Abstract
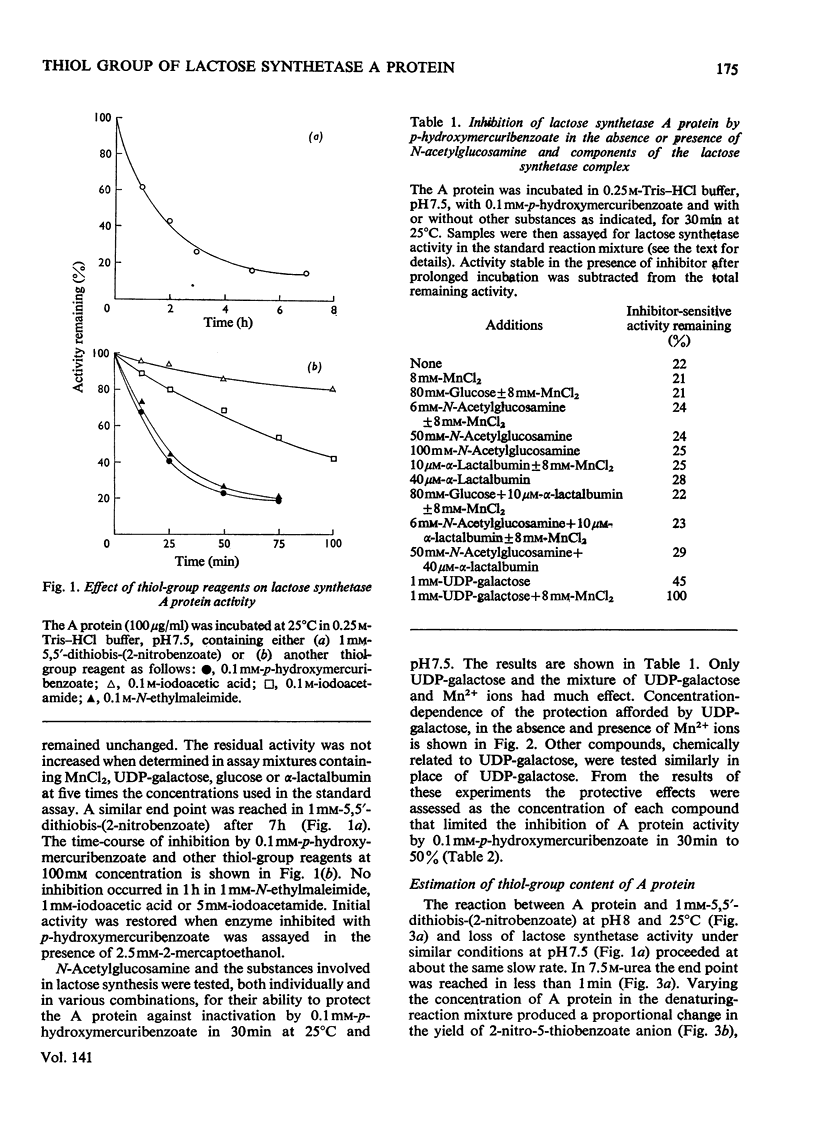
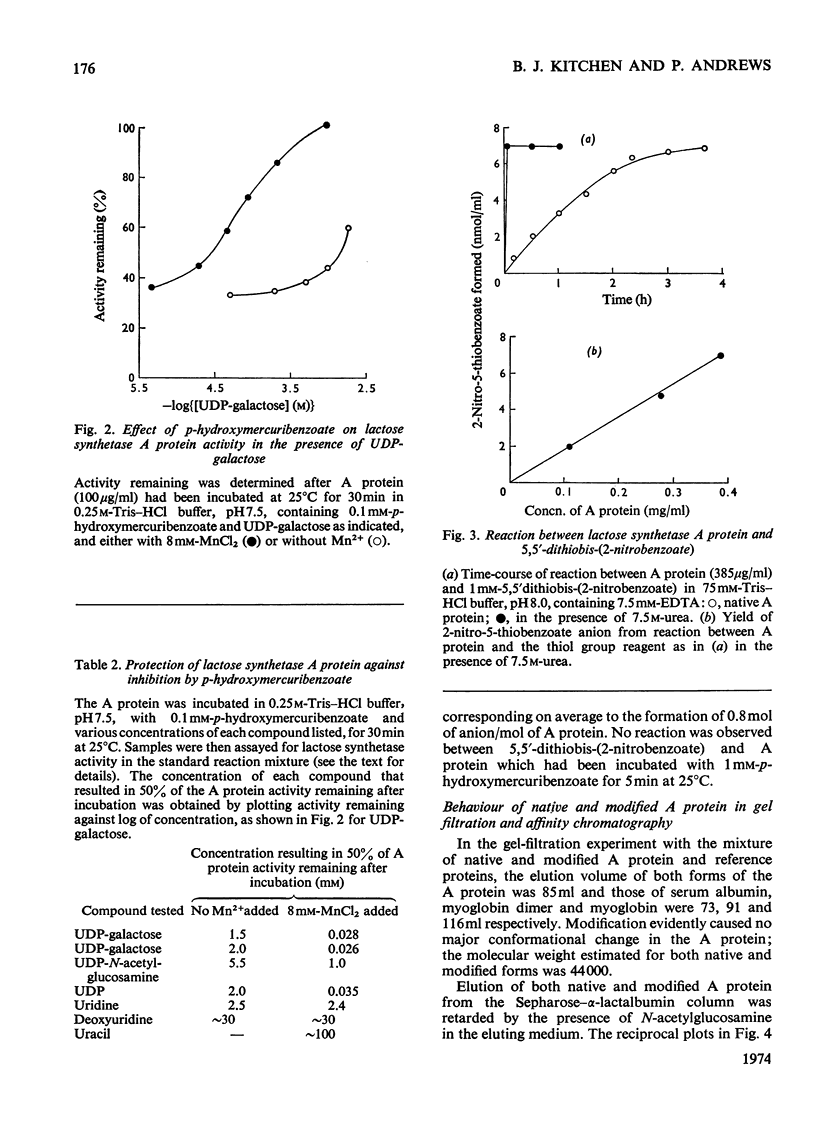
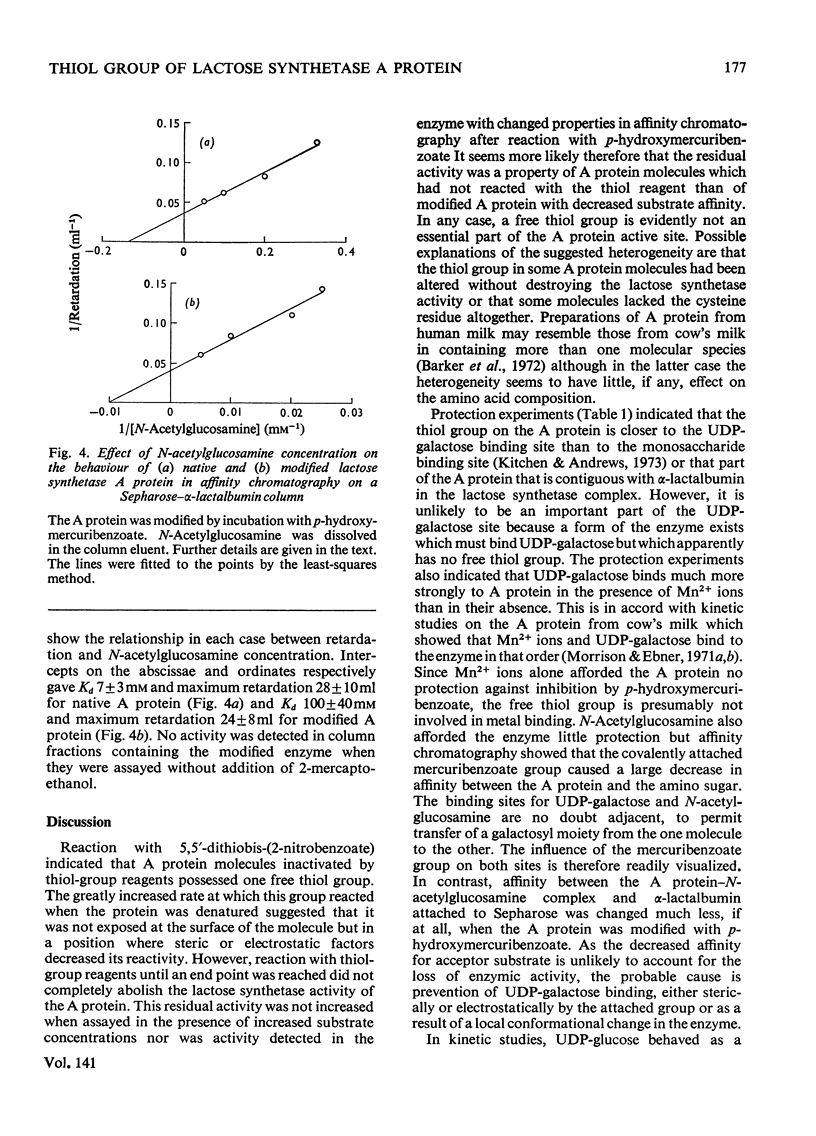
The lactose synthetase activity of A protein from human milk was much decreased but not abolished by reaction with thiol-group reagents. Protection experiments indicated that a free thiol group on the enzyme is situated near the UDP-galactose binding site and inactivation of the enzyme with p-hydroxymercuribenzoate was probably due to prevention of UDP-galactose binding. Affinity chromatography showed that the mercuribenzoate substituent also decreased the affinity of A protein for N-acetylglucosamine but complex-formation between A protein–N-acetylglucosamine and α-lactalbumin was relatively unaffected. UDP-galactose appears to be bound to the enzyme mainly through its pyrophosphate group with Mn2+ ion and through the cis hydroxyls of ribose, whereas its hexose moiety has little if any affinity for the enzyme. Lactose synthetase activity remaining after the reaction with thiol-group reagents indicates that a free thiol group is not an essential part of the A protein active site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Andrews P., Kitchen B. J., Winzor D. J. Use of affinity chromatography for the quantitative study of acceptor-ligand interactions: The lactose synthetase system. Biochem J. 1973 Dec;135(4):897–900. doi: 10.1042/bj1350897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Purification of lactose synthetase a protein from human milk and demonstration of its interaction with alpha-lactalbumin. FEBS Lett. 1970 Aug 31;9(5):297–300. doi: 10.1016/0014-5793(70)80382-9. [DOI] [PubMed] [Google Scholar]
- Babad H., Hassid W. Z. Soluble uridine diphosphate D-galactose: D-glucose beta-4-D-galactosyltransferase from bovine milk. J Biol Chem. 1966 Jun 10;241(11):2672–2678. [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Barman T. E. Purification and properties of bovine milk glyco-alpha-lactalbumin. Biochim Biophys Acta. 1970 Jul 27;214(1):242–244. doi: 10.1016/0005-2795(70)90094-2. [DOI] [PubMed] [Google Scholar]
- Brew K. Lactose synthetase: evolutionary origins, structure and control. Essays Biochem. 1970;6:93–118. [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck U., Denton W. L., Tanahashi N., Ebner K. E. The isolation and identification of the B protein of lactose synthetase as alpha-lactalbumin. J Biol Chem. 1967 Apr 10;242(7):1391–1397. [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. Resolution of a soluble lactose synthetase into two protein components and solubilization of microsomal lactose synthetase. J Biol Chem. 1966 Feb 10;241(3):762–764. [PubMed] [Google Scholar]
- COHN M., HUGHES T. R., Jr Nuclear magnetic resonance spectra of adenosine di- and triphosphate. II. Effect of complexing with divalent metal ions. J Biol Chem. 1962 Jan;237:176–181. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. K., Brodbeck U., Kiyosawa I., Mawal R., Colvin B., Ebner K. E. Alpha-lactalbumin and the lactose synthetase reaction. J Biol Chem. 1970 Apr 25;245(8):2103–2108. [PubMed] [Google Scholar]
- Fitzgerald D. K., McKenzie L., Ebner K. E. Galactosyl transferase activity in a variety of sources. Biochim Biophys Acta. 1971 Jun 16;235(3):425–428. doi: 10.1016/0005-2744(71)90282-8. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Klee C. B. The role of alpha-lactalbumin in lactose synthetase. Biochem Biophys Res Commun. 1970 Jun 5;39(5):833–841. doi: 10.1016/0006-291x(70)90398-0. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic investigations with N-acetylglucosamine as the galactosyl group acceptor. J Biol Chem. 1971 Jun 25;246(12):3977–3984. [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic investigations with glucose as the galactosyl group acceptor. J Biol Chem. 1971 Jun 25;246(12):3985–3991. [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]


