Abstract
Background
Esophageal strictures following esophageal atresia repair are a source of significant morbidity. To test new therapeutic approaches, we designed a piglet model of esophageal stricture by resecting variable lengths of esophagus with subsequent re‐anastomosis. This study describes the model and validates its physiologic impact by blinded analysis of the weight gains of the piglets.
Methods
A total of 24 two‐week old Pietrain piglets had esophageal resections performed, ranging from 0 to 5 cm, with the goal of inducing postoperative esophageal strictures. Postoperative body‐weights were evaluated by repeated analysis of variance followed by pairwise group‐comparisons based on estimated marginal means. In addition, body weight was modeled by linear‐mixed model regression. Different resection lengths were compared. The esophagi were evaluated postmortem for stricture.
Results
Of 24 operated piglets, 23 reached the endpoint, and 90% developed an esophageal stricture that was radiologically visible in a contrast study, as well as appreciable macroscopically in the necropsy. We found differences in pre‐ and postoperative body weights for all piglets (F (1, 18) = 298.54, p < 0.001), but no differences between resection lengths (F (4, 18) = 0.36, p = 0.837).
Conclusion
Our model of postoperative esophageal stricture offers the opportunity to investigate potential treatments for strictures associated with esophageal atresia, since it reliably induces strictures and results in minimal loss of animals. The similar body weight gain in all groups indicates that stricture is mainly the result of esophageal resection and re‐anastomosis, regardless of the length of the resected segment.
Keywords: esophageal atresia, esophageal surgery, postoperative complication, stricture, swine model
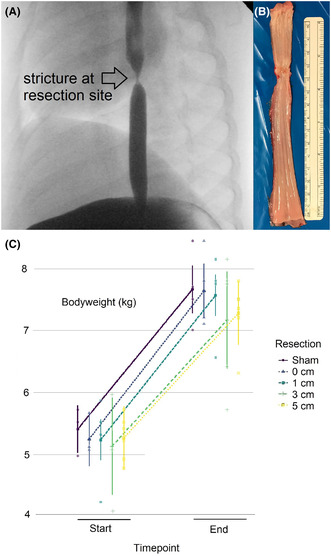
Esophageal strictures following esophageal atresia repair are a source of significant morbidity. To test new therapeutic approaches, we designed a piglet model of esophageal stricture by resecting variable lengths of esophagus with subsequent re‐anastomosis. This study describes the model and validates its physiologic impact by blinded analysis of the weight gains of the piglets. A total of 24 two‐week old Pietrain piglets had esophageal resections performed, ranging from zero to five cm, with the goal of inducing postoperative esophageal strictures. Postoperative body‐weights were evaluated by repeated analysis of variance followed by pairwise group‐comparisons based on estimated marginal means. In addition, body weight was modeled by linear‐mixed model regression. Different resection lengths were compared. The esophagi were evaluated postmortem for stricture. Of 24 operated piglets, 23 reached the endpoint, and 90% developed an esophageal stricture that was radiologically visible in contrast study (A), as well as appreciable macroscopically in the necropsy (B). We found differences in pre‐ and postoperative body weights for all piglets (F (1, 18) = 298.54, p < 0.001, C), but no differences between resection lengths (F (4, 18) = 0.36, p = 0.837). Our model of postoperative esophageal stricture offers the opportunity to investigate potential treatments for strictures associated with esophageal atresia, since it reliably induces strictures and results in minimal loss of animals. The similar body weight gain in all groups indicates that stricture is mainly the result of esophageal resection and re‐anastomosis, regardless of the length of the resected segment.
1. INTRODUCTION
Anastomotic strictures following esophageal atresia repair have been an ongoing problem since the first successful operation in 1941. 1 Strictures occur in at least 20% of patients, based on the population‐based French national register of esophageal atresia. 2 Strictures continue to play a role in up to 8% of adults who had an esophageal atresia repaired as a neonate, according to a Finnish population‐based study. 3 Due to its similarity to the human esophagus in terms of biomechanics, 4 , 5 and physiology, 6 , 7 the piglet is an excellent model for pediatric esophageal surgery, 8 which has been widely used for decades. 9 , 10 , 11 , 12 , 13 , 14 , 15
While gastroenterologists have successfully generated swine models of benign esophageal stricture by using common endoscopic interventions such as argon plasma coagulation, 16 and endoscopic mucosal 17 and submucosal resection, 18 piglet models of esophageal stricture following esophageal surgery are missing. Thus, possible interventions for esophageal strictures, such as endoscopic esophageal botulinum toxin injections have been tested in the native esophagus only. 19 , 20 For the investigation of potential therapeutic options for strictures associated with esophageal atresia, the availability of a reliable infant animal model is essential.
Our previous studies have shown that surgical division of the esophagus in piglets can produce scarring and subsequent stenosis. 21 Based on this knowledge, we planned to create a specific piglet model of esophageal stricture by standardized esophageal resection and re‐anastomosis. In this study, we describe the technical aspects of the proposed model, the effect of different resection lengths, and the physiologic effect of the experimental stricture on postoperative weight gain.
2. METHODS
We obtained 24 freshly weaned, two‐week‐old Pietrain piglets of both sexes (7 ♂; 17 ♀) from a local organic farmer licensed to sell swine for biomedical research as described before. 22 Individual pigs were randomized to one of five experimental groups (sham operation, as well as thoracotomy with resection of 9, 1, 3, and 5 cm of esophagus with subsequent anastomosis) based on pre‐defined random numbers from a random number generator. 23 To reduce possible bias by simple randomization, the experiments were conducted as two separate experiments 24 with starting days 6 weeks apart. We derived the sample size a priori by using the resource equation method 23 , 25 and calculated with four animals per group (E = 4x5–5 = 15). We expected surgery‐related fatalities, based on preceding reports in the literature for similar models, 12 , 26 , 27 in the groups with 3 and 5 cm resection and thus added one additional animal for 3 cm resection length, as well as 3 for 5 cm resection lengths to compensate for expected deaths. Classical blinding was not feasible because all experimenters were either involved in the operation or anesthesia and thus were aware of the group allocation. Piglet husbandry in our closed facility was described in detail elsewhere. 22 Briefly, piglets were kept in groups of five animals per box of 3.2 m2 equipped with appropriate enrichments. We used a 12‐h dark–light cycle with artificial lighting between 6 and 18 o'clock. Two infrared lamps per box were provided to ensure active warming at the resting period. Room temperatures were between 20.3°C and 21.7°C and relative humidity was regulated between 21.4% and 49%. The air within the facility was exchanged 13 times per hour. Drinking water was supplied ad libitum and piglets were fed three times a day with 180 g feeding slurry per animal (120 mL of hot water mixed with 60 g of piglet fodder (Protec pre, Denkavit, Warendorf, Germany)). Additional dry fodder of 30 g per animal was available between feeding times. We used a period of acclimatization to our husbandry conditions for a week 28 before starting the experiments.
Following adequate intramuscular premedication with ketamine (20 mg/kg, PZN 0771401, Ketamin Inresa, Inresa Arzneimittel, Freiburg im Breisgau, Germany) and xylazine (2 mg/kg, PZN 1400578860, Xylazin WDT, Wirtschaftsgenossenschaft Deutscher Tierärzte, Garbsen, Germany), general anesthesia was induced using propofol (2–8 mg/kg, PZN 16661502, Propofol 1% MCT, Fresenius Kabi, Bad Homburg vor der Höhe, Germany) and fentanyl (150 μg/kg, PZN 04795545, Fentanyl Janssen, Janssen Cilag, Neuss, Germany) after which the piglets had an oral endotracheal tube placed. General intravenous anesthesia was continued with propofol (5–50 mg/kg/h, PZN 16661502, Propofol 1% MCT, Fresenius Kabi, Bad Homburg vor der Höhe, Germany) and fentanyl (10–25 μg/kg/h, PZN 04795545, Fentanyl Janssen, Janssen Cilag, Neuss, Germany). The piglets' postoperative analgesia was based on intravenous buprenorphine (0.1 mg/kg, PZN 06318298, Temgesic, Indivior, Mannheim, Germany) followed by a transdermal patch of fentanyl (25 μg/h, PZN 0575114, Durogesic SMAT, Janssen Cilag, Neuss, Germany), applied at the end of the operation, and meloxicam (0.4 mg/kg, Metacam, Boehringer Ingelheim, Ingelheim, Germany) orally. In addition, the wound edges were infiltrated with the local anesthetic ropivacaine (10 mL 0.75%, PZN 08812708, Ropivacain 7.5 mg/mL, B. Braun Melsungen, Melsungen, Germany).
Intraoperatively, the esophagus was exposed via a right‐sided muscle‐sparing thoracotomy, and cut at the level of the carinal position with resection lengths of 0 (n = 4), 1 (n = 4), 3 (n = 5), and 5 (n = 7) cm. The esophagi were then re‐anastomosed with simple‐interrupted 4‐0 Polyglactin sutures. In the sham group (n = 4), the chest was kept open for the mean duration of the operation as determined in a pilot study. 22 The incision was closed in layers; skin was closed with intra‐cutaneous suturing followed by application of a sterile dressing. Postoperatively, piglets were kept fasting for the day of the operation and started on regular fodder the day after surgery. Piglets were followed for 14 postoperative days with daily monitoring of vital signs, body weight, and possible feeding difficulties. The interval of 14 days was chosen based on the description in previous research that stricture formation could be expected to be complete after this interval. 29 All body weights were obtained before feeding using a large animal scale with an accuracy of ±5 g.
Two weeks postoperatively, the piglets were euthanized using an overdose of pentobarbital (100 mg/kg, PZN 09110316, Euthadorm, CP‐Pharma, Burgdorf, Germany). Criteria for a premature termination of the experiment were a loss of 20% of body weight within 4 days or less, overwhelming systemic infections, severe behavioral problems such as self‐mutilation, central nervous abnormalities such as paresis or seizures, and bleeding complications. Before sacrifice and macroscopic investigation of the esophagus for strictures, radiographic examination of the esophagus was conducted using a fluoroscopy unit (Ziehm Vision, Ziehm Imaging GmbH, Nuremberg, Germany) by application of water‐soluble contrast (Isovist 300, Bayer Vital GmbH, Leverkusen, Germany) into the esophagus and digital acquisition of the contrast study.
Statistical analysis was conducted using R (RRID:SCR_001905) (version 3.5.3) with its basic stats4‐package (version 3.5.3) unless stated otherwise. 30 Statistical analysis of body weights was conducted blinded to group allocation. Differences in body weight between the start and the end of the experiment between the groups were analyzed by a repeated‐measures analysis of variance taking into account the main effects of the time point and resection length, and their interaction term. This analysis was based on the use of the afex‐package (version 1.0‐1). 31 Pairwise group comparisons following the repeated‐measures analysis of variance were conducted based on estimated marginal means by using the emmeans‐package (version 1.7.1‐1) 32 with post hoc adjustments of the false discovery rate according to the Benjamini‐Hochberg 33 method. To further explore the daily weight gain, we created a linear mixed effects model, in which we included resection length, postoperative days, and their cross‐level interaction as fixed effects while allowing the change over time to differ at random across the included swine. The model was fit by restricted maximum likelihood estimation. Influential data points in the model were assessed by using Cook's distance as calculated using the influence.ME‐package (version 0.9‐9). 34 The model was fit by using the lme4‐package (version 1.1‐27.1). 35 P‐values for model fit were obtained by likelihood‐ratio tests of the full model compared to the random intercepts model.
The datasets generated and analyzed during the current study are publicly available from Zenodo (https://doi.org.10.5281/ZENODO.5807805). 36
3. RESULTS
Of 24 piglets included in our study, 23 (96%) reached desired endpoints and were included in the subsequent analysis. We had no anesthesia‐related deaths. In one piglet with a 5 cm esophageal resection, the anastomosis was difficult to achieve due to the underlying tension, and ultimately failed: the piglet developed a fulminant mediastinitis and was sacrificed by an overdose of pentobarbital 28 h after the operation to avoid suffering, as per protocol. This animal was subsequently excluded from our analysis. In two animals, the experiment was prematurely terminated on postoperative day 12 due to weight loss of more than 20% compared to preoperative weight; one of the piglets was from the 0 cm group and the other from the 5 cm group. Another piglet from the 3 cm group experienced a systemic infection on the first postoperative day with fever, shivering, and wheezing breath sounds, and was subsequently empirically treated with ceftiofur (3 mg/kg/d, PZN 11283685, Excenel Flow, Zoetis Deutschland, Berlin, Germany) antibiotic coverage. At first, the piglet recovered and the symptoms subsided promptly, but the piglet experienced a breakthrough of infectious symptoms with fever and a toxic appearance, and refused to feed, mandating euthanasia on postoperative day 13. A third piglet from the 3 cm group developed a local infection of the thoracotomy wound on the ninth postoperative day, which was at first treated with local measures for 2 days, before adding enrofloxacin (7,5 mg/kg, PZN 11004277, Baytril, Elanco Deutschland, Monheim am Rhein, Germany) for the remaining 3 days duration of the experiment.
All piglets in the respective groups showed comparable postoperative weight gains that ranged from a mean weight gain of 2.04 kg in the 5 cm resection group to a mean weight gain of 2.42 kg in the 0 cm resection group, all of them were statistically different when comparing the body weights at the end and beginning of the experiment (p < 0.001) (Table 1). Although the average weight gain seemed to be lower for longer resection lengths, this was not the case for the comparison of estimated marginal means (estimated marginal means between −0.115 and 0.503, p > 0.899 for pairwise comparisons), indicating a similar mean postoperative weight gain irrespective of the extent of resection (Figure 1).
TABLE 1.
Body weights at the beginning and end of the experiment.
| Resection length | Body weight [kg] | Δ [kg] | p | |
|---|---|---|---|---|
| Beginning (CI) | End (CI) | |||
| Sham | 5.39 (4.85–5.92) | 7.66 (6.90–8.43) | 2.27 | <0.001 |
| 0 cm | 5.22 (4.69–5.76) | 7.64 (6.87–8.40) | 2.42 | <0.001 |
| 1 cm | 5.21 (4.68–5.75) | 7.56 (6.80–8.33) | 2.35 | <0.001 |
| 3 cm | 5.11 (4.63–5.59) | 7.16 (6.48–7.84) | 2.05 | <0.001 |
| 5 cm | 5.24 (4.81–5.68) | 7.28 (6.65–7.84) | 2.04 | <0.001 |
Note: Calculation of p‐values was based on contrasted estimated marginal means with correction for multiple testing according to Benjamini‐Hochberg. All piglets that reached the endpoint (n = 23) were included in the analysis. The ∆ of body weight was calculated by subtracting the mean body weight at the beginning from the mean body weight in the end in the respective group.
FIGURE 1.
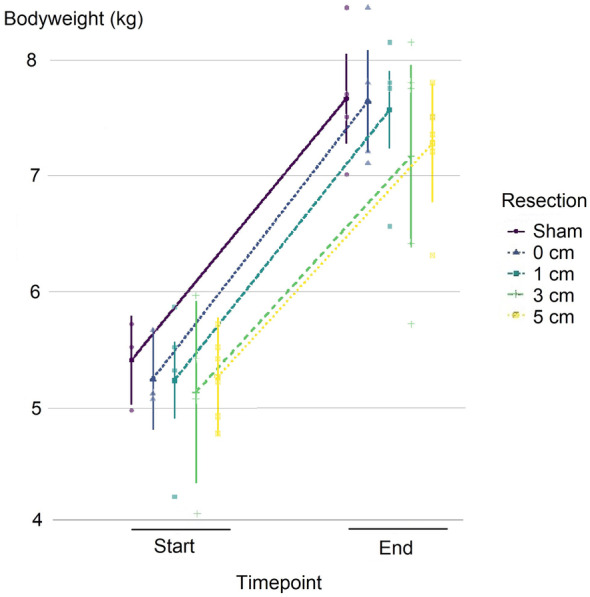
Estimated marginal means of the repeated‐measures analysis of variance are seen here for weight gains. Error bars represent 95% confidence intervals for the estimated marginal means within the groups of different resection lengths. All piglets that reached the endpoint (n = 23) were included in the analysis. Weight gain appears less steep for longer resection lengths, but the differences were not statistically significant.
Consequently, the repeated‐measures analysis of variance found a significant difference only in the time point of weight measurements (F (1, 18) = 298.54, p < 0.001), whereas neither resection length (F (4, 18) = 0.36, p = 0.837) nor their interaction (F (4, 19) = 0.386, p = 0.816) was found to have an effect in our experiments (Figure 1).
Despite similar developments in body weights across resection lengths, esophageal strictures developed in all but two piglets that underwent resection and anastomosis (Figure 2B), while none developed in the piglets in the sham group (Figure 2A). Strictures were also clearly visible in all piglets after resection and re‐anastomosis in postoperative contrast studies (Figure 3; Video S1), but not in the sham group (Figure 4; Video S2).
FIGURE 2.

Representative macroscopic depiction of an explanted sham esophagus (A) and of the esophageal stricture following 5 cm esophageal resection and re‐anastomosis (B). The stricture is marked by the arrow.
FIGURE 3.
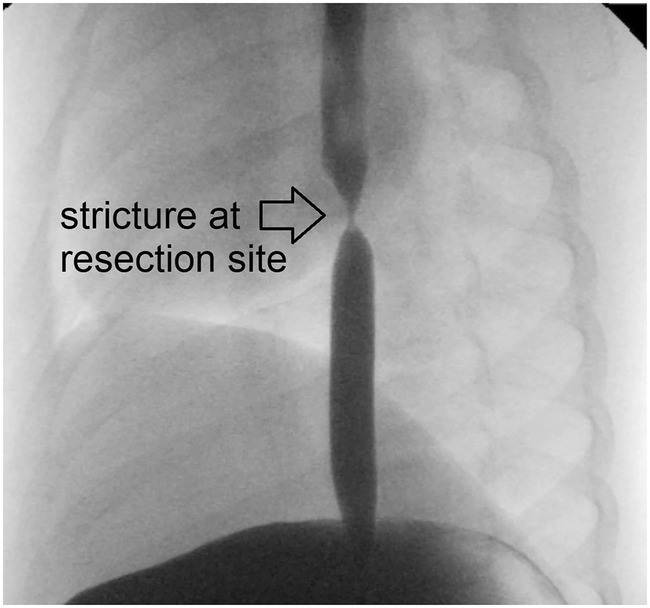
Postoperative stricture after esophagotomy and 5 cm segmental resection with reanastomosis (arrow). Although the contrast study passes distally into the stomach readily, explaining continuous postoperative weight gain, the stricture is well delineated and morphologically resembles strictures seen clinically after esophageal atresia repair.
FIGURE 4.
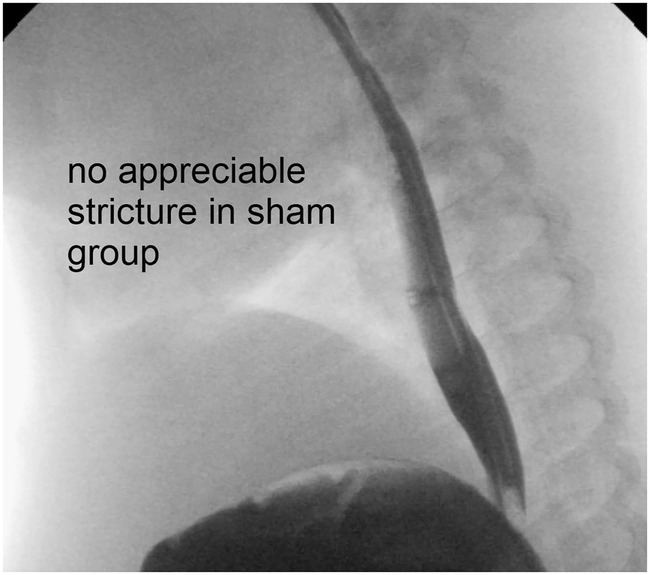
No stricture is seen in the sham group.
Although we did not perform a quantitative stricture analysis, stricture severity seemed to increase with longer resection segments (Figure 5).
FIGURE 5.
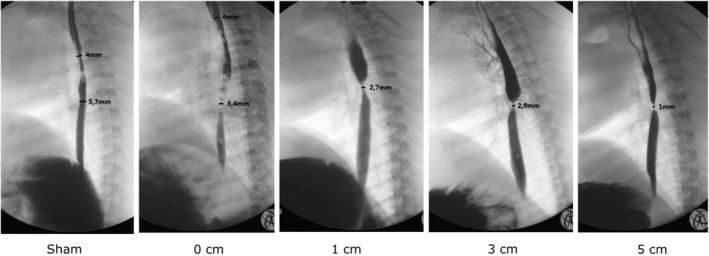
Subjectively, stricture severity seemed to increase with longer resection lengths on contrast radiography.
4. DISCUSSION
Strictures are a common problem of infants who undergo esophageal atresia repair, affecting more than 20% in prospective and multicentric 37 studies, as well as in large population‐based registries. 2 In some patients, the problem persists into adulthood. 3 Treatment of strictures includes bougienage, balloon dilatation, stent placement, injection of steroids, injection of botulinum toxin, or topical treatment with Mitomycin C, as well as resection and re‐anastomosis, 38 but there are very few objective data on long‐term success rates and thus the optimal management of such strictures. In order to experimentally assess these treatment options and their combination, a reliable, reproducible animal model of strictures in a young, growing organism is desperately needed. Large, adult animal models are not appropriate, because they do not adequately reflect the situation in the human infant. Therefore, research relevant for stricture treatment after esophageal atresia repair has been conducted on the native, unmanipulated esophagus only. 19 , 20 Based on clinical observations that have established a relationship between esophageal gap‐length and occurrence of postoperative stricture formation, 39 , 40 which possibly is linked to anastomotic tension, 2 , 41 , 42 , 43 we aimed to develop a piglet model of esophageal stricture following esophageal surgery. In 1972, Livaditis et al. first described a method 10 of reducing esophageal anastomotic tension in piglets. They used increasing resection lengths to induce higher anastomotic tension, and showed that this was linked to stricture formation. Takada et al. not only confirmed this observation, but also demonstrated tissue similarity between porcine and human esophagi when subjected to traction forces. 4
Consequently, swine are not only the species of choice to model the human esophagus, but also offer an appropriate means of increasing resection lengths to cause anastomotic tension and subsequent strictures. Findings to date indicate that increasing tension caused by increasing gap length represents the most important risk factor for stricture formation. 2 , 39 , 42 , 43 , 44 Because we used younger and smaller piglets than in preceding studies, we limited the esophageal resection lengths to 5 cm maximum, corresponding to approximately a third of the total esophageal length. This limit, first established by Parker & Brockington, 26 has been used for models of esophageal resection for more than 70 years and has recently been confirmed experimentally: if an esophagus is stretched to more than a third of its initial length, rupture of muscle fibers and capillaries occurs. 45 Earlier swine models of esophageal stricture, such as those obtained by excising circular segments of esophageal mucosa 4 , 9 , 10 have the disadvantage that successful stricture formation is unpredictable.
Before creating our model in the present form, we evaluated whether a Landrace piglet or a Minipig of a similar weight would be preferable: The Minipig offers the advantage of a more robust physiology due to their more advanced age at similar body weights to the human patient, 12 , 22 , 46 facilitating their perioperative care. In contrast, Landrace piglets not only have anatomic sizes similar to a human patient, but also model the more immature physiology of the patient more closely. 6 , 8 This makes their perioperative care more challenging and increases the risk of anesthesia‐related fatalities. 22 , 47 , 48
The best method to determine the correct esophageal length, either calculated from a formula 49 or empirically estimated by pH‐probes, 50 , 51 is highly debated. Because autopsy data are missing, 52 we conducted a pilot study to assess similarities between Landrace piglets, the Aachen Minipig 53 , 54 and humans, based on the comparison of organ weights. 22 This pilot study revealed that the Pietrain piglets' organ weights were similar to organ weights of humans of a similar age and weight, which was not the case for Aachen Minipigs of similar body weight. 22 Thus, the Pietran piglet model was favored to more accurately model the human patient. 22
Our model fared quite successfully: we had no anesthesia‐related deaths, and 90% of the operated piglets developed an esophageal stricture. The reliable induction of an esophageal stricture is crucial to limit the number of animals needed for further interventional studies: If fewer animals develop a stricture or die as a result of the intervention necessary to produce the model, more piglets are required, counteracting the principles of refinement, reduction, and replacement guiding animal experiments, and bringing into question the ethical justification for the study. 55 In the present study, we only assessed weight gain and did not quantitatively assess stricture severity directly. In addition, based on experimental design, and taking into account macroscopic and radiologic examinations, the present study might not be adequately powered to reliably assess stricture severity. Future studies with more animals and including a quantitative evaluation of stricture severity may eventually show a difference for resection length. Nevertheless, our model offers the opportunity to assess different treatment modalities for esophageal strictures due to its reliable stricture induction. Our results show that the planned endpoint of the study was reached in the vast majority of animals irrespective of the extent of esophageal resection.
Another crucial advantage of our model is the similar weight gain in all groups. This rather surprised us, but may serve a valuable purpose: Our experiment could not be blinded due to the limited number of staff in our small team, but the similar average weight gains between groups may allow blinding in future studies. Premature termination due to excessive weight loss would have been expected to occur frequently in the groups with larger resection lengths, but this was not the case. Therefore, any potential bias due to implicit guesses of group allocation by husbandry staff is reduced. The question of blinding is crucial, because a lack of blinding inflates effect sizes towards the desired outcome due to subjective bias, which is the reason why blinding has been included in the 10 essential items of the ARRIVE 2.0 guidelines. 56 Our piglet model thus offers the potential to reduce bias in experimental animal research into esophageal stricture treatment following esophageal surgery.
In the present study, participating staff were aware of the group allocation of the animals and thus only statistical analysis could be conducted in a blinded fashion by pseudonymisation of the individual piglets and their group allocation in the file that was used for statistical analysis. Another limitation of the study is our simple randomization method, which did not account for some covariates. A particular issue in our model description is the influence of sex: males were underrepresented in our study, and due to our simple randomization method, a male piglet was not randomized into the 5 cm resection group, precluding consideration of sex as a covariate in statistical analysis. However, the number of piglets in our study was limited to the number of piglets born during the study period at the local organic farm supplying us. Hence, the sex distribution in our study was determined by the sex of the piglets born during the study period and thereby their availability at the scheduled date for the surgery.
This model only evaluates the effect of the anastomosis and tension by increasing resection lengths on the anastomosis. However, there are many other factors that play a role in stricture formation, including anastomotic leaks and gastroesophageal reflux. The model is not designed to control for these factors. Since an anastomotic leak leads to infection, it is reasonable to assume that the two piglets that developed fever and were subsequently euthanized actually had an anastomotic leakage. Since the others did not develop fever, we believe that they did not have a leak and therefore assume that the piglets included in the final analysis did not experience anastomotic leakage. Regarding gastroesophageal reflux, this should be similar across all groups. However, it would be interesting to assess if different resection lengths are associated with more or less reflux in future experiments.
Notably, piglets are notoriously difficult to operate on at 2 weeks of life. Our operative success rate of 96% with only two subsequent cases of mediastinitis (<10%) and only one superficial wound infection (<5%) make this a robust model for esophageal atresia research.
Interestingly, 90% of our piglets showed some kind of stricture, a higher rate than is clinically noted after esophageal atresia repair (a stricture rate of around 50%). However, the higher stricture rate in our model may be secondary to the routine investigation that was performed after every anastomosis by protocol, even though the piglets were not symptomatic. In the clinical setting, only symptomatic strictures are discovered, since most pediatric surgeons do not perform routine endoscopy or contrast studies after the initial postoperative period until around 1 year of life. We suspect that subclinical strictures are equally as common as in our model, and that they may subside spontaneously by autobougienage by increasing oral intake of more textured foods.
5. CONCLUSIONS
We describe a novel, simple, and reproducible piglet model of postoperative esophageal strictures intended to simulate strictures after esophageal atresia repair. In our model, esophageal strictures are reliably induced after two postoperative weeks in 90% of operated animals. Our model is particularly suitable for blinded experimental research, because the body weight changes did not differ much between the groups with different esophageal resection lengths. Thus, our piglet model offers the opportunity to investigate potential therapeutic approaches for postoperative strictures after esophageal atresia repair.
AUTHOR CONTRIBUTIONS
Christina Oetzmann von Sochaczewski: Data curation; formal analysis; investigation; methodology; writing – original draft. Ann‐Kristin Riedesel: Formal analysis; investigation; visualization. Andreas Lindner: Data curation; formal analysis; investigation; methodology; project administration; writing – review and editing. Axel Heimann: Investigation; methodology; supervision; visualization. Arne Schröder: Data curation; formal analysis; investigation; methodology; visualization. Oliver J. Muensterer: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This research was funded intramurally and received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest. The present study was conducted without commercial funding.
ETHICS STATEMENT
The present study, including its pre‐specified study protocol, has been approved by the local animal welfare board (permit number G‐17‐1‐033). All experiments were performed in accordance to the relevant regulations and guidelines, namely the directive 2010/63/EU, the Council of Europe Convention ETS 123, the German national animal welfare act (TierSchG) and its subsequent statutory acts (TierSchVersV) as well as recommendations of the GV‐SOLAS. The study is compliant with the ARRIVE‐guidelines 2.0.
Supporting information
Video S1.
Video S2.
ACKNOWLEDGMENTS
We thank Evangelos Tagkalos for occasional intraoperative assistance. Open Access funding enabled and organized by Projekt DEAL.
von Sochaczewski CO, Riedesel A‐K, Lindner A, Heimann A, Schröder A, Muensterer OJ. A novel piglet model of esophageal stricture following variable segmental esophageal resection and re‐anastomosis. Anim Models Exp Med. 2024;7:936‐943. doi: 10.1002/ame2.12498
REFERENCES
- 1. Haight C, Towsley HA. Congenital atresia of the esophagus with tracheoesophageal fistula: extrapleural ligation of fistula and end‐to‐end anastomosis of esophageal segments. Surg Gynecol Obstet. 1943;76:672‐688. [Google Scholar]
- 2. Schneider A, Blanc S, Bonnard A, et al. Results from the French National Esophageal Atresia register: one‐year outcome. Orphanet J Rare Dis. 2014;9(1):206. doi: 10.1186/s13023-014-0206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sistonen SJ, Koivusalo A, Nieminen U, et al. Esophageal morbidity and function in adults with repaired esophageal atresia with tracheoesophageal fistula: a population‐based long‐term follow‐up. Ann Surg. 2010;251(6):1167‐1173. doi: 10.1097/SLA.0b013e3181c9b613 [DOI] [PubMed] [Google Scholar]
- 4. Takada Y, Kent G, Filler RM. Circular myotomy and esophageal length and safe esophageal anastomosis: an experimental study. J Pediatr Surg. 1981;16(3):343‐348. doi: 10.1016/S0022-3468(81)80692-6 [DOI] [PubMed] [Google Scholar]
- 5. Oetzmann von Sochaczewski C, Tagkalos E, Lindner A, et al. Esophageal biomechanics revisited: a tale of tenacity, anastomoses, and suture bite lengths in swine. Ann Thorac Surg. 2019;107(6):1670‐1677. doi: 10.1016/j.athoracsur.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 6. Glauser EM. Advantages of piglets as experimental animals in pediatric research. Exp Med Surg. 1966;24(2):181‐190. [PubMed] [Google Scholar]
- 7. Conrad MS, Johnson RW. The domestic piglet: an important model for investigating the neurodevelopmental consequences of early life insults. Annu Rev Anim Biosci. 2015;3(1):245‐264. doi: 10.1146/annurev-animal-022114-111049 [DOI] [PubMed] [Google Scholar]
- 8. Livaditis A, Jönsson L. The piglet in experimental pediatric surgery. Z Versuchstierkd. 1979;21(2):78‐82. [PubMed] [Google Scholar]
- 9. Livaditis A, Okmian L, Björck G, Ivemark B. Esophageal suture anastomosis: an experimental study in piglets. Scand J Thorac Cardiovasc Surg. 1969;3(2–3):163‐173. doi: 10.3109/14017436909131792 [DOI] [PubMed] [Google Scholar]
- 10. Livaditis A, Rådberg L, Odensjö G. Esophageal end‐to‐end anastomosis:reduction of anastomotic tension by circular myotomy. Scand J Thorac Cardiovasc Surg. 1972;6(2):206‐214. doi: 10.3109/14017437209134801 [DOI] [PubMed] [Google Scholar]
- 11. Nelson Ö, Okmian L. Healing of esophageal end‐to‐end anastomoses one, two and three weeks postoperatively. Z Kinderchir. 1976;19:25‐37. [Google Scholar]
- 12. Schaarschmidt K, Paschertz KW, Stratmann U, Ruprecht L, Willital GH, Unsüld E. Tracheoesophageal anastomosis, continent gastrostomy and oesophagostomy‐a new experimental model in minipiglets. Lab Anim. 1995;29(4):411‐419. doi: 10.1258/002367795780740131 [DOI] [PubMed] [Google Scholar]
- 13. Lorincz A, Langenburg SE, Knight CG, Gidell K, Rabah R, Klein MD. Robotically assisted esophago‐esophagostomy in newborn pigs. J Pediatr Surg. 2004;39(9):1386‐1389. doi: 10.1016/j.jpedsurg.2004.05.015 [DOI] [PubMed] [Google Scholar]
- 14. Sullins VF, Traum PK, French SW, Wu BM, Dunn JCY, Lee SL. A novel method of esophageal lengthening in a large animal model of long gap esophageal atresia. J Pediatr Surg. 2015;50(6):928‐932. doi: 10.1016/j.jpedsurg.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 15. Oetzmann von Sochaczewski C, Lindner A, Heimann A, et al. Beyond Magnamosis: a method to test Sutureless esophageal anastomotic devices in living swine by creating an esophageal bypass loop for natural Oral nutrition. J Laparoendosc Adv Surg Tech A. 2019;29(6):852‐855. doi: 10.1089/lap.2018.0778 [DOI] [PubMed] [Google Scholar]
- 16. Li L, Itani MI, Salimian KJ, et al. A patient‐like swine model of gastrointestinal fibrotic strictures for advancing therapeutics. Sci Rep. 2021;11(1):13344. doi: 10.1038/s41598-021-92628-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pauli EM, Schomisch SJ, Furlan JP, et al. Biodegradable esophageal stent placement does not prevent high‐grade stricture formation after circumferential mucosal resection in a porcine model. Surg Endosc. 2012;26(12):3500‐3508. doi: 10.1007/s00464-012-2373-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nonaka K, Miyazawa M, Ban S, et al. Different healing process of esophageal large mucosal defects by endoscopic mucosal dissection between with and without steroid injection in an animal model. BMC Gastroenterol. 2013;13(1):72. doi: 10.1186/1471-230X-13-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellebæk M, Qvist N, Schrøder H, Rasmussen L. Intramural injection with botulinum toxin type a in piglet esophagus. The influencer on maximum load and elongation: a dose response study. Eur J Pediatr Surg. 2015;26(3):282‐286. doi: 10.1055/s-0035-1551572 [DOI] [PubMed] [Google Scholar]
- 20. Dibbern C, Rose M, Ellebæk M, Qvist N. The effect of intramural botulinum toxin injections on the elongation of the piglet Oesophagus is time dependent. Eur J Pediatr Surg. 2017;27(1):56‐60. doi: 10.1055/s-0036-1593386 [DOI] [PubMed] [Google Scholar]
- 21. Muensterer OJ, Sterlin A, Oetzmann von Sochaczewski C, et al. An experimental study on magnetic esophageal compression anastomosis in piglets. J Pediatr Surg. 2020;55(3):425‐432. doi: 10.1016/j.jpedsurg.2019.04.029 [DOI] [PubMed] [Google Scholar]
- 22. Baumgart J, Deigendesch N, Lindner A, et al. Using multidimensional scaling in model choice for congenital oesophageal atresia: similarity analysis of human autopsy organ weights with those from a comparative assessment of Aachen Minipig and Pietrain piglets. Lab Anim. 2020;54(6):576‐587. doi: 10.1177/0023677220902184 [DOI] [PubMed] [Google Scholar]
- 23. Festing MFW, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43(4):244‐258. doi: 10.1093/ilar.43.4.244 [DOI] [PubMed] [Google Scholar]
- 24. von Kortzfleisch VT, Karp NA, Palme R, Kaiser S, Sachser N, Richter SH. Improving reproducibility in animal research by splitting the study population into several 'mini‐experiments'. Sci Rep. 2020;10(1):16579. doi: 10.1038/s41598-020-73503-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Festing MF. On determining sample size in experiments involving laboratory animals. Lab Anim. 2018;52(4):341‐350. doi: 10.1177/0023677217738268 [DOI] [PubMed] [Google Scholar]
- 26. Parker EF, Brockington WS. Esophageal resection with end‐to‐end anastomosis: experimental and clinical observations. Ann Surg. 1949;129(5):588‐603. [PMC free article] [PubMed] [Google Scholar]
- 27. Jönsson L, Friberg LG, Gatzinsky V, Jennische E, Sandin A, Abrahamsson K. Early regenerative response in the intrathoracic porcine esophagus—the impact of the inflammation. Artif Organs. 2014;38(6):439‐446. doi: 10.1111/aor.12216 [DOI] [PubMed] [Google Scholar]
- 28. Obernier JA, Baldwin RL. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J. 2006;47(4):364‐369. doi: 10.1093/ilar.47.4.364 [DOI] [PubMed] [Google Scholar]
- 29. Livaditis A, Ivemark B. Esophageal anastomosis in piglets: histologic and microangiographic aspects of the early phases of healing. Scand J Thorac Cardiovasc Surg. 1969;3(2–3):174‐180. doi: 10.3109/14017436909131793 [DOI] [PubMed] [Google Scholar]
- 30. R Core Team . R: A Language and Environment for Statistical Computing. Published online 2019. Accessed February 25, 2019. https://www.R‐project.org
- 31. Singmann H, Bolker B, Westfall J, et al. afex: Analysis of Factorial Experiments. Published online July 22, 2021. Accessed December 19, 2021. https://CRAN.R‐project.org/package=afex
- 32. Lenth RV, Buerkner P, Herve M, et al. emmeans: Estimated Marginal Means, aka Least‐Squares Means. Published online November 29, 2021. Accessed December 19, 2021. https://CRAN.R‐project.org/package=emmeans
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289‐300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 34. Nieuwenhuis R, Pelzer B, Grotenhuis M. influence.ME: Tools for Detecting Influential Data in Mixed Effects Models. Published online June 7, 2017. Accessed December 20, 2021. https://CRAN.R‐project.org/package=influence.ME
- 35. Bates D, Maechler M, Bolker B, et al. lme4: Linear Mixed‐Effects Models using “Eigen” and S4. Published online June 22, 2021. Accessed December 19, 2021. https://CRAN.R‐project.org/package=lme4
- 36. Oetzmann von Sochaczewski C, Riedesel AK, Lindner A, Heimann A, Schröder A, Muensterer OJ. Raw data for bodyweights of piglets used to establish the Mainz piglet model of oesophageal strictures. Published online December 28, 2021. doi: 10.5281/ZENODO.5807805 [DOI]
- 37. Vergouwe FWT, Spoel M, van Beelen NWG, et al. Longitudinal evaluation of growth in oesophageal atresia patients up to 12 years. Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F417‐F422. doi: 10.1136/archdischild-2016-311598 [DOI] [PubMed] [Google Scholar]
- 38. Angelino G, Tambucci R, Torroni F, De Angelis P, Dall'Oglio L. New therapies for esophageal strictures in children. Curr Opin Pediatr. 2021;33(5):503‐508. doi: 10.1097/MOP.0000000000001049 [DOI] [PubMed] [Google Scholar]
- 39. McKinnon LJ, Kosloske AM. Prediction and prevention of anastomotic complications of esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 1990;25(7):778‐781. doi: 10.1016/S0022-3468(05)80018-1 [DOI] [PubMed] [Google Scholar]
- 40. Upadhyaya VD, Gangopadhyaya AN, Gupta DK, et al. Prognosis of congenital tracheoesophageal fistula with esophageal atresia on the basis of gap length. Pediatr Surg Int. 2007;23(8):767‐771. doi: 10.1007/s00383-007-1964-0 [DOI] [PubMed] [Google Scholar]
- 41. Boyle EM, Irwin ED, Foker JE. Primary repair of ultra‐long‐gap esophageal atresia: results without a lengthening procedure. Ann Thorac Surg. 1994;57(3):576‐579. [DOI] [PubMed] [Google Scholar]
- 42. Michaud L, Guimber D, Sfeir R, et al. Sténose anastomotique après traitement chirurgical de lˈatrésie de lˈœsophage : fréquence, facteurs de risque et efficacité des dilatations œsophagiennes. Arch Pediatr. 2001;8(3):268‐274. doi: 10.1016/S0929-693X(00)00193-7 [DOI] [PubMed] [Google Scholar]
- 43. Serhal L, Gottrand F, Sfeir R, et al. Anastomotic stricture after surgical repair of esophageal atresia: frequency, risk factors, and efficacy of esophageal bougie dilatations. J Pediatr Surg. 2010;45(7):1459‐1462. doi: 10.1016/j.jpedsurg.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 44. Sillén U, Hagberg S, Rubenson A, Werkmäster K. Management of esophageal atresia: review of 16 years' experience. J Pediatr Surg. 1988;23(9):805‐809. doi: 10.1016/S0022-3468(88)80227-6 [DOI] [PubMed] [Google Scholar]
- 45. Saxena AK, Biro E, Sommer G, Holzapfel GA. Esophagus stretch tests: biomechanics for tissue engineering and possible implications on the outcome of esophageal atresia repairs performed under excessive tension. Esophagus. 2021;18(2):346‐352. doi: 10.1007/s10388-020-00769-y [DOI] [PubMed] [Google Scholar]
- 46. Oetzmann von Sochaczewski C, Deigendesch N, Lindner A, et al. Comparing Aachen Minipigs and Pietrain piglets as models of experimental pediatric urology to human reference data. Eur Surg Res. 2020;61(2–3):95‐100. doi: 10.1159/000511399 [DOI] [PubMed] [Google Scholar]
- 47. Koffeman GI, Hulscher JBF, Schoots IG, van Gulik TM, Heij HA, van Gemert WG. Intestinal lengthening and reversed segment in a piglet short bowel syndrome model. J Surg Res. 2015;195(2):433‐443. doi: 10.1016/j.jss.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 48. Kaska M, Blazej S, Turek Z, et al. The effect of three different surgical techniques for colon anastomosis on regional postoperative microperfusion: laser Doppler Flowmetry study in pigs. Clin Hemorheol Microcirc. 2018;68(1):61‐70. doi: 10.3233/CH-170297 [DOI] [PubMed] [Google Scholar]
- 49. Strobel CT, Byrne WJ, Ament ME, Euler AR. Correlation of esophageal lengths in children with height: application to the Tuttle test without prior esophageal manometry. J Pediatr. 1979;94(1):81‐84. doi: 10.1016/S0022-3476(79)80361-3 [DOI] [PubMed] [Google Scholar]
- 50. Putnam PE, Orenstein SR. Determining esophageal length from crown‐rump length. J Pediatr Gastroenterol Nutr. 1991;13(4):354‐359. [DOI] [PubMed] [Google Scholar]
- 51. Moreau B, Kambites S, Lévesque D. Esophageal length: esophageal Manometry remains superior to mathematical equations. J Pediatr Gastroenterol Nutr. 2013;57(2):236‐239. doi: 10.1097/MPG.0b013e3182952e50 [DOI] [PubMed] [Google Scholar]
- 52. Oetzmann von Sochaczewski C, Tagkalos E, Lindner A, et al. Bodyweight, not age, determines oesophageal length and breaking strength in rats. J Pediatr Surg. 2019;54(2):297‐302. doi: 10.1016/j.jpedsurg.2018.10.085 [DOI] [PubMed] [Google Scholar]
- 53. Pawlowsky K, Ernst L, Steitz J, et al. The Aachen Minipig: phenotype, genotype, hematological and biochemical characterization, and comparison to the Göttingen Minipig. Eur Surg Res. 2017;58(5–6):193‐203. [DOI] [PubMed] [Google Scholar]
- 54. Plotzki E, Heinrichs G, Kubícková B, Ulrich RG, Denner J. Microbiological characterization of a newly established pig breed, Aachen Minipigs. Xenotransplantation. 2016;23(2):159‐167. doi: 10.1111/xen.12233 [DOI] [PubMed] [Google Scholar]
- 55. Bailoo JD, Reichlin TS, Wurbel H. Refinement of experimental design and conduct in laboratory animal research. ILAR J. 2014;55(3):383‐391. doi: 10.1093/ilar/ilu037 [DOI] [PubMed] [Google Scholar]
- 56. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. doi: 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1.
Video S2.


