of depression [10]. According to pharmacopoeial standards, the raw material derived from Hypericum perforatum consists of the flowering tops of the shoots (Hyperici herba), which must meet specific compound content requirements [11].
Numerous studies have compared the chemical compositions of native and commercial samples [12,13]. For instance, ref. [14] examined how the origin and biological source influence the chemical makeup and biological properties of various St. John’s Wort species. The extracts from all samples demonstrated significant antioxidant potential; however, in most instances, the indigenous samples exhibited stronger effects than the original source. Variations in the levels of phenolic compounds, particularly flavonoids, were linked to the source of the drug, which impacted the observed activities. Nevertheless, further research is necessary to investigate how commercial varieties adapt to different climatic conditions and the subsequent effects on their phytochemical profiles.
In Europe, the main production of this crop takes place in Belarus, Germany, Italy, Poland, Romania, Switzerland, and Siberia. A significant portion of the total supply of Hypericum comes from the collection of wild plants, which has resulted in a troubling decrease in their natural populations [15,16]. Although the cultivation of Hypericum perforatum offers a lucrative opportunity for farmers, the lack of standardized agricultural practices for its growth poses a challenge. Furthermore, slow growth rates, low yields, and variable quality present considerable obstacles to its broader cultivation [17].
St. John’s Wort is a perennial shrub that can grow between 30 and 100 cm tall and is capable of reproducing through both vegetative and sexual means. Notable physical characteristics of Hypericum perforatum include its taproot system, woody stems, and rhizomes. The leaves are distinguished by translucent glands scattered throughout the lamina, and the plant produces cymes of yellow flowers that mature into dehiscent capsules filled with seeds [18].
The quality of St. John’s Wort can differ due to various factors, including the specific sub-species or varieties, the environmental conditions where it is grown, the timing of the harvest [19], and the cultivation techniques employed. Research indicates that there are notable genotypic and phenotypic variations among different St. John’s Wort populations [20]. To identify these genetic distinctions, scientists conduct morphological and biochemical analyses [21]. However, there is limited information on the cultivation and production of Hypericum, despite extensive documentation of the medicinal benefits associated with its aerial parts [22,23,24].
As mentioned above, St. John’s Wort is primarily recognized for its applications as a food additive and in medicinal contexts. Consequently, it is crucial to explore the potential for large-scale production utilizing organic farming methods. In this context, the incorporation of biostimulants—natural compounds or microbial products—merits consideration. These substances can greatly improve plant growth and their inherent processes when applied [25].
In recent years, seaweed extracts have gained increased attention as a category of biostimulants, largely due to various research studies [26,27]. As noted by [28], these seaweed-derived biostimulants contribute to improved crop yields, enhanced root development, flowering, resilience to stress, and better nitrogen uptake.
As previously noted, similar to many medicinal plants, there are no established cultivation protocols akin to those for traditional crops, despite their extensive use over the years. The impact of cultivation methods, including those applicable to organic farming, remains largely unknown for any species within the aromatic and medicinal plant category. Consequently, this research focused on cultivating two recognized varieties of Hypericum perforatum L. in Greece, employing both dry and fully irrigated treatments to explore the potential for utilizing all types of land, including those lacking water resources. Additionally, the study sought to investigate the application of biostimulants and their influence on overall performance regarding yield and quality during the second growing season, particularly since one of the varieties blooms biennially. Notably, biostimulants can be employed within organic production systems, underscoring their significance when these crops are intended as raw materials for pharmaceutical applications.
2. Materials and Methods
A comprehensive experiment that involved different varieties, different irrigation levels, and different biostimulant treatments was conducted. The study was designed to assess their effects on growth metrics, yield outputs, and the qualitative attributes of the St. John’s Wort varieties, including essential oil composition.
2.1. Experimental Site, Design, and Management
In May 2022, a field experiment was conducted at the experimental farm of the Agrotechnology Department at the University of Thessaly, situated in the Larissa region of Greece (latitude 39°37′34.0″ N, longitude 22°22′52.4″ E). The objective of this study was to examine the impact of irrigation and biostimulant application on the overall performance of two well-known varieties (Topaz and Taubertal) of St. John’s Wort, focusing on aspects such as crop development, drug yield, essential oil content, and the quality of essential oil within a typical Mediterranean soil–climatic setting.
A factorial split-split-plot design was used with six replicates (blocks) and twelve plots per replication. The main factor was the different testing varieties (V1: Topaz and V2: Taubertal). The main sub-factor was the different irrigation levels (I1: rainfed and I2: 100% reference evapotranspiration (ETo); using a drip irrigation system while the irrigation schedule was determined according to the Class A evaporation pan method [29] and irrigation was conducted on a weekly basis, aiming to replicate the actual conditions experienced by farmers during their irrigation practices). The main sub-sub-factor was the different biostimulant applications (B1: control; B2: seaweeds; auxin 1.0 mg/lt and cytokinin 0.031 mg/lt from Ecklonia maxima; application rate of 0.8 L per hectare per year; and B3: a specific formulation including 10% w/v of amino acids, 11.3% w/v of pure protein, 22% w/v of sucrose, and 10% w/v of UV filter; application rate of 1.6 L per hectare per year). The biostimulants were applied annually prior to the onset of flowering (in the final ten days of May and right after the prevailing rainfall), utilizing the specified amounts and employing a micro electric sprayer for precise application.
Certified seedlings of Hypericum perforatum L. (varieties Topaz and Taubertal) were transplanted into the field at a spacing of 60 cm between plants and 60 cm between rows. Each plot measured 2 m × 1.70 m and contained 12 plants in 3 rows. The findings presented pertain to the second growing season, during which both varieties exhibited blooming, noting that one of the varieties blooms every other growing year.
It is important to note that both varieties of plants re-germinated within the first ten days of March; however, there was a variation in the onset of flowering between them. Topaz began to flower earlier than Taubertal, resulting in an earlier harvest for Topaz. Consequently, Topaz was harvested around June 10, while Taubertal was harvested 20 days later, on June 29.
2.2. Soil Physical–Chemical Characteristics
The soil of the experimental field is a sandy loam (SL) and is characterized as semi-fertile to unfertile (organic matter of 1.3% at a depth of 0–30 cm). It is an alkaline nature (pH 7.6), with a bulk specific gravity of 1.3 g cm−3 and electrical conductivity of 0.2 ms/cm.
2.3. Plant Growing Characteristics and Yield
The height, flowering height, and overall yield were assessed at the ideal harvesting stage, which corresponds to full flowering, a stage known to maximize essential oil content. To prevent any border effects, the harvested flowers from each plot were taken from the inner row, specifically two plants per plot.
The aerial parts (flowers and upper leaves) were collected, weighed, and promptly transported to the laboratory, where they were air-dried in a shaded, well-ventilated area at ambient temperature (25–28 °C) until a constant weight was achieved. The dry weight was recorded for each plot to calculate the dry biomass yield per hectare and the distillation process followed.
Manual weeding was conducted every two weeks to manage weed competition, while there were no notable occurrences of pests or diseases; therefore, no pesticides were used (Figure 1).
Figure 1.
St. John’s Wort treatments.
2.4. Essential Oil Yield and Quality Characteristics
The dried samples were processed in a laboratory mill to achieve a consistent particle size of around 1 mm. Thereafter, the essential oil content was assessed using a Clevenger-type distillation apparatus. A total of 10 g of the dried plant material was subjected to hydro distillation with 250 mL of distilled water for 2.5 h. This process was conducted three times for each sample, and the essential oil content was calculated based on the dry weight of the plant material. The extracted essential oils were measured volumetrically and stored in amber glass vials in a refrigerator at 4 °C until further analysis [30,31]. Following storage, the essential oils were analyzed using gas chromatography coupled with mass spectrometry (GC–MS) on a fused silica DB-5 column. The relative content of each compound was determined as a percentage of the total chromatographic area, and the results are presented as the mean percentage of three replicates [31,32]. Compound identification was achieved by comparing retention indices (RIs) relative to n-alkanes (C7–C22) with literature data and matching spectra against mass spectrometry libraries (NIST 98, Willey) [33].
Moreover, the yield of the essential oil was calculated by multiplying the essential oil concentration by the dry biomass yield per hectare.
2.5. Meteorological Data and Statistical Analysis
Weather data, encompassing air temperature, radiation, humidity, wind speed, precipitation, and the class-A pan evaporation rate, were gathered from an automatic meteorological station situated near the experimental field. The data collected were subjected to an analysis of variance (ANOVA) for all measured and derived variables at designated time intervals, employing the GenStat statistical software (7th Edition). The differences in means for both the main effects and interaction effects were assessed using the LSD0.05 (least significant difference at 5%) test criteria [34]. This statistical evaluation enabled a thorough examination of the data, confirming that any observed differences among the studied variables were statistically significant and not due to random variation.
3. Results and Discussion
3.1. Meteorological Data
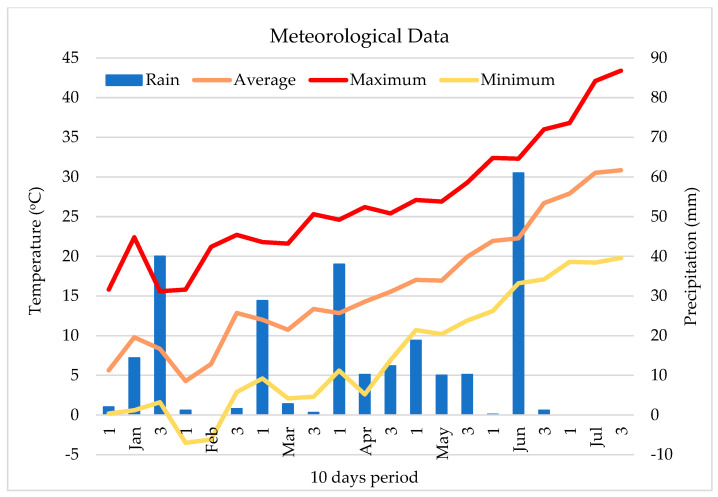
The Mediterranean climate characteristic of the research area is recognized for its warm summers and mild winters. In the experimental year under investigation, the average air temperature during the crop re-emergence phase ranged from 2.1 to 21.8 °C (average air temperature 11.9 °C). Throughout the crop growth period (re-emergence–beginning of flowering), the maximum air temperature reached 29.3 °C, while the minimum was recorded at 14 °C (average air temperature 20.8 °C; Figure 2). Thereafter, during the growing stages between the beginning of flowering until harvest, the maximum air temperature reached 29.3 °C, while the minimum was recorded at 14 °C (average air temperature 20.8 °C; Figure 2).
Figure 2.
Temperature (Max, Avg, Min) and precipitation from January to July 2023 (10-day intervals).
Precipitation is another vital environmental factor that greatly influences crop development and yield. In 2023, the region experienced 194.2 mm of rainfall during the growing season (re-emergence–harvest). Specifically, between this period, the monthly rainfall totals were recorded as 60.6 mm, 39 mm, 62.4 mm, and 0 mm for March to June, respectively.
These data highlight the occurrence of drought conditions during the summer months, particularly from April to June (Figure 2).
3.2. Plant Characteristics and Yield
As indicated in Table 1, there were statistically significant differences among the varieties examined for all three characteristics: plant diameter, inflorescence height, and plant height. Furthermore, the irrigation factor also demonstrated statistically significant differences concerning inflorescence height and plant height.
Table 1.
Plant characteristics (diameter, florescence height, plant height in cm) as affected by different varieties (V1, V2), different irrigation levels (I1, I2), different biostimulants (B1, B2, B3), and their interactions at final harvest.
| Plant Diameter (cm) | Florescence Height | Plant Height (cm) | Plant Diameter (cm) | Florescence Height | Plant Height (cm) | ||
|---|---|---|---|---|---|---|---|
| Varieties | Irrigation×Biostimulants | ||||||
| V1 | 52.7 | 55.8 | 80.7 | I1×B1 | 56.7 | 44.8 | 64.2 |
| V2 | 57.9 | 47.4 | 65.5 | I1×B2 | 56.1 | 45.5 | 66.4 |
| LSD0.05 | 3.81 | 5.91 | 8.15 | I1×B3 | 57.7 | 45.2 | 67.0 |
| Irrigation | I2×B1 | 56.1 | 58.6 | 80.9 | |||
| I1 | 56.7 | 45.2 | 65.9 | I2×B2 | 53.6 | 57.9 | 79.6 |
| I2 | 53.8 | 58.1 | 80.3 | I2×B3 | 51.7 | 57.7 | 80.4 |
| LSD0.05 | ns | 4.613 | 4.83 | LSD0.05 | ns | ns | ns |
| Biostimulants | Varities×Irrigation×Biostimulants | ||||||
| B1 | 56.4 | 51.7 | 72.5 | V1×I1×B1 | 54.2 | 45.6 | 66.9 |
| B2 | 54.9 | 51.7 | 73.0 | V1×I1×B2 | 54.1 | 48.3 | 73.2 |
| B3 | 54.6 | 51.4 | 73.7 | V1×I1×B3 | 54.8 | 47.9 | 72.1 |
| LSD0.05 | ns | ns | ns | V1×I2×B1 | 53.8 | 66.6 | 93.6 |
| Varieties×Irrigation | V1×I2×B2 | 49,8 | 63,8 | 87,5 | |||
| V1×I1 | 54.3 | 47.3 | 70.8 | V1×I2×B3 | 49.6 | 62.8 | 90.8 |
| V1×I2 | 51.0 | 64.4 | 90.6 | V2×I1×B1 | 59.2 | 44.1 | 61.4 |
| V2×I1 | 59.1 | 43.0 | 61.0 | V2×I1×B2 | 58.1 | 42.6 | 59.6 |
| V2×I2 | 56.6 | 51.7 | 70.0 | V2×I1×B3 | 60.2 | 42.4 | 61.9 |
| LSD0.05 | ns | ns | 8.59 | V2×I2×B1 | 58.5 | 50.5 | 68.2 |
| Varieties×Biostimulants | V2×I2×B2 | 57.5 | 52.1 | 71.7 | |||
| V1×B1 | 54.0 | 56.1 | 80.2 | V2×I2×B3 | 53.8 | 52.6 | 70.1 |
| V1×B2 | 51.9 | 56.0 | 80.4 | LSD0.05 | ns | ns | ns |
| V1×B3 | 52.2 | 55.3 | 81.4 | CV (%) | 9.3 | 11.6 | 11.9 |
| V2×B1 | 58.8 | 47.3 | 64.8 | ||||
| V2×B2 | 57.8 | 47.4 | 65.6 | ||||
| V2×B3 | 57.0 | 47.9 | 66.0 | ||||
| LSD0.05 | ns | ns | ns | ||||
LSD: least significant difference; ns: non-significant; V1: Topaz; V2: Taubertal; I1: rainfed; I2: 100% Eto; B1: control; B2: seaweeds, auxin 1.0 mg/lt and cytokinin 0.031 mg/lt from Ecklonia maxima; B3: specific formulation including 10% w/v of amino acids, 11.3% w/v of pure protein, 22% w/v of sucrose, and 10% w/v of UV filter.
Specifically, Variety 1 (Topaz) demonstrated a statistically significant superiority in both flowering height and overall plant height, with its final measurement exceeding that of Variety 2 by 15 cm. Conversely, Variety 2 (Taubertal) resulted in plants exhibiting a larger crown diameter, with a difference of nearly 5 cm, suggesting a greater capacity for spatial expansion (Table 1).
Concerning the irrigation factor, it was anticipated that the fully irrigated treatment (100% ETo) would produce the greatest values for both inflorescence height and overall plant height in comparison to the rainfed treatment. This anticipation is corroborated by meteorological data, which reveal a combination of recorded rainfall and elevated temperatures throughout the crop growth period. Notably, the total rainfall measured was 194.2 mm, while evaporation during the same timeframe reached 446.3 mm (data not presented). As illustrated in Table 1, the irrigation treatment led to plants that were, on average, 15 cm taller, with an average height of approximately 80 cm.
Finally, a statistically significant difference in plant height was observed regarding the interaction between the varieties and irrigation methods. Notably, the fully irrigated Variety 1 (Topaz) demonstrated the greatest plant height, measuring 90.6 cm, compared to the other treatments analyzed (Table 1).
The statistical analysis presented in Table 2 indicates that significant differences in production metrics, specifically fresh and dry weight, were observed solely among the examined cultivars.
Table 2.
Plant fresh and dry weight as affected by different varieties (V1, V2), different irrigation levels (I1, I2), different biostimulants (B1, B2, B3), and their interactions at final harvest.
| Fr.W (kg ha−1) |
D.W (kg ha−1) |
Fr.W (kg ha−1) |
D.W (kg ha−1) |
||
|---|---|---|---|---|---|
| Varieties | Irrigation×Biostimulants | ||||
| V1 | 8371 | 3137 | I1×B1 | 6285 | 2497 |
| V2 | 4522 | 1775 | I1×B2 | 6286 | 2407 |
| LSD0.05 | 1598 | 512.6 | I1×B3 | 5731 | 2215 |
| Irrigation | I2×B1 | 7412 | 2748 | ||
| I1 | 6101 | 2373 | I2×B2 | 6454 | 2410 |
| I2 | 6792 | 2539 | I2×B3 | 6509 | 2458 |
| LSD0.05 | ns | ns | LSD0.05 | ns | ns |
| Biostimulants | Varities×Irrigation×Biostimulants | ||||
| B1 | 6849 | 2622 | V1×I1×B1 | 8742 | 3382 |
| B2 | 6370 | 2409 | V1×I1×B2 | 8750 | 3222 |
| B3 | 6120 | 2337 | V1×I1×B3 | 7662 | 2856 |
| LSD0.05 | ns | ns | V1×I2×B1 | 9403 | 3500 |
| Varieties×Irrigation | V1×I2×B2 | 7329 | 2759 | ||
| V1×I1 | 8385 | 3154 | V1×I2×B3 | 8338 | 3102 |
| V1×I2 | 8356 | 3120 | V2×I1×B1 | 3829 | 1611 |
| V2×I1 | 3818 | 1593 | V2×I1×B2 | 3823 | 1593 |
| V2×I2 | 5227 | 1957 | V2×I1×B3 | 3801 | 1574 |
| LSD0.05 | ns | ns | V2×I2×B1 | 5421 | 1995 |
| Varieties×Biostimulants | V2×I2×B2 | 5579 | 2060 | ||
| V1×B1 | 9072 | 3441 | V2×I2×B3 | 4681 | 1815 |
| V1×B2 | 8039 | 2991 | LSD0.05 | ns | ns |
| V1×B3 | 8000 | 2979 | CV (%) | 35.1 | 32.6 |
| V2×B1 | 4625 | 1803 | |||
| V2×B2 | 4701 | 1826 | |||
| V2×B3 | 4241 | 1694 | |||
| LSD0.05 | ns | ns | |||
LSD: least significant difference; ns: non-significant; Fr.W: Fresh Weight; D.W: dry weight; V1: Topaz; V2: Taubertal; I1: rainfed; I2: 100% Eto; B1: control; B2: seaweeds, auxin 1.0 mg/lt and cytokinin 0.031 mg/lt from Ecklonia maxima; B3: a specific formulation including 10% w/v of amino acids, 11.3% w/v of pure protein, 22% w/v of sucrose, and 10% w/v of UV filter.
In particular, Variety 1 (Topaz) demonstrated the highest production levels, achieving fresh and dry weights of 8371 kg and 3137 kg per hectare, respectively. These figures are nearly double those of the second variety, Taubertal, as shown in Table 2.
Irrigation and biostimulants did not demonstrate a statistically significant impact on crop yield, as indicated in Table 2. The average yields for the irrigation factor and biostimulants, irrespective of the variety, were approximately 6500 kg per hectare for fresh weight and 2400 kg per hectare for dry weight.
Finally, the interaction analysis of the three factors did not demonstrate a statistically significant impact on crop yield, while it indicated that Variety 1 (Topaz) attained maximum dry weight production under full irrigation (100% ETo) conditions without the use of any biostimulant (control), resulting in a yield of 3500 kg per hectare.
3.3. Essential Oil Yield and Quality Characteristics Measurements
According to the ANOVA table below (Table 3), the essential oil content does not appear to vary significantly among the different varieties of St. John’s Wort. However, it is influenced by the irrigation and fertilization treatments, as well as their interactions.
Table 3.
The essential oil content (%) and essential oil yield (kg ha−1) as affected by the different varieties (V1, V2), the different irrigation levels (I1, I2), the different biostimulants (B1, B2, B3), and their interactions at the final harvest.
| Essential Oil Content (%) | Essential Oil Yield (kg ha−1) | Essential Oil Content (%) | Essential Oil Yield (kg ha−1) | ||
|---|---|---|---|---|---|
| Varieties | Irrigation×Biostimulants | ||||
| V1 | 0.128 | 397 | I1×B1 | 0.133 | 327 |
| V2 | 0.124 | 229 | I1×B2 | 0.087 | 214 |
| LSD0.05 | ns | 59.2 | I1×B3 | 0.048 | 115 |
| Irrigation | I2×B1 | 0.124 | 345 | ||
| I1 | 0.089 | 219 | I2×B2 | 0.200 | 468 |
| I2 | 0.163 | 407 | I2×B3 | 0.163 | 408 |
| LSD0.05 | 0.0126 | 84.8 | LSD0.05 | 0.0203 | 107.7 |
| Biostimulants | Varities×Irrigation×Biostimulants | ||||
| B1 | 0.128 | 336 | V1×I1×B1 | 0.173 | 431 |
| B2 | 0.144 | 341 | V1×I1×B2 | 0.095 | 304 |
| B3 | 0.105 | 362 | V1×I1×B3 | 0.067 | 185 |
| LSD0.05 | 0.0145 | ns | V1×I2×B1 | 0.127 | 443 |
| Varieties×Irrigation | V1×I2×B2 | 0.165 | 450 | ||
| V1×I1 | 0.096 | 307 | V1×I2×B3 | 0.185 | 566 |
| V1×I2 | 0.159 | 487 | V2×I1×B1 | 0.138 | 223 |
| V2×I1 | 0.082 | 131 | V2×I1×B2 | 0.078 | 124 |
| V2×I2 | 0.166 | 327 | V2×I1×B3 | 0.028 | 46 |
| LSD0.05 | ns | ns | V2×I2×B1 | 0.122 | 246 |
| Varieties×Biostimulants | V2×I2×B2 | 0.235 | 485 | ||
| V1×B1 | 0.127 | 437 | V2×I2×B3 | 0.142 | 250 |
| V1×B2 | 0.130 | 377 | LSD0.05 | 0.0274 | 137.2 |
| V1×B3 | 0.126 | 376 | CV (%) | 35.1 | 32.6 |
| V2×B1 | 0.130 | 235 | |||
| V2×B2 | 0.157 | 304 | |||
| V2×B3 | 0.085 | 148 | |||
| LSD0.05 | 0.0187 | 87.8 | |||
LSD: least significant difference; ns: non-significant; V1: Topaz; V2: Taubertal; I1: rainfed; I2: 100% Eto; B1: control; B2: seaweeds, auxin 1.0 mg/lt and cytokinin 0.031 mg/lt from Ecklonia maxima; B3: a specific formulation including 10% w/v of amino acids, 11.3% w/v of pure protein, 22% w/v of sucrose, and 10% w/v of UV filter.
Irrigation appears to have a beneficial impact on the essential oil percentage, nearly doubling its content. In terms of biostimulants, B2 seems to enhance the oil content significantly, whereas B3 shows a detrimental effect when compared to the control (Table 3).
According to the essential oil content analysis, Variety 2 (Taubertal) has the highest essential oil concentration at 0.235% when it is subjected to the irrigation regime and treated with Biostimulant 2 (B2: seaweeds, auxin at 1.0 mg/lt, and cytokinin at 0.031 mg/lt derived from Ecklonia maxima).
To calculate the essential oil production per hectare in kilograms, the percentage essential oil content must be multiplied by the yield of the crop (dry weight per hectare, see Table 2). The result will represent the yield of essential oil per hectare.
Table 3 illustrates the statistical evaluation of essential oil production concerning the study factors analyzed, which include varieties, irrigation, and biostimulants. The results indicate that only two of the three factors exhibit a statistically significant impact on essential oil yield per hectare. Specifically, when comparing the varieties, Topaz (V1) shows a statistically significant superiority over Taubertal (V2), with essential oil production capacities of approximately 397 kg per hectare and 229 kg per hectare, respectively.
The irrigation factor appears to have a similar impact, as treatments with irrigation led to higher yields. In particular, irrigated treatments (I2) can yield approximately 407 kg of essential oils per hectare, in contrast to the 219 kg per hectare observed in the rainfed treatments (I1).
The interaction of irrigation factor with biostimulants demonstrated a statistically significant advantage for the irrigated treatments that utilized the combination of the two biostimulants, B2 and B3, compared to all other treatments. This approach resulted in essential oil production reaching as high as 468 kg per hectare (I2B2; Table 3). In the case of the interaction of varieties with biostimulants, a statistically significant benefit was observed for the Topaz variety (V1), irrespective of the type of biostimulant used, achieving an essential oil yield of 407 kg per hectare (V1B1, Table 3).
The expected result, considering the previously mentioned factors, indicates that the treatment resulting in the greatest production of essential oil, amounting to 566 kg per hectare, is the irrigated treatment (I2) of the Topaz variety (V1) when paired with Biostimulant 3 (B3). This finding demonstrates a numerical advantage rather than a statistically significant difference when compared to the other biostimulant treatments (i.e., V1I2B1 is roughly equivalent to V1I2B2 and V1I2B3, as shown in Table 3).
The analysis of the essential oil obtained from the experimental treatments, conducted using gas chromatography coupled with mass spectrometry (GC–MS), revealed not only the primary compounds hypericone and pseudo-hypericin (which are not presented in the current study) but also identified an additional 14 compounds present in concentrations exceeding 1%. These compounds include 2-Methyl-Decane, 2-Methyl-Octane, (E)-β-Farnesene, α-Pinene, Benzyl Benzoate, 2-β-Funebrene, γ-Muurolene, (E)-β-Ocimene, Oxide-Caryophyllene, β-Pinene, (E)-Caryophyllene, γ-Himachelene, δ-Cadinene, and Spathulenol. The effects of the studied factors are detailed in the Anova table provided below (Table 4).
Table 4.
Essential oil characteristics (% components found using GC–MS; 2-Methyl-Decane, 2-Methyl-Octane, (E)-β-Farnesene, α-Pinene, Benzyl Benzoate, 2-β-Funebrene, γ-Muurolene, (E)-β-Ocimene, Oxide-Caryophyllene, β-Pinene, (E)-Caryophyllene, γ-Himachelene, δ-Cadinene, Spathulenol) as affected by different varieties (V1, V2), different irrigation levels (I1, I2), different biostimulants (B1, B2, B3), and their interactions at final harvest.
| 2-Methyl-Decane | 2-Methyl-Octane | (E)-β-Farnesene | α-Pinene | Benzyl Benzoate | 2-β-Funebrene | γ-Muurolene | (E)-β-Ocimene | Oxide-Caryophyllene | Β-Pinene | (E)-Caryophyllene | γ-Himachelene | δ-Cadinene | Spathulenol | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Varieties | ||||||||||||||
| V1 | 2.89 | 22.57 | 1.43 | 15.38 | 1.36 | 1.13 | 1.34 | 1.55 | 12.90 | 12.01 | 8.69 | 0.98 | 0.84 | 5.95 |
| V2 | 2.46 | 20.40 | 0.00 | 38.26 | 0.41 | 0.00 | 1.38 | 1.39 | 3.42 | 5.34 | 2.34 | 0.98 | 0.82 | 4.16 |
| LSD0.05 | ns | ns | 0.502 | 6.393 | 0.780 | 0.63 | ns | ns | 3.147 | 2.099 | 1.262 | ns | ns | 0.341 |
| Irrigation | ||||||||||||||
| I1 | 2.78 | 21.92 | 0.74 | 25.19 | 0.94 | 0.63 | 1.52 | 1.87 | 9.38 | 7.93 | 6.29 | 1.08 | 1.01 | 6.83 |
| I2 | 2.57 | 21.05 | 0.69 | 28.45 | 0.83 | 0.50 | 1.20 | 1.55 | 6.94 | 9.42 | 4.74 | 0.88 | 0.64 | 3.28 |
| LSD0.05 | ns | ns | ns | ns | ns | ns | 0.224 | ns | 1.306 | 1.067 | 1.113 | ns | 0.302 | 0.816 |
| Biostimulants | ||||||||||||||
| B1 | 2.63 | 22.73 | 0.66 | 28.11 | 0.73 | 0.46 | 1.13 | 1.60 | 6.25 | 8.83 | 5.69 | 0.95 | 0.71 | 4.76 |
| B2 | 2.45 | 19.76 | 0.85 | 25.90 | 1.13 | 0.74 | 1.46 | 1.29 | 10.91 | 7.78 | 6.12 | 1.03 | 0.92 | 5.96 |
| B3 | 2.94 | 21.97 | 0.64 | 26.45 | 0.80 | 0.49 | 1.50 | 1.51 | 7.32 | 9.41 | 4.74 | 0.97 | 0.84 | 4.45 |
| LSD0.05 | 0.353 | ns | 0.094 | ns | 0.264 | 0.129 | 0.244 | 0.173 | 1.288 | ns | 1.105 | ns | ns | 0.795 |
| Varieties×Irrigation | ||||||||||||||
| V1×I1 | 2.95 | 23.99 | 1.48 | 15.11 | 1.48 | 1.26 | 1.47 | 1.64 | 14.14 | 10.73 | 10.01 | 1.14 | 0.94 | 7.34 |
| V1×I2 | 2.83 | 21.14 | 1.37 | 15.65 | 1.25 | 1.00 | 1.21 | 1.45 | 11.67 | 13.28 | 7.38 | 0.83 | 0.73 | 4.57 |
| V2×I1 | 2.61 | 19.84 | 0.00 | 35.27 | 0.41 | 0.00 | 1.56 | 1.13 | 4.63 | 5.14 | 2.57 | 1.02 | 1.08 | 6.33 |
| V2×I2 | 2.31 | 20.96 | 0.00 | 41.26 | 0.42 | 0.00 | 1.20 | 1.64 | 2.21 | 5.55 | 2.1 | 0.94 | 0.555 | 2.00 |
| LSD0.05 | ns | ns | ns | ns | ns | ns | ns | 0.205 | ns | 1.664 | ns | ns | ns | ns |
| Varieties×Biostimulants | ||||||||||||||
| V1×B1 | 2.82 | 22.43 | 1.313 | 15.14 | 1.01 | 0.92 | 1.11 | 1.52 | 9.51 | 12.00 | 8.99 | 0.93 | 0.64 | 6.42 |
| V1×B2 | 2.37 | 19.14 | 1.693 | 13.75 | 2.01 | 1.47 | 1.78 | 1.32 | 18.69 | 10.30 | 10.04 | 1.12 | 1.19 | 7.86 |
| V1×B3 | 3.48 | 26.14 | 1.274 | 17.24 | 1.08 | 0.99 | 1.14 | 1.80 | 10.52 | 13.72 | 7.06 | 0.91 | 0.68 | 3.58 |
| V2×B1 | 2.44 | 23.04 | 0.00 | 41.08 | 0.46 | 0.00 | 1.14 | 1.68 | 2.99 | 5.67 | 2.39 | 0.968 | 0.79 | 3.10 |
| V2×B2 | 2.53 | 20.38 | 0.00 | 38.05 | 0.25 | 0.00 | 1.14 | 1.26 | 3.14 | 5.25 | 2.20 | 0.940 | 0.65 | 4.06 |
| V2×B3 | 2.40 | 17.79 | 0.00 | 35.65 | 0.53 | 0.00 | 1.85 | 1.22 | 4.13 | 5.11 | 2.42 | 1.02 | 1.00 | 5.33 |
| LSD0.05 | 0.549 | ns | 0.420 | ns | 0.592 | 0.516 | ns | 0.2146 | 2.421 | 1.927 | 1.414 | ns | 0.248 | 0.929 |
| Irrigation×Biostimulants | ||||||||||||||
| I1×B1 | 2.72 | 25.4 | 0.50 | 26.89 | 0.73 | 0.50 | 1.12 | 1.52 | 6.30 | 9.19 | 6.68 | 1.01 | 0.73 | 6.64 |
| I1×B2 | 2.49 | 19.07 | 0.89 | 24.79 | 1.14 | 0.83 | 1.67 | 1.13 | 11.82 | 7.81 | 6.09 | 1.08 | 1.17 | 7.28 |
| I1×B3 | 3.13 | 21.28 | 0.83 | 23.88 | 0.96 | 0.56 | 1.77 | 1.52 | 10.04 | 6.8 | 6.10 | 1.15 | 1.13 | 6.58 |
| I2×B1 | 2.55 | 20.07 | 0.82 | 29.33 | 0.74 | 0.43 | 1.13 | 1.68 | 6.21 | 8.48 | 4.70 | 0.88 | 0.70 | 2.88 |
| I2×B2 | 2.41 | 20.45 | 0.80 | 27.02 | 1.12 | 0.64 | 1.25 | 1.46 | 10.01 | 7.74 | 6.14 | 0.98 | 0.68 | 4.64 |
| I2×B3 | 2.75 | 22.65 | 0.44 | 29.01 | 0.65 | 0.43 | 1.23 | 1.50 | 4.61 | 12.03 | 3.38 | 0.78 | 0.56 | 2.33 |
| LSD0.05 | ns | ns | 0.133 | ns | ns | ns | ns | ns | 1.771 | 1.757 | 1.516 | ns | 0.321 | ns |
| Varities×Irrigation×Biostimulants | ||||||||||||||
| V1×I1×B1 | 2.76 | 27.91 | 1.00 | 16.24 | 0.86 | 1.00 | 1.03 | 1.41 | 8.30 | 12.90 | 10.37 | 0.84 | 0.42 | 8.64 |
| V1×I1×B2 | 2.35 | 19.46 | 1.78 | 14.36 | 2.01 | 1.66 | 1.91 | 1.41 | 18.79 | 10.71 | 10.02 | 1.34 | 1.37 | 8.02 |
| V1×I1×B3 | 3.76 | 24.62 | 1.67 | 14.71 | 1.56 | 1.12 | 1.48 | 2.10 | 15.34 | 8.57 | 9.63 | 1.24 | 1.03 | 5.34 |
| V1×I2×B1 | 2.89 | 16.95 | 1.63 | 14.03 | 1.16 | 0.85 | 1.19 | 1.63 | 10.72 | 11.09 | 7.60 | 1.01 | 0.85 | 4.20 |
| V1×I2×B2 | 2.39 | 18.53 | 1.60 | 13.14 | 2.01 | 1.28 | 1.64 | 1.24 | 18.59 | 9.88 | 10.05 | 0.91 | 1.01 | 7.69 |
| V1×I2×B3 | 3.20 | 27.65 | 0.88 | 19.77 | 0.59 | 0.85 | 0.81 | 1.49 | 5.69 | 18.87 | 4.49 | 0.57 | 0.33 | 1.82 |
| V2×I1×B1 | 2.68 | 22.90 | 0.00 | 37.53 | 0.60 | 0.00 | 1.21 | 1.63 | 4.29 | 5.47 | 2.98 | 1.18 | 1.03 | 4.63 |
| V2×I1×B2 | 2.62 | 18.69 | 0.00 | 35.21 | 0.26 | 0.00 | 1.42 | 0.84 | 4.85 | 4.91 | 2.16 | 0.82 | 0.97 | 6.54 |
| V2×I1×B3 | 2.51 | 17.95 | 0.00 | 33.06 | 0.36 | 0.00 | 2.05 | 0.93 | 4.73 | 5.03 | 2.57 | 1.05 | 1.23 | 7.81 |
| V2×I2×B1 | 2.21 | 23.19 | 0.00 | 44.63 | 0.32 | 0.00 | 1.08 | 1.74 | 1.69 | 5.87 | 1.81 | 0.75 | 0.54 | 1.56 |
| V2×I2×B2 | 2.43 | 22.07 | 0.00 | 40.89 | 0.23 | 0.00 | 0.86 | 1.68 | 1.43 | 5.60 | 2.24 | 1.06 | 0.34 | 1.59 |
| V2×I2×B3 | 2.29 | 17.64 | 0.00 | 38.25 | 0.70 | 0.00 | 1.65 | 1.51 | 3.52 | 5.19 | 2.27 | 1.00 | 0.78 | 2.84 |
| LSD0.05 | ns | ns | 0.384 | ns | 0.628 | ns | ns | 0.326 | 2.829 | 2.561 | ns | 0.686 | 0.390 | 1.424 |
| CV (%) | 15.3 | 17.2 | 15.3 | 15.8 | 34.4 | 26.5 | 20.7 | 13.6 | 18.2 | 18.3 | 23.2 | 24.8 | 25.1 | 18.2 |
LSD: least significant difference; ns: non-significant; V1: Topaz; V2: Taubertal; I1: rainfed; I2: 100% Eto; B1: control; B2: seaweeds, auxin 1.0 mg/lt and cytokinin 0.031 mg/lt from Ecklonia maxima; B3: a specific formulation including 10% w/v of amino acids, 11.3% w/v of pure protein, 22% w/v of sucrose, and 10% w/v of UV filter.
It appears that all three factors—variety, irrigation levels, and biostimulants—have a statistically significant impact, depending on the compounds analyzed. Among the thirteen compounds studied, nine (2-methyl-decane, (ε)-β-phamesene, benzyl benzoate, 2-β-Funebrene, γ-Muurolene, osimene (ε-β), Caryophyllene oxide, (E)-Caryophyllene, and spathulenol) show a statistically significant effect related to the various biostimulants. Additionally, seven compounds (γ-Muurolene, Caryophyllene oxide, β-pinene, (E)-Caryophyllene, δ-Cadinene, and spathulenol) are significantly affected by the different irrigation levels, while seven compounds ((ε)-β-phamesene, α-pinene, benzyl benzoate, 2-β-Funebrene, Caryophyllene oxide, β-pinene, (E)-Caryophyllene, and Spathulenol) demonstrate significant variation across the different varieties.
All of the examined factors influence the composition of the essential oils and the percentage of each component. It is essential to note that the Taubertal variety does not contain “(E)-β-Farnesene” and “2-β-Funebrene”, while there is a significant reduction (over 50%) in the levels of “Oxide-Caryophylene”, “β-Pinene”, and “Benzyl Benzoate.” In contrast, the content of “α-Pinene” in the Taubertal variety is 2.5 times higher. This finding emphasizes the importance of genetic factors and the necessity for meticulous planning in crop cultivation and product application.
The data presented in Table 4 clearly illustrate that, aside from the genetic material (plants) utilized, varying cultivation practices can lead to differences in the composition of the essential oil produced, even when the same genotype (variety) is involved. In the context of biostimulant application, it is evident that B2 (comprising seaweeds, auxin at 1.0 mg/lt, and cytokinin at 0.031 mg/lt sourced from Ecklonia maxima) significantly increases the concentrations of “(E)-β-Farnesene”, “Benzyl Benzoate”, “2-β-Funebrene”, “Oxide-Caryophyllene”, and “Spathulenol”; in some cases, almost doubling the concentration, while concurrently reducing the levels of “(E)-β-Ocimene” with a reduction of about 20% compared to the control.
Finally, it is evident from Table 4 that enhanced irrigation leads to a statistically significant increase of approximately 18% in the percentage of “β pinene” when compared to the rainfed treatment. In contrast, the compounds γ-Muurolene, Caryophyllene oxide, (E)-Caryophyllene, δ-Cadinene, and Spathulenol show a statistically significant decrease in their percentages with increased irrigation, exhibiting reductions ranging from 15% to 50% relative to the rainfed treatment.
4. Discussion
Hypericum perforatum originates from Europe and Asia, where it thrives under specific ecological conditions [35,36]. The rising industrial and medicinal demand for this plant necessitates substantial production volumes [16,37]. Consequently, its cultivation is essential to satisfy both industrial and biotechnological needs. This research provides initial insights into the adaptation of cultivated Hypericum perforatum to the climatic and soil conditions found in Greece, a representative Mediterranean nation.
The existing global literature regarding the impacts of cultivating medicinal plants remains insufficient [38,39,40], with particularly limited data available for the Mediterranean region, especially concerning St. John’s Wort. The aim of this study is to initiate a series of experiments.
Most prior research has concentrated on Hypericum perforatum development under regulated environmental conditions. For instance, ref. [41] examined the impact of light quality and intensity on the growth of Hypericum perforatum L. In a similar vein, ref. [42] investigated how nickel pollution affected Hypericum perforatum L. growth and its secondary metabolite profile. Additionally, ref. [43] studied how varying light quality affects growth and secondary metabolite production in the adventitious root cultivation of Hypericum perforatum.
Studies contacted in Turkey and in Jordan [44,45,46] recorded the flowering of Hypericum perforatum between April and September, which agrees with the results of the current study. One other growth characteristic is plant height, which varies according to different studies. Ref. [47] reported a height range of 45 to 99 cm, whereas [48] indicated that the height can vary between 25 and 44 cm and 55 and 80 cm, depending on the conditions of establishment. The abovementioned range also agrees with the plant height that was found for the different examined factors of this study.
While there is a lack of field studies specifically examining the cultivation of St. John’s Wort and the impact of irrigation and biostimulant use on its yield, the existing literature [49,50,51,52,53,54] indicates a beneficial effect of irrigation on the yields of various aromatic and medicinal plants, similar to the findings of the current research. In the case of biostimulant effects, numerous studies have indicated that the application of biostimulants leads to a notable enhancement in the growth characteristics of various aromatic and medicinal plants, including increases in plant height, leaf count, and both fresh and dry weight such as in the results of the current study [55,56,57,58,59]. Several researchers [60,61,62,63] have suggested that these beneficial effects may stem from enhanced cell elongation, improved cellular membrane permeability, or increased nitrogen absorption, all of which contribute to vigorous root and foliage development. However, it is also noted in the literature that, despite the advantages, the application of biostimulants can lead to a significant decrease in the fresh and dry weights of plants, roots, and chlorophyll b content in certain cases [64].
H. perforatum L. essential oil is primarily derived from the aerial parts of the plant [65]. Numerous studies have documented the chemical variability of H. perforatum, indicating the presence of chemotypes that lack certain compounds [66,67], which aligns with the findings of the current research. Additionally, this study highlights the significance of plant variety and cultivation methods, which can result in statistically significant variations in both the yield of essential oil and the composition of its various components. The literature [68,69,70] indicates that H. perforatum essential oil contains a variety of compounds in significant amounts, including β-pinene (18.32%), α-pinene (5.56–30.92%), δ-Cadinene (0.0–22.58%), and caryophyllene (15.26%), while several compounds are present in lesser quantities, such as (E)-β-caryophyllene, δ-Cadinene, (E)-β-Ocimene, 2-methyldecane, germacrene-δ, and 2-methyl octane, which align with the focus of this research.
The differences in the chemical composition of the essential oils derived from medicinal plants, influenced by several factors as highlighted in this research, represent a major challenge for the modern herbal industry. These compositional differences may restrict the use of specific oils in industrial applications or necessitate their application in alternative products [71,72,73,74,75].
Reference is specifically made to certain substances, including α- and β-pinene, which are significant constituents of the monoterpene family and are frequently present in the essential oils of numerous plants. They are associated with a broad spectrum of pharmacological effects, including modulation of antibiotic resistance, antitumor activity, antimicrobial effects, antimalarial properties, antioxidant capabilities, anti-inflammatory actions, anti-Leishmania effects, and analgesic properties [76]. The diverse biological activities of these phytochemicals facilitate their application in numerous fields, such as fungicides, flavoring agents, fragrances, and both antiviral and antimicrobial products [77]. Additionally, α- and β-pinene are integral components in certain renal and hepatic medications [78]. Their antibacterial properties are attributed to their toxic effects on cellular membranes. Furthermore, studies have indicated that these compounds exhibit inhibitory effects against breast cancer and leukemia [79]. Consequently, higher concentrations of α- and β-pinene in essential oils correlate with enhanced efficacy in these properties. Specifically, treatments that increase α-pinene concentrations include the V2×I2×B1 treatment, while the V1×I2×B3 treatment is effective for β-pinene.
Caryophyllene oxide is an oxygenated terpenoid that typically arises as a metabolic byproduct of Caryophyllene. This compound is recognized for being non-toxic and non-sensitizing, making it a popular choice as a preservative in food, pharmaceuticals, and cosmetics, as well as serving as an insecticide. Its potential application as a cancer treatment is bolstered by findings that indicate a lack of genotoxicity and its effective absorption through cellular membranes. As a result, higher concentrations found in essential oils are associated with improved efficacy in these roles. Notably, treatments that elevate its concentration include V1×I1×B2 and V1×I2×B2.
Finally, a review of the existing literature reveals that Spathulenol exhibits significant bioactive properties, including anticholinesterase activity, antinociceptive and anti-hyperalgesic effects, and anti-mycobacterial properties [80,81,82]. Recent research has also identified Spathulenol as an eco-friendly insecticide effective against aphids [83]. Notably, higher concentrations of Spathulenol found in essential oils correlate with enhanced effectiveness in these applications, with specific treatments such as V1×I1×B2 and V1×I2×B2 leading to increased concentrations.
Therefore, by analyzing the most effective combination of treatments with components at their optimal percentages, it is determined that the treatments V1×I1×B2, V1×I1×B3, and V1×I2×B3 are the most suitable options.
5. Conclusions
This study marks the first investigation into the cultivation of the medicinal plant Hypericum perforatum under the climatic conditions of a Mediterranean country, specifically Greece. The research focused on the impact of irrigation and biostimulants on two distinct genotypes (varieties) of the plant, assessing both quantitative factors (such as height, drug yield, and essential oil yield) and qualitative characteristics (including essential oil content and composition). The findings highlight the importance of customized irrigation and biostimulant approaches in enhancing both the yield and quality of balsam cultivars during their second growing season, setting a foundation for future advancements in sustainable agricultural methods.
The findings indicate that the genetic material used plays a significant role in the performance of the plant, influencing percentage characteristics such as production potential, height, and flowering, as well as the composition of the essential oil. Notably, one of the two varieties blooms biennially, resulting in either a complete absence of certain compounds in the essential oil or their presence in very limited quantities.
Furthermore, the research revealed that irrigation positively affects the overall yield of the balsam plant, enhancing production while simultaneously reducing the levels of several essential oil compounds. However, a notable increase in β-pinene content was observed during this study.
Regarding biostimulants, their effects appear to vary based on their specific composition, with some contributing to an increase in essential oil compound content while others may lead to a decrease.
These findings represent some of the first data from a field experiment on the cultivation of H. perforatum in a Mediterranean climate. Continued research is essential and could focus on native populations from the surrounding area (to explore different genetic materials), varying irrigation levels, additional biostimulants, fertilization, and other factors that may influence crop yield and alter the quality characteristics, such as the essential oil composition of H. perforatum.
Acknowledgments
The authors would like to thank Onoufrios Haralampous (Department of Digital Systems, University of Thessaly, Larissa, Greece) for providing access to the department’s weather station.
Author Contributions
Conceptualization, A.T., K.D.G., N.G.D. and E.W-K.; data curation, A.T., K.D.G., E.Z. and D.B.; formal analysis, A.T., K.D.G. and D.B.; investigation, A.T., K.D.G., N.G.D. and E.W-K.; methodology, A.T., K.D.G., S.P., N.G.D. and E.W.-K.; resources, A.T., E.Z., S.P. and E.W.-K.; writing—original draft preparation, A.T. and K.D.G.; writing—review and editing, A.T., K.D.G., N.G.D. and E.W.-K.; supervision, K.D.G., N.G.D. and E.W.-K. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zobayed S.M.A., Afreen F., Kozai T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’swort. Plant Physiol. Biochem. 2005;43:977–984. doi: 10.1016/j.plaphy.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Russo E., Scicchitano F., Whalley B.J., Mazzitello C., Ciriaco M., Esposito S., Patanè M., Upton R., Pugliese M., Chimirri S., et al. Hypericum perforatum: Pharmacokinetic, mechanism of action, tolerability, and clinical drug–drug interactions. Phytother. Res. 2014;28:643–655. doi: 10.1002/ptr.5050. [DOI] [PubMed] [Google Scholar]
- 3.Wolfle U., Seelinger G., Schempp C.M. Topical application of St. Johnʼs wort (Hypericum perforatum) Planta Med. 2014;80:109–120, 02/03. doi: 10.1055/s-0033-1351019. [DOI] [PubMed] [Google Scholar]
- 4.Crockett S.L., Robson N.K.B. Taxonomy and chemotaxonomy of the genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011;5:1–13. [PMC free article] [PubMed] [Google Scholar]
- 5.Shasmita B.S., Mishra P., Mishra P., Samal M., Mohapatra D., Monalisa K., Kumar Naik S. Recent advances in tissue culture and secondary metabolite production in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2023;154:13–28. doi: 10.1007/s11240-023-02525-3. [DOI] [Google Scholar]
- 6.Ersoy E., Eroglu Ozkan E., Boga M., Mat A. Evaluation of in vitro biological activities of three Hypericum species (H. calycinum, H. confertum, and H. perforatum) from Turkey. S. Afr. J. Bot. 2020;130:141–147. doi: 10.1016/j.sajb.2019.12.017. [DOI] [Google Scholar]
- 7.Sarikurkcu C., Locatelli M., Tartaglia A., Ferrone V., Juszczak A.M., Ozer M.S., Tepe B., Tomczyk M. Enzyme and Biological activities of the water extracts from the plants Aesculus hippocastanum, Olea europaea and Hypericum perforatum that are used as folk remedies in Turkey. Molecules. 2020;25:1202. doi: 10.3390/molecules25051202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nobakht S.Z., Akaberi M., Mohammadpour A.H., Moghadam A.T., Emami S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran. J. Basic Med. Sci. 2022;25:1045. doi: 10.22038/IJBMS.2022.65112.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linde K., Berner M.M., Kriston L. St John’s wort for major depression. Cochrane Database Syst. Rev. 2008;2008:CD000448. doi: 10.1002/14651858.CD000448.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dell’Aica I., Caniato R., Biggin S., Garbisa S. Matrix proteases, green tea and St. John’s Wort: Biomedical research catches up with folk medicine. Clin. Chim. Acta. 2007;381:69–77. doi: 10.1016/j.cca.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Agapouda A., Bookera A., Tivadar Kiss T.D., Hohmannc J., Heinricha M., Csupor D. Quality control of Hypericum perforatum L. analytical challenges and recent progress. J. Pharm. Pharmacol. 2019;71:15–37. doi: 10.1111/jphp.12711. [DOI] [PubMed] [Google Scholar]
- 12.Bruni R., Sacchetti G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s wort (Hypericum perforatum L. Hypericaceae/Guttiferae) Molecules. 2009;14:682–725. doi: 10.3390/molecules14020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozin B., Kladar N., Grujic N., Anackov G., Samojlik I., Gavaric N., Conic B.S. Impact of Origin and Biological Source on Chemical Composition, Anticholinesterase and Antioxidant Properties of Some St. John’s Wort Species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules. 2013;18:11733–11750. doi: 10.3390/molecules181011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çırak C., Bertoli A., Pistelli L., Seyis F. Essential oil composition and variability of Hypericum perforatum from wild populations of northern Turkey. Pharm. Biol. 2010;48:906–914. doi: 10.3109/13880200903311136. [DOI] [PubMed] [Google Scholar]
- 15.Lazzara S., Militello M., Carrubba A., Napoli E., Saia S. Arbuscular mycorrhizal fungi altered the hypericin, pseudohypericin, and hyperforin content in flowers of Hypericum perforatum grown under contrasting P availability in a highly organic substrate. Mycorrhiza. 2017;27:345–354. doi: 10.1007/s00572-016-0756-6. [DOI] [PubMed] [Google Scholar]
- 16.Lazzara S., Carrubba A., Napoli E. Cultivating for the industry: Cropping experiences with Hypericum perforatum L. in a Mediterranean environment. Agriculture. 2021;11:446. doi: 10.3390/agriculture11050446. [DOI] [Google Scholar]
- 17.Mañero F.J.G., Algar E., Martin Gomez M.S., Saco Sierra M.D., Solano B.R. Elicitation of secondary metabolism in Hypericum perforatum by rhizosphere bacteria and derived elicitors in seedlings and shoot cultures. Pharm. Biol. 2012;50:1201–1209. doi: 10.3109/13880209.2012.664150. [DOI] [PubMed] [Google Scholar]
- 18.Velingkar V.S., Gupta G.L., Hegde N.B. A current update on phytochemistry, pharmacology and herb–drug interactions of Hypericum perforatum. Phytochem. Rev. 2017;16:725–744. doi: 10.1007/s11101-017-9503-7. [DOI] [Google Scholar]
- 19.Verotta L. Hypericum perforatum, a source of neuroactive leads structures. Curr. Top. Med. Chem. 2003;3:187–201. doi: 10.2174/1568026033392589. [DOI] [PubMed] [Google Scholar]
- 20.Walker L., Sirvent T., Gibson D., Vance N. Regional differences in hypericin and pseudo hypericin concentrations and five morphological traits among Hypericum perforatum plants in the north western United States. Can. J. Bot. 2001;79:1248–1255. [Google Scholar]
- 21.Rahnavrd A. Genetic and biochemical diversity of Hypericum perforatum L. Grown in the caspian climate of Iran. Appl. Ecol. Environ. Res. 2017;15:665–675. doi: 10.15666/aeer/1501_665675. [DOI] [Google Scholar]
- 22.Caraci F., Crupi R., Drago F., Spina E. Metabolic drug interactions between antidepressants and anticancer drugs: Focus on selective serotonin reuptake inhibitors and Hypericum extract. Curr. Drug Metab. 2011;12:570–577. doi: 10.2174/138920011795713706. [DOI] [PubMed] [Google Scholar]
- 23.Saddiqe Z., Naeem I., Maimoona A. A review of the antibacterial activity of Hypericum perforatum. J. Ethnopharmacol. 2010;131:511–521. doi: 10.1016/j.jep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Birt D.F., Widrlechner M.P., Hammer K.D., Hillwig M.L., Wei J., Kraus G.A., Murphy P.A., McCoy J.A., Wurtele E.S., Neighbors J.D., et al. Hypericum in infection: Identification of anti-viral and anti-inflammatory constituents. Pharm. Biol. 2009;47:774–782. doi: 10.1080/13880200902988645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakhin O.I., Lubyanov A.A., Yakhin I.A., Brown P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017;54:23–27. doi: 10.3389/fpls.2016.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.du Jardin P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015;53:3555–3654. doi: 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- 27.Khan W., Rayirath U.P., Subramanian S., Jithesh M.N., Rayorath P., Hodges D.M., Critchley A.T., Craigie J.S., Norrie J., Prithiviraj B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009;45:112–134. doi: 10.1007/s00344-009-9103-x. [DOI] [Google Scholar]
- 28.Ali O., Ramsubhag A., Jayaraman J. Biostimulatory Activities of Ascophyllum nodosum Extract in Tomato and Sweet Pepper Crops in a Tropical Environment. PLoS ONE. 2019;14:0216710. doi: 10.1371/journal.pone.0216710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FAO . The International Support Programme for Irrigation Water Management Land and Water Development Division. FAO; Rome, Italy: 1986. pp. 1–74. [Google Scholar]
- 30.Giannoulis K.D., Evangelopoulos V., Gougoulias N., Wogiatzi Ε. Lavender organic cultivation yield and essential oil can be improved by using bio-stimulants. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020;70:648–656. doi: 10.1080/09064710.2020.1833974. [DOI] [Google Scholar]
- 31.Tsivelika N., Sarrou E., Gusheva K., Pankou C., Koutsos T., Chatzopoulou P., Mavromatis A. Phenotypic variation of wild Chamomile (Matricaria chamomilla L.) populations and their evaluation for medicinally important essential oil. Biochem. Syst. Ecol. 2018;80:21–28. doi: 10.1016/j.bse.2018.06.001. [DOI] [Google Scholar]
- 32.Sarrou E., Tsivelika N., Chatzopoulou P., Tsakalidis G., Menexes G., Mavromatis A. Conventional breeding of Greek oregano (Origanum vulgare ssp. hirtum) and development of improved cultivars for yield potential and essential oil quality. Euphytica. 2017;213:104. [Google Scholar]
- 33.Adams R.P. Identification of Volatile Oil Components by Gas Chromatography/Mass Spectroscopy. Allured publishing Co.; Chicago, IL, USA: 1995. [Google Scholar]
- 34.Steel R.G.D., Torrie J.H. Principles and Procedures of Statistics. A Biometrical Approach. 2nd ed. McGraw-Hill, Inc.; New York, NY, USA: 1982. p. 633. [Google Scholar]
- 35.Bonari G., Monaci F., Nannoni F., Angiolini C., Protano G. Trace Element Uptake and Accumulation in the Medicinal Herb Hypericum perforatum L. Across Different Geolithological Settings. Biol. Trace Elem. Res. 2019;189:267–276. doi: 10.1007/s12011-018-1453-4. [DOI] [PubMed] [Google Scholar]
- 36.Kwiecie’n I., Nicosia N., Ekiert H. Cultivation of Hypericum perforatum (St. John’s Wort) and Biotechnological Approaches for Improvement of Plant Raw Material Quality. In: Ekiert H.M., Ramawat K.G., Arora J., editors. Medicinal Plants: Domestication, Biotechnology and Regional Importance, Sustainable Development and Biodiversity. Springer International Publishing; Cham, Switzerland: 2021. pp. 253–291. [Google Scholar]
- 37.Rizzo P., Altschmied L., Ravindran B.M., Rutten T., D’Auria J.C. The Biochemical and Genetic Basis for the Biosynthesis of Bioactive Compounds in Hypericum perforatum L., One of the Largest Medicinal Crops in Europe. Genes. 2020;11:1210. doi: 10.3390/genes11101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Gao G., Wu Z., Wen X., Zhong H., Zhong Z., Yang C., Bian F., Gai X. Responses of soil nutrients and microbial communities to intercropping medicinal plants in moso bamboo plantations in subtropical China. Environ. Sci. Pollut. Res. 2020;27:2301–2310. doi: 10.1007/s11356-019-06750-2. [DOI] [PubMed] [Google Scholar]
- 39.Duc N.H., Vo A.T., Haddidi I., Daood H., Posta K. Arbuscular Mycorrhizal Fungi Improve Tolerance of the Medicinal Plant Eclipta prostrata (L.) and Induce Major Changes in Polyphenol Profiles under Salt Stresses. Front. Plant Sci. 2021;11:612299. doi: 10.3389/fpls.2020.612299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., Zheng X., Wei X., Kai Z., Xu Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021;11:23015. doi: 10.1038/s41598-021-02521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura T., Zobayed S.M.A., Kozai T., Goto E. Medicinally Important Secondary Metabolites and Growth of Hypericum perforatum L. Plants as Affected by Light Quality and Intensity. Environ. Control. Biol. 2007;45:113–120. doi: 10.2525/ecb.45.113. [DOI] [Google Scholar]
- 42.Murch S.J., Haq K., Rupasinghe H.P.V., Saxena P.K. Nickel contamination affects growth and secondary metabolite composition of St. John’s wort (Hypericum perforatum L.) Environ. Exp. Bot. 2003;49:251–257. doi: 10.1016/S0098-8472(02)00090-4. [DOI] [Google Scholar]
- 43.Sobhani Najafabadi A., Khanahmadi M., Ebrahimi M., Moradi K., Behroozi P., Noormohammadi N. Effect of different quality of light on growth and production of secondary metabolites in adventitious root cultivation of Hypericum perforatum. Plant Signal. Behav. 2019;14:1640561. doi: 10.1080/15592324.2019.1640561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Çirak C., Radusiene J., Janulis V., Ivanauskas L. Pseudohypericin and Hyperforin in Hypericum perforatum from Northern Turkey: Variation among Populations, Plant Parts and Phenological Stages. J. Integr. Plant Biol. 2008;50:575–580. doi: 10.1111/j.1744-7909.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 45.Al Rifaee M.K., Haddad N., Aburjai T. Domestication of Wild Hypericum triquetrifolium Populations Under Semi-Arid Environment of Jordan: Cultivation Potential and Breeding Perspectives. J. Herbs Spices Med. Plants. 2010;16:51–62. doi: 10.1080/10496475.2010.481934. [DOI] [Google Scholar]
- 46.Bertoli A., Cirak C., Leonardi M., Seyis F., Pistelli L. Morphogenetic changes in essential oil composition of Hypericum perforatum during the course of ontogenesis. Pharm. Biol. 2011;49:741–751. doi: 10.3109/13880209.2010.545826. [DOI] [PubMed] [Google Scholar]
- 47.Ceylan A., Bayram E., Arabaci O., Marquard R.A., Ozay N., Geren H. Breeding and determination of suitable chemotype in st. John’s Wort (Hypericum perforatum L.) populations the flora of Aegean region. J. Agric. 2002;42:33–44. [Google Scholar]
- 48.Pluhar Z., Rehak O., Nemeth E. Comparative investigation on Hypericum perforatum L. populations of different origin. Int. J. Horticult. Sci. 2000;6:56–60. doi: 10.31421/IJHS/6/1/68. [DOI] [Google Scholar]
- 49.Chaski C., Giannoulis K.D., Alexopoulos A.A., Petropoulos S.A. Biostimulant Application Alleviates the Negative Effects of Deficit Irrigation and Improves Growth Performance, Essential Oil Yield and Water-Use Efficiency of Mint Crop. Agronomy. 2023;13:2182. doi: 10.3390/agronomy13082182. [DOI] [Google Scholar]
- 50.Kumar D., Kumar R., Singh A.K., Verma K., Singh K.P., Nilofer, Kumar A., Singh V., Kaur P., Singh A., et al. A novel and economically viable agro-technique for enhancing productivity and resource use efficiency in menthol mint (Mentha arvensis L.) Ind. Crops Prod. 2021;162:113233. doi: 10.1016/j.indcrop.2020.113233. [DOI] [Google Scholar]
- 51.Giannoulis K.D., Kamvoukou C.A., Gougoulias N., Wogiatzi E. Matricaria chamomilla L. (German chamomile) flower yield and essential oil affected by irrigation and nitrogen fertilization. Emir. J. Food Agric. 2020;32:328–335. doi: 10.9755/ejfa.2020.v32.i5.2099. [DOI] [Google Scholar]
- 52.Zheljazkov V.D., Cantrell C.L., Astatkie T., Hristov A. Yield, content, and composition of peppermint and spearmints as a function of harvesting time and drying. J. Agric. Food Chem. 2010;58:11400–11407. doi: 10.1021/jf1022077. [DOI] [PubMed] [Google Scholar]
- 53.Ghamarnia H., Mousabeygi F., Arji I. Lemon Balm (Melissa officinalis L.) Water Requirement, Crop Coefficients Determination and SIM Dual Kc Model Implementing. Eur. J. Med. Plants. 2014;5:281–296. doi: 10.9734/EJMP/2015/14138. [DOI] [Google Scholar]
- 54.Baghalian K., Abdoshah S., Farahnaz K.S., Paknejad F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.) Plant Physiol. Biochem. 2011;49:201–207. doi: 10.1016/j.plaphy.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Youssef A.A., Aziz A.A., Talaat I.M. Influence of some antioxidants on growth, flowerheads and essential oil content of Matricaria chamomilla, L. plants. Ann. Agric. Sci. Moshtohor. 2005;43:823–832. [Google Scholar]
- 56.Orlita A., Gorycka M.S., Paszkiewicz M., Malinski E., Kumirska J., Sied M., Stepnowski P., Lojkowska E. Application of chitin and chitosan as elicitors of coumarins and furoquinolone alkaloids in Ruta graveolens L. (common rue) J. Biotechnol. Appl. Biochem. 2008;51:91–960. doi: 10.1042/BA20070200. [DOI] [PubMed] [Google Scholar]
- 57.Yin H., Fretté X.C., Christensen L.P., Grevsen K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Majorana vulgare ssp. hirtum) J. Agric. Food Chem. 2012;60:136–143. doi: 10.1021/jf204376j. [DOI] [PubMed] [Google Scholar]
- 58.El-Bassiony A.M., Fawzy Z.F., Zaki M.F., El-Nemr M.A. Increasing productivity of two sweet fennel cultivars by foliar spraying of some bio and organic compounds. Middle East J. Appl. Sci. 2014;4:794–801. [Google Scholar]
- 59.El-Khateeb M.A., El-Attar A.B., Nour R.M. Application of plant biostimulants to improve the biological responses and essential oil production of marjoram (Majorana hortensis, Moench) plants. Middle East J. Agric. Res. 2017;6:928–941. [Google Scholar]
- 60.Shaddad L.M.A., Radi A.F., Abdel-Rahman A.M., Azooz M.M. Response of seeds of Lupines termis and Vicia faba to the interactive effect of salinity and ascorbic acid on pyridoxine. Plant Soil. 1990;122:177–183. doi: 10.1007/BF02851972. [DOI] [Google Scholar]
- 61.Nardi S., Pizzeghello D., Muscolo A., Vianello A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002;34:1527–1536. doi: 10.1016/S0038-0717(02)00174-8. [DOI] [Google Scholar]
- 62.Mahfouz S.A., Sharaf Eldin M.A. Effect of mineral vs. biofertilizer on growth, yield, and essential oil content of fennel (Foeniculum vulgare Mill) Int. Agrophys. 2007;21:361–366. [Google Scholar]
- 63.Kalyanasundaram B., Kumar T.S., Kumar S., Swaminathan V. Effect of N, P, with biofertilizers and vermicompost on growth and physiological characteristics of sweet flag (Acorus calamus L.) Adv. Plant Sci. 2008;21:323–326. [Google Scholar]
- 64.Hanafy M.S., Ashour H.A., Sedek F.M. Effect of some biostimulants and micronutrients on growth, yield and essential oil production of Majorana hortensis plants. Int. J. Environ. 2018;7:37–52. [Google Scholar]
- 65.Morshedloo M.R., Ebadi A., Maggi F., Fattahi R., Yazdani D., Jafari M. Chemical characterization of the essential oil compositions from Iranian populations of Hypericum perforatum L. Ind. Crop Prod. 2015;76:565–573. doi: 10.1016/j.indcrop.2015.07.033. [DOI] [Google Scholar]
- 66.Martonfi P., Repcak M., Ciccarelli D., Garbari F. Hypericum perforatum L.—Chemotype without rutin from Italy. Biochem. Syst. Ecol. 2001;29:659–661. doi: 10.1016/S0305-1978(00)00094-6. [DOI] [PubMed] [Google Scholar]
- 67.Avato P., Guglielmi G. Determination of major constituents in St. John’s wort under different extraction conditions. Pharm. Biol. 2004;42:83–89. doi: 10.1080/13880200490505663. [DOI] [Google Scholar]
- 68.Crockett S.L. Essential oil and volatile components of the genus Hypericum (Hypericaceae) Nat. Prod. Commun. 2010;5:1493–1506. doi: 10.1177/1934578X1000500926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arpag O.F., Duran N., Açikgül F.C., Türkmen M. Comparison of Minimum inhibitory concentrations of Hypericum perforatum L. Essential oils, 0.2% chlorhexidine and 10% povidone-iodine over Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. J. Essent. Oil-Bear. Plants. 2020;23:1192–1205. doi: 10.1080/0972060X.2020.1867007. [DOI] [Google Scholar]
- 70.Schepetkin I.A., Ozek G., Ozek T., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. Chemical composition and immunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules. 2021;26:3652. doi: 10.3390/molecules26123652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Günes S., Tıhmınlıoglu F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017;102:933–943. doi: 10.1016/j.ijbiomac.2017.04.080. [DOI] [PubMed] [Google Scholar]
- 72.Egri O., Erdemir N. Production of Hypericum perforatum oil-loaded membranes for wound dressing material and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2019;47:1404–1415. doi: 10.1080/21691401.2019.1596933. [DOI] [PubMed] [Google Scholar]
- 73.Clarke K., Porter R., Facey P., Yee T., Thoms-Rodriguez C. Chemical composition and biological activities of Pimentarichardii. Flavour Fragr. J. 2021;36:272–279. doi: 10.1002/ffj.3642. [DOI] [Google Scholar]
- 74.Silva A.R., Taofiq O., Ferreira I.C.F.R., Barros L. Hypericum genus cosmeceutical application—A decade comprehensive review on its multifunctional biological properties. Ind. Crop Prod. 2021;159:113053. doi: 10.1016/j.indcrop.2020.113053. [DOI] [Google Scholar]
- 75.Guleken Z., Depciuch J., Ege H., İlbay G., Kalkandelen C., Ozbeyli D., Bulut H., Sener G., Tarhan N., ErdemKuruca S. Spectrochemical and biochemical assay comparison study of the healing effect of the Aloe vera and Hypericum perforatum loaded nanofiber dressings on diabetic wound. Spectrochim. Acta Mol. Biomol. Spectrosc. 2021;254:119639. doi: 10.1016/j.saa.2021.119639. [DOI] [PubMed] [Google Scholar]
- 76.da Silva A.C., Lopes P.M., de Azevedo M.M., Costa D.C., Alviano C.S., Alviano D.S. Biological activities of alpha-pinene and beta-pinene enantiomers. Molecules. 2012;17:6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sybilska D., Kowalczyk J., Asztemborska M., Ochocka R.J., Lamparczyk H. Chromatographic studies of the enantiomeric composition of some therapeutic compositions applied in the treatment of liver and kidney diseases. J. Chromatogr. A. 1994;665:67–73. doi: 10.1016/0021-9673(94)87033-0. [DOI] [PubMed] [Google Scholar]
- 78.Alma M.H., Nitz S., Kollmannsberger H., Digrak M., Efe F.T., Yilmaz N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish pistachio (Pistacia vera L.) J. Agric. Food Chem. 2004;52:3911–3914. doi: 10.1021/jf040014e. [DOI] [PubMed] [Google Scholar]
- 79.Zhou J.Y., Tang F.D., Mao G.G., Bian R.L. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol. Sin. 2004;25:480–484. [PubMed] [Google Scholar]
- 80.Karakaya S., Yilmaz S.V., Özdemir Ö., Koca M., Pınar N.M., Demirci B., Yıldırım K., Sytar O., Turkez H., Baser K.H.C. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae) J. Essent. Oil Res. 2020;32:512–525. [Google Scholar]
- 81.Dos Santos E., Radai J.A.S., do Nascimento K.F., Formagio A.S.N., de Matos Balsalobre N., Ziff E.B., Castelon Konkiewitz E., Kassuya C.A.L. Contribution of spathulenol to the anti-nociceptive effects of Psidium guineense. Nutr. Neurosci. 2020;25:812–822. doi: 10.1080/1028415X.2020.1815330. [DOI] [PubMed] [Google Scholar]
- 82.de Jesús Dzul-Beh A., García-Sosa K., Uc-Cachón A.H., Bórquez J., Loyola L.A., Barrios-García H.B., Peña-Rodríguez L.M., Molina-Salinas G.M. In vitro growth inhibition and bactericidal activity of spathulenol against drugresistant clinical isolates of Mycobacterium tuberculosis. Rev. Bras. Farmacogn. 2019;29:798–800. doi: 10.1016/j.bjp.2019.06.001. [DOI] [Google Scholar]
- 83.Benelli G., Pavela R., Drenaggi E., Desneux N., Maggi F. Phytol, (E)-nerolidol and spathulenol from Stevia rebaudiana leaf essential oil as effective and eco-friendly botanical insecticides against Metopolophium dirhodum. Ind. Crops Prod. 2020;155:112844. doi: 10.1016/j.indcrop.2020.112844. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.