Abstract
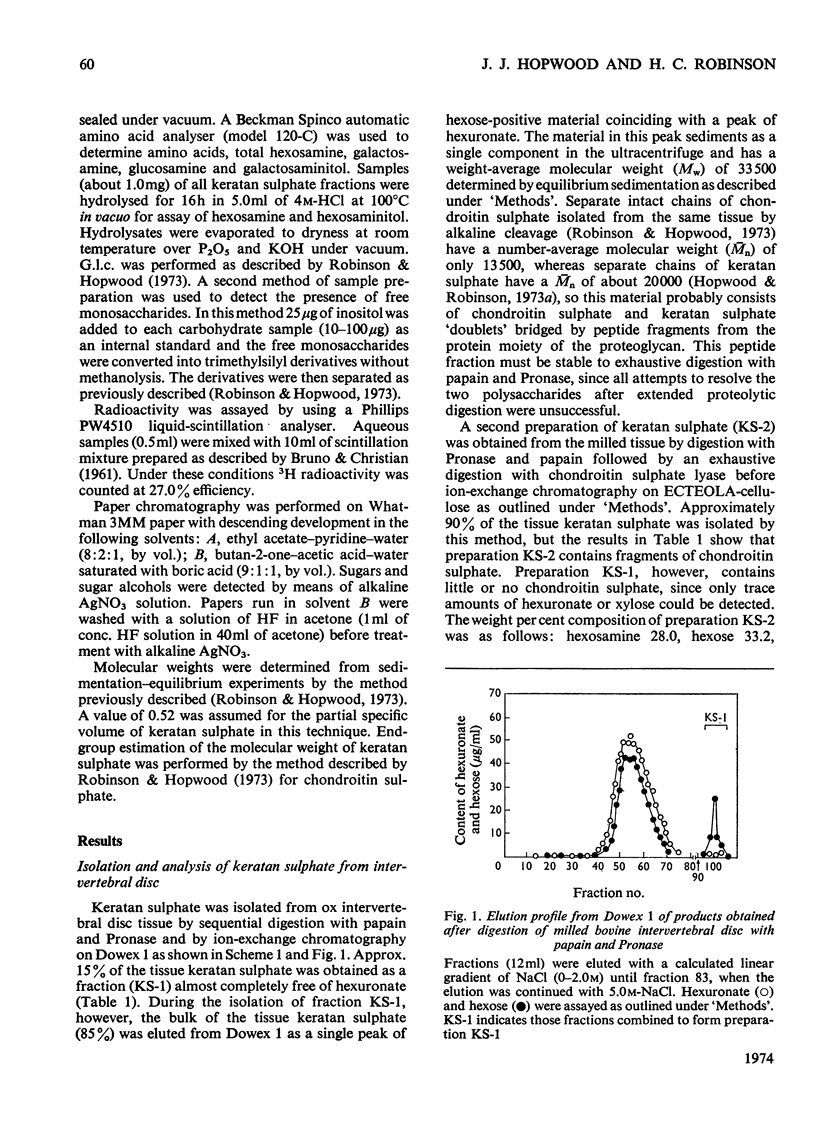
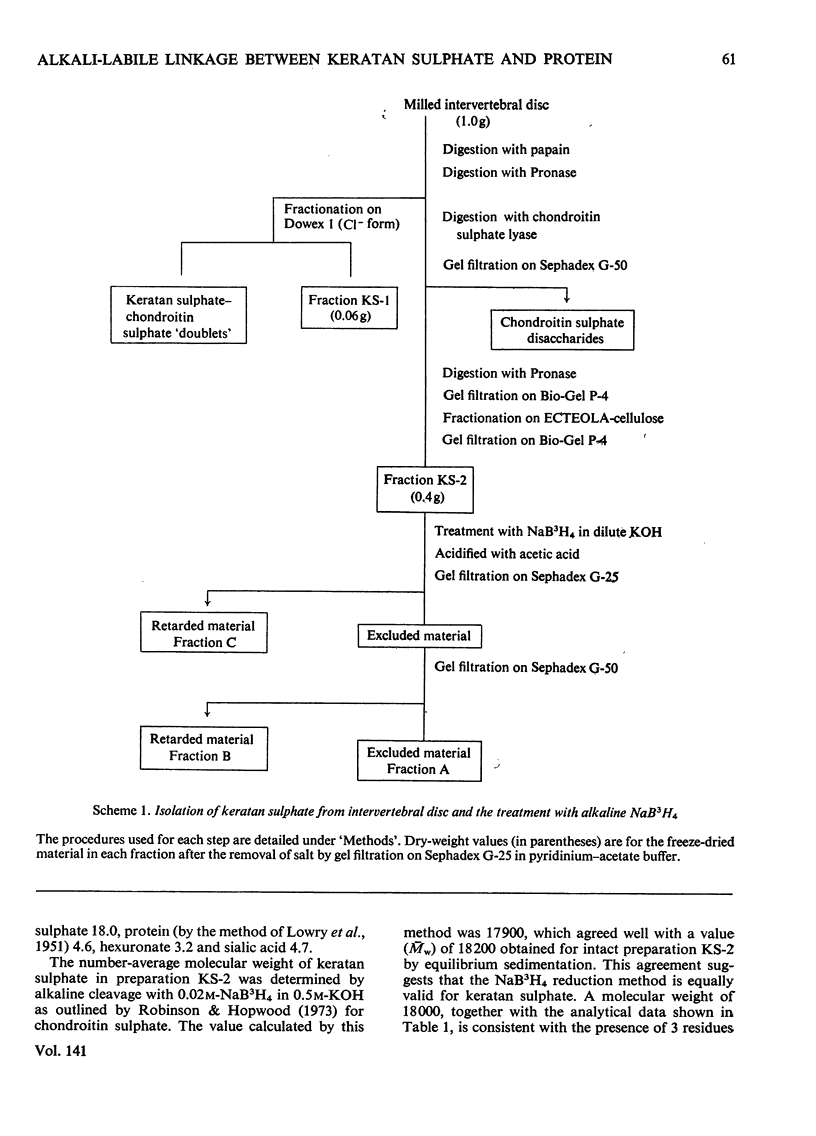
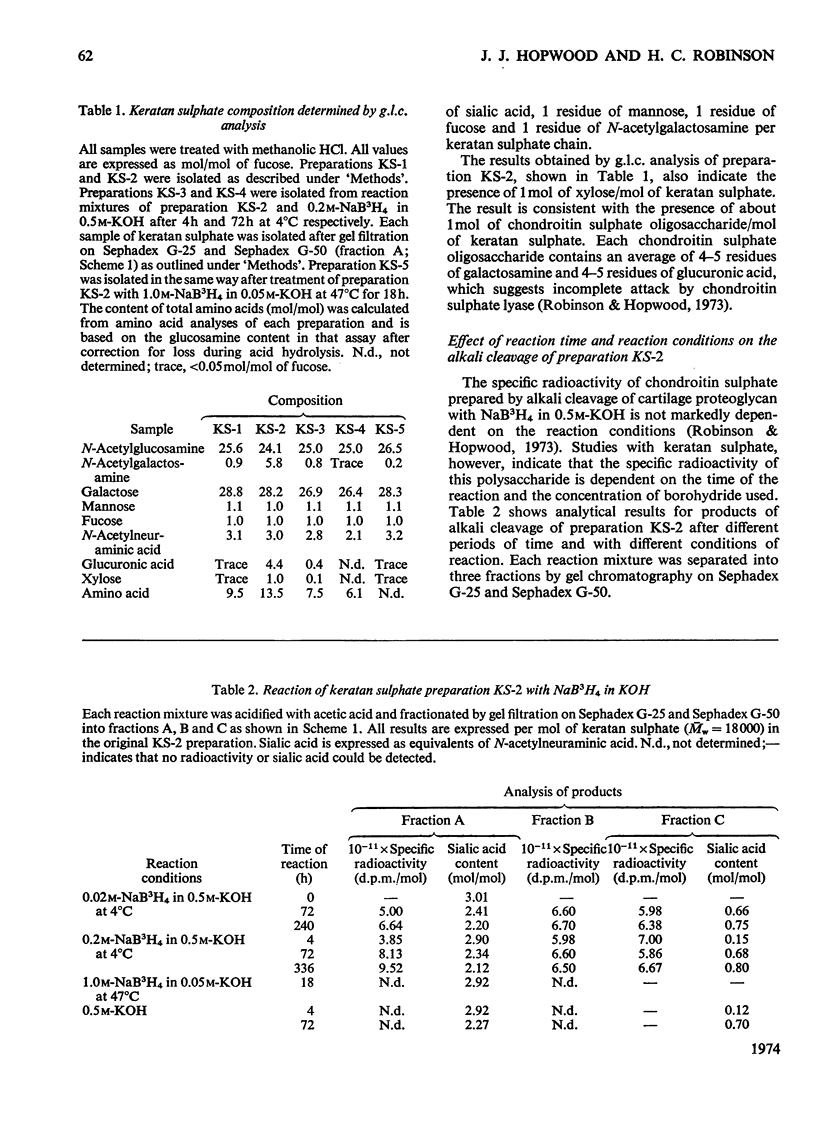
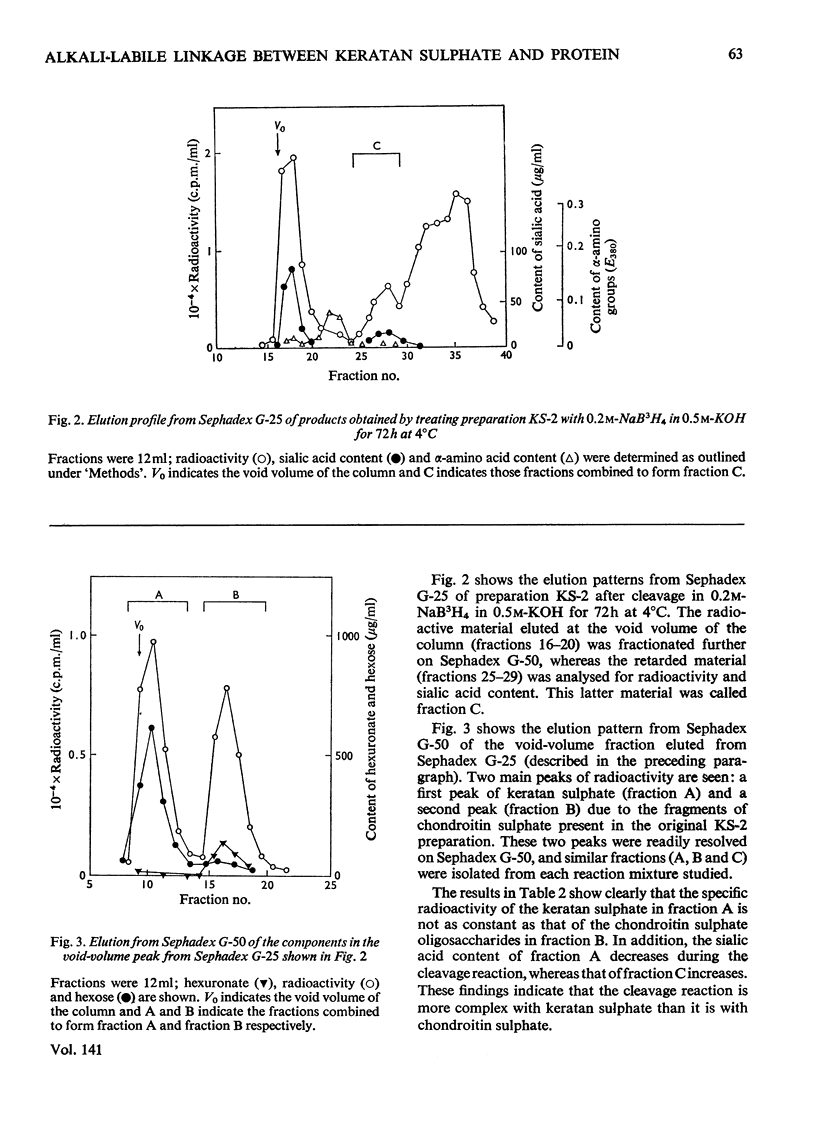
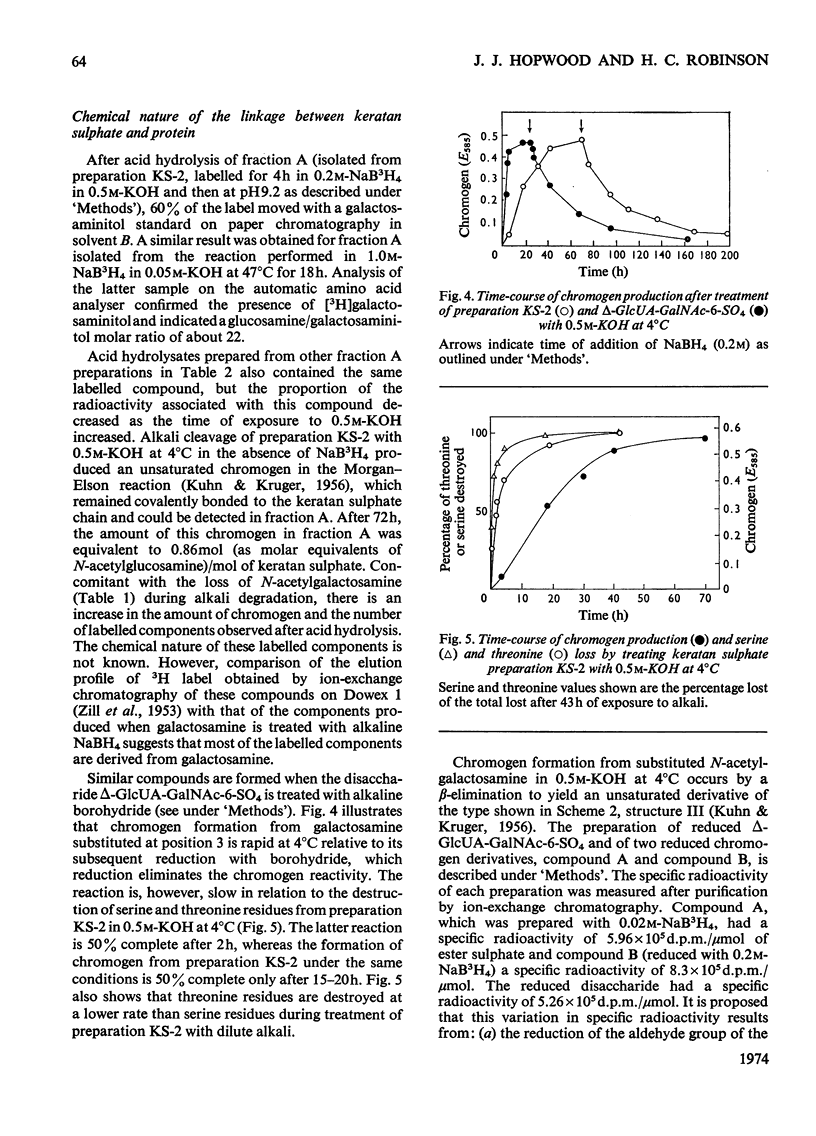
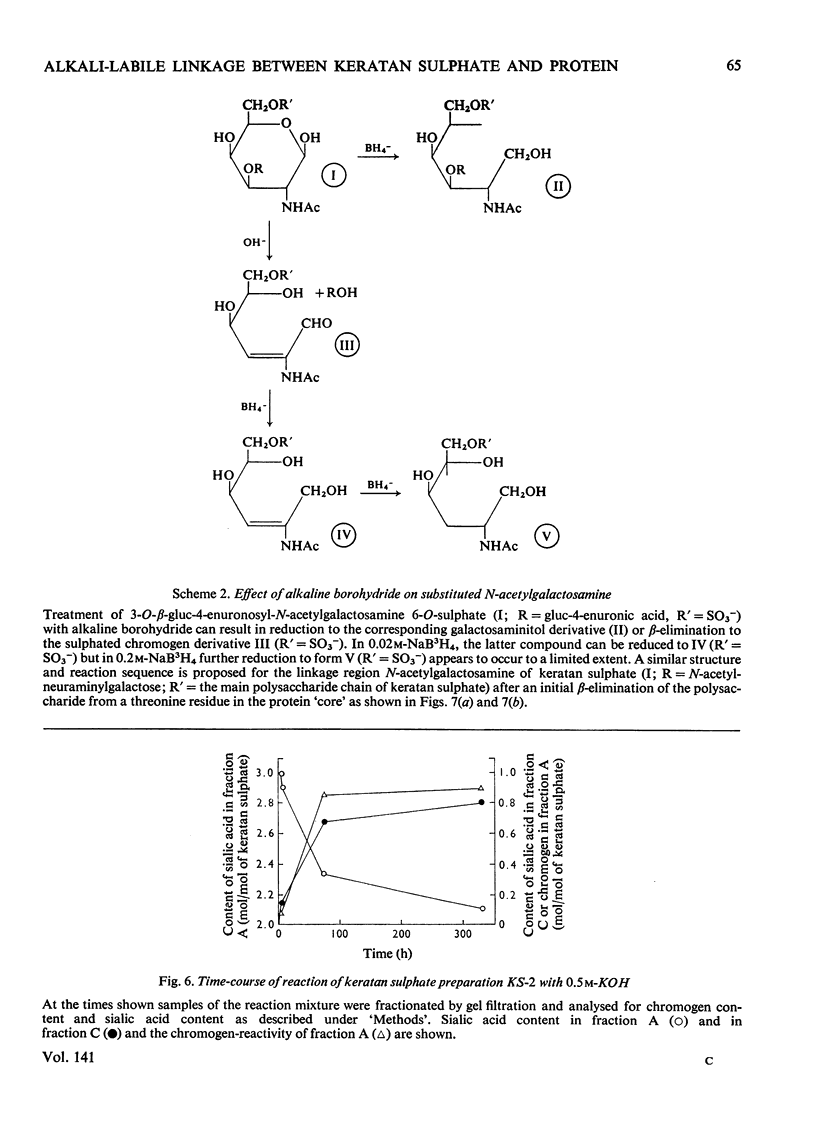
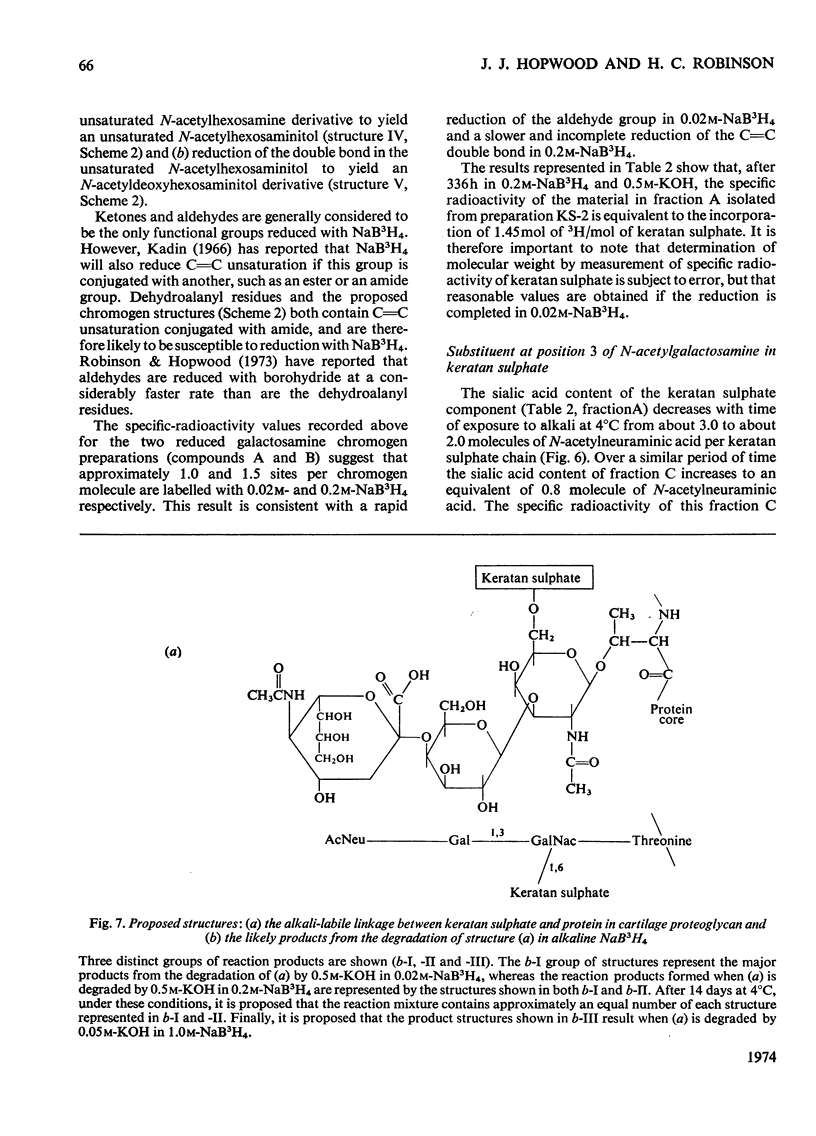
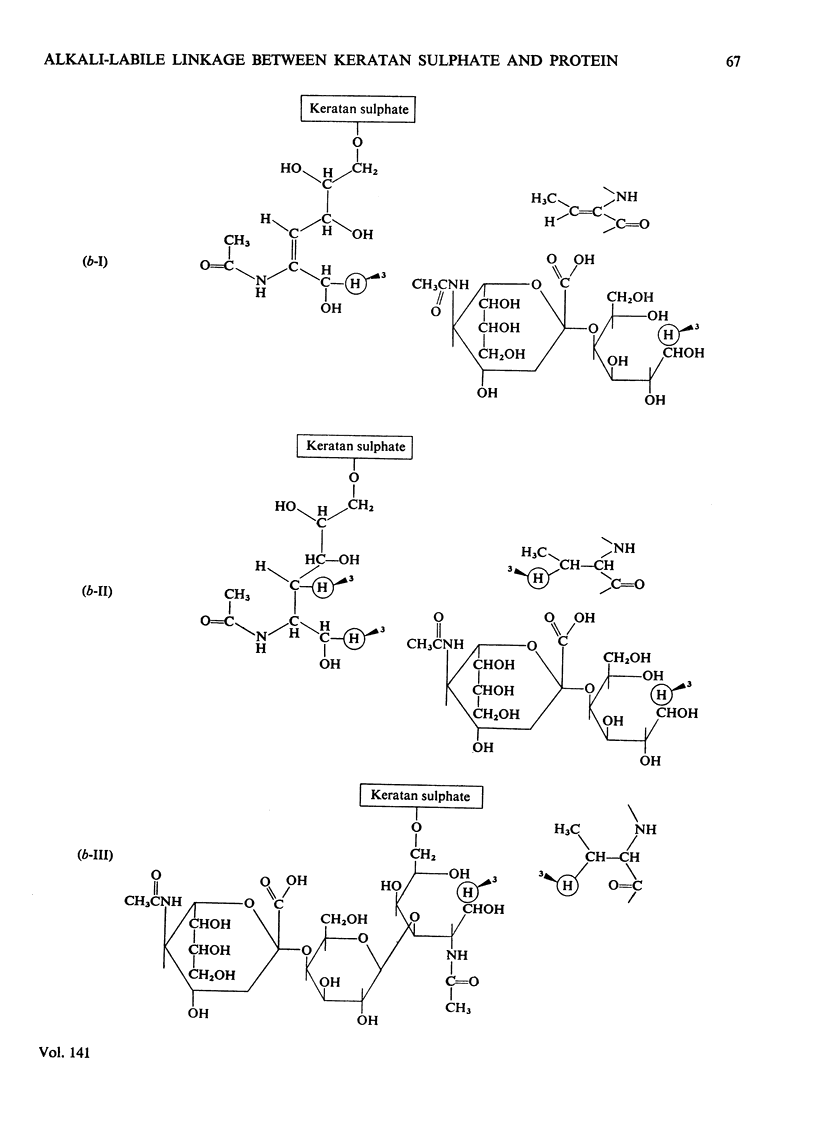
Keratan sulphate was isolated from adult intervertebral disc in 90% yield by sequential digestion of the whole tissue with papain, Pronase and Proteus vulgaris chondroitin sulphate lyase. Treatment of this preparation with alkali cleaved a glycosidic bond between N-acetylgalactosamine and threonine and produced, by an alkali-catalysed `peeling' reaction, an unsaturated derivative of N-acetylgalactosamine which reacted as a chromogen in the Morgan–Elson reaction, but remained covalently bonded to the keratan sulphate chain. This derivative was reduced and labelled by alkaline NaB3H4. The substituent at position 3 of N-acetylgalactosamine in the keratan sulphate–protein linkage was identified as a disaccharide, N-acetylneuraminylgalactose, which was isolated from the reaction mixture after alkali treatment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray B. A., Lieberman R., Meyer K. Structure of human skeletal keratosulfate. The linkage region. J Biol Chem. 1967 Jul 25;242(14):3373–3380. [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D. Hyaluronidase digestion and alkaline treatment of bovine tracheal cartilage proteoglycans. Isolation and characterisation of different keratan sulfate proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):193–207. doi: 10.1016/0005-2795(72)90191-2. [DOI] [PubMed] [Google Scholar]
- Helting T., Rodén L. Biosynthesis of chondroitin sulfate. I. Galactosyl transfer in the formation of the carbohydrate-protein linkage region. J Biol Chem. 1969 May 25;244(10):2790–2798. [PubMed] [Google Scholar]
- Helting T., Rodén L. Biosynthesis of chondroitin sulfate. II. Glucuronosyl transfer in the formation of the carbohydrate-protein linkage region. J Biol Chem. 1969 May 25;244(10):2799–2805. [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The molecular-weight distribution of glycosaminoglycans. Biochem J. 1973 Dec;135(4):631–637. doi: 10.1042/bj1350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathan R. H., Adamany A. Comparison of human MM, NN, and MN blood group antigens. J Biol Chem. 1967 Apr 25;242(8):1716–1722. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Layug E. J., Gruezo F. Immunochemical studies on blood groups. XXXIV. Structures of some oligosaccharides produced by alkaline degradation of blood group A, B, and H substances. Biochemistry. 1966 May;5(5):1489–1501. doi: 10.1021/bi00869a007. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Licerio E. [Immunochemical studies on blood groups. 38. Structures and activities of oligosaccharides produced by alkaline degradation of blood-group Lewis-a substance. Proposed structure of the carbohydrate chains of human blood-group A, B, H, Le-a, and Le-b substances]. Biochemistry. 1968 Aug;7(8):2976–2990. doi: 10.1021/bi00848a039. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Cifonelli J. A. Comparative biochemistry of keratosulfates. J Biol Chem. 1965 Nov;240(11):4140–4145. [PubMed] [Google Scholar]
- Meyer K. Biochemistry and biology of mucopolysaccharides. Am J Med. 1969 Nov;47(5):664–672. doi: 10.1016/0002-9343(69)90162-4. [DOI] [PubMed] [Google Scholar]
- Nitecki D. E., Stoltenberg I. M., Goodman J. W. Qualitative and quantitative determination of mixtures of amino acids using 2,4,6-trinitrobenzenesulfonic acid. Anal Biochem. 1967 May;19(2):344–350. doi: 10.1016/0003-2697(67)90170-4. [DOI] [PubMed] [Google Scholar]
- Pedrini V. Electrophoretic heterogeneity of proteinpolysaccharides. J Biol Chem. 1969 Mar 25;244(6):1540–1546. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Robinson H. C., Dorfman A. The sulfation of chondroitin sulfate in embryonic chick cartilage epiphyses. J Biol Chem. 1969 Jan 25;244(2):348–352. [PubMed] [Google Scholar]
- Robinson H. C., Hopwood J. J. The alkaline cleavage and borohydride reduction of cartilage proteoglycan. Biochem J. 1973 Jul;133(3):457–470. doi: 10.1042/bj1330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- SENO N., MEYER K., ANDERSON B., HOFFMAN P. VARIATIONS IN KERATOSULFATES. J Biol Chem. 1965 Mar;240:1005–1010. [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telser A., Robinson H. C., Dorfman A. The biosynthesis of chondroitin-sulfate protein complex. Proc Natl Acad Sci U S A. 1965 Sep;54(3):912–919. doi: 10.1073/pnas.54.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. Configuration of the ketosidic bond of sialic acid. J Biol Chem. 1969 Mar 10;244(5):1306–1313. [PubMed] [Google Scholar]


