Abstract
It is difficult to distinguish Parkinson’s disease (PD) in the early stage from those of various disorders including atypical Parkinson’s syndrome (APS), vascular parkinsonism (VP), and even essential tremor (ET), because of the overlap of symptoms. Other, more challenging problems will arise when Parkinson’s disease develops into Parkinson’s disease dementia (PDD) in the middle and late stages. At this time, the differential diagnosis of PDD and DLB becomes thorny. These complicate the diagnostic process for PD, which traditionally heavily relies on symptomatic assessment and treatment response. Recent advances have identified several biomarkers in the blood and cerebrospinal fluid (CSF), including α-synuclein, lysosomal enzymes, fatty acid-binding proteins, and neurofilament light chain, whose concentration differs in PD and the related diseases. However, not all these molecules can effectively discriminate PD from related disorders. This review advocates for a paradigm shift toward biomarker-based diagnosis to effectively distinguish between PD and similar conditions. These biomarkers may reflect the diversity that exist among different diseases and provide an effective way to accurately understand their mechanisms. This review focused on blood and CSF biomarkers of PD that may have differential diagnostic value and the related molecular measurement methods with high diagnostic performance due to emerging technologies.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10571-024-01523-z.
Keywords: Parkinson’s disease, Atypical Parkinsonian disorders, Dementia and movement disorders, Differential diagnosis, Biomarkers
Introduction
There are many diseases that overlap with the symptoms of Parkinson’s disease (PD). After excluding the external causes of injury, infection, and poisoning, these three types of diseases, including atypical Parkinson’s syndrome (APS), vascular parkinsonism (VP), and essential tremor (ET), are difficult to distinguish from PD during the early stage and easily misdiagnosed due to the unknown course of onset (Jabbari et al. 2020; Yu et al. 2023; Yoo et al. 2023). In addition, more than 80% of patients suffering long-term PD will develop into PD dementia (PDD); it is very hard to differentiate from dementia with Lewy bodies (DLB) when PD develops into PDD in the middle and late stages.
APS includes progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), and multiple system atrophy (MSA) (Anastassiadis et al. 2024). VP does not belong to the category of APS because of its vascular lesions of relative speciality (Holm et al. 2023). ET has no definite pathological changes in the nervous system, but abnormal pathway activity in the brain still exists, so it is listed separately (Kosmowska and Wardas 2021). DLB and PDD are collectively referred to as Lewy body disease or synucleinopathy (Harris 2023). Unclear distinction between PD and the aforementioned diseases may result in indiscriminate clinical pathology studies or clinical trials (Hirschberg et al. 2023).
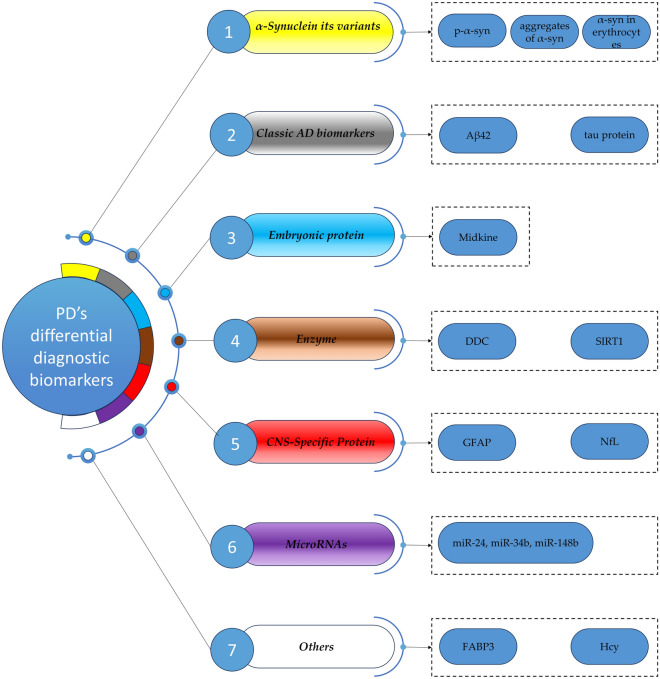
Through an extensive literature search, we found that several blood and cerebrospinal fluid (CSF) biomarkers significantly differ between PD and other related diseases (Fig. 1). These molecules may be defined as differential diagnostic biomarkers of PD (Quadalti et al. 2021). While these biomarkers have significant statistical differences in the study of groups, the traits of discrimination may need to be demonstrated in many ways. The differential diagnostic biomarkers of PD may be used as reference for clinical pathological studies and clinical trials, because differentiating these diseases is the first step for such studies (Dutta et al. 2021).
Fig. 1.
Flow diagram of the different types of PD’s differential diagnostic biomarkers. CNS central nervous system, DDC DOPA decarboxylase, GFAP glial fibrillary acidic protein, NfL neurofilament light chain protein, FABP3 fatty acid-binding protein 3, heart type, Hcy homocysteine
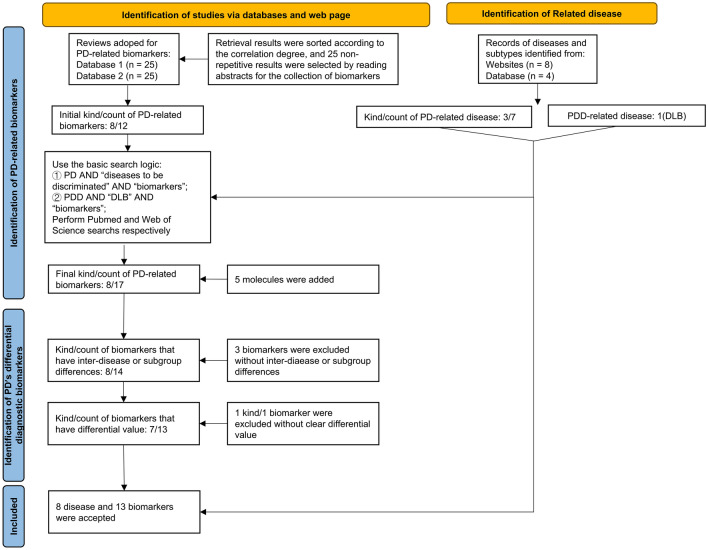
Search Strategy and Selection Criteria
Through a literature review, we first identified 6 diseases that require differentiation from PD (see search query). Next, we screened for diagnostic biomarkers using the search statements (“Parkinson’s disease” OR “PD”) AND (“biomarker”) on PubMed and Web of Science. We initially obtained 11 PD-related biomarkers (see search query) and selected review articles to obtain a comprehensive biomarkers selection. Based on the biomarkers identified, we used the search formulas (“Parkinson’s Disease” OR PD) AND ((“Atypical Parkinson’s syndrome” OR APS OR “Atypical parkinsonism disorder” OR APD) OR (“progressive supranuclear palsy” OR PSP) OR (“corticobasal syndrome” OR CBS) OR (“multiple system atrophy” OR MSA) OR (“vascular parkinsonism” OR VP) OR (“dementia with Lewy bodies” OR DLB) OR (“essential tremor” OR ET)) AND ((“α-Synuclein” OR “α-Syn”) OR (“DOPA Decarboxylase” OR “DDC”) OR (“Amyloid beta” OR “Aβ”) OR “tau protein” OR “Exosomes” OR (“Neurofilament Light Protein” OR “NfL”) OR “MicroRNAs” OR (“FABP3” OR “Fatty acid-binding protein 3, heart type”) OR (“DJ-1” OR “Parkinson’s disease protein 7”) OR (“CRP” OR “C-Reactive Protein”) OR (“Serum amyloid A” OR “SAA”) (“YKL-40” OR “chitinase-3-like-1” OR “CHI3L1” OR “human cartilage glycoprotein-39” OR “HC-gp39”)) to retrieve targeted literature while selecting studies that were appropriate to meet our research objectives. After screening abstracts of top 500 articles (sorted by best match), 160 articles were identified for further in-depth reading. Midkine (MK) and Kallikrein 10 were mentioned in a separate study, but no similar results suggested their value for the purpose we needed. Also, lysosomal enzymes (multiple) were molecules not obtained in the earliest search, and no study further elaborated their roles between PD and related diseases. Ultimately, 104 articles were included in this review.
Proteins Specific to Central Nervous System
α-Synuclein (α-Syn) and Its Variants
α-Syn is detectable in both CSF and plasma and is the most widely researched biomarker of PD (Tsao et al. 2022; Estaun-Panzano et al. 2023; Tofaris 2022). Phosphorylation of the Ser129 site results in phosphorylated α-syn (PS-129), while pro-aggregating forms of α-syn, such as oligomeric α-syn (o-α-syn), are also found in CSF and blood (Ma et al. 2024; Constantinides et al. 2021; Zubelzu et al. 2022; Chen et al. 2022a, b). Meanwhile, the pathogenic β-sheet seed is the pathological conformation of α-syn and can be detected in serum (Okuzumi et al. 2023).
Total α-Syn in CSF
Several studies and meta-analyses have confirmed that when compared to the control group, the total α-syn (t-α-syn) levels in the CSF are consistently lower in PD, MSA, PSP, CBS, and VP groups, with no significant differences among them (Zubelzu et al. 2022; Constantinides et al. 2017; Førland et al. 2020; Koníčková et al. 2023; Aerts et al. 2012). Therefore, t-α-syn levels in CSF cannot differentiate between PD and APS.
Phosphorylated α-Syn
Utilizing a Bead-based Luminex assay (with a sensitivity of 9 pg/mL), researchers measured the concentration of pS129 in the CSF of patients with PD, MSA, and PSP, revealing differences among them. To differentiate between the different diseases, ROC analysis following the discovery phase indicated that pS-129/t-α-syn was superior to pS-129 alone, with a specificity of ≥ 80%. The sensitivity among the three different Parkinsonian disease groups was as follows: PD vs MSA, 40%; PD vs PSP, 72%; and MSA vs. PSP, 63% (Wang et al. 2012). However, with the latest detection technology, this result may no longer reliable (Dutta et al. 2021; Silva et al. 2024). In the future, more targeted experimental method of large sample are needed to determine the distribution of phosphorylated α-syn in different diseases.
Oligomeric α-Syn
Notably, earlier studies have shown higher levels of o-α-syn in patients with PD compared to patients with PSP (Tokuda et al. 2010). But recent researches revealed that levels of o-α-syn in PD and other Parkinsonian syndromes do not differ significantly even though it is elevated compared to that of the control group (Eusebi et al. 2017; Majbour et al. 2020; Luo et al. 2024).
Pathogenic β-Sheet Seed
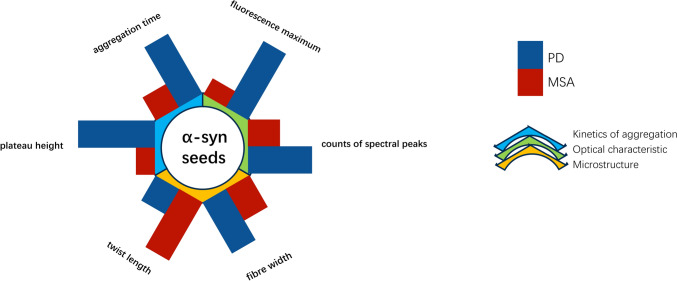
Aggregates of α-syn, including propagative α-syn seeds, showed high diagnostic performance in differentiating between PD and MSA (Siderowf et al. 2023; Painous et al. 2024; Parnetti et al. 2019; Goolla et al. 2023; Shahnawaz et al. 2020). Amplified seeds maintain disease-specific properties, allowing for the differentiation of samples from individuals with PD and MSA (Painous et al. 2024). Okuzumi et al.’ study (2023) suggested that the rate of negative results of IP/RT-QuIC in patients with MSA was significantly higher than that in patients with PD. Additionally, their study also examined the distinctive morphological features of seeds in various diseases. The fibril morphology of products derived from IP/RT-QuIC of serum α-syn seeds in patients with synucleinopathies could differentiate PD, DLB, and MSA, allowing for further research in this area (Fig. 2). A study found that the intensity of the signal in MSA was greater than that in PD when aggregation was performed in a specific buffered solution, indicating that α-syn seed aggregation from various diseases require different conditions for optimal detection (Martinez-Valbuena et al. 2022). These results suggest that our follow-up study can focus on the structural diversity and disease specificity of α-syn seeds.
Fig. 2.
α-Syn seeds show a specificity of deeper aspects. The differences in three dimensions make this biomarker show extremely high specificity in discriminating PD and MSA. PD Parkinson’s disease, MSA multiple system atrophy, plateau height: the peak at which the interaction of amyloid-binding dyes and α-syn aggregates ultimately reaches, aggregation time: speed of interaction between amyloid-binding dyes and α-syn aggregates, fluorescence maximum: the final intensity of fluorescence when the binding peak is reached, counts of spectral peaks: the wavelengths corresponding to the peaks of the derived two seed fibrils in the spectrum, fiber width: fibrils width in Cryo-ET (cryo-electron tomography), twist length: the length between the twisted nodes, reflecting the number of twists, the longer the length, the lower the twist frequency
α-Syn in Erythrocytes
While pathological α-syn aggregations primarily localize in the central nervous system, peripheral α-syn concentrations, particularly in erythrocytes, are higher than those in the CSF (Barbour et al. 2008). It is reported that more than 99% of the α-syn in the blood are in red blood cells (Miglis and Muppidi 2020). α-Syn in erythrocytes are reportedly excellent biomarkers for diagnosing PD (Yu et al. 2023, 2022).
Total α-syn levels in erythrocytes are higher in patients with ET than in those with PD. Moreover, the proportion of aggregated α-syn levels to t-α-syn levels in erythrocytes is markedly lower in patients with ET than in those with PD and HCs. ROC curve analysis showed that the ratios of aggregated α-syn to monomeric α-syn concentrations performed well in distinguishing patients with ET from those with PD and HCs, with an AUC of 0.892, sensitivity of 86.67%, and specificity of 97.96% for patients with ET vs. HCs. For ET vs. PD, the AUC was 0.817, with a sensitivity of 80.00% and specificity of 81.25% (Yu et al. 2023).
Glial Fibrillary Acidic Protein (GFAP)
Studies revealed that plasma GFAP levels in patients with PDD were higher than those in HCs, PD with mild cognitive impairment (PD-MCI), and PD with normal cognition (Bartl et al. 2023; Tang et al. 2023; Lin et al. 2023; Che et al. 2024). This may indicate that GFAP is associated with the progression of dementia in PD.
Neurofilament Light Chain (NfL)
NfL in CSF (cNfL) and plasma (pNfL) is a marker for neuronal damage that may potentially be used to distinguish between clinically similar conditions, such as frontotemporal dementia from AD and PD from APS (Quadalti et al. 2021).
Among Parkinsonian syndromes, the mean cNfL levels were higher in MSA, PSP, and CBS when compared with PD (Bridel et al. 2019).
Some studies have compared serum NfL levels in PD and ET (Hansson et al. 2017). Huang et al. (2022) reported that serum NfL concentrations in patients with PD (16.6 ± 3.5 pg/mL) were significantly higher than that in patients with ET (12.2 ± 2.4 pg/mL) and HCs (11.8 ± 2.4 pg/mL) (both p < 0.01, effect sizes = 1.47 and 1.60, respectively). When the cut-off was set at 13.65 pg/mL, the sensitivity and specificity of distinguishing between PD and ET were 76.7% and 84.1%, respectively, with an AUC of 0.854 (Fig. 3).
Fig. 3.
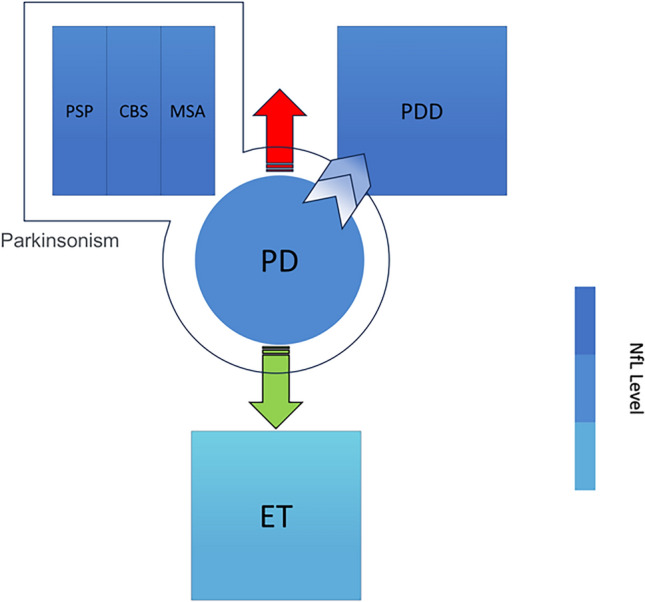
Concentrations of NfL in PD were considerably different from those in APS, PDD, and ET, with NfL concentrations higher in APS and PDD than in PD and the opposite in APS (VP is not included). This may suggest a different pathogenesis of its disease at the molecular level. NfL Neurofilament light protein, PD Parkinson’s disease, APS atypical Parkinson’s syndrome, PSP progressive supranuclear palsy, CBS corticobasal syndrome, MSA multiple system atrophy, PDD Parkinson’s disease dementia, ET essential tremor
Classic Alzheimer’s Disease (AD) Biomarkers
Amyloid-beta-Aβ42, tau protein-τT, and phosphorylated tau protein-τP-181 are classical biomarkers of AD (Sung et al. 2023). Notably, their significance in Parkinson’s syndrome has been re-recognized.
When compared to patients with PD, τT/Aβ42 ratio was increased in patients with MSA (Constantinides et al. 2017, 2021). An elevated τT/Aβ42 ratio effectively differentiated MSA from PD, with an optimal cut-off value of 0.344 that yielded a sensitivity of 0.71 and specificity of 0.93 (Constantinides et al. 2017).
Despite being a hallmark of AD, tau proteins are also found in the brains of patients with PD and DLB (Shim et al. 2022). A study aimed at distinguishing AD, DLB, and PD revealed that t-tau levels were higher in the DLB group than in the control and PD groups, but the differences were not statistically significant. Meanwhile, the t-tau/t-α-syn ratio had a better performance than standalone markers. For AD vs. DLB, the AUC increased from 0.66 for t-tau alone (70% sensitivity and 68% specificity) to 0.74 for the t-tau/t-α-syn ratio (55% sensitivity and 95% specificity) (Førland et al. 2020).
Inflammatory Markers
C-Reactive Protein (CRP)
CRP concentrations in the CSF are higher in patients with PD and PDD than in patients with PD without dementia and HCs (Lindqvist et al. 2013; Hall et al. 2018; Qiu et al. 2019). However, distinguishing between patients with PD and DLB or other neurodegenerative diseases (NDDs) based on CRP levels was not feasible.
YKL-40
YKL-40 is a biomarker of AD dementia and shows great potential in identifying AD dementia and other types of dementia, but so far there is no literature to explain its potential in identifying PDD and DLB (target literature of this review). The results showed that the contents of PDD and DLB were lower than those of AD dementia, but there was no difference (Morenas-Rodríguez et al. 2019; Mavroudis et al. 2021; Amin et al. 2022; Paolini Paoletti et al. 2023; Gautam and Singh 2024). A meta-analysis reached the opposite conclusion to that of a single study which showed that YKL-40 can significantly distinguish PD from MSA with a large effect size. However, the authors also point out that due to the small number of studies included in YKL-40, they cannot be regarded as promising biomarkers at present (Xiang et al. 2022).
Serum Amyloid A (SAA)
Hall et al.’s (2018) research measured the difference of SAA content in different diagnostic groups, and the results showed that the SAA level of PDD and MSA patients was higher than that of the control group. Furthermore, SAA levels were higher in PDD and MSA patients than in non-dementia PD patients. However, like other inflammatory markers, it is difficult to distinguish PD from other diagnostic groups.
Extracellular Vesicles
Pathological Proteins in Exosomes
Exosomes themselves do not have disease specificity; however, α-syn and its variants, tau and its variants can be detected in exosomes. Here is a detailed description of the nerve vesicles of central origin. Vesicles leaking out of the CNS in peripheral blood and CSF have been used to distinguish PD and MSA (Taha and Bogoniewski 2024; Taha and Hornung et al. 2023; Yan et al. 2024).
Dutta et al.’s (2021) study confirmed that α-syn concentrations in exosomes were markedly lower in the control group and significantly higher in the MSA group compared to the PD group. They created a ratio using α-syn concentrations of putative oligodendroglial exosomes and putative neuronal exosomes with good sensitivity in distinguishing PD and MSA. By incorporating this ratio along with the α-syn and total exosome concentrations, a multinomial logistic model successfully distinguished PD from MSA, with an area under the curve (AUC) of 0.902, sensitivity of 89.8%, and specificity of 86.0% after application to an independent validation cohort.
Meloni et al. (2023) investigated neural-derived extracellular vesicles (NDEVs) isolated from the blood. Analysis of NDEVs revealed a significant increase in o-α-syn levels in PD compared to APS (CBD and PSP). Additionally, levels of tau aggregates in NDEVs were significantly elevated in APS compared to PD (p < 0.0001). ROC analysis showed that the concentration of NDEVs of both oligomeric o-α-syn and tau aggregates exhibited an “excellent” power of classification that effectively distinguished PD from APS. For o-α-syn, the AUC was 0.817 (95% confidence interval (CI): 0.732–0.885; p < 0.0001), sensitivity of 78.6%, and specificity of 77.5%. For tau aggregates, the AUC was 0.856 (95% CI 0.776–0.915; p < 0.0001), sensitivity of 90.0%, and specificity of 75.7%.
Taha et al. (2023) were the first to measure pS129-α-syn levels in neuronal extracellular vesicles (nEVs) and oligodendroglial extracellular vesicles (oEVs). They reported that nEV pS129-α-syn concentrations were highest in healthy controls (HC) followed by PD and MSA, but the differences were not statistically significant. Conversely, oEV concentrations of pS129-α-syn were also highest in HC followed by PD and MSA and were significantly higher in both disease groups. Additionally, their study revealed that the oEV/nEV pS129-α-syn ratio increased in the order of HC < PD < MSA. Furthermore, they also measured total tau, pT181-tau (tau phosphorylated at Thr181) in nEVs and oEVs, and/or serum neurofilament light protein (NfL) levels. Due to the detection sensitivity, pT181-tau was detected in few samples. Other results were similar to experiments involving plasma or CSF.
MicroRNAs in Secreted Vesicles
MicroRNAs, which are small non-coding RNAs with 20–22 nucleotides, play a critical role in several mechanisms underlying the pathogenesis of various neurodegenerative diseases, including PD (Maiese 2022; Guévremont et al. 2023). More importantly, they can be detected in serum (Wamelen et al. 2020).
Among microRNAs, miR-30c and miR-148b are specific to individuals with PD, whereas miR-24, miR-223, and miR-324-3p are present in patients with both PD and MSA when compared with healthy individuals (Villar-Menéndez et al. 2014). Three microRNAs of miR-24, miR-34 b, and miR-148b were found significantly up-regulated in MSA compared with PD (Annamaria Vallelunga et al. 2014). Of course, these conclusions from the findings of high-throughput assays of RNA have not been validated by extensive sample measurements of larger samples.
Enzyme Protein
DOPA Decarboxylase (DDC)
A primary pathological feature of PD is the degeneration of dopaminergic neurons in the substantia nigra (Stoker and Greenland 2018). DDC is a diagnostic marker of dopaminergic dysfunction and can be detected in CSF (Painous et al. 2024).
Several studies have attempted to reveal the differences in DDC between PD and APS (Paslawski et al. 2023; Pereira et al. 2023). CSF levels of DDC may potentially be useful in differentiating among degenerative parkinsonisms (PD vs. APS) (Paslawski et al. 2023).
Kallikreins
Kallikreins, which is a subgroup of serine proteases, play various physiological roles. Recent research has emphasized their involvement in carcinogenesis, highlighting several kallikreins as promising candidates for novel biomarkers in cancer and other diseases. This supports the potential utility of kallikreins in clinical diagnostics and therapeutic targeting (Kalinska et al. 2016).
Kallikrein 10 has exhibited specific changes in APS compared to PD and controls; unfortunately, these changes were not elaborated (Paslawski et al. 2023).
Lysosomal Enzyme
Lysosomal enzyme is one of the markers of PD, and its various isoforms have little discriminatory power. We have found some potential lysosomal enzymes, GCase, Arylsulfatase A (ASA), cathepsin D, and β-hexosaminidase, which are related to the degradation of α-synuclein in patients with PD (Angelopoulou et al.2020). Someone needs to further measure the content of lysosomal enzyme isoforms in different PD, which is an unfinished direction.
NAD-Dependent Deacetylase Sirtuin-1 (SIRT1)
SIRT1 can regulate a variety of physiological and pathological processes, including inflammation, oxidative stress, metabolism, cell proliferation, cell differentiation, apoptosis, and so on (Li et al. 2020a, b). Li et al.’s (2023) outcome showed that the serum SIRT1 level of VP patients was significantly lower than that of PD, and SIRT1 was related to the severity of the disease, which could be used as an index to distinguish PD from VP.
Embryonic Protein
Midkine (MK)
MK is predominantly expressed during midgestation in embryogenesis, but its presence in normal adult brains is minimal. However, MK may recently play a role in various adult brain pathologies (Neumaier et al. 2023).
MK has demonstrated significant diagnostic potential as its levels were notably higher in patients with PD compared to those with APS (Paslawski et al. 2023).
DJ-1 (Aka Parkinson’s Disease Protein 7)
Regarding the discriminatory role of DJ-1 in APS, the most original research literature may be: The DJ-1 concentration in cerebrospinal fluid does not differentiate among Parkinsonian syndromes (2012). Similar subsequent small sample studies also came to the same conclusion, that is, DJ-1 has low value in distinguishing PD from various APS (Xiang et al. 2022; Guo et al. 2024; Gautam et al. 2024).
Other
Fatty Acid-Binding Protein 3, Heart Type (FABP3)
FABP3 is a small cytosolic protein that plays a role in lipid transport (Chiasserini et al. 2017). In the brain, FABP3 plays a regulatory role in the lipid composition of the membrane, suggesting a potential involvement in synapse formation and in the activity of cholinergic and glutamatergic neurons (Parnetti et al. 2019).
Elevated FABP3 levels have been detected in the serum of individuals diagnosed with DLB and PDD (Kawahata et al. 2023). Additionally, FABP3 levels were higher in patients with DLB than in those with PDD. This suggests the potential of FABP3 as a distinctive biomarker for DLB (Kawahata and Fukunaga 2023).
Similar to DLB, FABP3 levels were higher in patients with AD than in those with PD and other neurological disorders (p < 0.001). Notably, a combination of p-tau, FABP3, and α-syn successfully differentiated patients with AD from those with PDD, yielding an AUC of 0.96 (Chiasserini et al. 2017).
Plasma homocysteine (Hcy)
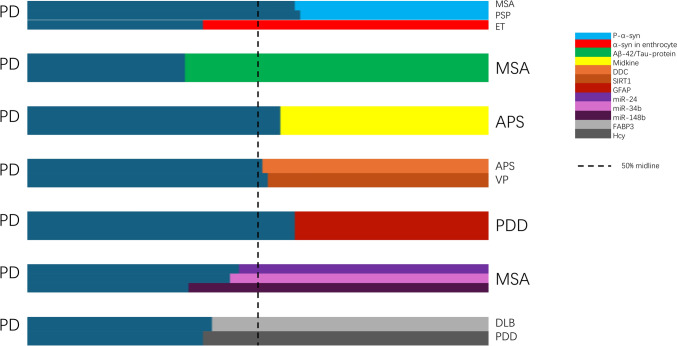
In one study, Hcy levels were measured in patients with PDD and PD without dementia (PDwoD) as well as in HCs. Results showed that individuals with PDD demonstrated higher Hcy levels than PDwoD and HCs (Song et al. 2013). In our final search, Hcy was not found to have the ability to distinguish PD from related diseases (Sharma et al. 2015; Liu et al. 2023; Zhou 2024) (Fig. 4).
Fig. 4.
The relative proportion of statistically significant content reported in the key literatures (represented by mean content of biomarkers). PD Parkinson’s disease, MSA multiple system atrophy, PSP progressive supranuclear palsy, ET essential tremor, APS atypical Parkinson’s syndrome, PDD Parkinson’s disease dementia, DLB dementia with Lewy bodies, DDC DOPA decarboxylase, SIRT1 NAD-dependent deacetylase sirtuin-1, GFAP glial fibrillary acidic protein, NfL neurofilament light chain protein, FABP3 fatty acid-binding protein 3, heart type, Hcy homocysteine
Emerging Technologies
At present, the research on PD’s biomarkers has been carried out from quantification to pathological protein conformation; the existing detection methods are constantly being improved in the direction of high specificity and sensitivity with high efficiency. The importance of novel technologies in identifying biomarkers has been emphasized as these advancements provided increased discriminatory capabilities (Parnetti et al. 2019). Highly accurate methods are changing previously known but non-significant findings.
In short, from the perspective of technological evolution, the development of targeted detection methods is an inevitable requirement for the differential diagnosis of PD. Follow-up technologies may involve many steps, be time-consuming, focus on scientific research targets, and be difficult to apply to patients, but technology is always a process of accumulation. After all, 20 years ago, no one would have thought of the high efficiency brought by the automation of testing technology.
Mass Spectrometry
Mass spectrometry-based assays may be useful in characterizing and quantifying forms of protein molecules, and now detection of different pathological isomers can even be accomplished by it.
Capillary Electrophoresis-Mass Spectrometry(CE-MS) and Its Improved Version
CE-MS is a protein determination technique that combines chromatographic techniques with MS to improve resolution power and is a widely used method for proteomics analysis (Valeriia et al. 2021). CE-MS proved to be an excellent platform for peptide analysis because of its multi-dimensionality, rapidity, and high sensitivity (Gomes et al. 2019; Pero-Gascon et al. 2020).
On-line aptamer affinity solid-phase extraction direct mass spectrometry (AA-SPE-MS), as a simplification and improvement over CE-MS, is an on-line sample preparation method because they allow automation to minimize sample processing and improve analytical throughput. Overall, the method is repeatable between 0.025 and 5 μg/ml with satisfactory linear results which has a 1000 × LOD than the original CE-MS (Salim et al. 2023). But at the same time, this improvement sacrifices a certain degree of specificity.
Cyclic Ion Mobility-Mass Spectrometry (CIM-MS)
Traditional methods are difficult to distinguish between different structural a-syn protein quantitative information, whereas CIM-MS can be used to detect analytes exhibiting subtle differences in higher-order structures. This method can determine the relative amounts of three disease-associated α-syn variants directly from artificial CSF (aCSF) (Makey et al. 2024).
Seed Amplification Assay (SAA)
SAA is inspired by the transmission process of prions and is used to detect misfolded protein aggregates (Fernandes Gomes et al. 2023). The core idea of this method is to use the intrinsic self-replicative characteristics of misfolded α-syn aggregates (seeds) to proliferate in vitro (Concha-Marambio et al. 2023). SAA methodologies related to Aβ, tau, and α-syn have been expanded by researchers. The method has been employed to identify misfolded α-syn aggregates in the CSF and peripheral tissues (Ma et al. 2024). What has been demonstrated is the high sensitivity and specificity of SAA in the detection of α-syn in CSF of PD patients. Pathologically soluble α-syn is present in neuron-derived EVs (NEs), and these vesicles can be isolated and captured in peripheral blood. The use of these vesicles in peripheral blood to detect pathological α-syn is a conceptual approach. Research of Annika Kluge et al. (2022) and Kluge and Iranzo (2024) provides the proof of concept and process basis for this idea. They analyzed the structural and functional characteristics of pathological α-syn in EVs, not just the content, and the experimental results can reliably distinguish PD and non-PD control groups, but there is no clear answer whether the pathological conformation is different in different APS and whether it can be distinguished from DLB. In studies that only quantify total α-syn in EVs, distinguishing PD from APS seems difficult and shows diametrically opposite conclusions (Hong et al. 2021). Moreover, researchers found that different sources of α-syn seeds have different optimal signal display conditions, making it possible to develop specific SAAs for a single disease such as MSA (Martinez-Valbuena et al. 2022). We hope to have the opportunity to perform individual measurements in patients with several APS, VP, DLB, and ET.
Platform Technology from Protein Misfolding Cyclic Amplification (PMCA) to Its Modified Version: Real-Time Quaking-Induced Conversion(RT-QuIC)
A lot of platform-related technologies for detection of final SAA product have been extended. PMCA and its evolutionary body RT-QuIC, including immunoprecipitation-based RT-QuIC (IP/RT-QuIC), which enables the detection of pathogenic α-syn seeds in the serum of individuals with synucleinopathy (Okuzumi et al. 2023) have shown high specificity (almost 100%) and sensitivity (> 90%) (Shahnawaz et al. 2017; Fairfoul et al. 2016). They can distinguish PD from other NDDs according to variations in α-syn aggregates. Their reliability in detection and the adaptability of RT-QuIC across different tissues and biological fluids have allowed this technique to become the benchmark when investigating the aggregation of α-syn in humans (Goolla et al. 2023).
Surround Optical Fiber Immunoassay (SOFIA)
This technique does not use any seed polymerization or amplification, thus eliminating the possibility of cross-contamination, and is based on immunocapture detection, combined with a uniquely designed highly sensitive fiber laser-induced fluorescence detection protocol (Chang et al.2009; Soares et al.2021).
HANdai Amyloid Burst Inducer Technique
The HANdai Amyloid Burst Inducer technique has been suggested as a viable alternative to the PMCA and RT-QuIC for assessing pro-aggregating proteins in biofluids due to its faster assay speed than PMCA and RT-QuIC (Umemoto et al. 2014; Parnetti et al. 2019). It is currently being studied as a technique for measuring pro-aggregating forms of α-syn.
Updates of ELISA
Novel ELISA assays have been developed by Majbour et al. (2016). Their method expanded the detection limit of α-syn to 50 pg/mL, which is 20-fold lower than that detected in human CSF. Meanwhile, the detection limits of pS129 and recombinant o-α-syn were expanded to 20 pg/mL and 10 pg/mL, respectively. To exceed these limits, technologies, such as novel photochemical, electrochemical, and crystal biosensors should be utilized (Jabbari et al. 2020).
Thus, its combination with electrochemical methods extends a range of ultra-high-precision quantitative methods. Based on the total α-synuclein assay, a modified Luminex assay, namely the Bead-based Luminex assays, was developed. Its sensitivity is approximately 9 pg/mL, providing a highly precise method for measuring pS129 (Wang et al. 2012). Detection systems supported by the Simoa Bead technology can accurately quantify low-concentration proteins and peptides at the level of fM with excellent reproducibility. Kawahata et al.(2023) used this technique to quantify FABP levels. Currently, Meso Scale Discovery electrochemiluminescence technology has been gradually used to detect molecular markers of neurodegenerative diseases and has shown a higher sensitivity (Zhao et al. 2020).
For phosphorylated α-syn, some researchers have screened antibodies to specifically quantify the ps129 in human CSF, and have reached a different conclusion from previous studies. The ELISA method provides extreme sensitivity and consistency (Silva et al. 2024). This suggests that we need to reconsider the possible sources of phosphorylated proteins and the consistency of different detection methods.
Conclusions
This review focused on the blood and CSF markers in PD as they are easily acquired, non-invasive, and in proximity to the brain in contrast to brain tissue biopsy or urine tests (Parnetti et al. 2019). Thus, facilitating the integration of clinical and scientific research for these biomarkers is essential.
The differences between t-α-syn, o-α-syn, pS-129, aggregates of α-syn, Kallikrein 10, τT/Aβ42 ratio, α-syn concentrations in exosomes, NDEV concentrations of both o-α-syn and tau aggregates, the oEV/nEV pS129-α-syn ratio, and cNfL levels between PD and APS have been revealed. Future diagnostic studies on PD should focus on the differentially expressed molecules in this disease. miR-30c, miR-148b, miR-24, miR-223, and miR-324-3p exhibit specificity in identifying patient with PD with MSA. FABP3, the t-tau/t-a-syn ratio, total α-syn levels in erythrocytes, and serum NfL concentrations may distinguish PDD from other dementias and movement disorders. Total α-syn levels in erythrocytes and serum NfL levels have distinctly different concentrations in ET compared to PD (Supplementary Table 1).
Currently, diagnosing PD primarily depends on clinical symptoms, and the relationship between symptoms and prognosis has been partially established (Armstrong and Okun 2020). However, the differences between biomarkers among different neurodegenerative diseases should make us consider the occurrence and development of this disease at a more specific level. Differential diagnostic biomarkers of PD in the blood and CSF represent its unique onset and evolution, which should be further researched for insights into its etiology and pathogenesis (Kelly et al. 2023).
Clinical pathological studies and clinical trials should accurately classify study participants (Lin et al. 2020). The emergence of biomarkers makes it feasible to accurately identify patients and allows for a more reliable reference for distinguishing PD from other diseases (Tolosa et al. 2021). This is a practical significance of PD’s differential diagnostic biomarkers.
Several biomarkers in PD and related diseases have obvious differences, but only a few have excellent performance by relying on high-precision testing methods. The combination of multiple biomarkers or clinical signs can increase the ability to discriminate between diseases (Quadalti et al. 2021; Dutta et al. 2021; Meloni et al. 2023; Taha et al. 2023; Kawahata et al. 2023). Developing a combined detection method based on multiple biomarkers may not be an urgent need for PD, which is an incurable disease; however, if patients can be accurately classified at disease onset, improving the efficiency of future scientific research and follow-up research in a large population will be beneficial.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The manuscript has been carefully reviewed by an experienced editor whose first language is English and who specializes in editing papers written by scientists whose native language is not English.
Author Contributions
Study design was performed by YW and writing of the original draft and visualization was performed by JM. Data collection was performed by ZT, YqW, JZ, ZW and LH. Data acquisition and critical revision of the manuscript was performed by SL. Project administration was carried out by YW. All authors have read and approved the final version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
Author LH was employed by ICON Plc. The remaining authors have no relevant financial or nonfinancial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
All authors have read and approved the final version of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aerts MB, Esselink RA, Abdo WF, Bloem BR, Verbeek MM (2012) CSF α-synuclein does not differentiate between Parkinsonian disorders. Neurobiol Aging 33:430.e1-430.e3. 10.1016/j.neurobiolaging.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Amin J, Erskine D, Donaghy PC, Surendranathan A, Swann P, Kunicki AP, Boche D, Holmes C, McKeith IG, O’Brien JT, Teeling JL, Thomas AJ (2022) Inflammation in dementia with Lewy bodies. Neurobiol Dis 168:105698. 10.1016/j.nbd.2022.105698 [DOI] [PubMed] [Google Scholar]
- Anastassiadis C, Martinez-Valbuena I, Vasilevskaya A, Thapa S, Hadian M, Morales-Rivero A, Mora-Fisher D, Salvo C, Taghdiri F, Sato C, Moreno D, Anor CJ, Misquitta K, Couto B, Tang-Wai DF, Lang AE, Fox SH, Rogaeva E, Kovacs GG, Tartaglia MC (2024) CSF α-synuclein seed amplification assay in patients with atypical Parkinsonian disorders. Neurology 103(6):e209818. 10.1016/j.nbd.2022.10569810.1212/WNL.0000000000209818 [DOI] [PubMed] [Google Scholar]
- Angelopoulou E, Paudel YN, Villa C, Piperi C (2020) Arylsulfatase A (ASA) in Parkinson’s disease: from pathogenesis to biomarker potential. Brain Sci. 10.3390/brainsci10100713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. JAMA 323:548–560. 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5:55–59. 10.1159/000112832 [DOI] [PubMed] [Google Scholar]
- Bartl M, Dakna M, Schade S, Otte B, Wicke T, Lang E, Starke M, Ebentheuer J, Weber S, Toischer K, Schnelle M, Sixel-Döring F, Trenkwalder C, Mollenhauer B (2023) Blood markers of inflammation, neurodegeneration, and cardiovascular risk in early Parkinson’s disease. Mov Disord 38:68–81. 10.1002/mds.29257 [DOI] [PubMed] [Google Scholar]
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFL Group, Alvarez-Cermeño JC, Andreasson U, Axelsson M, Bäckström DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, Christensen T, Fialová L, Forsgren L, Frederiksen JL, Gisslén M, Gray E, Gunnarsson M, Hall S, Hansson O, Herbert MK, Jakobsson J, Jessen-Krut J, Janelidze S, Johannsson G, Jonsson M, Kappos L, Khademi M, Khalil M, Kuhle J, Landén M, Leinonen V, Logroscino G, Lu CH, Lycke J, Magdalinou NK, Malaspina A, Mattsson N, Meeter LH, Mehta SR, Modvig S, Olsson T, Paterson RW, Pérez-Santiago J, Piehl F, Pijnenburg YAL, Pyykkö OT, Ragnarsson O, Rojas JC, Romme Christensen J, Sandberg L, Scherling CS, Schott JM, Sellebjerg FT, Simone IL, Skillbäck T, Stilund M, Sundström P, Svenningsson A, Tortelli R, Tortorella C, Trentini A, Troiano M, Turner MR, van Swieten JC, Vågberg M, Verbeek MM, Villar LM, Visser PJ, Wallin A, Weiss A, Wikkelsø C, Wild EJ (2019) Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 76:1035–1048. 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed]
- Chang B, Gray P, Piltch M, Bulgin MS, Sorensen-Melson S, Miller MW, Davies P, Brown DR, Coughlin DR, Rubenstein R (2009) Surround optical fiber immunoassay (SOFIA): an ultra-sensitive assay for prion protein detection. J Virol Methods 159(1):15–22. 10.1016/j.jviromet.2009.02.019 [DOI] [PubMed] [Google Scholar]
- Che N, Ou R, Li C, Zhang L, Wei Q, Wang S, Jiang Q, Yang T, Xiao Y, Lin J, Zhao B, Chen X, Shang H (2024) Plasma GFAP as a prognostic biomarker of motor subtype in early Parkinson’s disease. NPJ Parkinsons Dis 10(1):48. 10.1038/s41531-024-00664-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Gu X, Wang X (2022a) α-synuclein in Parkinson’s disease and advances in detection. Clin Chim Acta 529:76–86. 10.1016/j.cca.2022.02.006 [DOI] [PubMed] [Google Scholar]
- Chen WR, Chen JC, Chang SY, Chao CT, Wu YR, Chen CM, Chou C (2022b) Phosphorylated α-synuclein in diluted human serum as a biomarker for Parkinson’s disease. Biomed J 45:914–922. 10.1016/j.bj.2021.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasserini D, Biscetti L, Eusebi P, Salvadori N, Frattini G, Simoni S, De Roeck N, Tambasco N, Stoops E, Vanderstichele H, Engelborghs S, Mollenhauer B, Calabresi P, Parnetti L (2017) Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimers Res Ther 9:52. 10.1186/s13195-017-0276-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha-Marambio L, Pritzkow S, Shahnawaz M, Farris CM, Soto C (2023) Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat Protoc 18(4):1179–1196. 10.1038/s41596-022-00787-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides VC, Paraskevas GP, Emmanouilidou E, Petropoulou O, Bougea A, Vekrellis K, Evdokimidis I, Stamboulis E, Kapaki E (2017) CSF biomarkers beta-amyloid, tau proteins and a-synuclein in the differential diagnosis of Parkinson-plus syndromes. J Neurol Sci 382:91–95. 10.1016/j.jns.2017.09.039 [DOI] [PubMed] [Google Scholar]
- Constantinides VC, Majbour NK, Paraskevas GP, Abdi I, Safieh-Garabedian B, Stefanis L, El-Agnaf OM, Kapaki E (2021) Cerebrospinal fluid alpha-synuclein species in cognitive and movements disorders. Brain Sci 11:119. 10.3390/brainsci11010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Hornung S, Kruayatidee A, Maina KN, Del Rosario I, Paul KC, Wong DY, Duarte Folle A, Markovic D, Palma JA, Serrano GE, Adler CH, Perlman SL, Poon WW, Kang UJ, Alcalay RN, Sklerov M, Gylys KH, Kaufmann H, Fogel BL, Bronstein JM, Ritz B, Bitan G (2021) α-synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol 142:495–511. 10.1007/s00401-021-02324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaun-Panzano J, Arotcarena ML, Bezard E (2023) Monitoring α-synuclein aggregation. Neurobiol Dis 176:105966. 10.1016/j.nbd.2022.105966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi P, Giannandrea D, Biscetti L, Abraha I, Chiasserini D, Orso M, Calabresi P, Parnetti L (2017) Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 32:1389–1400. 10.1002/mds.27110 [DOI] [PubMed] [Google Scholar]
- Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, Joachim C, Esiri M, Evetts SG, Rolinski M, Baig F, Ruffmann C, Wade-Martins R, Hu MTM, Parkkinen L, Green AJE (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3:812–818. 10.1002/acn3.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Gomes B, Farris CM, Ma Y, Concha-Marambio L, Lebovitz R, Nellgård B, Dalla K, Constantinescu J, Constantinescu R, Gobom J, Andreasson U, Zetterberg H, Blennow K (2023) α-Synuclein seed amplification assay as a diagnostic tool for Parkinsonian disorders. Parkinsonism Relat Disord 117:105807. 10.1016/j.parkreldis.2023.105807 [DOI] [PubMed] [Google Scholar]
- Førland MG, Tysnes OB, Aarsland D, Maple-Grødem J, Pedersen KF, Alves G, Lange J (2020) The value of cerebrospinal fluid α-synuclein and the tau/α-synuclein ratio for diagnosis of neurodegenerative disorders with Lewy pathology. Eur J Neurol 27:43–50. 10.1111/ene.14032 [DOI] [PubMed] [Google Scholar]
- Gautam G, Singh H (2024) Biomarkers in dementia research. In: Moradikor N, Chatterjee I, Mohamed W (eds) Nutrition in brain aging and dementia. Springer, Singapore, pp 93–107. 10.1007/978-981-97-4117-5_4
- Gomes FP, Yates JR (2019) Recent trends of capillary electrophoresis-mass spectrometry in proteomics research. Mass Spectrom Rev 38(6):445–460. 10.1002/mas.21599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goolla M, Cheshire WP, Ross OA, Kondru N (2023) Diagnosing multiple system atrophy: current clinical guidance and emerging molecular biomarkers. Front Neurol 14:1210220. 10.3389/fneur.2023.1210220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guévremont D, Roy J, Cutfield NJ, Williams JM (2023) MicroRNAs in Parkinson’s disease: a systematic review and diagnostic accuracy meta-analysis. Sci Rep 13:16272. 10.1038/s41598-023-43096-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Zhou L, Xiong M, Xiong J, Huang J, Li Y, Zhang G, Chen G, Wang Z-H, Xiao T, Hu D, Bao A, Zhang Z (2024) N-homocysteinylation of DJ-1 promotes neurodegeneration in Parkinson’s disease. Aging Cell 23(5):e14124. 10.1111/acel.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Janelidze S, Surova Y, Widner H, Zetterberg H, Hansson O (2018) Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical Parkinsonian disorders. Sci Rep 8(1):13276. 10.1038/s41598-018-31517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K, Swedish BioFINDER Study (2017) Blood-based NfL: a biomarker for differential diagnosis of Parkinsonian disorder. Neurology 88:930–937. 10.1212/WNL.0000000000003680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E (2023) Identifying Lewy body disease before symptoms. JAMA 330:686. 10.1001/jama.2023.13621 [DOI] [PubMed] [Google Scholar]
- Hirschberg Y, Valle-Tamayo N, Dols-Icardo O, Engelborghs S, Buelens B, Vandenbroucke RE, Vermeiren Y, Boonen K, Mertens I (2023) Proteomic comparison between non-purified cerebrospinal fluid and cerebrospinal fluid-derived extracellular vesicles from patients with Alzheimer’s, Parkinson’s and Lewy body dementia. J Extracell Vesicles 12:e12383. 10.1002/jev2.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm H, Gundersen V, Dietrichs E (2023) Vascular parkinsonism. Tidsskr Nor Laegeforen 143. 10.4045/tidsskr.22.0539 [DOI] [PubMed]
- Hong Z, Tian C, Stewart T, Aro P, Soltys D, Bercow M, Sheng L, Borden K, Khrisat T, Pan C, Zabetian CP, Peskind ER, Quinn JF, Montine TJ, Aasly J, Shi M, Zhang J (2021) Development of a sensitive diagnostic assay for Parkinson disease quantifying α-synuclein-containing extracellular vesicles. Neurology 96(18):e2332–e2345. 10.1212/WNL.0000000000011853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Huang C, Zhang Q, Shen T, Sun J (2022) Serum NFL discriminates Parkinson disease from essential tremor and reflect motor and cognition severity. BMC Neurol 22:39. 10.1186/s12883-022-02558-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari E, Holland N, Chelban V, Jones PS, Lamb R, Rawlinson C, Guo T, Costantini AA, Tan MMX, Heslegrave AJ, Roncaroli F, Klein JC, Ansorge O, Allinson KSJ, Jaunmuktane Z, Holton JL, Revesz T, Warner TT, Lees AJ, Zetterberg H, Russell LL, Bocchetta M, Rohrer JD, Williams NM, Grosset DG, Burn DJ, Pavese N, Gerhard A, Kobylecki C, Leigh PN, Church A, Hu MTM, Woodside J, Houlden H, Rowe JB, Morris HR (2020) Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurol 77:377–387. 10.1001/jamaneurol.2019.4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinska M, Meyer-Hoffert U, Kantyka T, Potempa J (2016) Kallikreins—the melting pot of activity and function. Biochimie 122:270–282. 10.1016/j.biochi.2015.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahata I, Fukunaga K (2023) Pathogenic impact of fatty acid-binding proteins in Parkinson’s disease-potential biomarkers and therapeutic targets. Int J Mol Sci 24:17037. 10.3390/ijms242317037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahata I, Sekimori T, Oizumi H, Takeda A, Fukunaga K (2023) Using fatty acid-binding proteins as potential biomarkers to discriminate between Parkinson’s disease and dementia with Lewy bodies: exploration of a novel technique. Int J Mol Sci 24:13267. 10.3390/ijms241713267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Moyeed R, Carroll C, Luo S, Li X (2023) Blood biomarker-based classification study for neurodegenerative diseases. Sci Rep 13:17191. 10.1038/s41598-023-43956-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge A, Iranzo A (2024) Biofluid detection of pathological α-synuclein in the prodromal phase of synucleinopathies. Journal of Parkinson’s Disease 14(s2):S323–S331. 10.3233/JPD-230429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge A, Bunk J, Schaeffer E, Drobny A, Xiang W, Knacke H, Bub S, Lückstädt W, Arnold P, Lucius R, Berg D, Zunke F (2022) Detection of neuron-derived pathological α-synuclein in blood. Brain 145(9):3058–3071. 10.1093/brain/awac115 [DOI] [PubMed] [Google Scholar]
- Koníčková D, Menšíková K, Klíčová K, Chudáčková M, Kaiserová M, Přikrylová H, Otruba P, Nevrlý M, Hluštík P, Hényková E, Kaleta M, Friedecký D, Matěj R, Strnad M, Novák O, Plíhalová L, Rosales R, Colosimo C, Kaňovský P (2023) Cerebrospinal fluid and blood serum biomarkers in neurodegenerative proteinopathies: a prospective, open, cross-correlation study. J Neurochem 167:168–182. 10.1111/jnc.15944 [DOI] [PubMed] [Google Scholar]
- Kosmowska B, Wardas J (2021) The pathophysiology and treatment of essential tremor: the role of adenosine and dopamine receptors in animal models. Biomolecules. 10.3390/biom11121813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gu C, Zhu M, Li D, Chen L, Zhu X (2020a) Correlations between blood lipid, serum cystatin C, and homocysteine levels in patients with Parkinson’s disease. Psychogeriatrics 20:180–188. 10.1111/psyg.12483 [DOI] [PubMed] [Google Scholar]
- Li X, Feng Y, Wang X-X, Truong D, Wu Y-C (2020b) The critical role of SIRT1 in parkinson’s disease: mechanism and therapeutic considerations. Aging Dis 11(6):1608–1622. 10.14336/AD.2020.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang C, Xu W, Chen J, Tuo J, Wen Y, Huang Z, Zeng R (2023) Serum Sirtuin1 level decreases in Parkinson’s disease and vascular parkinsonism: a prospective observational study. Clin Neurol Neurosurg 225:107595. 10.1016/j.clineuro.2023.107595 [DOI] [PubMed] [Google Scholar]
- Lin CH, Chiu SI, Chen TF, Jang JR, Chiu MJ (2020) Classifications of neurodegenerative disorders using a multiplex blood biomarkers-based machine learning model. Int J Mol Sci 21:6914. 10.3390/ijms21186914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Ou R, Li C, Hou Y, Zhang L, Wei Q, Pang D, Liu K, Jiang Q, Yang T, Xiao Y, Zhao B, Chen X, Song W, Yang J, Wu Y, Shang H (2023) Plasma glial fibrillary acidic protein as a biomarker of disease progression in Parkinson’s disease: a prospective cohort study. BMC Med 21(1):420. 10.1186/s12916-023-03120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, Hansson O (2013) Cerebrospinal fluid inflammatory markers in Parkinson’s disease—associations with depression, fatigue, and cognitive impairment. Brain Behav Immun 33:183–189. 10.1016/j.bbi.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Liu Y, Gou M, Guo X (2023) Features of plasma homocysteine, vitamin B12, and folate in Parkinson’s disease: an updated meta-analysis. J Integr Neurosci 22(5):115. 10.31083/j.jin2205115 [DOI] [PubMed] [Google Scholar]
- Luo H, Yu X, Li P, Hu J, Li W, Li X, Chen M, Yu S (2024) Different neurotoxicity and seeding activity between α-synuclein oligomers formed in plasma of patients with Parkinson’s disease and multiple system atrophy. Neuroscience. 10.1016/j.neuroscience.2024.08.006 [DOI] [PubMed] [Google Scholar]
- Ma Z-L, Wang Z-L, Zhang F-Y, Liu H-X, Mao L-H, Yuan L (2024) Biomarkers of Parkinson’s disease: from basic research to clinical practice. Aging Dis 15:1813–1830. 10.14336/AD.2023.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2022) Biomarkers for Parkinson’s disease and neurodegenerative disorders: a role for non-coding RNAs. Curr Neurovasc Res 19(2):127–130. 10.2174/1567202619666220602125806 [DOI] [PubMed] [Google Scholar]
- Majbour NK, Vaikath NN, van Dijk KD, Ardah MT, Varghese S, Vesterager LB, Montezinho LP, Poole S, Safieh-Garabedian B, Tokuda T, Teunissen CE, Berendse HW, van de Berg WDJ, El-Agnaf OMA (2016) Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson’s disease. Mol Neurodegener 11:7. 10.1186/s13024-016-0072-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majbour NK, Aasly JO, Hustad E, Thomas MA, Vaikath NN, Elkum N, van de Berg WDJ, Tokuda T, Mollenhauer B, Berendse HW, El-Agnaf OMA (2020) CSF total and oligomeric α-Synuclein along with TNF-α as risk biomarkers for Parkinson’s disease: a study in LRRK2 mutation carriers. Transl Neurodegener 9(1):15. 10.1186/s40035-020-00192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makey DM, Gadkari VV, Kennedy RT, Ruotolo BT (2024) Cyclic ion mobility-mass spectrometry and tandem collision induced unfolding for quantification of elusive protein biomarkers. Anal Chem 96(15):6021–6029. 10.1021/acs.analchem.4c00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Valbuena I, Visanji NP, Kim A, Lau HHC, So RWL, Alshimemeri S, Gao A, Seidman MA, Luquin MR, Watts JC, Lang AE, Kovacs GG (2022) Alpha-synuclein seeding shows a wide heterogeneity in multiple system atrophy. Transl Neurodegener 11:7. 10.1186/s40035-022-00283-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroudis I, Chowdhury R, Petridis F, Karantali E, Chatzikonstantinou S, Balmus IM, Luca IS, Ciobica A, Kazis D (2021) YKL-40 as a potential biomarker for the differential diagnosis of Alzheimer’s disease. Medicina (Kaunas). 10.3390/medicina58010060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni M, Agliardi C, Guerini FR, Zanzottera M, Bolognesi E, Picciolini S, Marano M, Magliozzi A, Di Fonzo A, Arighi A, Fenoglio C, Franco G, Arienti F, Saibene FL, Navarro J, Clerici M (2023) Oligomeric α-synuclein and tau aggregates in NDEVs differentiate Parkinson’s disease from atypical Parkinsonisms. Neurobiol Dis 176:105947. 10.1016/j.nbd.2022.105947 [DOI] [PubMed] [Google Scholar]
- Miglis MG, Muppidi S (2020) Synuclein in red blood cells: a potential biomarker for multiple system atrophy, and other updates on recent autonomic research. Clin Auton Res 30(2):107–109. 10.1007/s10286-020-00680-7 [DOI] [PubMed] [Google Scholar]
- Morenas-Rodríguez E, Alcolea D, Suárez-Calvet M, Muñoz-Llahuna L, Vilaplana E, Sala I, Subirana A, Querol-Vilaseca M, Carmona-Iragui M, Illán-Gala I, Ribosa-Nogué R, Blesa R, Haass C, Fortea J, Lleó A (2019) Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci Rep 9(1):7803. 10.1038/s41598-019-44173-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier EE, Rothhammer V, Linnerbauer M (2023) The role of midkine in health and disease. Front Immunol 14:1310094. 10.3389/fimmu.2023.1310094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuzumi A, Hatano T, Matsumoto G, Nojiri S, Ueno SI, Imamichi-Tatano Y, Kimura H, Kakuta S, Kondo A, Fukuhara T, Li Y, Funayama M, Saiki S, Taniguchi D, Tsunemi T, McIntyre D, Gérardy JJ, Mittelbronn M, Kruger R, Uchiyama Y, Nukina N, Hattori N (2023) Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat Med 29:1448–1455. 10.1038/s41591-023-02358-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painous C, Fernández M, Pérez J, de Mena L, Cámara A, Compta Y (2024) Fluid and tissue biomarkers in Parkinson’s disease: immunodetection or seed amplification? Central or peripheral? Parkinsonism Relat Disord 121:105968. 10.1016/j.parkreldis.2023.105968 [DOI] [PubMed] [Google Scholar]
- Paolini Paoletti F, Gaetani L, Bellomo G, Chipi E, Salvadori N, Montanucci C, Mancini A, Filidei M, Nigro P, Simoni S, Tambasco N, Di Filippo M, Parnetti L (2023) CSF neurochemical profile and cognitive changes in Parkinson’s disease with mild cognitive impairment. NPJ Parkinson’s Dis 9(1):68. 10.1038/s41531-023-00509-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, Calabresi P (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18:573–586. 10.1016/S1474-4422(19)30024-9 [DOI] [PubMed] [Google Scholar]
- Paslawski W, Khosousi S, Hertz E, Markaki I, Boxer A, Svenningsson P (2023) Large-scale proximity extension assay reveals CSF midkine and DOPA decarboxylase as supportive diagnostic biomarkers for Parkinson’s disease. Transl Neurodegener 12:42. 10.1186/s40035-023-00374-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Kumar A, Hall S, Palmqvist S, Stomrud E, Bali D, Parchi P, Mattsson-Carlgren N, Janelidze S, Hansson O (2023) DOPA decarboxylase is an emerging biomarker for Parkinsonian disorders including preclinical Lewy body disease. Nat Aging 3:1201–1209. 10.1038/s43587-023-00478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero-Gascon R, Benavente F, Minic Z, Berezovski MV, Sanz-Nebot V (2020) On-line aptamer affinity solid-phase extraction capillary electrophoresis-mass spectrometry for the analysis of blood α-synuclein. Anal Chem 92(1):1525–1533. 10.1021/acs.analchem.9b04802 [DOI] [PubMed] [Google Scholar]
- Qiu X, Xiao Y, Wu J, Gan L, Huang Y, Wang J (2019) C-Reactive protein and risk of Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 10:384. 10.3389/fneur.2019.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadalti C, Calandra-Buonaura G, Baiardi S, Mastrangelo A, Rossi M, Zenesini C, Giannini G, Candelise N, Sambati L, Polischi B, Plazzi G, Capellari S, Cortelli P, Parchi P (2021) Neurofilament light chain and alpha-synuclein RT-QuIC as differential diagnostic biomarkers in Parkinsonisms and related syndromes. NPJ Parkinsons Dis 7:93. 10.1038/s41531-021-00232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim H, Pont L, Giménez E, Benavente F (2023) On-line aptamer affinity solid-phase extraction direct mass spectrometry for the rapid analysis of α-synuclein in blood. Anal Chim Acta 1256:341149. 10.1016/j.aca.2023.341149 [DOI] [PubMed] [Google Scholar]
- Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C, Mollenhauer B, Soto C (2017) Development of a biochemical diagnosis of Parkinson disease by detection of alpha-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 74:163–172. 10.1001/jamaneurol.2016.4547 [DOI] [PubMed] [Google Scholar]
- Shahnawaz M, Mukherjee A, Pritzkow S, Mendez N, Rabadia P, Liu X, Hu B, Schmeichel A, Singer W, Wu G, Tsai AL, Shirani H, Nilsson KPR, Low PA, Soto C (2020) Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578:273–277. 10.1038/s41586-020-1984-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Tiwari M, Tiwari RK (2015) Hyperhomocysteinemia: impact on neurodegenerative diseases. Basic Clin Pharmacol Toxicol 117(5):287–296. 10.1111/bcpt.12424 [DOI] [PubMed] [Google Scholar]
- Shim KH, Kang MJ, Youn YC, An SSA, Kim S (2022) Alpha-synuclein: a pathological factor with Abeta and tau and biomarker in Alzheimer’s disease. Alzheimers Res Ther 14:201. 10.1186/s13195-022-01150-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LMA, Hutten SJ, Sherer T, Marek K, Soto C, Parkinson’s Progression Markers Initiative (2023) Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol 22:407–417. 10.1016/S1474-4422(23)00109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AM, Hickford ES, Cutler P (2024) An immunoassay for the quantification of phosphorylated α-synuclein at serine 129 in human cerebrospinal fluid. Bioanalysis. 10.1080/17576180.2024.2407718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MS, Vidal M, Santos NF, Costa FM, Marques C, Pereira SO, Leitão C (2021) Immunosensing based on optical fiber technology: recent advances. Biosensors (Basel). 10.3390/bios11090305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IU, Kim JS, Park IS, Kim YD, Cho HJ, Chung SW, Lee KS (2013) Clinical significance of homocysteine (hcy) on dementia in Parkinson’s disease (PD). Arch Gerontol Geriatr 57:288–291. 10.1016/j.archger.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Stoker TB, Greenland JC (2018) Parkinson’s disease: pathogenesis and clinical aspects. Codon Publications, Brisbane [PubMed] [Google Scholar]
- Sung YJ, Yang C, Norton J, Johnson M, Fagan A, Bateman RJ, Perrin RJ, Morris JC, Farlow MR, Chhatwal JP, Schofield PR, Chui H, Wang F, Novotny B, Eteleeb A, Karch C, Schindler SE, Rhinn H, Johnson ECB, Oh HSH, Rutledge JE, Dammer EB, Seyfried NT, Wyss-Coray T, Harari O, Cruchaga C (2023) Proteomics of brain, CSF, and plasma identifies molecular signatures for distinguishing sporadic and genetic Alzheimer’s disease. Sci Transl Med 15:eabq5923. 10.1126/scitranslmed.abq5923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha HB (2023) Rethinking the reliability and accuracy of biomarkers in CNS-originating EVs for Parkinson’s disease and multiple system atrophy. Front Neurol 14:1192115. 10.3389/fneur.2023.1192115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha HB, Bogoniewski A (2024) Analysis of biomarkers in speculative CNS-enriched extracellular vesicles for parkinsonian disorders: a comprehensive systematic review and diagnostic meta-analysis. J Neurol 271:1680–1706. 10.1007/s00415-023-12093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha HB, Hornung S, Dutta S, Fenwick L, Lahgui O, Howe K, Elabed N, Del Rosario I, Wong DY, Duarte Folle A, Markovic D, Palma JA, Kang UJ, Alcalay RN, Sklerov M, Kaufmann H, Fogel BL, Bronstein JM, Ritz B, Bitan G (2023) Toward a biomarker panel measured in CNS-originating extracellular vesicles for improved differential diagnosis of Parkinson’s disease and multiple system atrophy. Transl Neurodegener 12:14. 10.1186/s40035-023-00346-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Han L, Li S, Hu T, Xu Z, Fan Y, Liang X, Yu H, Wu J, Wang J (2023) Plasma GFAP in Parkinson’s disease with cognitive impairment and its potential to predict conversion to dementia. NPJ Parkinson’s Disease 9(1):23. 10.1038/s41531-023-00447-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK (2022) Initiation and progression of α-synuclein pathology in Parkinson’s disease. Cell Mol Life Sci 79:210. 10.1007/s00018-022-04240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SAS, Kasai T, Ishigami N, Tamaoka A, Nakagawa M, El-Agnaf OMA (2010) Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75(20):1766–1772. 10.1212/WNL.0b013e3181fd613b [DOI] [PubMed] [Google Scholar]
- Tolosa E, Garrido A, Scholz SW, Poewe W (2021) Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol 20:385–397. 10.1016/S1474-4422(21)00030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H-H, Huang C-G, Wu Y-R (2022) Detection and assessment of alpha-synuclein in Parkinson disease. Neurochem Int 158:105358. 10.1016/j.neuint.2022.105358 [DOI] [PubMed] [Google Scholar]
- Umemoto A, Yagi H, So M, Goto Y (2014) High-throughput analysis of ultrasonication-forced amyloid fibrillation reveals the mechanism underlying the large fluctuation in the lag time. J Biol Chem 289:27290–27299. 10.1074/jbc.M114.569814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallelunga A, Ragusa M, Di Mauro S, Iannitti T, Pilleri M, Biundo R, Weis L, Di Pietro C, De Iuliis A, Nicoletti A, Zappia M, Purrello M, Antonini A (2014) Identification of circulating microRNAs for the differential diagnosis of Parkinson’s disease and multiple system atrophy. Front Cell Neurosci 8:156. 10.3389/fncel.2014.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wamelen DJ, Taddei RN, Calvano A, Titova N, Leta V, Shtuchniy I, Jenner P, Martinez-Martin P, Katunina E, Chaudhuri KR (2020) Serum uric acid levels and non-motor symptoms in Parkinson’s disease. J Parkinsons Dis 10:1003–1010. 10.3233/JPD-201988 [DOI] [PubMed] [Google Scholar]
- Valeriia O, Kuzyk Govert W, Somsen Rob, Haselberg Ana Valéria, Colnaghi Simionato (2021) Separation Techniques Applied to Omics Sciences From Principles to Relevant Applications CE-MS for Proteomics and Intact Protein Analysis Springer International Publishing Cham 51–86
- Villar-Menéndez I, Porta S, Buira SP, Pereira-Veiga T, Díaz-Sánchez S, Albasanz JL, Ferrer I, Martín M, Barrachina M (2014) Increased striatal adenosine A2a receptor levels is an early event in Parkinson’s disease-related pathology and it is potentially regulated by miR-34b. Neurobiol Dis 69:206–214. 10.1016/j.nbd.2014.05.030 [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, Srulijes K, Trojanowski JQ, Lee VMY, Siderowf AD, Hurtig H, Litvan I, Schiess MC, Peskind ER, Masuda M, Hasegawa M, Lin X, Pan C, Galasko D, Goldstein DS, Jensen PH, Yang H, Cain KC, Zhang J (2012) Phosphorylated α-synuclein in Parkinson’s disease. Sci Transl Med 4:121ra20. 10.1126/scitranslmed.3002566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Cong S, Tan X, Ma S, Liu Y, Wang H, Cong S (2022) A meta-analysis of the diagnostic utility of biomarkers in cerebrospinal fluid in Parkinson’s disease. NPJ Parkinson’s Dis 8(1):165. 10.1038/s41531-022-00431-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Jiang C, Janzen A, Barber TR, Seger A, Sommerauer M, Davis JJ, Marek K, Hu MT, Oertel WH, Tofaris GK (2024) Neuronally derived extracellular vesicle α-synuclein as a serum biomarker for individuals at risk of developing Parkinson disease. JAMA Neurol 81:59–68. 10.1001/jamaneurol.2023.4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SW, Ha S, Lyoo CH, Kim Y, Yoo JY, Kim JS (2023) Exploring the link between essential tremor and Parkinson’s disease. NPJ Parkinsons Dis 9:134. 10.1038/s41531-023-00577-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Liu G, Li Y, Arkin E, Zheng Y, Feng T (2022) Erythrocytic alpha-synuclein species for Parkinson’s disease diagnosis and the correlations with clinical characteristics. Front Aging Neurosci 14:827493. 10.3389/fnagi.2022.827493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Liu G, Zheng Y, Huang G, Feng T (2023) Erythrocytic alpha-synuclein as potential biomarker for the differentiation between essential tremor and Parkinson’s disease. Front Neurol 14:1173074. 10.3389/fneur.2023.1173074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Li Y, Niu M, Li G, Luo N, Zhou L, Kang W, Liu J (2020) SNCA hypomethylation in rapid eye movement sleep behavior disorder is a potential biomarker for Parkinson’s disease. J Parkinsons Dis 10:1023–1031. 10.3233/JPD-201912 [DOI] [PubMed] [Google Scholar]
- Zhou L (2024) Homocysteine and Parkinson’s disease. CNS Neurosci Ther 30(2):e14420. 10.1111/cns.14420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubelzu M, Morera-Herreras T, Irastorza G, Gómez-Esteban JC, Murueta-Goyena A (2022) Plasma and serum alpha-synuclein as a biomarker in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord 99:107–115. 10.1016/j.parkreldis.2022.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.