Abstract
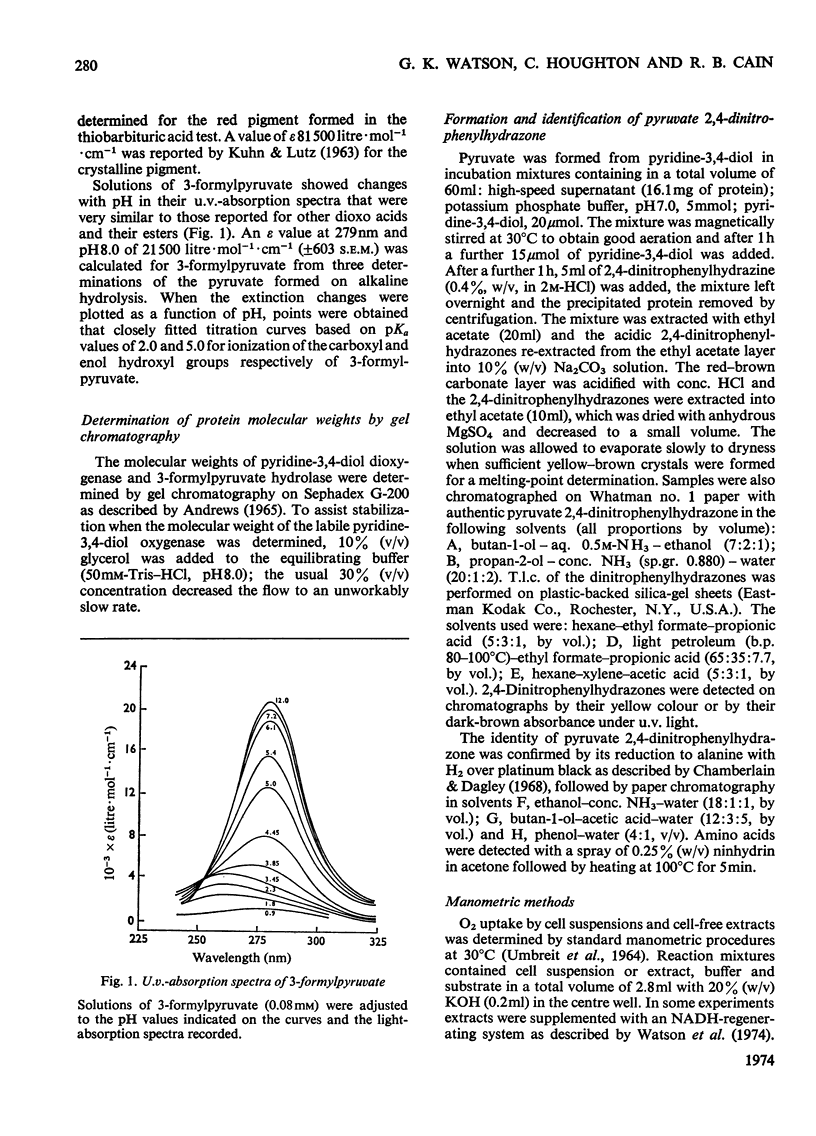
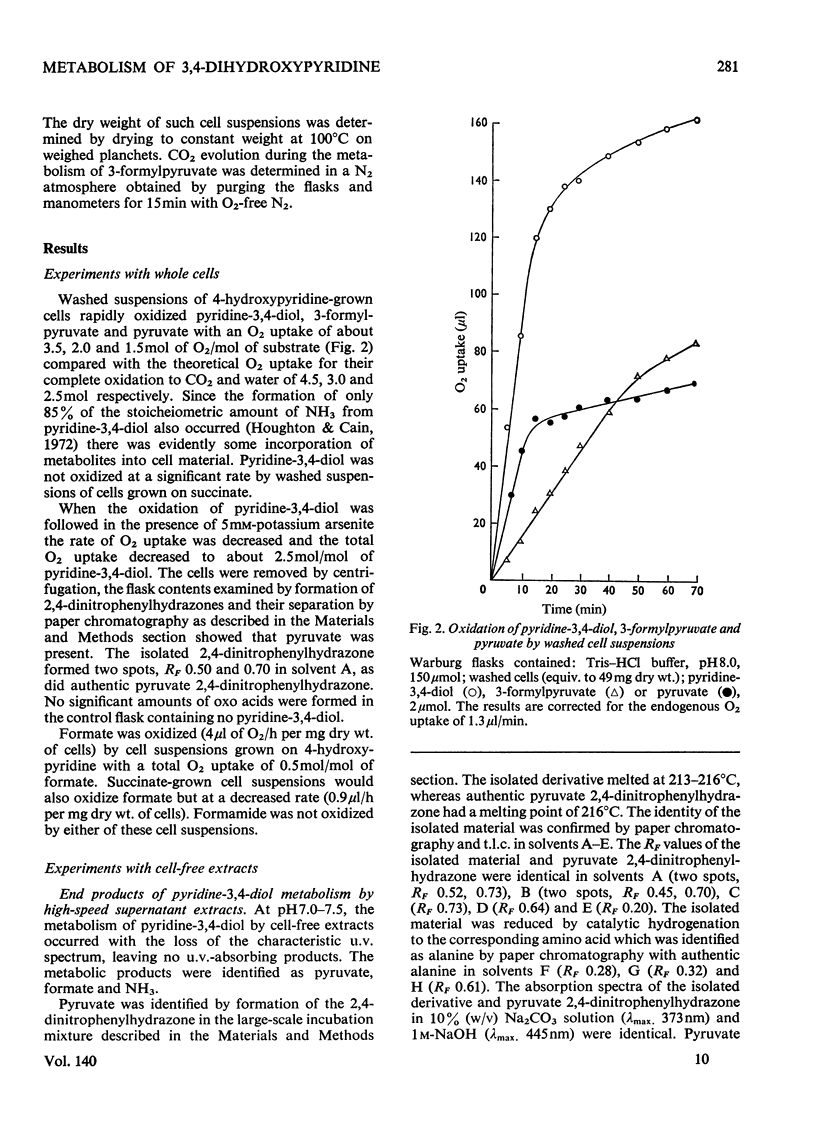
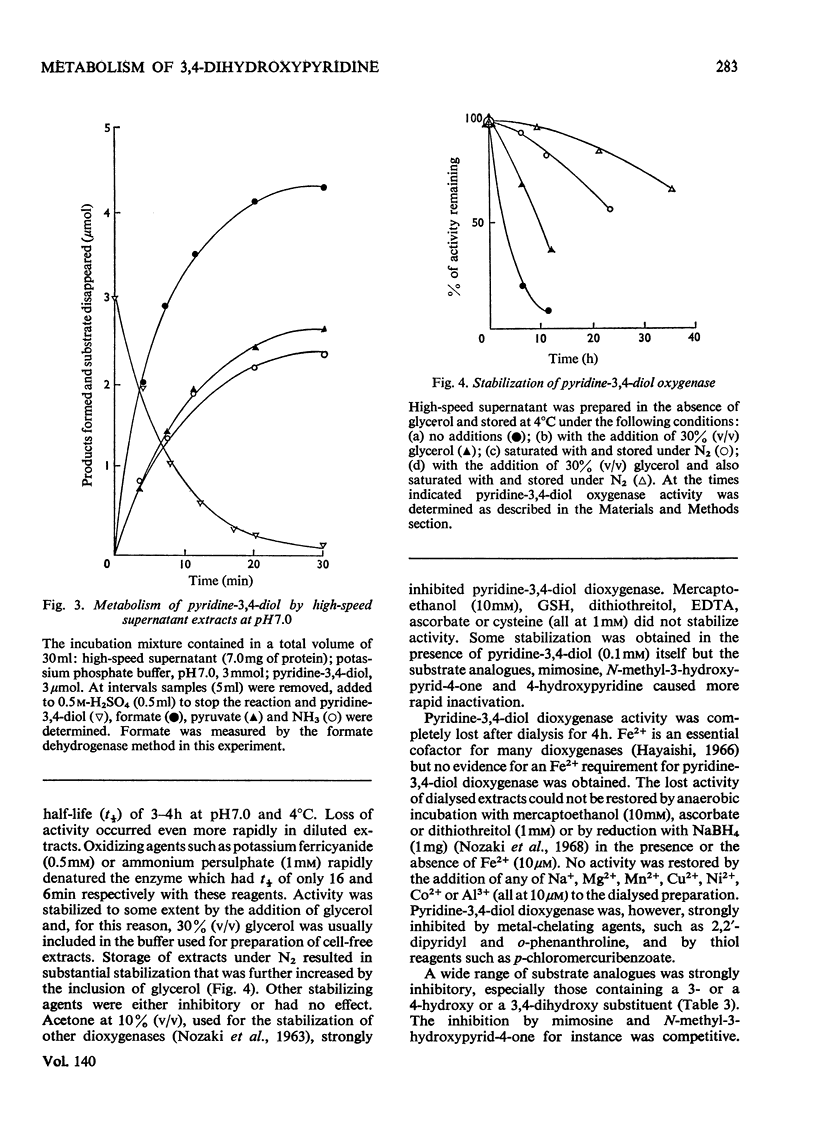
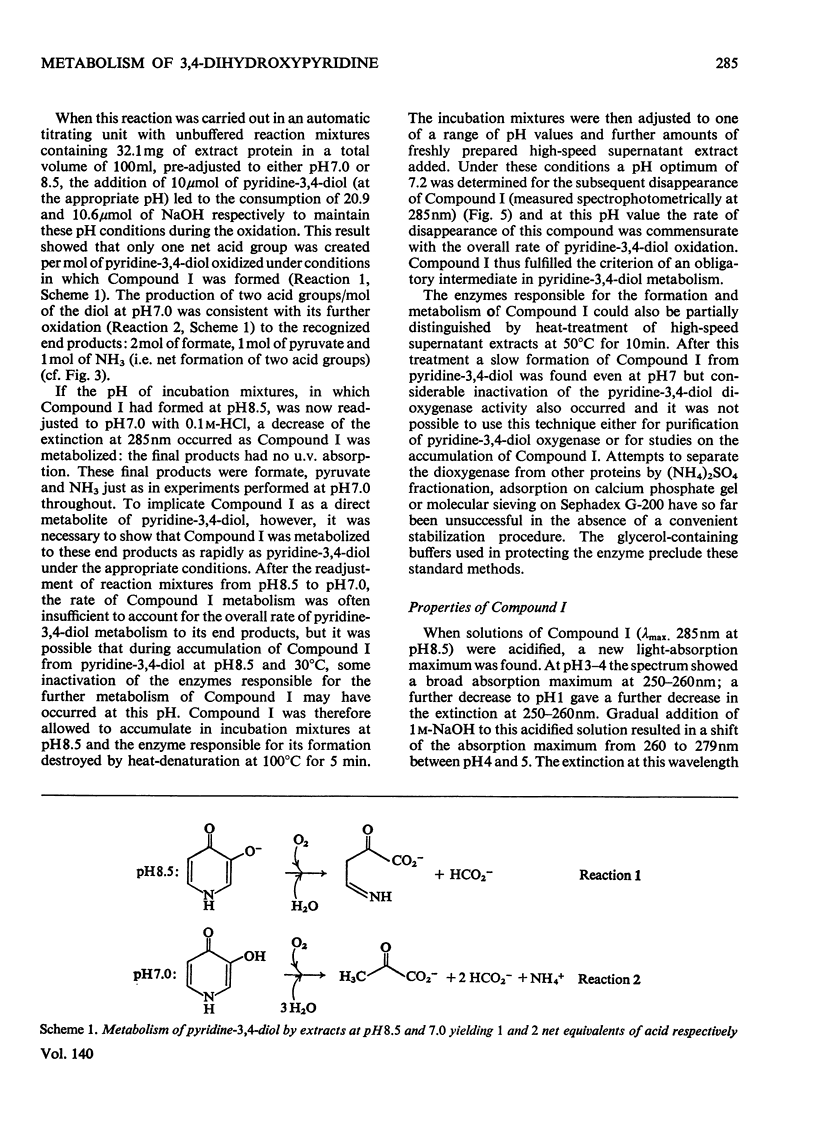
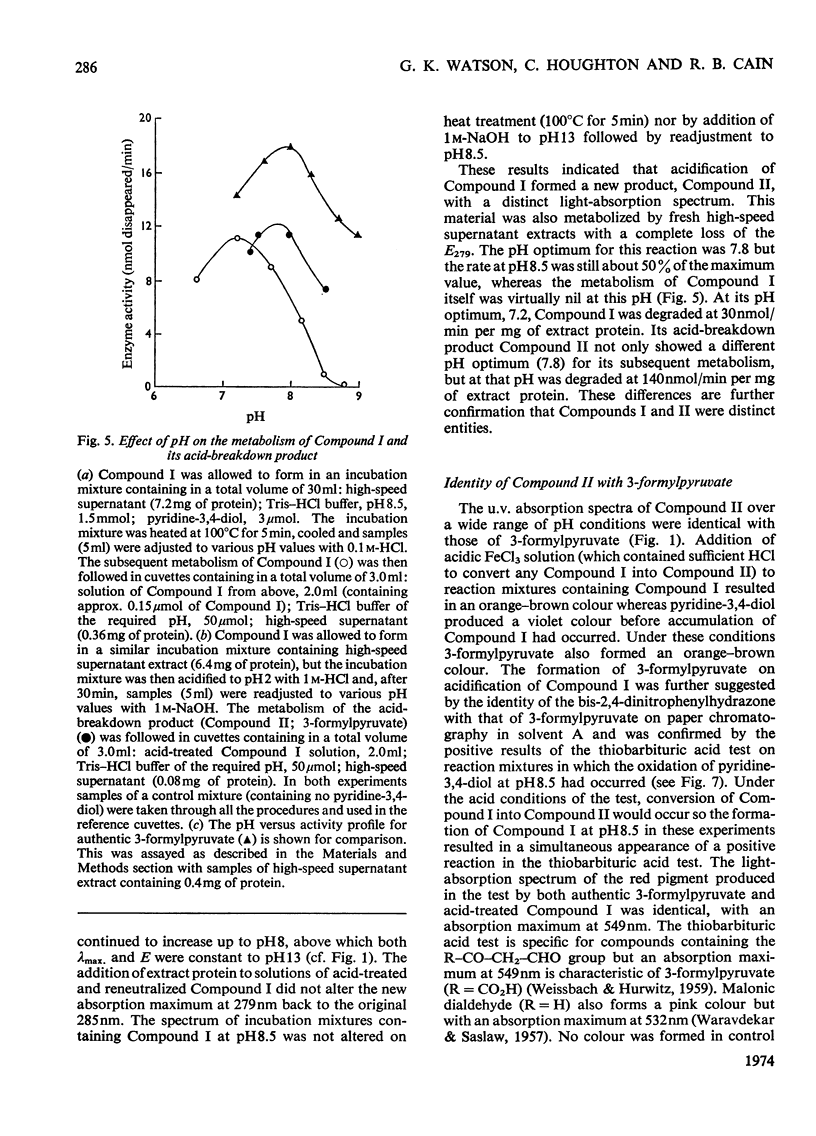
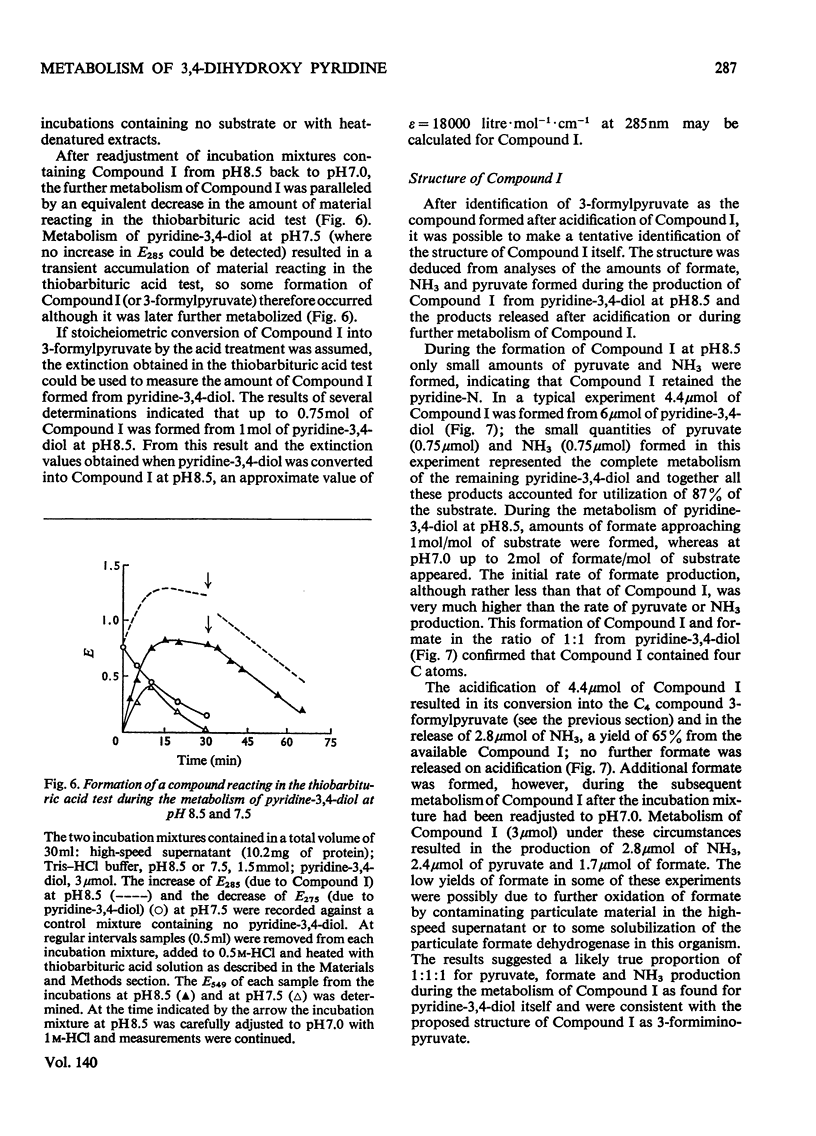
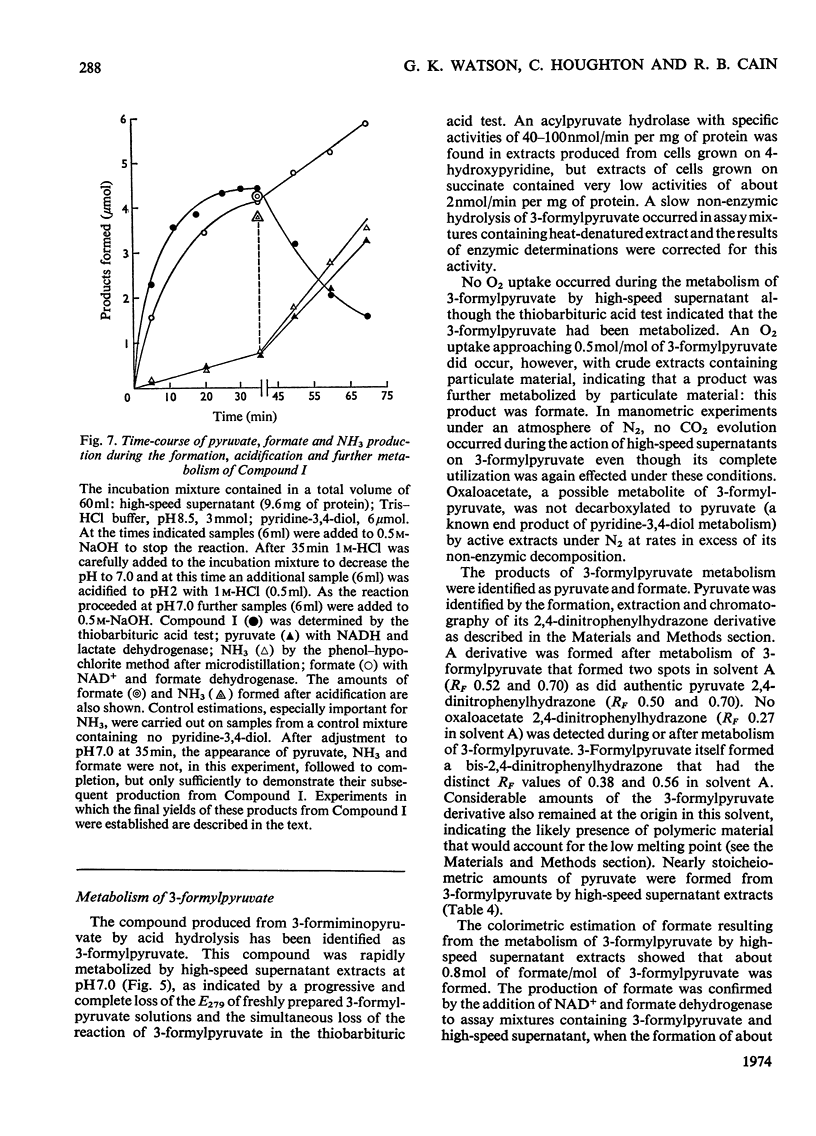
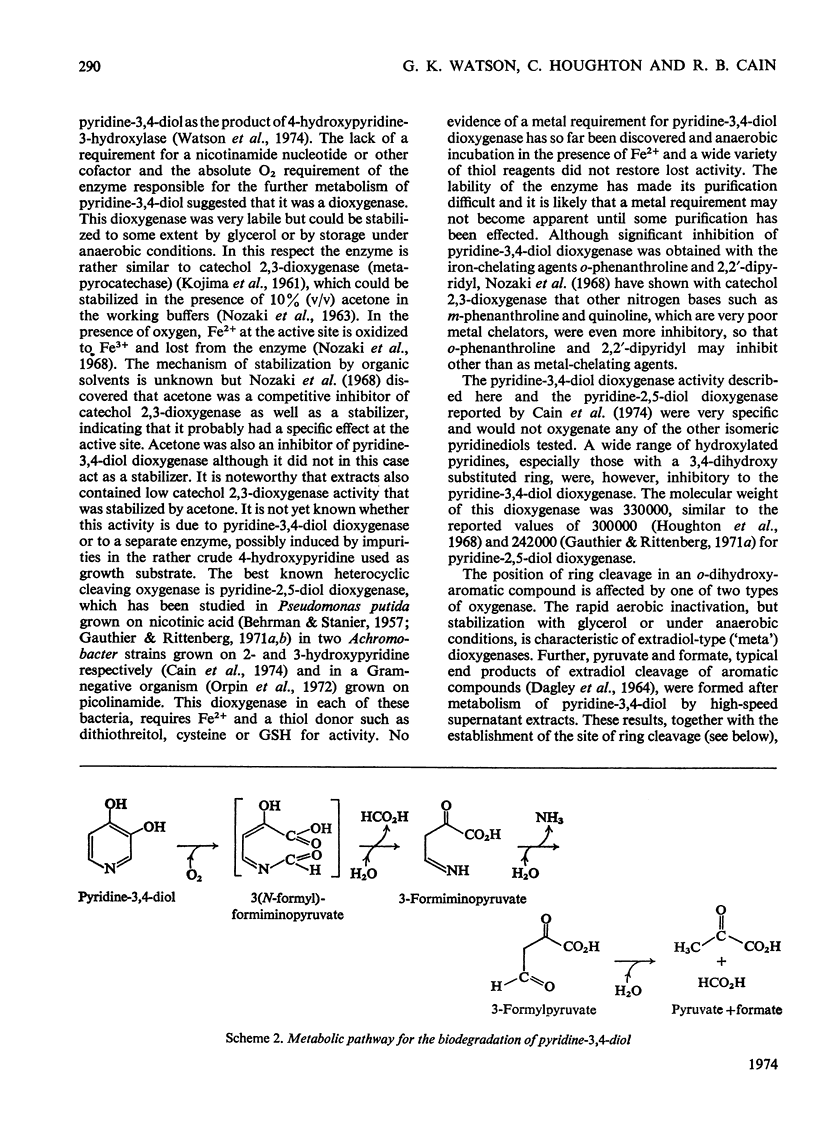
1. Pyridine-3,4-diol (3,4-dihydroxypyridine, 3-hydroxypyrid-4-one), an intermediate in 4-hydroxypyridine metabolism by an Agrobacterium sp (N.C.I.B. 10413), was converted by extracts into 1mol of pyruvate, 2mol of formate and 1mol of NH3 at pH7.0. 2. Formate, but not the alternative likely product formamide, was further oxidized fivefold faster by 4-hydroxypyridine-grown washed cells than by similar organisms grown on succinate. 3. The oxidation of pyridine-3,4-diol by crude extracts at pH8.5 required 1mol of O2/mol of substrate, produced 1mol of acid and led to the formation of formate and a new compound with an extinction maximum of 285nm (Compound I). This step was believed to be mediated by a new labile dioxygenase (t½=4h at pH7.0, 4°C) cleaving the pyridine ring between C-2 and C-3. 4. Many of the properties of this pyridine-3,4-diol dioxygenase paralleled those of the extradiol (`meta') oxygenases of aromatic-ring cleavage. The extreme lability of the enzyme has so far precluded extensive purification. 5. Compound I showed changes in the u.v.-absorption spectrum with pH but after acidification it was converted into a new product, 3-formylpyruvate, with an extinction maximum now at 279nm. 6. Both Compound I and 3-formylpyruvate were metabolized by extracts but at very different rates. The slower rate of metabolism of Compound I was nevertheless consistent with that of pyridine-3,4-diol metabolism. 7. On acidification Compound I released about 0.65mol of NH3 and has been identified as 3-formiminopyruvate. 8. 3-Formylpyruvate was hydrolysed to formate and pyruvate (Km 2μm) by an acylpyruvate hydrolase active against several other dioxo homologues. The activity of this enzyme was much lower in extracts of succinate-grown cells.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEHRMAN E. J., STANIER R. Y. The bacterial oxidation of nicotinic acid. J Biol Chem. 1957 Oct;228(2):923–945. [PubMed] [Google Scholar]
- Cain R. B., Houghton C., Wright K. A. Microbial metabolism of the pyridine ring. Metabolism of 2- and 3-hydroxypyridines by the maleamate pathway in Achromobacter sp. Biochem J. 1974 May;140(2):293–300. doi: 10.1042/bj1400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain E. M., Dagley S. The metabolism of thymol by a Pseudomonas. Biochem J. 1968 Dec;110(4):755–763. doi: 10.1042/bj1100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., CHAPMAN P. J., GIBSON D. T., WOOD J. M. DEGRADATION OF THE BENZENE NUCLEUS BY BACTERIA. Nature. 1964 May 23;202:775–778. doi: 10.1038/202775a0. [DOI] [PubMed] [Google Scholar]
- Gauthier J. J., Rittenberg S. C. The metabolism of nicotinic acid. I. Purification and properties of 2,5-dihydroxypyridine oxygenase from Pseudomonas putida N-9. J Biol Chem. 1971 Jun 10;246(11):3737–3742. [PubMed] [Google Scholar]
- Gauthier J. J., Rittenberg S. C. The metabolism of nicotinic acid. II. 2,5-dihydroxypyridine oxidation, product formation, and oxygen 18 incorporation. J Biol Chem. 1971 Jun 10;246(11):3743–3748. [PubMed] [Google Scholar]
- Hayaishi O. Crystalline oxygenases of pseudomonads. Bacteriol Rev. 1966 Dec;30(4):720–731. doi: 10.1128/br.30.4.720-731.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton C., Cain R. B. Microbial metabolism of the pyridine ring. Formation of pyridinediols (dihydroxypyridines) as intermediates in the degradation of pyridine compounds by micro-organisms. Biochem J. 1972 Dec;130(3):879–893. doi: 10.1042/bj1300879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON P. A., JONES-MORTIMER M. C., QUAYLE J. R. USE OF A PURIFIED BACTERIAL FORMATE DEHYDROGENASE FOR THE MICRO-ESTIMATION OF FORMATE. Biochim Biophys Acta. 1964 Aug 26;89:351–353. doi: 10.1016/0926-6569(64)90225-1. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJIMA Y., ITADA N., HAYAISHI O. Metapyrocatachase: a new catechol-cleaving enzyme. J Biol Chem. 1961 Aug;236:2223–2228. [PubMed] [Google Scholar]
- KUHN R., LUTZ P. UBER FORMYL-BRENZTRAUBENSAEURE UND DEN FARBSTOFF DER WARREN-REAKTION. Biochem Z. 1963;338:554–560. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOZAKI M., KAGAMIYAMA H., HAYAISHI O. METAPYROCATECHASE. I. PURIFICATION, CRYSTALLIZATION AND SOME PROPERTIES. Biochem Z. 1963;338:582–590. [PubMed] [Google Scholar]
- Nozaki M., Ono K., Nakazawa T., Kotani S., Hayaishi O. Metapyrocatechase. II. The role of iron and sulfhydryl groups. J Biol Chem. 1968 May 25;243(10):2682–2690. [PubMed] [Google Scholar]
- Orpin C. G., Knight M., Evans W. C. The bacterial oxidation of picolinamide, a photolytic product of Diquat. Biochem J. 1972 May;127(5):819–831. doi: 10.1042/bj1270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN P. R., SHIGEURA H. T., SPRECHER M., SPRINSON D. B., DAVIS B. D. The biosynthesis of shikimic acid from D-glucose. J Biol Chem. 1956 May;220(1):477–497. [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A method of estimation of 2-deoxyribose. Biochim Biophys Acta. 1957 May;24(2):439–439. doi: 10.1016/0006-3002(57)90224-x. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Watson G. K., Houghton C., Cain R. B. Microbial metabolism of the pyridine ring. The hydroxylation of 4-hydroxypyridine to pyridine-3,4-diol (3,4-dihydroxypyridine) by 4-hydroxypyridine-3-hydroxylase. Biochem J. 1974 May;140(2):265–276. doi: 10.1042/bj1400265. [DOI] [PMC free article] [PubMed] [Google Scholar]


