Abstract
Abstract
Chiral diaryl alcohols, such as (4-chlorophenyl)(pyridin-2-yl)methanol, are important intermediates for pharmaceutical synthesis. However, using alcohol dehydrogenases (ADHs) in the asymmetric reduction of diaryl ketones to produce the corresponding alcohols is challenging due to steric hindrance in the substrate binding pockets of the enzymes. In this study, the steric hindrance of the ADH from Geotrichum candidum NBRC 4597 (G. candidum acetophenone reductase, GcAPRD) was eliminated by simultaneous site-directed mutagenesis of Phe56 (in the large pocket) and Trp288 (in the small pocket). As a result, two double mutants, Phe56Ile/Trp288Ala, and Phe56Ala/Trp288Ala, exhibited much higher specific activities towards 2-(4′-chlorobenzoyl)pyridine (4.5 μmol/min/mg and 3.4 μmol/min/mg, respectively) than the wild type (< 0.2 μmol/min/mg). In whole-cell-catalyzed asymmetric reductions of diaryl ketones, Phe56Ile/Trp288Ala significantly increased the isolated yields, which were over 90% for the reactions of most of the tested substrates. Regarding enantioselectivity, Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala, and Trp288Ala generally exhibited similar selectivity to produce (R)-alcohols with up to 97% ee.
Key points
• Phe56 in Geotrichum reductase (GcAPRD) was mutated to eliminate steric hindrance.
• Mutation at Phe56 increased enzymatic activity and expanded substrate specificity.
• Phe56Ile/Trp288Ala showed high activity and (R)-selectivity towards diaryl ketones.
Graphical Abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s00253-024-13375-0.
Keywords: Alcohol dehydrogenase, Asymmetric reduction, Diaryl ketone, Chiral diaryl alcohol, Site-directed mutagenesis, Enzyme engineering
Introduction
Enantiopure alcohols, such as diaryl alcohols, are important intermediates for pharmaceutical synthesis. Among these alcohols, (S)-phenyl(pyridin-2-yl)methanol has analgesic and anticonvulsant properties (Li et al. 2021; Özdemir and Şahin 2024); (S)-(4-chlorophenyl)(pyridin-2-yl)methanol is an important intermediate for the synthesis of antihistamines, bepotastine (Chen et al. 2021; Li et al. 2021) and carbinoxamine (Xu et al. 2020; Li et al. 2021). Using alcohol dehydrogenases (ADHs), which belong to the large family of NAD(P)H-dependent oxidoreductases, in the asymmetric reduction of ketones is an environmentally friendly and straightforward method to produce enantiopure alcohols (Hou et al. 2020; Koesoema et al. 2020b; Hall 2021; Sardauna et al. 2023). Now biocatalytic asymmetric reduction of ketones by ADHs has attracted increasing attention as an alternative to chemical synthesis, which typically uses expensive metal catalysts or resolving agents. However, due to steric hindrance in substrate binding pockets of ADHs, their application in the reduction of diaryl ketones is often obstructed by low activity (Hou et al. 2020; Wu et al. 2020). The similarity in the size of the two aryl groups also makes the asymmetric reduction challenging, since they are difficult to be discriminated by the binding pockets (Wang et al. 2018; Xu et al. 2020). As a result, only a few ADHs have been demonstrated to catalyze the reduction of diaryl ketones, such as ADH from Candida glabrata (CgADH) (Sun et al. 2022), ADH from Lactobacillus kefiri (LkADH) (Wu et al. 2020, 2021), and ADH from Kluyveromyces polyspora (KpADH) (Xu et al. 2018, 2020; Zhang et al. 2022, 2024a). However, these ADHs belong to the short-chain dehydrogenase/reductase (SDR) family (Sun et al. 2022; Shanbhag 2023; Yuan et al. 2024). To the best of our present knowledge, using an ADH from medium-chain dehydrogenase/reductase (MDR) family to catalyze the reduction of diaryl ketones has been less studied, and the related studies mainly focus on ADH from Thermoanaerobacter brockii (TbSADH) (Liu et al. 2019; Qu et al. 2019, 2022; Chen et al. 2021; Jiang et al. 2022).
To develop a variety of biocatalysts for the reduction of challenging diaryl ketones, further studies on enzyme mutagenesis are required to eliminate steric hindrance. An ADH from Geotrichum candidum NBRC 4597 (G. candidum acetophenone reductase, GcAPRD, PDB ID: 6ISV) belonging to the MDR family is a suitable target since it has been reported to present high thermostability (Nakata et al. 2010; Yamamoto et al. 2013; T.sriwong et al. 2020; T.sriwong et al. 2022) and non-aqueous solvent tolerance (Nakamura et al. 1999; Yamamoto et al. 2013). Furthermore, following Prelog’s rule, this enzyme exhibited excellent (S)-enantioselectivity in the asymmetric reduction of various ketones, consistent with observations from other Prelog ADH-catalyzed reductions (Prelog 1964; Koesoema et al. 2019a, b, 2020a, b, c; Shanbhag 2023; Zhang et al. 2024b), for example, ee > 99% (S) for the reduction of 3-hexanone (Koesoema et al. 2019b). GcAPRD has small and large pockets in the substrate binding site (Fig. 1), and steric hindrance in the small pocket can be eliminated by mutating Trp288 to Ala, Val, or other small amino acids (Koesoema et al. 2019a). Then, the Trp288 mutants exhibited expanded substrate specificity towards aliphatic ketones and altered enantioselectivity (Koesoema et al. 2019a, 2020b). For example, Trp288Val was able to catalyze the reduction of 4-octanone (propyl butyl ketone) (ee 87% (R)), which was not catalyzed by the wild type (Koesoema et al. 2019a, 2020b).
Fig. 1.
Mutants constructed and substrates/products used in this study. aThe absolute configuration (S/R) is determined by the Cahn–Ingold–Prelog priority rule. Therefore, when the phenyl group enters the large pocket, and the methyl or pyridyl group enters the small pocket, (S)-1b-(S)-11b and (R)-12b-(R)-19b are formed, respectively
To investigate the reduction of diaryl ketones by GcAPRD, mutagenesis targeting the amino acids in the large pocket is necessary for expanding substrate specificity and improving activity. Based on the crystal structure of GcAPRD, the large pocket is composed of Ser47, His50, Ile51, Phe56, His66, Asn119, Leu122, and Leu264 (Koesoema et al. 2019b). Among these residues, Phe56 is located at the entrance of the large pocket. In this study, Phe56 was mutated to smaller amino acids based on preliminary docking simulation studies using diphenyl ketone as a substrate. First, four GcAPRD Phe56 single mutants (Phe56Ala, Phe56Val, Phe56Ile, and Phe56His) were constructed, considering the Van der Waals volumes and hydrophobicity of the amino acids, and were used for the reduction of acetophenone and its analogs (1a-11a) (Fig. 1). As a result, Phe56Ile demonstrated high activity and enantioselectivity (ee 99% (S)). Next, the double mutants Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala were constructed, and these double mutants and single mutants were used for the reduction of 2-benzoylpyridine analogs (12a-19a) (Fig. 1). As a result, improved activities towards diaryl ketones were observed, especially in the case of Phe56Ile/Trp288Ala. Regarding enantioselectivity, the two double mutants and Trp288Ala exhibited similar (R)-enantioselectivity to produce alcohols with up to 97% ee.
Materials and methods
Chemicals
Most chemicals are purchased from Nacalai Tesque (Kyoto, Japan), Wako (Osaka, Japan), Tokyo Chemical Industry (Tokyo, Japan), Bio-Rad (California, USA), and Sigma-Aldrich (St. Louis, USA). Primers for inverse PCR were obtained from Toyobo (Osaka, Japan) and Thermo Fisher Scientific (Tokyo, Japan). Substrates 1a-12a and 18a were purchased from Nacalai Tesque (Kyoto, Japan), while other substrates 13a-17a and 19a were synthesized by Grignard reactions following the literature method (Tao et al. 2012). Regarding racemic alcohol standards, rac-1b was purchased from Nacalai Tesque (Kyoto, Japan), while rac-2b and rac-6b-11b were previously prepared by our group (Koesoema et al. 2020a). Other racemic alcohol standards, rac-3b-5b and rac-12b-19b, were synthesized by sodium borohydride reduction of the corresponding ketones. Detailed information about the synthesis of 13a-17a, 19a, rac-3b-5b, and rac-12b-19b is provided in the Supplementary information (Sects. 1 and 2).
Preparation of GcAPRD mutants by site-directed mutagenesis
GcAPRD wild type and Trp288Ala were prepared according to previously reported procedures (Yamamoto et al. 2013; Koesoema et al. 2019a). Using the pET-21b (+)-GcAPRD-His or pET-21b (+)-GcAPRD-His-Trp288Ala plasmid prepared in the previous study as templates, the plasmids for GcAPRD Phe56 mutants and double mutants were constructed by inverse PCR as reported previously (Koesoema et al. 2019a). The primer sequences are listed in Supplementary Table 1. Transformation of the resulting plasmids into Escherichia coli Rosetta™(DE3)pLysS (Novagen, USA), cultivation of the E. coli, overexpression of the enzyme using isopropyl β-d-1-thiogalactopyranoside (IPTG) induction, preparation of the cell-free extract, and enzyme purification were done as reported previously (Koesoema et al. 2019b). The protein concentration was measured by the Bradford method using bovine serum albumin (BSA) as the standard. A standard curve was made using different concentrations of BSA (0.125, 0.25, and 0.5 mg/mL). One milliliter of Bradford reagent was added to 20 μL of purified enzyme solution. The absorbance at 595 nm was measured after incubating the mixture at room temperature for 3 min. The SDS-PAGE of the mutants is shown in Supplementary Fig. 1.
Enzymatic activity assays
All enzymatic assays were performed at 37 °C in triplicate by measuring the absorbance at 340 nm for 132 s using a UV–Vis spectrophotometer (UV-1900, Shimadzu, Japan) to determine the decrease in NADH concentration during ketone reduction. Activity was measured in 100 mM HEPES–NaOH buffer (pH 7.2, 1.0 mL) containing 1a-11a (1.25 mM) or 12a-19a (1 mM), NADH (0.3 mM), and purified enzyme (0.2–13.7 μg).
Analytical-scale asymmetric reduction of 1a-11a by GcAPRD Phe56Ile
Analytical-scale reductions of 1a-11a were performed in HEPES–NaOH buffer (100 mM pH 7.2, 3.0 mL) consisting of NAD+ (0.2 mM), 2-propanol (15% v/v), 1a-11a (10 mM), and purified GcAPRD Phe56Ile (the amount of enzyme was calculated from the relative activity to achieve initial reaction rate of 3 µmol/min). Reductions were done at 30 °C with shaking at 200 rpm for 3 h. 270 μL of the reaction mixture was extracted with diethyl ether for conversion and enantioselectivity excess (ee) determination by chiral GC analysis with a flame ionization detector (GC-14B, Shimadzu, Japan) equipped with a chiral column (CP-Chirasil-Dex-CB, 0.32 mm × 0.25 μm × 50 m, Agilent, USA). The GC analysis conditions and retention times of the ketones, as well as R and S enantiomers, are listed in Supplementary Table 2.
Preparative-scale asymmetric reduction of 5a by GcAPRD Phe56Ile
The preparative-scale reduction was performed in HEPES–NaOH buffer (100 mM pH 7.2, 50 mL) consisting of NAD+ (46 mg), 2-propanol (1.5 mL), 5a (52.2 mg, 0.34 mmol), and purified GcAPRD Phe56Ile (15.17 mg/mL, 135 μL) at 30 °C with shaking at 200 rpm for 18 h. The product was extracted with diethyl ether three times, dried over MgSO4, and evaporated under reduced pressure. Silica gel column chromatography (hexane: ethyl acetate, 4:1) was performed to give (S)-5b. The product was characterized by 1H-NMR analysis, and ee was determined by chiral GC analysis. The absolute configuration was determined by comparing the optical rotation sign of the product with that reported in the literature. The 1H-NMR spectrum matched those reported in the literature (Liu et al. 2021). Detailed information is provided in the Supplementary information (Sect. 3).
Asymmetric reduction of diaryl ketones by GcAPRD Trp288Ala, Phe56Ile/Trp288Ala, and Phe56Ala/Trp288Ala
Reductions of 12a-19a were performed in HEPES–NaOH buffer (100 mM pH 7.2, 3.0 mL) consisting of 2-propanol (15% v/v for Trp288Ala and Phe56Ile/Trp288Ala or 5% v/v for Phe56Ala/Trp288Ala), a substrate (10.1–16.7 mM), and whole cells (0.5 g wet weight) at 30 °C with a shaking speed of 250 rpm for 3 h. The products were extracted with diethyl ether three times and evaporated under reduced pressure. Silica gel column chromatography (hexane: ethyl acetate, 2:1) was performed to give the corresponding products, 12b-19b. The products were characterized by 1H-NMR analysis, and ee was determined by chiral HPLC analysis (LC-20AD with SPD-20A UV–Vis Detector, Shimadzu, Japan), equipped with a chiral column (CHIRALPAKⓇ IA-3, 4.6 mm × 3 μm × 250 mm, Daicel, Japan). The HPLC analysis conditions and the retention times of the R and S enantiomers are listed in Supplementary Table 3. The 1H-NMR spectra were in agreement with those reported in the literature for 12b-16b (Nian et al. 2019), 17b (Liu et al. 2019), and 18b-19b (Nian et al. 2019). Detailed information is provided in the Supplementary information (Sect. 4).
Results
Activity of GcAPRD Phe56 mutants towards acetophenone and its analogs
Phe56 in the large pocket in the GcAPRD substrate binding site was mutated to smaller amino acids, Ala, Val, Ile, and His, to eliminate the steric hindrance. Thus, single mutants (Phe56Ala, Phe56Val, Phe56Ile, and Phe56His) were constructed, overexpressed in E. coli, and purified, and their reduction activities towards acetophenone 1a as a representative substrate was investigated. As listed in Table 1, among the tested enzymes, Phe56Ile exhibited the highest specific activity (48.8 μmol/min/mg) towards 1a. Based on this result, Phe56Ile was selected as a promising candidate for the subsequent substrate specificity study.
Table 1.
The specific activity of GcAPRD wild type and Phe56 mutants towards 1a
| Enzyme | Wild typea | Phe56Ala | Phe56Val | Phe56Ile | Phe56His |
|---|---|---|---|---|---|
| Specific activity towards 1a (μmol/min/mg) | 22.2 | 7.2 | 27.6 | 48.8 | 10.5 |
aThe preparative-scale reduction of 1a by the wild type was published previously, but not those for the relative activity
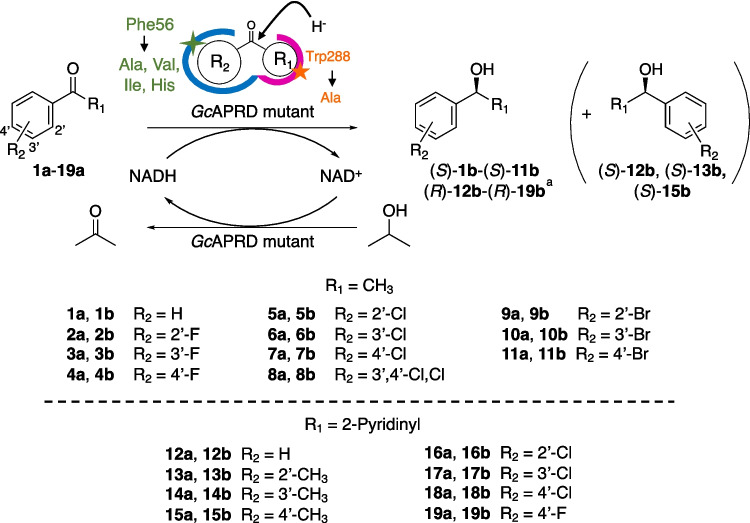
Then, the reduction activity of Phe56Ile towards 2a-11a was investigated. The specific activity towards 1a was set to 100% to calculate the relative activities towards 2a-11a (Fig. 2). Phe56Ile exhibited improved activities on all the tested substrates compared to the wild type. Moreover, Phe56Ile exhibited a correlation between the position of the halogenated substituent and the activity; significantly increased activities of the 2′-chloro- or 2′-bromo-substituted compounds, 5a and 9a, compared to 1a were observed (Supplementary Table 4).
Fig. 2.
The relative activity of (A) wild typea and (B) Phe56Ile towards 1a-11a. aThe preparative-scale reduction of these substrates by the wild type were published previously, but not those for the relative activity
Asymmetric reduction of acetophenone and its analogs by Phe56Ile
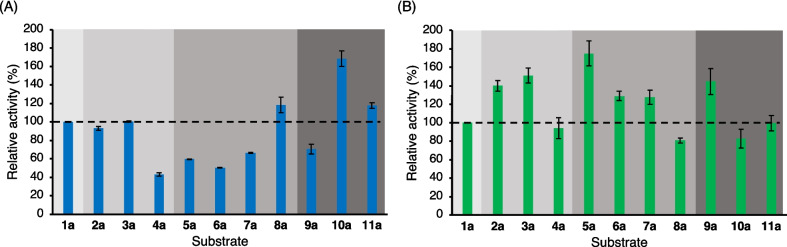
Following the analysis of activity, asymmetric reductions of 1a-11a on an analytical-scale by purified Phe56Ile were performed to confirm the conversion and enantioselectivity. 2-Propanol (15% v/v) was used as an auxiliary substrate for cofactor regeneration and as a cosolvent to facilitate the dissolution of ketones. The results are shown in Fig. 3. The reduction proceeded with more than 98% conversion for all tested substrates. Moreover, the enantioselectivities were > 99% ee (S) following Prelog’s rule (Prelog 1964), independent of the size and position of the halogenated substituents. To further investigate the versatility of Phe56Ile in organic synthesis, a preparative-scale reduction was conducted using 5a, as the highest relative activity was obtained with 5a among all tested substrates. The reaction resulted in > 99% conversion, 76.5% isolated yield, and > 99% ee (S).
Fig. 3.
Analytical-scale reductions of 1a-11a by GcAPRD Phe56Ile: (A) conversion and (B) enantioselectivity. The reaction conditions are described in the “Materials and methods,” section “Analytical-scale asymmetric reduction of 1a-11a by GcAPRD Phe56Ile”
Activity of GcAPRD mutants towards diaryl ketones
With the excellent performance of Phe56Ile towards 1a-11a, Phe56 in the large pocket and Trp288 in the small pocket were mutated to construct the double mutants, Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala. The two double mutants, as well as the wild type, Phe56Ile, Phe56Ala, and Trp288Ala were overexpressed in E. coli, purified, and the reduction activity towards 2-(4′-chlorobenzoyl)pyridine (18a) as a representative substrate was investigated. The specific activities are listed in Table 2. Compared to the wild type (< 0.2 μmol/min/mg), Trp288Ala increased the activity by more than sevenfold to 1.5 μmol/min/mg. Further mutagenesis in the large pocket to construct the double mutants, Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala, increased the specific activity more than 23-fold and 17-fold to 4.5 and 3.4 μmol/min/mg, respectively, although the Phe56 mutation alone did not show a significant change in activity.
Table 2.
The specific activity of GcAPRD wild type and mutants towards 18a
| Enzyme | Wild type | Phe56Ile | Phe56Ala | Trp288Ala | Phe56Ile/Trp288Ala | Phe56Ala/Trp288Ala |
|---|---|---|---|---|---|---|
| Specific activity (μmol/min/mg) | < 0.2 | < 0.1 | < 0.1 | 1.5 | 4.5 | 3.4 |
Asymmetric reduction of diaryl ketones by GcAPRD Trp288Ala, Phe56Ile/Trp288Ala, and Phe56Ala/Trp288Ala
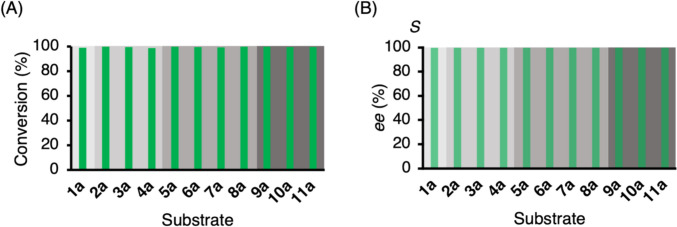
Following activity analysis, asymmetric reductions of 12a-19a by whole cells harboring Trp288Ala, Phe56Ile/Trp288Ala, or Phe56Ala/Trp288Ala were performed to confirm isolated yields and enantioselectivities. The reaction also used 2-propanol as an auxiliary substrate and cosolvent, similar to the single mutant cases. The results are shown in Fig. 4. Using Trp288Ala, isolated yields exceeding 80% were obtained for the reduction of 15a-17a, while yields for the reactions of other substrates ranged from 58 to 80%. Compared to Trp288Ala, Phe56Ile/Trp288Ala significantly increased the yields for the reactions of most of the tested substrates. Specifically, the yields of 15b and 18b increased to 96%, and other products, except for 14b, were obtained with yields above or around 90%. Phe56Ile/Trp288Ala not only improved activity (initial reaction rate) towards 18a (Table 2) but also demonstrated high performance in the reduction reactions of 3 h. On the other hand, although Phe56Ala/Trp288Ala exhibited higher enzymatic activity towards 18a than Trp288Ala (Table 2), higher isolated yields were not achieved, resulting in only up to 81% yield.
Fig. 4.
Reductions of 12a–19a by GcAPRD mutants: (A) yield and (B) ee for the reaction by Trp288Ala, (C) yield and (D) ee for the reaction by Phe56Ile/Trp288Ala, and (E) yield and (F) ee for the reaction by Phe56Ala/Trp288Ala. The reaction conditions are described in the “Materials and methods,” section “Asymmetric reduction of diaryl ketones by GcAPRD Trp288Ala, Phe56Ile/Trp288Ala, and Phe56Ala/Trp288Ala”
Regarding enantioselectivity, all three mutants generally produced (R)-alcohols following Prelog’s rule (Prelog 1964). For example, (R)-alcohols were produced by Trp288Ala in the reductions of meta-substituted 14a and 17a with ee of 91 and 97%, respectively. Phe56Ile/Trp288Ala also demonstrated high (R)-enantioselectivity for 14a and 17a, while the highest enantioselectivity of Phe56Ile/Trp288Ala was observed in the reduction of 16a. The enantioselectivity for 16a was also high when using the other double mutant, Phe56Ala/Trp288Ala. On the other hand, (S)-alcohols were produced from the reduction of 12a and 15a by Trp288Ala and Phe56Ile/Trp288Ala. When comparing Trp288Ala with Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala, significant improvements in enantioselectivity were observed for the reduction of 2′-substituted substrates 13a and 16a.
Discussion
Based on the previous research on the reduction of ketones by ADHs, there are three factors affecting the activity, yield, and, enantioselectivity of ADH-catalyzed reactions: substrate binding pocket sizes (Qin et al. 2018; Koesoema et al. 2019a; Wu et al. 2020), hydrophobicity of amino acids in the binding pockets (Qin et al. 2018; Wu et al. 2021), and substituent properties of substrates (Qin et al. 2018; Koesoema et al. 2019a). These factors collectively determine the catalytic performance.
First, the sizes of the binding pockets of GcAPRD wild type and Trp288Ala were considered to determine their suitability for diaryl ketone reductions. In our previous study, the GcAPRD wild type exhibited excellent (S)-enantioselectivity for the reduction of halogenated acetophenones (Koesoema et al. 2019a), whereas Trp288Ala exhibited (R)-enantioselectivity (Koesoema et al. 2020a). This demonstrates that the small binding pocket of the wild type is suitable for a methyl group but too small for an aryl group (Koesoema et al. 2019a), whereas the enlarged small pocket in Trp288Ala is suitable for an aryl group (Koesoema et al. 2019a, 2020a), which provides a basis for Trp288Ala, but not the wild type, to exhibit catalytic activity toward diaryl ketones. As expected, Trp288Ala exhibited higher activity towards 18a (1.5 μmol/min/mg) compared to the wild type (less than 0.2 μmol/min/mg) (Table 2).
Since the phenyl ring and the pyridine ring are similar in size, achieving high enantioselectivity is challenging (Wang et al. 2018; Xu et al. 2020). Nevertheless, depending on the substituent, GcAPRD mutants can reduce diaryl ketones with high enantioselectivity. For example, Trp288Ala exhibited high (R)-enantioselectivity for 3′-substituted substrates, yielding 91% ee (R) for 14b and 97% ee (R) for 17b. This observation suggests that the substituted phenyl group, rather than the pyridyl group, effectively enters the large pocket. This result aligns with the fact that 3′-substituted phenyl groups have a higher tendency to enter the large pocket than 2′- or 4′-substituted phenyl groups in the reduction of substituted acetophenones by Trp288Ala in previous studies (Koesoema et al. 2020a).
Further mutation of Phe56 at the entrance of the large pocket to smaller amino acids, creating mutants Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala, enlarged the large binding pocket by eliminating steric hindrance. Therefore, Phe56Ile/Trp288Ala exhibited the highest activity towards 18a (4.5 μmol/min/mg) among all the tested enzymes. The higher activity of Phe56Ala/Trp288Ala towards 18a (3.4 μmol/min/mg) than that of Trp288Ala is also attributed to the increased size of the large binding pocket. The literature search on mutations at position Phe56 or the associated loop region of MDR was conducted, and the alignment results of GcAPRD and several MDRs are shown in Supplementary Figs. 2 and 3. This position or the associated loop region has been mutated to design ADH-A mutant (Phe43Thr/Tyr54Gly/Leu119Tyr/Phe282Trp) to oxidize 1-phenylpropane-(1R,2S)-diol regioselectivity (Maurer et al. 2018), to design cpADH5 (Cys57Val/Trp286Ser) to catalyze methyl 3-hexanoate (Ensari et al. 2018), and to design Lactococcus lactis alcohol dehydrogenase (LlAdhA) mutant (Tyr50Phe/Asn110Ser/Ile212Thr/Leu264Val) to reduce isobutyraldehyde to isobutanol (Liu et al. 2012) (Supplementary Fig. 3B). To the best of our knowledge, this position has not been mutated to expand the substrate specificity towards diaryl ketones. Previous studies only focused on another loop for the reduction of diaryl ketones to design TbSADH mutants (Ala85Gly/Ile86Leu and Ala85Gly/Ile86Ala/Gln101Ala) (Liu et al. 2019; Qu et al. 2019, 2022; Chen et al. 2021; Jiang et al. 2022) (Supplementary Fig. 3B). These indicated that the loop in the large binding pocket was found to be targeted in this study to control the activity and enantioselectivity of MDR towards diaryl ketones.
Furthermore, the studies on ADH from Thermoanaerobacter ethanolicus (TeSADH, identical in sequence to TbSADH) mutants mainly focused on mutations at position Trp110, which was mutated to Ala or Gly to enlarge the large binding pocket and enable the reduction of 2-tetralones that could not be reduced by the wild type (Musa et al. 2007; Bsharat et al. 2017). This strategy aligns with our approach of mutating Phe56 in GcAPRD to Ile or Ala to expand substrate specificity by enlarging the large binding pocket. Although Phe56 in GcAPRD and Trp110 in TeSADH are not related in the amino acid sequence, they occupy a similar space when the structures are compared (Supplementary Fig. 3C).
The mutation of Phe56 to Ile or Ala was especially effective in overcoming the limitation of the reaction of the 2′-substituted phenyl compounds. Phe56Ile exhibited especially higher activities towards 2′-chloro or 2′-bromo substituted compounds (5a and 9a) than the wild type (Fig. 2, Supplementary Table 4). Moreover, Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala exhibited stricter (R)-enantioselectivity for 2′-substituted diaryl ketones (13a and 16a) than Trp288Ala. For example, the ee of (R)-16b increased from 31% for Trp288Ala to 88% for Phe56Ile/Trp288Ala and 86% for Phe56Ala/Trp288Ala (Fig. 3). These results demonstrate that enlarging the large binding pocket can effectively eliminate the steric hindrance caused by the 2′-substituted phenyl group. Moreover, these results also demonstrated that matching the hydrophobicity of the substituent and the substrate binding site is important. As the halogen or methyl substitutes are strongly hydrophobic, the high hydrophobicity (Mozhaev et al. 1988; Bagchi 2013) of the large pocket in Phe56Ile is more favorable to 2′-chloro or 2′-bromo acetophenone and methyl or chloro-substituted 2-benzoylpyridines than that in the wild type. Importantly, these results may pave the way for solving the general problem in various biocatalysts of the steric hindrance caused by the 2′-substitution of the phenyl group at the α-position (Xu et al. 2018; Hoshino et al. 2020; Otsu et al. 2020; Wu et al. 2020, 2021; Zhang et al. 2022).
The electronegativity of the substituent on the substrates also affects the enantioselectivity of the reactions of diaryl ketones. When the substituent is located at the 2′- or 4′-position of the phenyl ring, there is an increasing tendency of (R)-enantioselectivity as the electronegativity of the substituent increases. For example, in the cases of 4′-substituted substrates, 15a has an electron-donating methyl group, while 18a and 19a contain an electron-withdrawing chloro or fluoro group. The enantioselectivity for these substrates were reversed from S (or close to racemic) to R with increasing electronegativity. Trp288Ala showed 42% ee (S) for 15b but achieved 68% ee (R) for 18b and 81% ee (R) for 19b. Phe56Ile/Trp288Ala exhibited a similar trend, with 29% ee (S) for 15b, 46% ee (R) for 18b, and 58% ee (R) for 19b. For Phe56Ala/Trp288Ala, the enantioselectivities were 9% ee (R) for 15b, 84% ee (R) for 18b, and 88% ee (R) for 19b. For 2′-substituted substrates, when the substituent was changed from methyl group (13a) to chloro group (16a), the (R)-enantioselectivities were increased. For Phe56Ile/Trp288Ala, ee (R) increased from 48% (13b) to 88% (16b). For Phe56Ala/Trp288Ala, ee (R) increased from 75% (13b) to 86% (16b). Notably, for Trp288Ala, ee reversed from 30% (S) (13b) to 31% (R) (16b). These results demonstrated that the electronegativity of the substituent on 2′- and 4′-positions of the phenyl group is critical to the enantioselectivity of GcAPRD mutants towards 2′-benzoylpyridine analogs, which is consistent with other previous studies of KpADH (Zhang et al. 2022).
In conclusion, a new mutation site, Phe56, in the large binding pocket of GcAPRD has been identified as important for eliminating steric hindrance. The new GcAPRD mutant, Phe56Ile, exhibited improved activity and excellent (S)-enantioselectivity for acetophenone and its halogenated analogs, as the entrance of the large pocket was enlarged through mutagenesis. Moreover, GcAPRD Trp288Ala exhibited catalytic activity towards 2-(4’-chlorobenzoyl)pyridine (18a). Therefore, Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala with further enhanced activities were constructed. In whole-cell-catalyzed reductions of diaryl ketones by Phe56Ile/Trp288Ala, significant increases in isolated yields of over 90% were obtained for most of the tested substrates. Moreover, excellent enantioselectivity of up to 97% ee (R) for the reduction of diaryl ketones was achieved. These results demonstrate that the GcAPRD mutants obtained in this study are promising biocatalysts for the preparation of valuable chiral diaryl alcohols, providing support for future immobilization studies of GcAPRD mutants and their applications in flow reactions.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
ZT conceptualized, performed experiments, analyzed the data, wrote the manuscript, and acquired the funding. YT performed experiments. AAK conceptualized. TM supervised the project, reviewed the manuscript, and acquired the funding. All authors read and approved the manuscript.
Funding
This work was supported by the JSPS KAKENHI (Grant Number 22K05187) and JST SPRING (Japan Grant Number JPMJSP2106 and JPMJSP2180).
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bagchi B (2013) The hydrophobic effect. In: Water in biological and chemical processes: from structure and dynamics to function. Cambridge University Press, 215–242
- Bsharat O, Musa MM, Vieille C, Oladepo SA, Takahashi M, Hamdan SM (2017) Asymmetric reduction of substituted 2-tetralones by Thermoanaerobacter pseudoethanolicus secondary alcohol dehydrogenase. ChemCatChem 9:1487–1493. 10.1002/cctc.201601618 [Google Scholar]
- Chen Q, Guo M, Bi Y, Qu G, Sun Z, Wang Y, Luo G (2021) Whole-cell biocatalytic synthesis of S -(4-chlorophenyl)-(pyridin-2-yl) methanol in a liquid–liquid biphasic microreaction system. Bioresour Technol 330:125022. 10.1016/j.biortech.2021.125022 [DOI] [PubMed] [Google Scholar]
- Ensari Y, Dhoke GV, Davari MD, Ruff AJ, Schwaneberg U (2018) A comparative reengineering study of cpADH5 through iterative and simultaneous multisite saturation mutagenesis. ChemBioChem 19:1563–1569. 10.1002/cbic.201800159 [DOI] [PubMed] [Google Scholar]
- Hall M (2021) Enzymatic strategies for asymmetric synthesis. RSC Chem Biol 2:958–989. 10.1039/d1cb00080b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Yamabe E, Hawari MA, Tamura M, Kanamaru S, Yoshida K, Koesoema AA, Matsuda T (2020) Oxidation of aromatic and aliphatic aldehydes to carboxylic acids by Geotrichum candidum aldehyde dehydrogenase. Tetrahedron 76:131387. 10.1016/j.tet.2020.131387 [Google Scholar]
- Hou J, Xu G, Ni Y (2020) Stereochemistry in asymmetric reduction of bulky-bulky ketones by alcohol dehydrogenases. ACS Catal 10:10954–10966. 10.1021/acscatal.0c02646 [Google Scholar]
- Jiang Y, Li X, Liu B, Tong F, Qu G, Sun Z (2022) Engineering the hydrogen transfer pathway of an alcohol dehydrogenase to increase activity by rational enzyme design. Mol Catal 530:112603. 10.1016/j.mcat.2022.112603 [Google Scholar]
- Koesoema AA, Sugiyama Y, T.sriwong K, Xu Z, Verina S, Standley DM, Senda M, Senda T, Matsuda T (2019) Reversible control of enantioselectivity by the length of ketone substituent in biocatalytic reduction. Appl Microbiol Biotechnol 103:9529–9541. 10.1007/S00253-019-10206-5 [DOI] [PubMed] [Google Scholar]
- Koesoema AA, Sugiyama Y, Xu Z, Standley DM, Senda M, Senda T, Matsuda T (2019) Structural basis for a highly (S)-enantioselective reductase towards aliphatic ketones with only one carbon difference between side chain. Appl Microbiol Biotechnol 103:9543–9553. 10.1007/s00253-019-10093-w [DOI] [PubMed] [Google Scholar]
- Koesoema AA, Standley DM, Ohshima S, Tamura M, Matsuda T (2020) Control of enantioselectivity in the enzymatic reduction of halogenated acetophenone analogs by substituent positions and sizes. Tetrahedron Lett 61:151820. 10.1016/j.tetlet.2020.151820 [Google Scholar]
- Koesoema AA, Standley DM, Senda T, Matsuda T (2020) Impact and relevance of alcohol dehydrogenase enantioselectivities on biotechnological applications. Appl Microbiol Biotechnol 104:2897–2909. 10.1007/s00253-020-10440-2 [DOI] [PubMed] [Google Scholar]
- Koesoema AA, Standley DM, T.sriwong K, Tamura M, Matsuda T (2020) Access to both enantiomers of substituted 2-tetralol analogs by a highly enantioselective reductase. Tetrahedron Lett 61:151682. 10.1016/j.tetlet.2020.151682 [Google Scholar]
- Li D, Lou Y, Xu J, Chen X, Lin X, Wu Q (2021) Electronic effect-guided rational design of Candida antarctica lipase B for kinetic resolution towards diarylmethanols. Adv Synth Catal 363:1867–1872. 10.1002/adsc.202001367 [Google Scholar]
- Liu X, Bastian S, Snow CD, Brustad EM, Saleski TE, Xu J, Meinhold P, Arnold FH (2012) Structure-guided engineering of Lactococcus lactis alcohol dehydrogenase LlAdhA for improved conversion of isobutyraldehyde to isobutanol. J Biotechnol 164:188–195. 10.1016/j.jbiotec.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Qu G, Li J, Fan W, Ma J, Xu Y, Nie Y, Sun Z (2019) Conformational dynamics-guided loop engineering of an alcohol dehydrogenase: capture, turnover and enantioselective transformation of difficult-to-reduce ketones. Adv Synth Catal 361:3182–3190. 10.1002/adsc.201900249 [Google Scholar]
- Liu J, Li W, Li Y, Liu Y, Ke Z (2021) Selective C-alkylationbetween alcohols catalyzed by N-heterocyclic carbene molybdenum. Chem - An Asian J 16:3124–3128. 10.1002/asia.202100959 [DOI] [PubMed] [Google Scholar]
- Maurer D, Enugala TR, Hamnevik E, Bauer P, Lüking M, Petrović D, Hillier H, Kamerlin SCL, Dobritzsch D, Widersten M (2018) Stereo- and regioselectivity in catalyzed transformation of a 1,2-disubstituted vicinal diol and the corresponding diketone by wild type and laboratory evolved alcohol dehydrogenases. ACS Catal 8:7526–7538. 10.1021/acscatal.8b01762 [Google Scholar]
- Mozhaev VV, Berezin IV, Martinek K (1988) Structure-stability relationship in proteins: fundamental tasks and strategy for the development of stabilized enzyme catalysts for biotechnology. Crit Rev Biochem Mol Biol 23:235–281. 10.3109/10409238809088225 [DOI] [PubMed] [Google Scholar]
- Musa MM, Ziegelmann-Fjeld KI, Vieille C, Zeikus JG, Phillips RS (2007) Asymmetric reduction and oxidation of aromatic ketones and alcohols using W110A secondary alcohol dehydrogenase from Thermoanaerobacter ethanolicus. J Org Chem 72:30–34. 10.1021/jo0616097 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Inoue Y, Matsuda T, Misawa I (1999) Stereoselective oxidation and reduction by immobilized Geotrichum candidum in an organic solvent. J Chem Soc - Perkin Trans 1:2397–2402. 10.1039/a900936a [Google Scholar]
- Nakata Y, Fukae T, Kanamori R, Kanamaru S, Matsuda T (2010) Purification and characterization of acetophenone reductase with excellent enantioselectivity from Geotrichum candidum NBRC 4597. Appl Microbiol Biotechnol 86:625–631. 10.1007/S00253-009-2329-5 [DOI] [PubMed] [Google Scholar]
- Nian S, Ling F, Chen J, Wang Z, Shen H, Yi X, Yang Y, She Y, Zhong W (2019) Highly enantioselective hydrogenation of non-ortho-substituted 2-pyridyl aryl ketones via iridium-f-diaphos catalysis. Org Lett 21:5392–5396. 10.1021/acs.orglett.9b01415 [DOI] [PubMed] [Google Scholar]
- Otsu M, Suzuki Y, Koesoema AA, Hoang HN, Tamura M, Matsuda T (2020) CO2-expanded liquids as solvents to enhance activity of Pseudozyma antarctica lipase B towards ortho-substituted 1-phenylethanols. Tetrahedron Lett 61:152424. 10.1016/j.tetlet.2020.152424 [Google Scholar]
- Özdemir A, Şahin E (2024) A multi-response nonlinear programming model with an inscribed design to optimize bioreduction conditions of (S)-phenyl(pyridin-2-yl)methanol by Leuconostoc pseudomesenteroides N13. Arab J Sci Eng 49:8225–8235. 10.1007/s13369-024-08773-5 [Google Scholar]
- Prelog V (1964) Specification of the stereospecificity of some oxido-reductases by diamond lattice sections. Pure Appl Chem 9:119–130. 10.1351/pac196409010119 [Google Scholar]
- Qin F, Qin B, Zhang W, Liu Y, Su X, Zhu T, Ouyang J, Guo J, Li Y, Zhang F, Tang J, Jia X, You S (2018) Discovery of a switch between Prelog and anti-Prelog reduction toward halogen-substituted acetophenones in short-chain dehydrogenase/reductases. ACS Catal 8:6012–6020. 10.1021/acscatal.8b00807 [Google Scholar]
- Qu G, Bi Y, Liu B, Li J, Han X, Liu W, Jiang Y, Qin Z, Sun Z (2022) Unlocking the stereoselectivity and substrate acceptance of enzymes: proline-induced loop engineering test. Angew Chemie - Int Ed 61:e202110793. 10.1002/anie.202110793 [DOI] [PubMed] [Google Scholar]
- Qu G, Liu B, Jiang Y, Nie Y, Yu H, Sun Z (2019) Laboratory evolution of an alcohol dehydrogenase towards enantioselective reduction of difficult-to-reduce ketones. Bioresour Bioprocess 6:18. 10.1186/s40643-019-0253-9 [Google Scholar]
- Sardauna AE, Abdulrasheed M, Nzila A, Musa MM (2023) Biocatalytic asymmetric reduction of prochiral bulky-bulky ketones. Mol Catal 541:113099. 10.1016/j.mcat.2023.113099 [Google Scholar]
- Shanbhag AP (2023) Stairway to stereoisomers: engineering short- and medium-chain ketoreductases to produce chiral alcohols. ChemBioChem 24:e202200687. 10.1002/cbic.202200687 [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhang J, Yin D, Xu G, Ni Y (2022) Novel alcohol dehydrogenase CgADH from Candida glabrata for stereocomplementary reduction of bulky-bulky ketones featuring self-sufficient NADPH regeneration. ACS Sustain Chem Eng 10:13722–13732. 10.1021/acssuschemeng.2c03872 [Google Scholar]
- T.sriwong K, Koesoema AA, Matsuda T (2020) Organic-inorganic nanocrystal reductase to promote green asymmetric synthesis. RSC Adv 10:30953–30960. 10.1039/d0ra03160g [DOI] [PMC free article] [PubMed] [Google Scholar]
- T.sriwong K, Kamogawa R, Castro Issasi CS, Sasaki M, Matsuda T (2022) Geotrichum candidum acetophenone reductase immobilization on reduced graphene oxide: a promising biocatalyst for green asymmetric reduction of ketones. Biochem Eng J 177:108263. 10.1016/j.bej.2021.108263 [Google Scholar]
- Tao X, Li W, Ma X, Li X, Fan W, Xie X, Ayad T, Ratovelomanana-Vidal V, Zhang Z (2012) Ruthenium-catalyzed enantioselective hydrogenation of aryl-pyridyl ketones. J Org Chem 77:612–616. 10.1021/jo202204j [DOI] [PubMed] [Google Scholar]
- Wang Y, Dai W, Liu Y, Zhang Z, Zhou J, Xu G, Ni Y (2018) Fine tuning the enantioselectivity and substrate specificity of alcohol dehydrogenase from Kluyveromyces polysporus by single residue at 237. Catal Commun 108:1–6. 10.1016/j.catcom.2018.01.012 [Google Scholar]
- Wu K, Yang Z, Meng X, Chen R, Huang J, Shao L (2020) Engineering an alcohol dehydrogenase with enhanced activity and stereoselectivity toward diaryl ketones: reduction of steric hindrance and change of the stereocontrol element. Catal Sci Technol 10:1650–1660. 10.1039/c9cy02444a [Google Scholar]
- Wu K, Yan J, Wang X, Yin X, Shi G, Yang L, Li F, Huang J, Shao L (2021) Efficient synthesis of bepotastine and cloperastine intermediates using engineered alcohol dehydrogenase with a hydrophobic pocket. Appl Microbiol Biotechnol 105:5873–5882. 10.1007/S00253-021-11413-9 [DOI] [PubMed] [Google Scholar]
- Xu G, Wang Y, Tang M, Zhou J, Zhao J, Han R, Ni Y (2018) Hydroclassified combinatorial saturation mutagenesis: Reshaping substrate binding pockets of KpADH for enantioselective reduction of bulky-bulky ketones. ACS Catal 8:8336–8345. 10.1021/acscatal.8b02286 [Google Scholar]
- Xu G, Dai W, Wang Y, Zhang L, Sun Z, Zhou J, Ni Y (2020) Molecular switch manipulating Prelog priority of an alcohol dehydrogenase toward bulky-bulky ketones. Mol Catal 484:110741. 10.1016/j.mcat.2019.110741 [Google Scholar]
- Yamamoto T, Nakata Y, Cao C, Sugiyama Y, Asanuma Y, Kanamaru S, Matsuda T (2013) Acetophenone reductase with extreme stability against a high concentration of organic compounds or an elevated temperature. Appl Microbiol Biotechnol 97:10413–10421. 10.1007/S00253-013-4801-5 [DOI] [PubMed] [Google Scholar]
- Yuan Q, Ma L, Kong W, Liu J, Zhang S, Yan J, Bai J, He Y, Zhou L, Liu Y, Jiang Y (2024) Enzymatic synthesis of chiral alcohols using ketoreductases. Catal Rev 1–40. 10.1080/01614940.2024.2313603
- Zhang J, Zhou J, Xu G, Ni Y (2022) Stereodivergent evolution of KpADH for the asymmetric reduction of diaryl ketones with para-substituents. Mol Catal 524:112315. 10.1016/J.MCAT.2022.112315 [Google Scholar]
- Zhang L, Dai W, Rong S, Schwaneberg U, Xu G, Ni Y (2024) Engineering diaryl alcohol dehydrogenase KpADH reveals importance of retaining hydration shell in organic solvent tolerance. Protein Sci 33:e4933. 10.1002/pro.4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sun Z, Xu G, Ni Y (2024) Classification and functional origins of stereocomplementary alcohol dehydrogenases for asymmetric synthesis of chiral secondary alcohols: a review. Int J Biol Macromol 270:132238. 10.1016/j.ijbiomac.2024.132238 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information.