Abstract
Background
The Inflammatory burden Index (IBI) is an effective predictor for a range of malignancies. However, the significance of IBI in esophageal squamous cell carcinoma (ESCC) needs to be further verified. The aim of this study was to verify the predictive power of IBI in ESCC undergoing radical resection.
Methods
The current retrospective study, which comprised 408 ESCC patients randomized into either the primary or validation cohort, evaluated the relationships between IBI, clinical characteristics, and cancer-specific survival (CSS). Additionally, the nomogram model was also constructed and verified.
Results
The IBI is significantly related to tumor length, vessel invasion, perineural invasion, and TNM stage. Compared to other hematological indices, the decision curve analyses (DCA) and receiver operating characteristic curve (ROC) confirmed the higher prognostic value of IBI, indicating the better clinical applicability. In patients with high IBI compared to the low IBI cohort, the 5-year CSS was considerably worse (total: 27.0% vs. 59.1%, P < 0.001; primary: 25.0% vs. 58.9%, P < 0.001; validation: 31.7% vs. 59.7%, P = 0.002). The IBI was shown to be an independent parameter by multivariate analyses (primary: HR = 2.352, P < 0.001; validation: HR = 1.683, P = 0.045). Finally, with the C-index of 0.675 (0.656–0.695) in the primary set and 0.662 (0.630–0.694) in the validation set for CSS in ESCC, an IBI-based nomogram was created and validated.
Conclusion
The predictive significance of IBI in ESCC patients undergoing radical resection was validated by this investigation. IBI may be utilized for preoperative evaluation of ESCC as it was found to be substantially correlated with prognosis.
Keywords: Cancer-specific survival, Recursive partitioning analysis, Inflammatory burden index, Esophageal squamous cell carcinoma, Prognosis
Subject terms: Cancer, Cancer prevention, Gastrointestinal cancer, Tumour biomarkers
Introduction
Esophageal cancer (EC), a malignant tumor that is aggressive and poses a major risk to public health, is not uncommon worldwide, including in China1,2. One of the main variables influencing the outcomes of EC is the tumor-related pathological stage (TNM stage), which is commonly used to assess the survival3. Sometimes, however, the TNM stage alone may not be adequate to determine a patient’s prognosis. Therefore, additional indicators may also need to be further investigated. The growing trend of using genetic biomarker detection to determine the EC prognosis is nevertheless constrained by the cost and inconvenience of testing4,5. To better improve patient prognosis and inform therapeutic decision-making, it is crucial to more accurately estimate the prognosis of EC prior to treatment using a range of simple, inexpensive, and effective prognostic indicators.
Systemic inflammatory response (SIR), the most representative tumor-host interaction, plays critical roles in cancer progression and prognosis6,7. A growing body of research indicates that the prognosis of various malignancies is closely related to a number of hematological indices that represent host SIR, such as albumin (ALB), C-reactive protein (CRP), monocytes (MONs), neutrophils (NEUs), platelets (PLTs), and lymphocytes (LYMs)8–10. Additionally, an increasing number of integrative indices with higher sensitivity and specificity are also being developed by researchers based on these SIR-related indices, such as CRP to ALB ratio (CAR), systemic immune inflammation index (SII), PLTs to LYMs ratio (PLR), LYMs to MONs ratio (LMR), and NEUs to LYMs ratio (NLR). The outcomes demonstrate that these indices have a higher prognostic role for several malignancies, including EC11–14. However, for assessing the prognosis of EC, it is unclear which combination of SIR-related indices is useful. Thus, it is of great clinical significance to explore an indicator that fully reflects the SIR to better predict the prognosis in those with EC.
It has been demonstrated that a novel inflammatory burden index (IBI) that takes SIR into account is a more accurate predictor of cancer15. The IBI was created with the intention of helping the authors forecast the prognosis of cancer as well as evaluate the inflammatory burden associated with various malignancies. The IBI’s predictive significance has since been verified in a number of cancer cases16–19. However, there is a limited understanding of IBI’s prognostic significance in patients with EC20. Therefore, this study sought to evaluate the prognostic role of preoperative IBI in esophageal squamous cell carcinoma (ESCC) with radical resection. Additionally, a predictive IBI-based nomogram was also constructed and validated to predict individual survival in patients with ESCC.
Materials and methods
Study design and inclusion and exclusion criteria
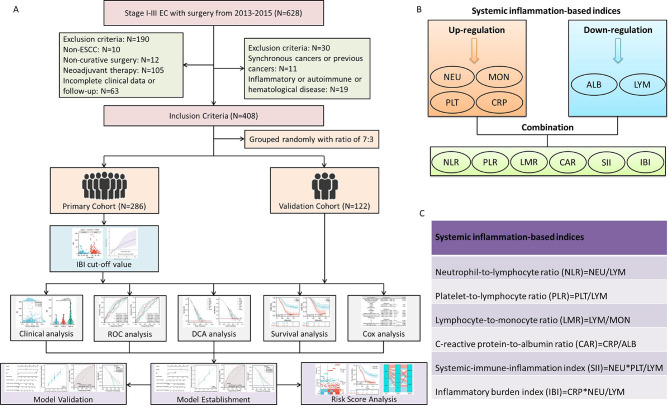
Retrospective medical records were gathered from 2013 to 2015 for 628 ESCC patients who underwent radical resection. The flow chart of study design and inclusion and exclusion criteria is shown in Fig. 1A. Following a thorough screening process, a total number of 408 cases were finally enrolled and randomly split into either the primary or validation cohort at a ratio of 7:3. The ethics committee approved this study and waived the informed consent due to the retrospective nature (IRB.2021-4).
Fig. 1.
The flow chart of patient selection and study design (A). An overview of the IBI as well as other hematological indices calculation (B). The definitions of the integrated hematological indices (C).
Therapy and follow-up
This study employed surgical procedures such as subtotal minimally invasive or open esophagectomy combined with two-field lymphadenectomy, as reported previously, by the Ivor Lewis or McKeown procedure21. Due to the potential impact of neoadjuvant therapy on peripheral indicators, patients undergoing such treatment were not included in the present study. Adjuvant treatment following radical resection in EC was then optional and mostly decided by pathological findings22,23. After treatment, patients were checked regularly: first 2 years: every 3 months, next 3 to 5 years: every 6 months, and after 5 years: once a year. Data from this study were last followed up in December 2020.
Laboratory collection and definition
From the medical records, the hematological indices, clinical and demographic features were retrospectively retrieved. In this investigation, the eighth AJCC/UICC TNM classification was used24. Preoperative laboratory indicators were gathered a week before surgery. As previously mentioned, the IBI was formulated as CRP × NEU/LYM15–20. Figure 1B displays an overview of the IBI as well as other hematological indices calculations. Figure 1C displays the definitions of the integrated hematological indices based on previously published research11–14.
Statistical analysis
SPSS 20.0, R 4.1.2, and Medcalc 17.6 were used to evaluate the statistical data. Based on the death/survival of cancer-specific survival (CSS), the receiver operating characteristic curve (ROC) was used to compare the areas under the curve (AUCs) between IBI and other indices. A time-independent ROC and AUC verified the IBI’s prognostic capabilities. Decision curve analysis (DCA), a tool for assessing the clinical value of various prediction models, was also utilized to analyze the difference between IBI and other hematological indices by measuring net income at various probability thresholds. Using the restricted cubic spline (RCS) model, the appropriate threshold for IBI was obtained by analyzing the non-linear relationship between IBI and CSS. RCS regression uses third-order polynomials joined at knot points to model non-linear relationships, which is highly relevant to Cox regression model analysis. Cox regress analyses were used to identify parameters in CSS that had 95% confidence intervals (CIs) and hazard ratios (HRs). Kaplan-Meier curves were used to compare the variations in CSS. By assessing discrimination and calibration in the primary and validation cohorts, a nomogram model was created and verified. If the P value was less than 0.05, the results were considered statistically significant.
Results
Characteristics comparison between two cohorts
The characteristics as well as hematological indices of the two cohorts were displayed in Table 1 along with a number of hematological indicators. Since there was no discernible difference between the two cohorts, the validation cohort’s findings may offer more reliable validation for the primary cohort.
Table 1.
Clinical characteristics of ESCC patients in the primary and validation cohorts.
| Total (n = 408) | Primary cohort (n = 286) | Validation cohort (n = 122) | P-value | |
|---|---|---|---|---|
|
Age (mean ± SD, years) Sex (female/male, n, %) Tumor location (U/M/L, n, %) Differentiation (W/M/P, n, %) Vessel invasion (yes/no, n, %) Perineural invasion (yes/no, n, %) Tumor length (mean ± SD, cm) pTNM stage (I/II/III, n, %) Adjuvant therapy (yes/no, n, %) NEU (mean ± SD, 109/L) LYM (mean ± SD, 109/L) MON (mean ± SD, 109/L) PLT (mean ± SD, 109/L) ALB (mean ± SD, d/dL) CRP (mean ± SD, mg/L) NLR (mean ± SD, range) PLR (mean ± SD, range) LMR (mean ± SD, range) CAR (mean ± SD, range) |
59.6 ± 7.7 132(32.4)/276(67.6) 26(6.4)/191(46.8)/191(46.8) 63(15.4)/264(64.7)/81(19.9) 67(16.4)/341(83.6) 78(19.1)/330(80.9) 4.03 ± 1.80 137(33.6)/131(32.1)/140(34.3) 119(29.2)/289(70.8) 4.36 ± 1.41 1.57 ± 0.47 0.51 ± 0.18 220.9 ± 67.2 4.09 ± 0.43 5.34 ± 5.60 2.90 ± 0.98 150.3 ± 59.8 3.26 ± 0.94 1.33 ± 1.43 |
59.2 ± 8.0 93(32.5)/193(67.5) 18(6.3)/129(45.1)/139(48.6) 40(14.0)/189(66.1)/57(19.9) 47(16.4)/239(83.6) 58(20.3)/228(79.7) 4.05 ± 1.87 95(33.2)/90(31.5)/101(35.3) 84(29.4)/202(70.6) 4.41 ± 1.53 1.59 ± 0.49 0.52 ± 0.18 217.8 ± 68.9 4.07 ± 0.46 5.52 ± 6.08 2.93 ± 1.09 148.1 ± 61.1 3.22 ± 0.89 1.39 ± 1.58 |
60.5 ± 7.1 39(32.0)/83(68.0) 8(6.6)/62(50.8)/52(42.6) 23(18.9)/75(61.5)/24(19.8) 20(16.4)/102(83.6) 20(16.4)/102(83.6) 3.99 ± 1.64 42(34.4)/41(33.6)/39(32.0) 35(28.7)/87(71.3) 4.24 ± 1.07 1.54 ± 0.40 0.49 ± 0.16 228.1 ± 62.7 4.15 ± 0.34 4.93 ± 4.27 2.82 ± 0.65 155.6 ± 56.6 3.36 ± 1.03 1.20 ± 1.04 |
0.125 0.913 0.533 0.450 0.992 0.361 0.786 0.803 0.890 0.246 0.383 0.139 0.158 0.081 0.327 0.276 0.245 0.167 0.221 |
|
SII (mean ± SD, range) IBI (mean ± SD, range) |
648.1 ± 305.4 15.9 ± 19.2 |
648.2 ± 327.8 16.7 ± 21.2 |
647.9 ± 246.3 14.2 ± 13.5 |
0.993 0.224 |
Abbreviation: ESCC: esophageal squamous cell carcinoma; SD: standard deviation; U/M/L: upper/middle/lower; W/M/P; well/moderate/poor; pTNM: pathological tumor node metastasis; NEU: neutrophil; LYM: lymphocyte; MON: monocyte; PLT: platelet; ALB: albumin; CRP: c-reactive protein; NLR: NEU to LYM ratio; PLR: PLT to LYM ratio; LMR: LYM to MON ratio; CAR: CRP to ALB ratio; SII: systemic immune-inflammation index; IBI: inflammatory burden index.
Characteristics grouped by IBI
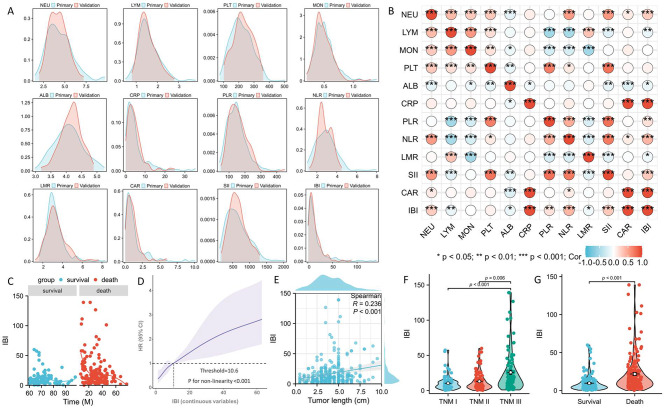
Figure 2A displays the distribution of all the indices. Figure 2B displays the correlation heat map for the primary cohort. The CSS and IBI exhibited a nonlinear connection (Fig. 2C), thus dividing patients into two separate cohorts after establishing an IBI threshold of 10.6 by RCS (Fig. 2D). Table 2 displays the baseline features and hematological indices sorted by IBI. Individuals exhibiting elevated IBI had longer tumor length, more vessel and perineural invasion, and a more advanced TNM stage. Figure 2E displays a positive correlation between IBI and tumor length. Patients with a high level of IBI were also found to have a considerably greater tumor stage (Fig. 2F) and death risk (Fig. 2G), according to the violin plots.
Fig. 2.
The distribution of all the indices in two cohorts (A). The correlation heat map for all SIR-related indices (B). A nonlinear connection between CSS and IBI (C). An IBI threshold of 10.6 by RCS (D). A positive correlation between IBI and tumor length (E). IBI grouped by tumor stage (F) and death risk (G).
Table 2.
Clinical characteristics grouped by IBI in the primary and validation cohorts.
| Primary Cohort (n = 286) Low-IBI (n = 146) High-IBI (n = 140) P-value |
Validation Cohort (n = 122) Low-IBI (n = 62) High-IBI (n = 60) P-value |
|
|---|---|---|
|
Age (≤ 60/>60, years, n) Sex (female/male, n) Tumor location (U/M/L, n) Differentiation (W/M/P, n) Vessel invasion (yes/no, n) Perineural invasion (yes/no, n) Tumor length (≤ 3.0/>3.0, n) pTNM stage (I/II/III, n) |
77(52.7)/69(47.3) 92(65.7)/48(34.3) 0.026 46(31.5)/100(68.5) 47(33.6)/93(66.4) 0.709 10(6.8)/60(41.1)/76(52.1) 8(5.7)/69(49.3)/63(45.0) 0.379 24(16.4)/94(64.4)/28(19.2) 16(11.4)/95(67.9)/29(20.7) 0.473 16(11.0)/130(89.0) 31(22.1)/109(77.9) 0.011 20(13.7)/126(86.3) 38(27.1)/102(72.9) 0.005 65(44.5)/81(55.5) 35(25.0)/105(75.0) 0.001 57(39.0)/52(35.6)/37(25.3) 38(27.1)/38(27.1)/64(45.8) 0.001 |
34(54.8)/28(45.2) 32(53.3)/28(46.7) 0.868 22(35.5)/40(64.5) 17(28.3)/43(71.3) 0.397 4(6.5)/25(40.3)/33(53.2) 4(6.7)/37(61.7)/19(31.6) 0.048 12(19.4)/36(58.1)/14(22.5) 11(18.3)/39(65.0)/10(16.7) 0.671 5(8.1)/57(91.9) 15(25.0)/45(75.0) 0.012 6(9.7)/56(90.3) 14(23.3)/46(76.7) 0.042 26(41.9)/36(58.1) 15(25.0)/45(75.0) 0.048 28(45.2)/18(29.0)/16(25.8) 14(23.4)/23(38.3)/23(38.3) 0.039 |
| Adjuvant therapy (yes/no, n) | 40(27.4)/106(72.6) 44(31.4)/96(68.6) 0.454 | 42(67.7)/20(32.3) 45(75.0)/15(25.0) 0.376 |
Abbreviation: ESCC: esophageal squamous cell carcinoma; IBI: inflammatory burden index; U/M/L: upper/middle/lower; W/M/P; well/moderate/poor; pTNM: pathological tumor node metastasis.
Prognostic comparison between IBI and other indices
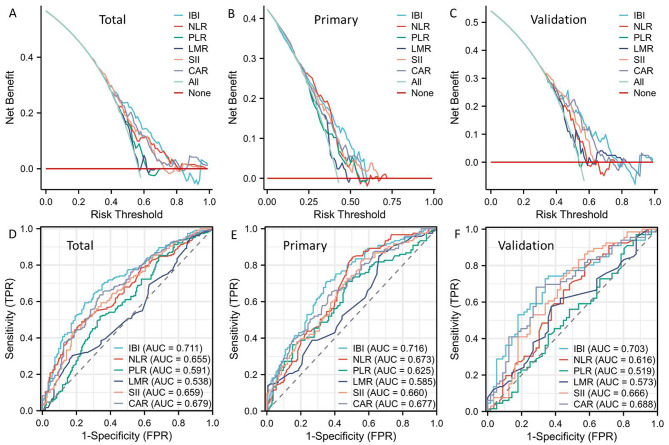
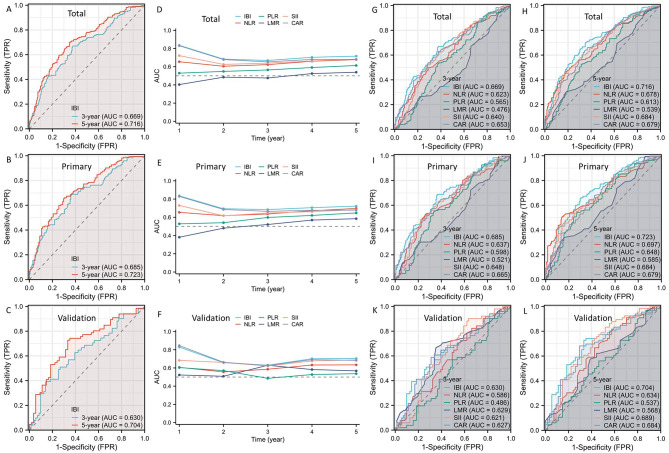
DCAs and ROCs between IBI and other traditional indices (SII, CAR, PLR, NLR, and LMR) were conducted in order to have a better understanding of the predictive utility of IBI. The DCAs supported the higher prognostic value of IBI in comparison to other hematological indices, indicating the IBI’s potential for improved clinical application (Fig. 3A-B). When compared to other traditional indices, IBI exhibited the greatest AUC based on the ROCs, suggesting a stronger predictive capacity (Fig. 3D-F). In order to better represent the prognostic value of IBI, we further adopted time-dependent ROC curves (Fig. 4A-C) and AUC curves (Fig. 4D-F). Compared to other hematological indices, the results also showed the prognostic advantages of IBI (Fig. 4G-L).
Fig. 3.
To better understand the prognostic value of IBI, DCAs (A-C) and ROCs (D-F) between IBI and other conventional indices were compared.
Fig. 4.
Time-dependent ROC curves (A-C) and AUC curves (D-F). The results also showed the prognostic advantages of IBI (G-L).
Kaplan-Meier curves of CSS and Cox analysis
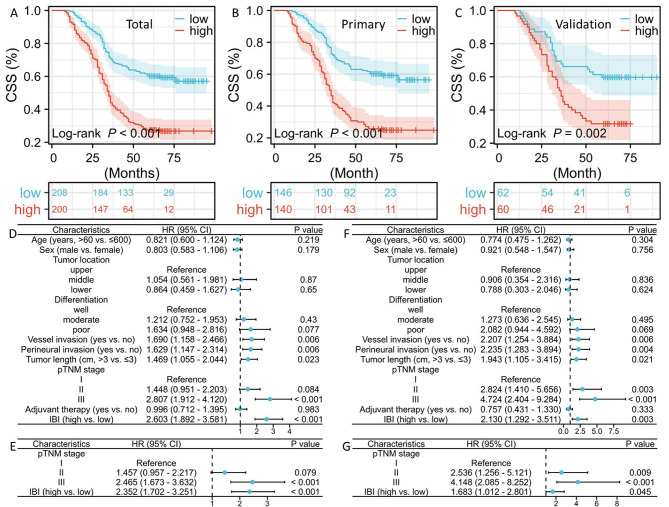
In patients with high IBI compared to the low IBI cohort, the 5-year CSS was considerably worse (total: 27.0% vs. 59.1%, P < 0.001, Fig. 5A; primary: 25.0% vs. 58.9%, P < 0.001, Fig. 5B; validation: 31.7% vs. 59.7%, P = 0.002, Fig. 5C). Prominent prognostic variables from the univariate analyses with regard to CSS, including TNM stage, perineural and vessel invasion, tumor length, and IBI were then recruited for further multivariate analyses in the primary cohort (Fig. 5D). IBI was shown to be an independent parameter in the primary cohort (HR = 2.352, P < 0.001; Fig. 5E). The similar results of IBI in Cox analyses were also obtained in the validation cohort (HR = 1.683, P = 0.045; Fig. 5F-G).
Fig. 5.
The 5-year CSS in total (A), primary (B), and validation (C) cohort. Univariate (D) and multivariate (E) Cox analyses in the primary cohort. Univariate (F) and multivariate (G) Cox analyses in the validation cohort.
Nomogram establishment and validation
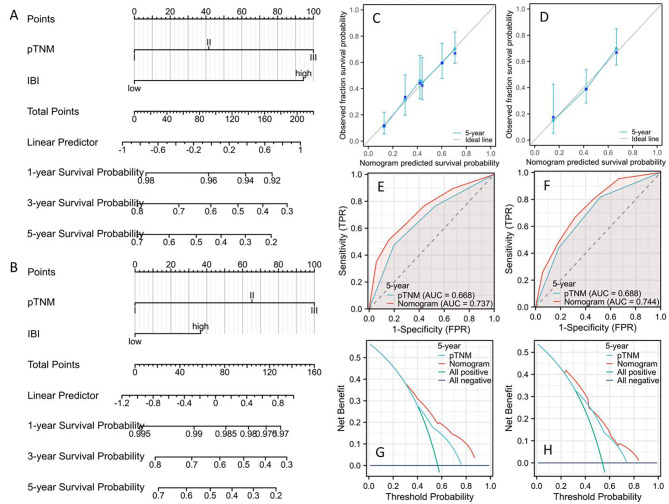
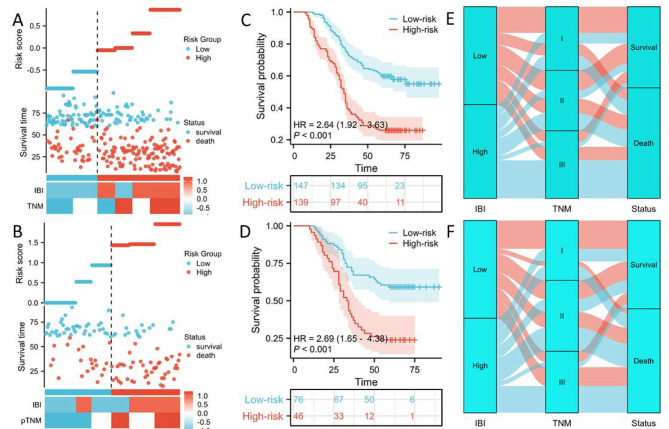
To forecast 1-, 3-, and 5-year CSS, two predictive nomograms made up of two independent parameters (TNM stage and IBI) were created and validated in the primary and validation cohorts, respectively (Fig. 6A-B). The C-indexes for CSS were 0.675 (0.656–0.695) and 0.662 (0.630–0.694) in the primary and validation cohorts, respectively. Compared to two cohorts of the individual 5-year CSS, the results showed satisfactory levels of agreement (Fig. 6C-D). The nomogram had better prediction of 5-year CSS on account of the ROCs (Fig. 6E-F) and DCAs (Fig. 6G-J). The nomogram model was used as the foundation for risk stratification, dividing the population into high-risk and low-risk categories (Fig. 7A-B). There was a noticeable variation in CSS between the two risk categories, as seen in Fig. 7C-D. The Sankey diagram in respect to risk stratification is shown in Fig. 7E-F.
Fig. 6.
Nomograms in primary (A) and validation (B) cohort. The CCAs showed satisfactory levels of agreements in the primary (C) and validation cohort (D). The ROCs indicated a higher prognostic ability in the primary (E) and validation cohort (F). The DCAs displayed a better clinical applicability in the primary (G) and validation cohort (H).
Fig. 7.
Risk was categorized into high-risk and low-risk groups in the primary (A) and validation cohort (B). The 5-year CSS between the two groups in the primary (C) and validation cohort (D). Sankey diagram with relation to risk stratification in the primary (E) and validation cohort (F).
Discussion
To stratify patients, at present, optimize treatments, and forecast survival in ESCC, the TNM stage is the most widely utilized tool3. Nevertheless, one drawback of the TNM stage mentioned above is that it only takes into account the features of cancer, excluding host factors like SIR that could have an impact on the prognosis of the disease25. A growing body of research indicates that the prognosis for EC has been predicted and validated using a number of SIR-related factors10,13,14. Consequently, future prognostic improvement will be guided by more improved and composite SIR-related indices. The predictive impact of IBI was validated in this investigation, and it was found to be much stronger than that of other traditional prognostic indices. Compared with patients in the low IBI set, this study also revealed a worse CSS in patients with high IBI. IBI then functions as an independent parameter in two cohorts.
Tumor-related inflammation is strongly associated with tumor occurrence, development, invasion, and metastasis because it is widely thought to be the immune system’s reaction to tumor cells6,7. The SIR, the most representative tumor-host interaction, is thought to be a hallmark of tumors, which can remarkably accelerate tumor growth by changing the tumor microenvironment, which in turn affects stromal cell renewal rate and polarized immune cell immunosuppressive capacity26,27. As markers of tumor aggressiveness and treatment response, SIR-related indices can help customize therapeutic approaches because they show the relationship between the tumor microenvironment and the host immune response28.
The IBI, one of the most creative and promising prognostic indices, thoroughly assesses inflammatory burden in cancer patients. In a prospective multicenter research involving 6359 cancer patients, the first thorough examination of IBI was presented and indicated that IBI was an independent high-risk variable associated with short-term outcomes, nutritional status, and life functions15. In response to the above study, researchers had noted that the lack of external validation of IBI was not conducive to clinical application. Therefore, they proved that IBI was of great significance to the prognosis through external verification in locally advanced gastric cancer, and therefore believe that IBI will provide a more personalized reference for prognostic monitoring16. Based on the findings of a study including 93 gastric cancer patients who had multimodal therapy, a high IBI was linked to an increasing risk of death and postoperative complications17. A multicenter prospective study aimed to compare the prognostic value of existing SIR-related indices in non-small cell lung cancer patients, suggesting that a high level of IBI was linked to a high incidence of cachexia, death risk, and 90-day complications18. Additionally, IBI was also found to be able to predict a poor prognosis in a research involving 701 patients who had hepatocellular carcinoma resection19.
Nevertheless, there haven’t been many pertinent results of IBI in ESCC. Furthermore, the superiority of IBI over its constituents and other traditional indices in predicting the prognosis of ESCC remains unclear. Recently, a study analyzed the associations between IBI and prognosis in EC20. Between the current study and the prior study, there were a few discrepancies. First of all, the preceding study included a range of EC forms and treatments, and these intricate variables might have an impact on the result. Secondly, this study’s results were created in the primary set and validated in the validation set, correspondingly, and its samples were larger than those of the previous study. Thirdly, no additional conventional indices were included in the prior study for comparison. In order to ascertain the IBI’s superiority in the present investigation, prognostic values were compared between the IBI and additional classical indices. Notably, IBI demonstrated the highest predictive power and clinical application on account of ROCs and DCAs, making it the best option for SIR-related prognostic stratification in ESCC. Fourthly, the hematological indices listed above may not be the same for other cancers because ESCC has unique characteristics and its patients are typically malnourished. Finally, the current study constructed and validated a predictive nomogram for survival prediction based on IBI. The findings of our study shed new light on the prognostic importance of IBI in ESCC.
With the inclusion of three crucial parameters–LYMs, NEUs, and CRP–the IBI has become a useful instrument for evaluating the intricacy of the inflammatory process. By releasing a variety of pro-inflammatory cytokines, CRP can induce SIR and ultimately cause the death of cancer patients by slowly depleting vital protein components in the host29. Research has additionally revealed a strong association between CRP, cancer stage, and inflammatory response30. NEUs have a pro-tumor effect by attracting immune-suppressive cells to the tumor microenvironment31. Additionally, by releasing extracellular traps through cytotoxic lymphocyte release, tumor-associated NEUs shield tumor cells from toxic death, hence encouraging tumor angiogenesis32. Likely to impair the growth and invasion of tumor cells, LYMs can penetrate the tumor microenvironment, which is why they are frequently employed as an indicator of immunological competence33. As an efficient defense against tumor cells, LYMs, on the other hand, play a part in immune regulation within the tumor microenvironment34. The IBI is linked to both SIR status and tumor-related variables, suggesting that it is a more reliable prognostic indicator than other traditional indices. This should make it easier for supervising clinicians to use IBI to make preliminary assessments of patients’ clinical status and to focus more on probable complications and early hospitalization prognoses35,36.
It is important to recognize a few of this study’s strengths. First of all, the findings verified that individuals with ESCC who had higher baseline values of IBI also had higher tumor stages and poorer prognoses. Secondly, the superiority was ascertained by comparing the prognostic values of IBI with other traditional indices. Interestingly, IBI demonstrated the best predictive capability in terms of CSS among all the most often used SIR-related indices. Thirdly, the great predictive accuracy and low cost and convenience of calculating the IBI from regular laboratory tests suggest that it will likely be highly useful in ESCC daily clinical practice. Fourthly, it is hypothesized that IBI could reduce potential biases and improve the utility of prognosis. IBI is more accurate and has greater clinical relevance when compared to other hematological indices. Adjuvant therapy may be necessary for patients in an advanced stage, and greater monitoring may be necessary for those in an earlier stage.
Currently, there are a large number of studies on imaging and minimal residual disease (MRD) in cancer prediction. Radiomics is a non-invasive technology that involves extracting quantitative features from medical pictures, selecting features using specific procedures, and analyzing correlations with clinical data for classification or prediction37. In EC, radiomics has been shown to better predict pathological reactions such as pathological full response, complications, recurrence, and prognosis38,39. MRD refers to the small amount of cancer cells that remain in the body following cancer treatment. These remaining cancer cells have either failed to respond or are resistant to treatment40. Some studies have looked into the role of ctDNA-based MRD surveillance in the early treatment and prognosis of EC41,42. MRD is currently a hot research topic, but it has not been fully promoted due to its price and technology. Therefore, more clinical study findings are needed to determine whether MRD may be used as a prognostic predictor of EC.
It is important to take into account some of the study’s limitations. To begin with, potential bias was unavoidable in this single-center retrospective research. Secondly, IBI, a useful and straightforward index obtained from peripheral blood, might be impacted in different status, restricting the application. Thirdly, the fact that individuals who underwent neoadjuvant therapy were not included in this study may have limited the findings. Therefore, additional perspective studies are required to demonstrate the predictive validity of IBI.
Conclusion
In summary, in patients with ESCC who underwent radical resection, IBI was verified as a useful and straightforward index. Preoperative evaluation may benefit from the relationship between IBI and the tumor’s stage and prognosis.
Author contributions
JF and QC contributed and designed the current study. QZ, LW, and JF drafted the manuscript. LW and XY contributed to data collect. JF and YX interpreted and analyzed the data. JF and QC reviewed the manuscript for important intellectual content critically. All authors contributed to the article and approved the final manuscript as submitted version.
Funding
This study was supported by Zhejiang TCM Science and Technology Project (2021ZB034 and 2022ZB051).
Data availability
All data are available upon request. Further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Helsinki Declaration and approved by the Ethics Committee of Zhejiang Cancer Hospital (IRB-2021-4).
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jifeng Feng, Email: fengjf@zjcc.org.cn.
Qixun Chen, Email: Chenqix@yeah.net.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71 (3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Uhlenhopp, D. J. et al. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin. J. Gastroenterol.13 (6), 1010–1021 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Betancourt-Cuellar, S. L. et al. Esophageal Cancer: tumor-node-metastasis staging. Radiol. Clin. North. Am.59 (2), 219–229 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Dinh, H. Q. et al. Integrated single-cell transcriptome analysis reveals heterogeneity of esophageal squamous cell carcinoma microenvironment. Nat. Commun.12 (1), 7335 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pusung, M., Zeki, S. & Fitzgerald, R. Genomics of esophageal cancer and biomarkers for early detection. Adv. Exp. Med. Biol.908, 237–263 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Cazares, D. et al. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front. Endocrinol. (Lausanne). 13, 929572 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandia, R. & Munjal, A. Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol.119, 199–245 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Hsueh, C. et al. The prognostic value of preoperative neutrophils, platelets, lymphocytes, monocytes and calculated ratios in patients with laryngeal squamous cell cancer. Oncotarget8 (36), 60514–60527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikkelsen, M. K. et al. Systematic review and meta-analysis of C-reactive protein as a biomarker in breast cancer. Crit. Rev. Clin. Lab. Sci.59 (7), 480–500 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Wang, J. et al. Pretreatment plasma fibrinogen and serum albumin levels predict therapeutic efficacy of concurrent radiochemotherapy for esophageal squamous cell cancer. Front. Oncol.12, 1021214 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, N. et al. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Med. (Baltim).101 (3), e28617 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou, J. et al. Correlation between preoperative peripheral blood NLR, PLR, LMR and prognosis of patients with head and neck squamous cell carcinoma. BMC Cancer. 23 (1), 1247 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang, Y., Xiao, G. & Wang, R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res.11, 4185–4200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obata, Y. et al. The preoperative systemic immune-inflammation index is associated with an unfavorable prognosis for patients undergoing curative resection of esophageal squamous cell carcinoma after neoadjuvant therapy. Surg. Today. 53 (8), 964–972 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Xie, H. et al. Inflammatory burden as a prognostic biomarker for cancer. Clin. Nutr.41 (6), 1236–1243 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Ding, P. et al. The inflammatory burden index: a promising prognostic predictor in patients with locally advanced gastric cancer. Clin. Nutr.42 (2), 247–248 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Pelc, Z. et al. Prognostic value of inflammatory burden index in advanced gastric cancer patients undergoing multimodal treatment. Cancers (Basel). 16 (4), 828 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie, H. et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J. Cachexia Sarcopenia Muscle. 14 (2), 869–878 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song, R. et al. Prognostic value of inflammation-immunity-nutrition score and inflammatory burden index for hepatocellular carcinoma patients after hepatectomy. J. Inflamm. Res.15, 6463–6479 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin, C. et al. Clinical significance of the preoperative inflammatory burden index in esophageal cancer. Oncology10.1159/000535727 (2023). Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, T. et al. Effectiveness and safety of minimally invasive Ivor Lewis and McKeown oesophagectomy in Chinese patients with stage IA-IIIB oesophageal squamous cell cancer: a multicentre, non-interventional and observational study. Interact. Cardiovasc. Thorac. Surg.30 (6), 812–819 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Ni, W. et al. Postoperative adjuvant therapy versus surgery alone for stage IIB-III esophageal squamous cell carcinoma: a phase III randomized controlled trial. Oncologist26 (12), e2151–e2160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, L. et al. Adjuvant therapeutic modalities following three-field lymph node dissection for stage II/III esophageal squamous cell carcinoma. J. Cancer. 8 (11), 2051–2059 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice, T. W. et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis. Esophagus. 29 (8), 897–905 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.linav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 13 (11), 759–771 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Anastasiou, D. Tumour microenvironment factors shaping the cancer metabolism landscape. Br. J. Cancer. 116 (3), 277–286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denk, D. & Greten, F. R. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. 8 (11), 901–914 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Nallasamy, P. et al. Tumor microenvironment enriches the stemness features: the architectural event of therapy resistance and metastasis. Mol. Cancer. 21 (1), 225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, Y. et al. The effect of acupuncture on the expression of inflammatory factors TNF-α, IL-6,IL-1 and CRP in cerebral infarction: a protocol of systematic review and meta-analysis. Med. (Baltim).98 (24), e15408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Král, Z. et al. Systemic inflammatory response with high CRP values as the dominant symptom of multiple myeloma. Vnitr Lek. 65 (1), 37–44 (2019). [PubMed] [Google Scholar]
- 31.Masucci, M. T., Minopoli, M. & Carriero, M. V. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front. Oncol.9, 1146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Que, H. et al. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta Rev. Cancer. 1877 (5), 188762 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Ye, L. et al. Tumor-infiltrating immune cells act as a marker for prognosis in colorectal cancer. Front. Immunol.10, 2368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, B. et al. Tumor-infiltrating lymphocytes: warriors fight against tumors powerfully. Biomed. Pharmacother. 132, 110873 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Song, Z. et al. Inflammatory burden index: association between novel systemic inflammatory biomarkers and prognosis as well as in-hospital complications of patients with aneurysmal subarachnoid hemorrhage. J. Inflamm. Res.16, 3911–3921 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du, M. et al. Association between inflammatory burden index and unfavorable prognosis after endovascular thrombectomy in acute ischemic stroke. J. Inflamm. Res.16, 3009–3017 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambin, P. et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur. J. Cancer. 48 (4), 441–446 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang, Z. et al. CT-based radiomic signatures for prediction of pathologic complete response in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. J. Radiat. Res.60 (4), 538–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rishi, A. et al. Pretreatment CT and 18 F-FDG PET-based radiomic model predicting pathological complete response and loco-regional control following neoadjuvant chemoradiation in oesophageal cancer. J. Med. Imaging Radiat. Oncol.65 (1), 102–111 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Coakley, M., Garcia-Murillas, I. & Turner, N. C. Molecular residual disease and adjuvant Trial Design in Solid tumors. Clin. Cancer Res.25 (20), 6026–6034 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Azad, T. D. et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology158 (3), 494–505 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egyud, M. et al. Detection of circulating tumor DNA in plasma: a potential biomarker for esophageal adenocarcinoma. Ann. Thorac. Surg.108 (2), 343–349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request. Further inquiries can be directed to the corresponding author.