Abstract
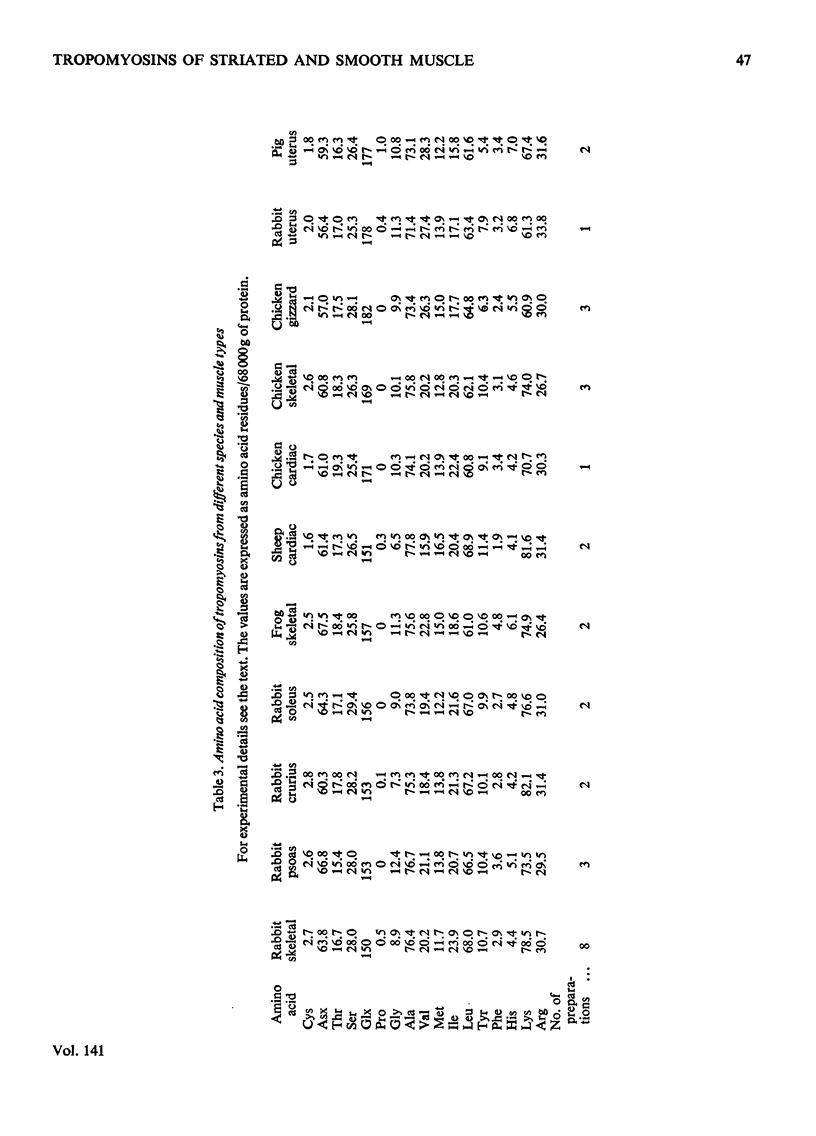
1. On electrophoresis in dissociating conditions the tropomyosins isolated from skeletal muscles of mammalian, avian and amphibian species migrated as two components. These were comparable with the α and β subunits of tropomyosin present in rabbit skeletal muscle. 2. The α and β components of all skeletal-muscle tropomyosins contained 1 and 2 residues of cysteine per 34000g respectively. 3. The ratio of the amounts of α and β subunit present in skeletal muscle tropomyosins was characteristic for the muscle type. Muscle consisting of slow red fibres contained a greater proportion of β-tropomyosin than muscles consisting predominantly of white fast fibres. 4. Mammalian and avian cardiac muscle tropomyosins consisted of α-tropomyosin only. 5. Mammalian and avian smooth-muscle tropomyosins differed both chemically and immunologically from striated-muscle tropomyosins. 6. Antibody raised against rabbit skeletal α-tropomyosin was species non-specific, reacting with all other striated muscle α-tropomyosin subunits tested. 7. Antibody raised against rabbit skeletal β-tropomyosin subunit was species-specific.
Full text
PDF

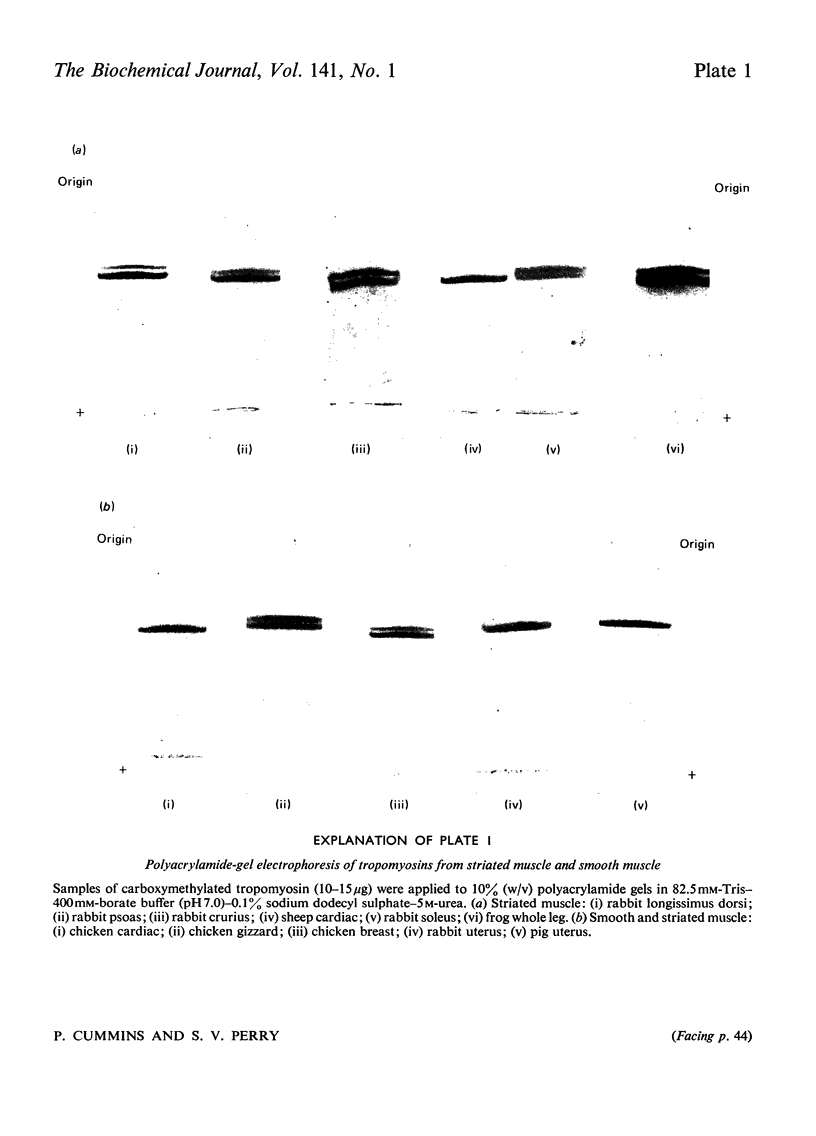
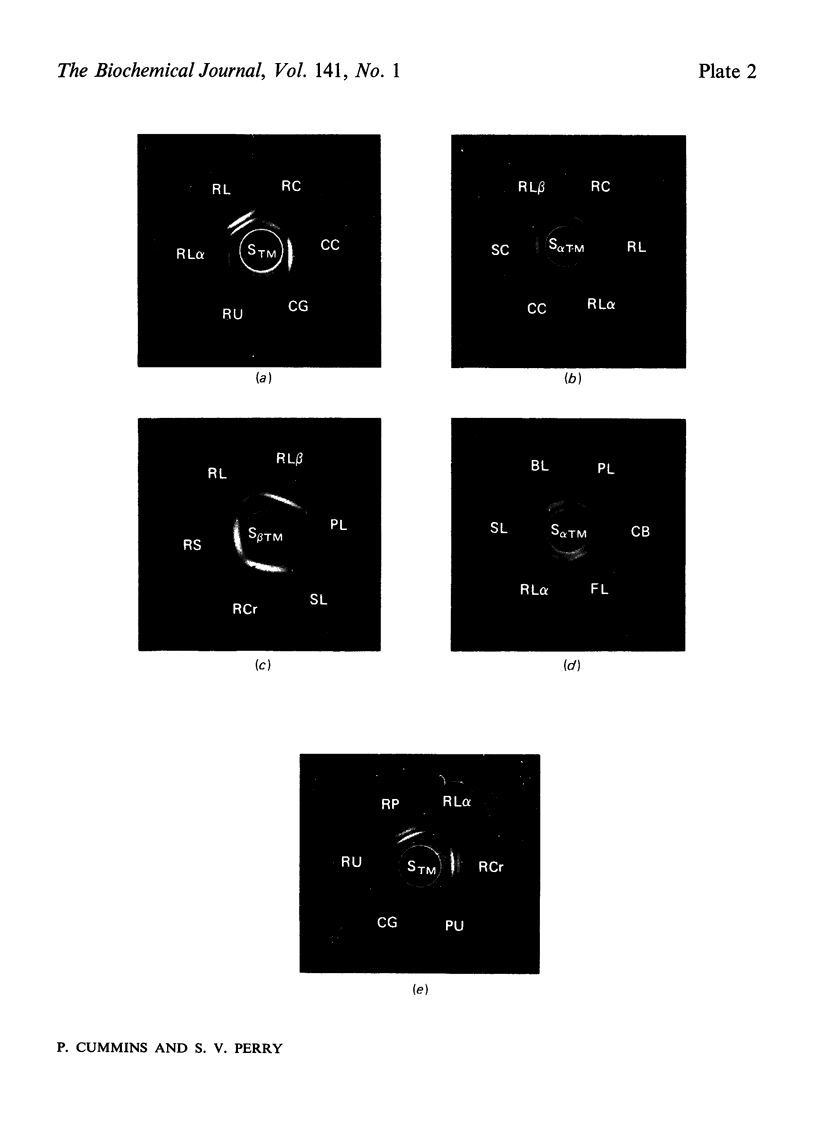

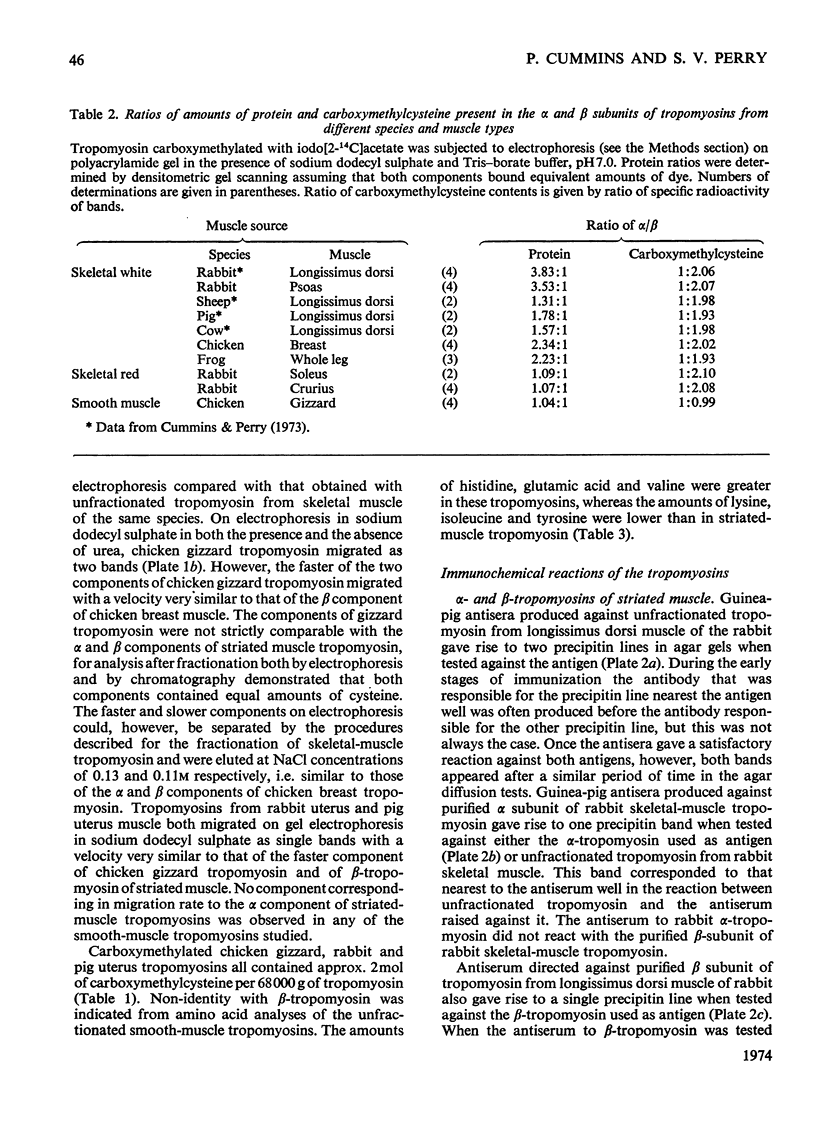


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY K., RUEGG J. C. Further chemical studies on the tropomyosins of lamellibranch muscle with special reference to Pecten maximus. Biochim Biophys Acta. 1960 Feb 26;38:239–245. doi: 10.1016/0006-3002(60)91237-3. [DOI] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsten M. E. Tropomyosin from smooth muscle of the uterus. Biochemistry. 1968 Mar;7(3):960–967. doi: 10.1021/bi00843a012. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cohen C., Longley W. Tropomyosin paracrystals formed by divalent cations. Science. 1966 May 6;152(3723):794–796. doi: 10.1126/science.152.3723.794. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi T., Hayashi Y. Studies on muscle differentiation. II. Antigenicity of tropomyosin from frog skeletal muscles. Dev Growth Differ. 1970 Dec;12(3):151–178. doi: 10.1111/j.1440-169x.1970.00151.x. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. Chemical evidence for chain heterogeneity in rabbit muscle tropomyosin. Biochem Biophys Res Commun. 1970 Nov 25;41(4):987–994. doi: 10.1016/0006-291x(70)90182-8. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The regulatory proteins of the myofibril. Characterization and properties of the inhibitory factor (troponin B). Biochem J. 1971 Jul;123(3):367–377. doi: 10.1042/bj1230367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender P. M. Muscle fibrils: Solubilization and gel electrophoresis. FEBS Lett. 1971 Sep 15;17(1):106–110. doi: 10.1016/0014-5793(71)80575-6. [DOI] [PubMed] [Google Scholar]
- Sodek J., Hodges R. S., Smillie L. B., Jurasek L. Amino-acid sequence of rabbit skeletal tropomyosin and its coiled-coil structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3800–3804. doi: 10.1073/pnas.69.12.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods E. F. Comparative physicochemical studies on vertebrate tropomyosins. Biochemistry. 1969 Nov;8(11):4336–4344. doi: 10.1021/bi00839a017. [DOI] [PubMed] [Google Scholar]