Abstract
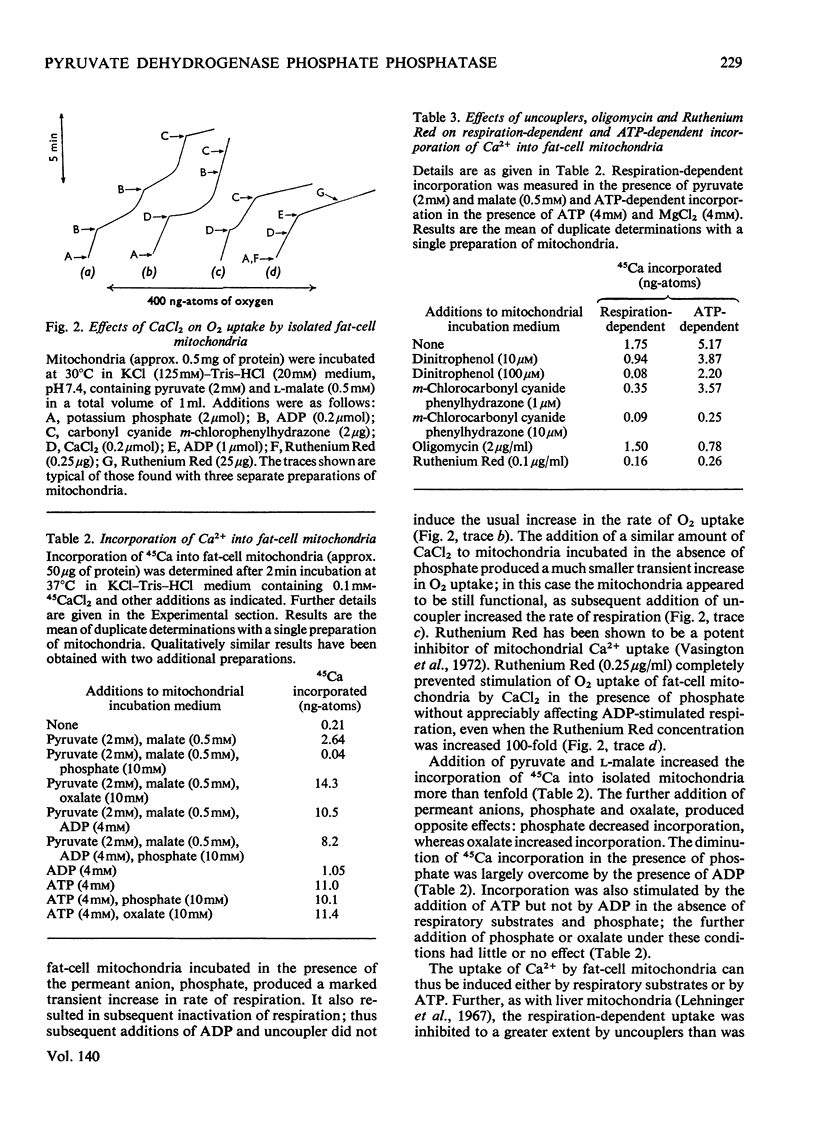
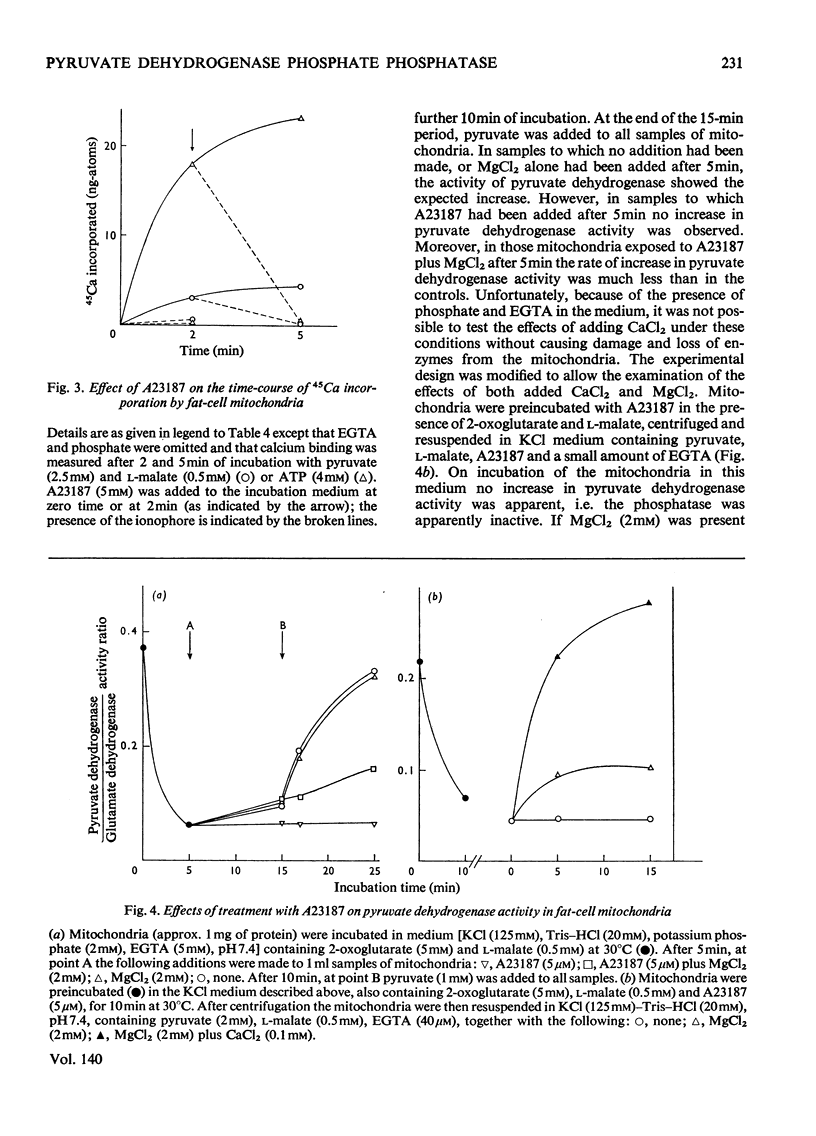
The metal-ion requirement of extracted and partially purified pyruvate dehydrogenase phosphate phosphatase from rat epididymal fat-pads was investigated with pig heart pyruvate dehydrogenase [32P]phosphate as substrate. The enzyme required Mg2+ (Km 0.5mm) and was activated additionally by Ca2+ (Km 1μm) or Sr2+ and inhibited by Ni2+. Isolated fat-cell mitochondria, like liver mitochondria, possess a respiration- or ATP-linked Ca2+-uptake system which is inhibited by Ruthenium Red, by uncouplers when linked to respiration, and by oligomycin when linked to ATP. Depletion of fat-cell mitochondria of 75% of their total magnesium content and of 94% of their total calcium content by incubation with the bivalent-metal ionophore A23187 leads to complete loss of pyruvate dehydrogenase phosphate phosphatase activity. Restoration of full activity required addition of both MgCl2 and CaCl2. SrCl2 could replace CaCl2 (but not MgCl2) and NiCl2 was inhibitory. The metal-ion requirement of the phosphatase within mitochondria was thus equivalent to that of the extracted enzyme. Insulin activation of pyruvate dehydrogenase in rat epididymal fat-pads was not accompanied by any measurable increase in the activity of the phosphatase in extracts of the tissue when either endogenous substrate or 32P-labelled pig heart substrate was used for assay. The activation of pyruvate dehydrogenase in fat-pads by insulin was inhibited by Ruthenium Red (which may inhibit cell and mitochondrial uptake of Ca2+) and by MnCl2 and NiCl2 (which may inhibit cell uptake of Ca2+). It is concluded that Mg2+ and Ca2+ are cofactors for pyruvate dehydrogenase phosphate phosphatase and that an increased mitochondrial uptake of Ca2+ might contribute to the activation of pyruvate dehydrogenase by insulin.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cittadini A., Scarpa A., Chance B. Calcium transport in intact Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Jan 2;291(1):246–259. doi: 10.1016/0005-2736(73)90416-1. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H., Rodbard D., Levine D. Mathematical theory of cross-reactive radioimmunoassay and ligand-binding systems of equilibrium. Anal Biochem. 1972 Feb;45(2):530–556. doi: 10.1016/0003-2697(72)90216-3. [DOI] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Hormonal regulation of pyruvate dehydrogenase. Metabolism. 1971 Jan;20(1):43–53. doi: 10.1016/0026-0495(71)90058-8. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Selective inhibition of the transmembrane Ca conductivity of mammalian myocardial fibres by Ni, Co and Mn ions. Pflugers Arch. 1973 Jan 22;338(2):115–123. doi: 10.1007/BF00592747. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M., Pask H. T., Randle P. J. Mechanisms regulating adipose-tissue pyruvate dehydrogenase. Biochem J. 1972 Sep;129(3):763–773. doi: 10.1042/bj1290763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- ROSSI C. S., LEHNINGER A. L. STOICHIOMETRY OF RESPIRATORY STIMULATION, ACCUMULATION OF CA++ AND PHOSPHATE, AND OXIDATIVE PHOSPHORYLATION IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3971–3980. [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., Pask H. T., Severson D. L. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;(39):75–88. [PubMed] [Google Scholar]
- Randle P. J., Denton R. M. Rate control by insulin and its mechanism. Symp Soc Exp Biol. 1973;27:401–428. [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Sica V., Cuatrecasas P. Effects of insulin, epinephrine, and cyclic adenosine monophosphate on pyruvate dehydrogenase of adipose tissue. Biochemistry. 1973 Jun 5;12(12):2282–2291. doi: 10.1021/bi00736a016. [DOI] [PubMed] [Google Scholar]
- Vasington F. D., Gazzotti P., Tiozzo R., Carafoli E. The effect of ruthenium red on Ca 2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta. 1972 Jan 21;256(1):43–54. doi: 10.1016/0005-2728(72)90161-2. [DOI] [PubMed] [Google Scholar]
- Weiss L., Löffler G., Schirmann A., Wieland O. Control of pyruvate dehydrogenase interconversion in adipose tissue by insulin. FEBS Lett. 1971 Jun 24;15(3):229–231. doi: 10.1016/0014-5793(71)80318-6. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Wilkinson J. R., Hamill R. L., Horng J. S. Effects of antibiotic ionophore, A23187, on oxidative phosphorylation and calcium transport of liver mitochondria. Arch Biochem Biophys. 1973 Jun;156(2):578–585. doi: 10.1016/0003-9861(73)90308-1. [DOI] [PubMed] [Google Scholar]


