Abstract
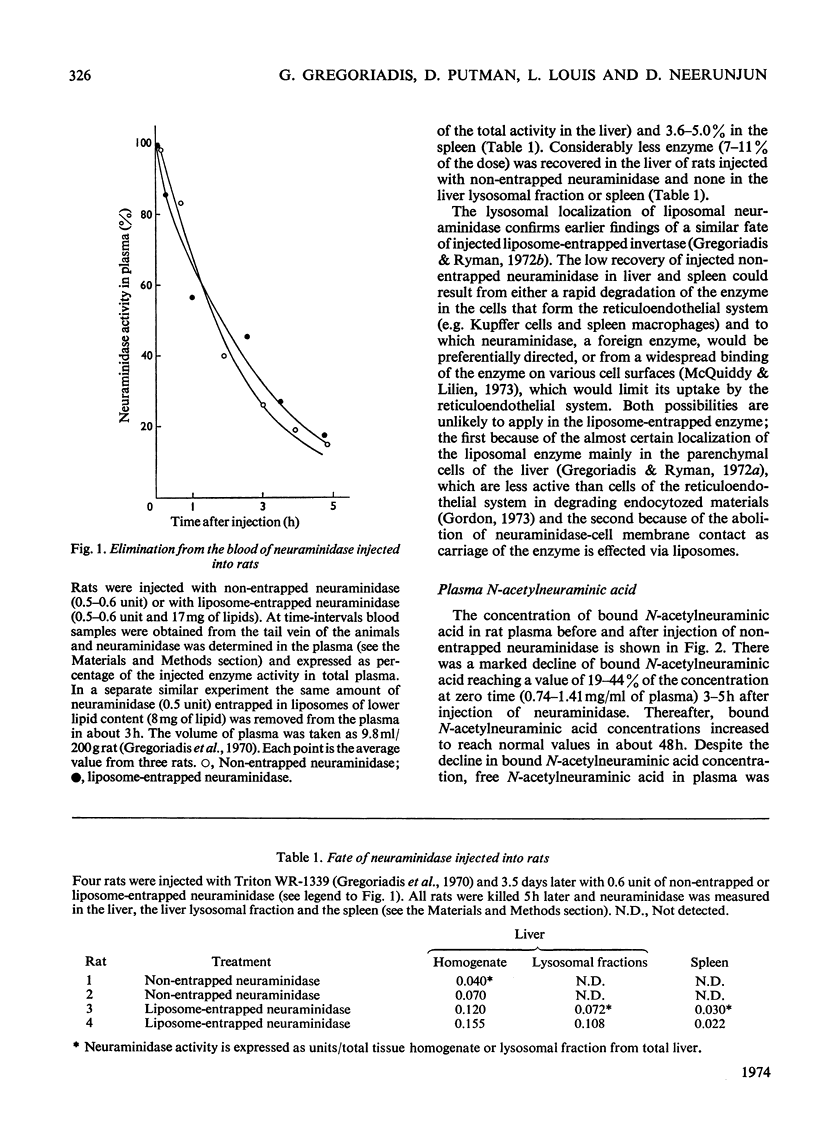
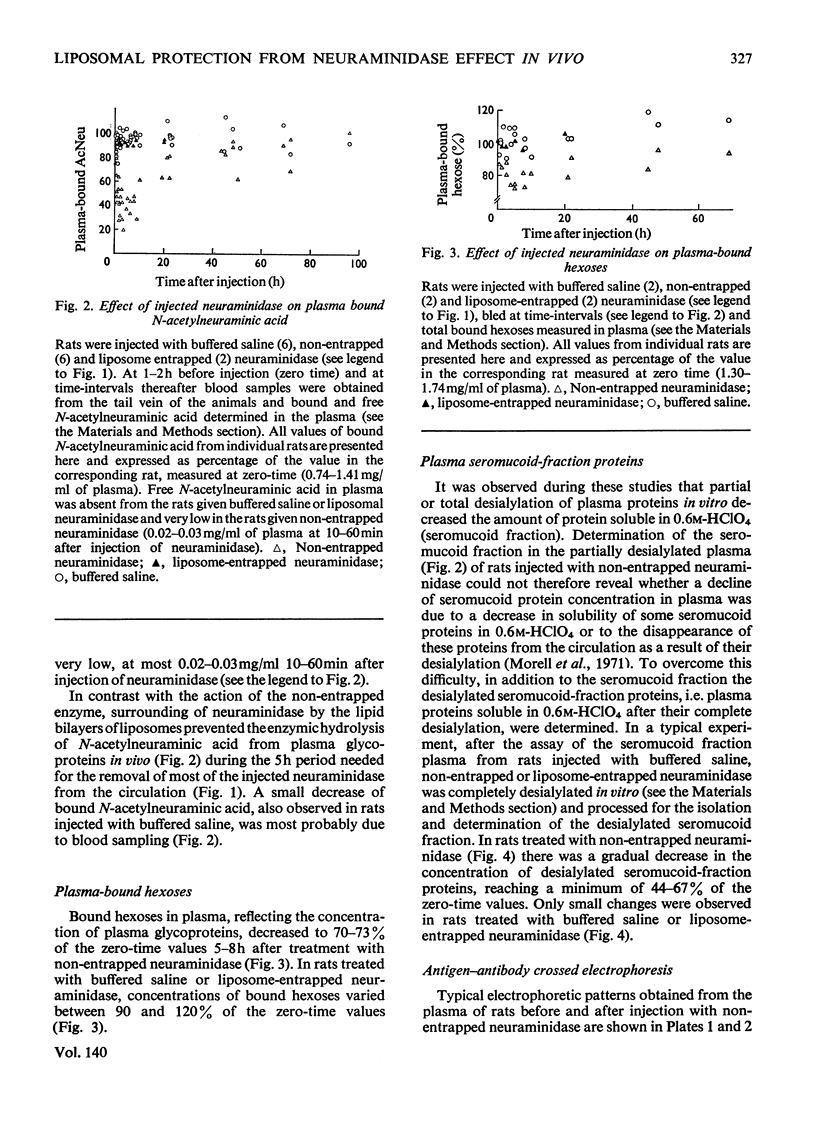
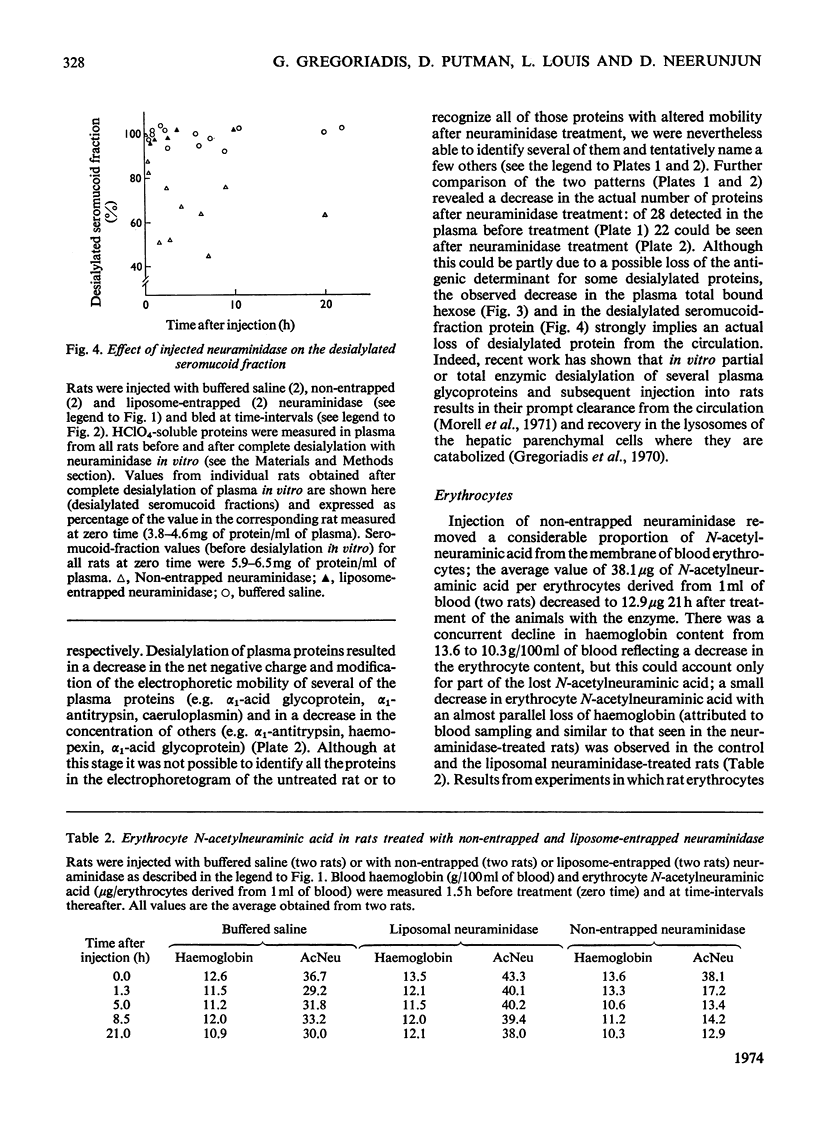
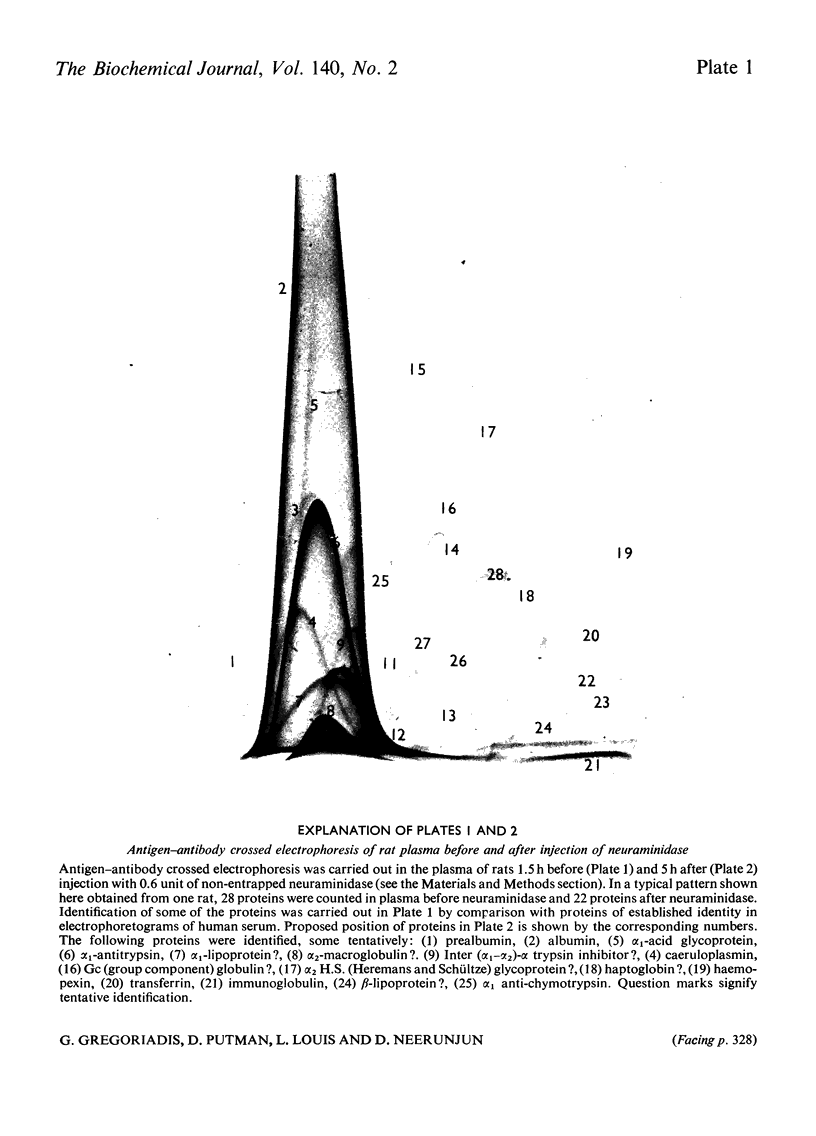
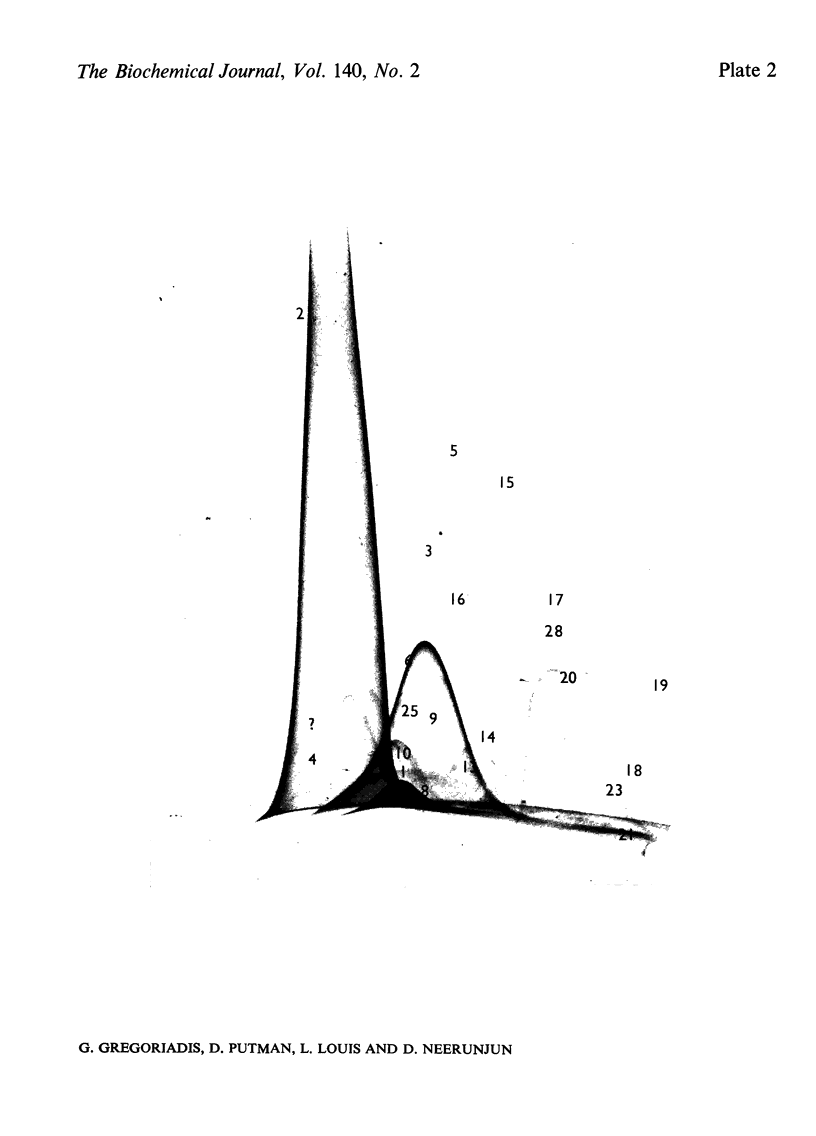
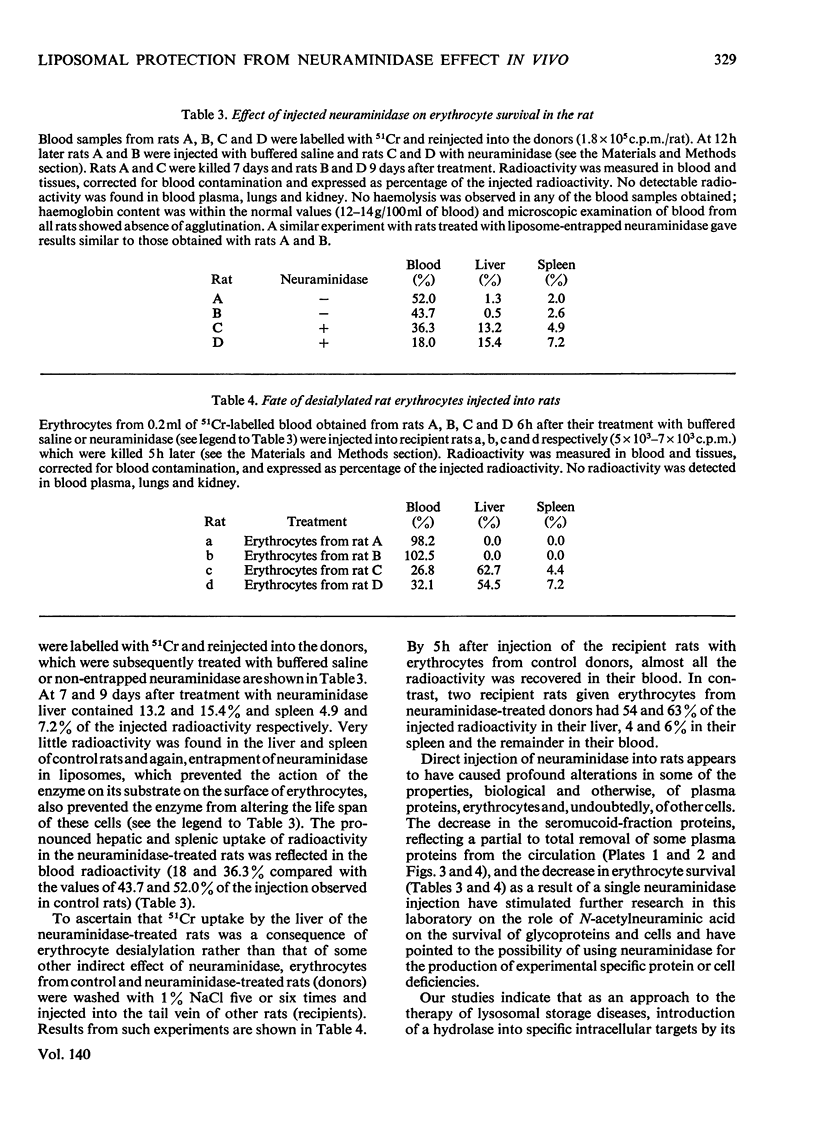
Non-entrapped and liposome-entrapped Clostridium perfringens neuraminidase (0.5–0.6 unit) was injected into rats and its fate as well as its effect on plasma and erythrocyte N-acetylneuraminic acid was investigated. The following observations were made. (1) Although removal of both non-entrapped and liposome-entrapped neuraminidase from the circulation was completed within 5h after injection, their recovery in tissues was distinctly different; 7–10% of the injected non-entrapped enzyme was found in the liver and none in the liver lysosomal fraction or the spleen. In contrast, 20–26% of the liposome-entrapped enzyme was found in the liver of which 60–69% was in the lysosomal fraction. Spleen contained 3.6–5.0% of the enzyme. (2) The presence of the non-entrapped neuraminidase in blood led to the extensive desialylation of plasma and to a decrease in the concentration or total removal from the circulation of some of the plasma glycoproteins. (3) Injection of non-entrapped neuraminidase also led to the partial desialylation of erythrocytes the life span of which was diminished and their uptake by the liver and spleen augmented. (4) Entrapment of neuraminidase in liposomes before its injection prevented the enzyme from acting on its substrate in plasma or on the erythrocyte surface, and values obtained for plasma glycoproteins and erythrocyte survival were similar to those observed in control rats. (5) Entrapment in liposomes of therapeutic hydrolases intended for the degradation of substances stored within the tissue lysosomes of patients with storage diseases could prevent the potentially hazardous enzymic action of hydrolases in blood and at the same time direct the enzymes to the intracellular sites where they are needed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady R. O. The adnormal biochemistry of inherited disorders of lipid metabolism. Fed Proc. 1973 Jun;32(6):1660–1667. [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Davies D. R., Spurr E. D., Versey J. B. Modifications to the technique of two-dimensional immunoelectrophoresis. Clin Sci. 1971 May;40(5):411–417. doi: 10.1042/cs0400411. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Buckland R. A. Enzyme-containing liposomes alleviate a model for storage disease. Nature. 1973 Jul 20;244(5412):170–172. doi: 10.1038/244170a0. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. Drug entrapment in liposomes. FEBS Lett. 1973 Nov 1;36(3):292–296. doi: 10.1016/0014-5793(73)80394-1. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Leathwood P. D., Ryman B. E. Enzyme entrapment in liposomes. FEBS Lett. 1971 Apr;14(2):95–99. doi: 10.1016/0014-5793(71)80109-6. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Morell A. G., Sternlieb I., Scheinberg I. H. Catabolism of desialylated ceruloplasmin in the liver. J Biol Chem. 1970 Nov 10;245(21):5833–5837. [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Fate of protein-containing liposomes injected into rats. An approach to the treatment of storage diseases. Eur J Biochem. 1972 Jan 21;24(3):485–491. doi: 10.1111/j.1432-1033.1972.tb19710.x. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Lysosomal localization of -fructofuranosidase-containing liposomes injected into rats. Biochem J. 1972 Aug;129(1):123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McQuiddy P., Lilien J. E. The binding of exogenously added neuraminidase to cells and tissues in culture. Biochim Biophys Acta. 1973 Feb 16;291(3):774–779. doi: 10.1016/0005-2736(73)90480-x. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Trouet A. Immunisation de lapins par des lysosomes hépatiques de rats traités au Triton WR 1339. Arch Int Physiol Biochim. 1964 Sep;72(4):698–700. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]