Abstract
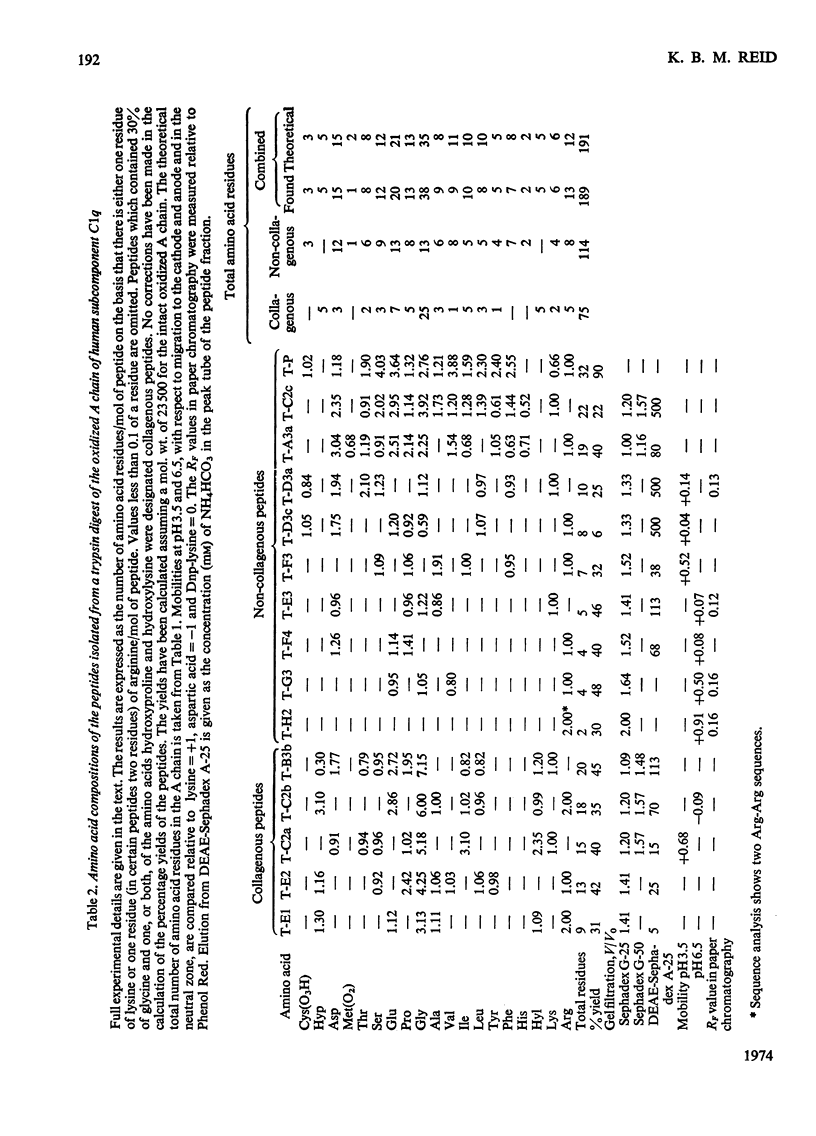
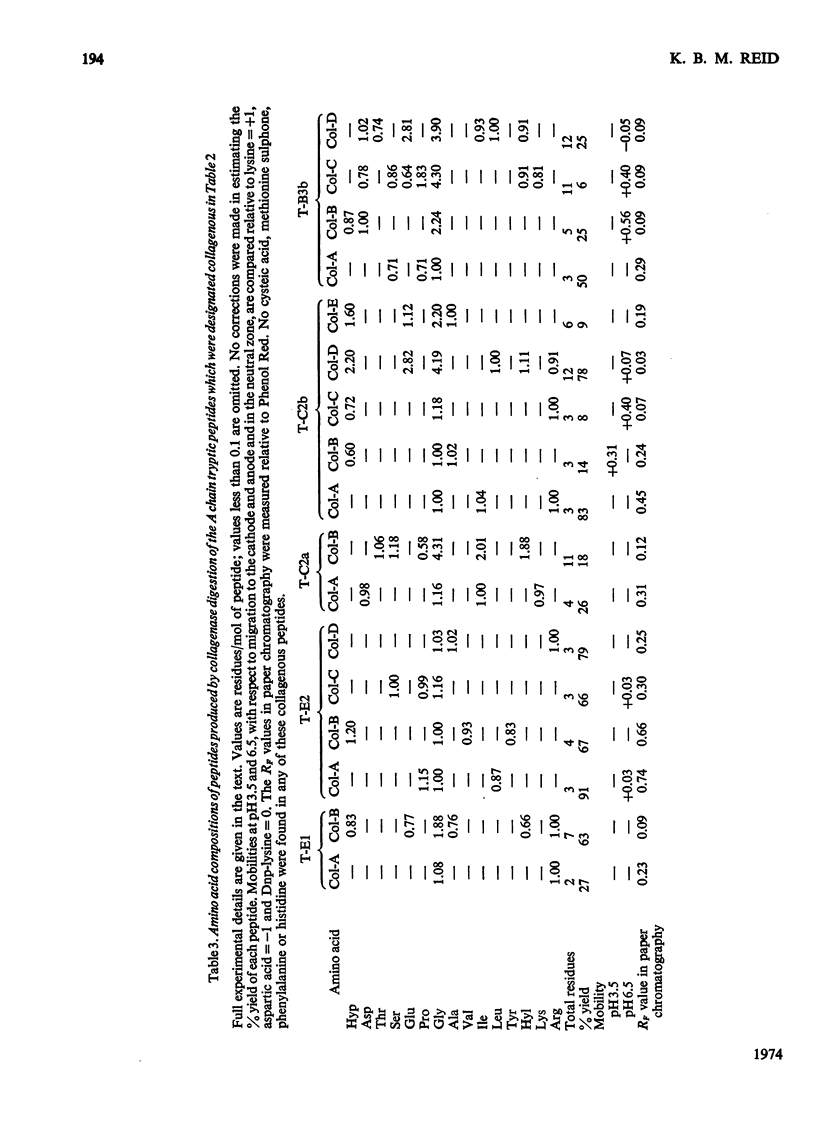
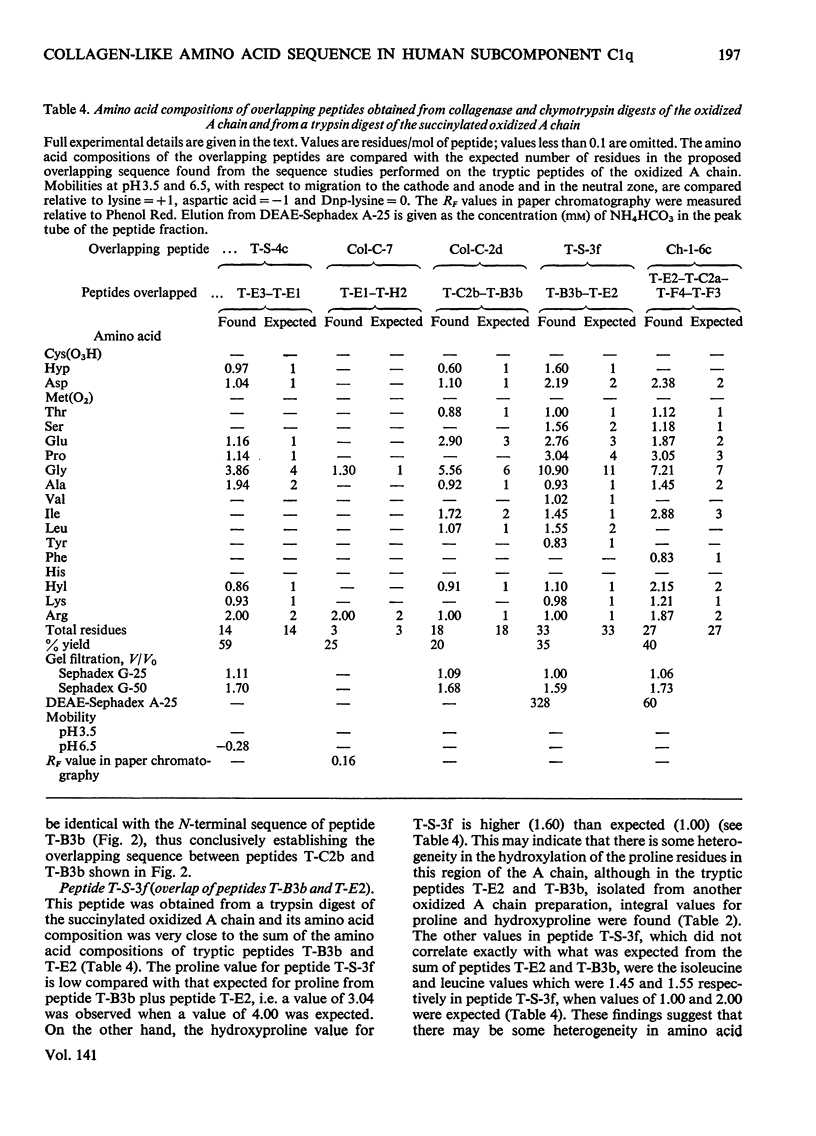
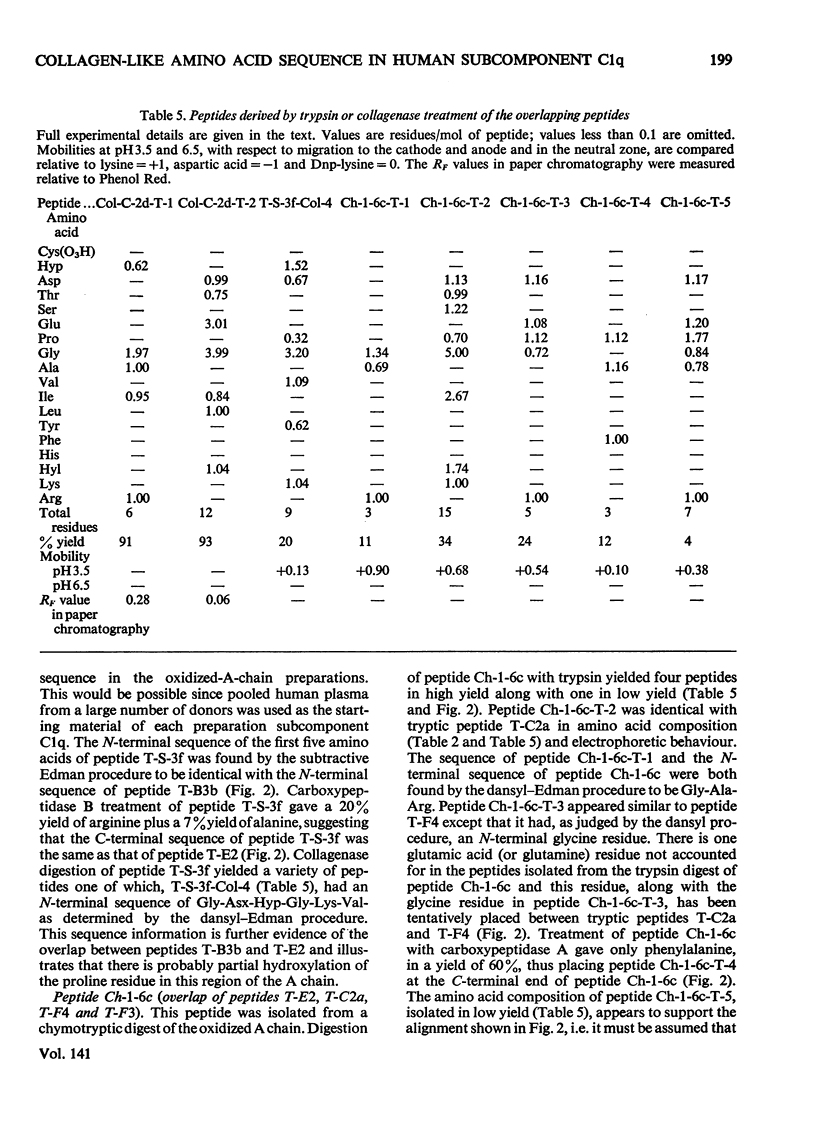
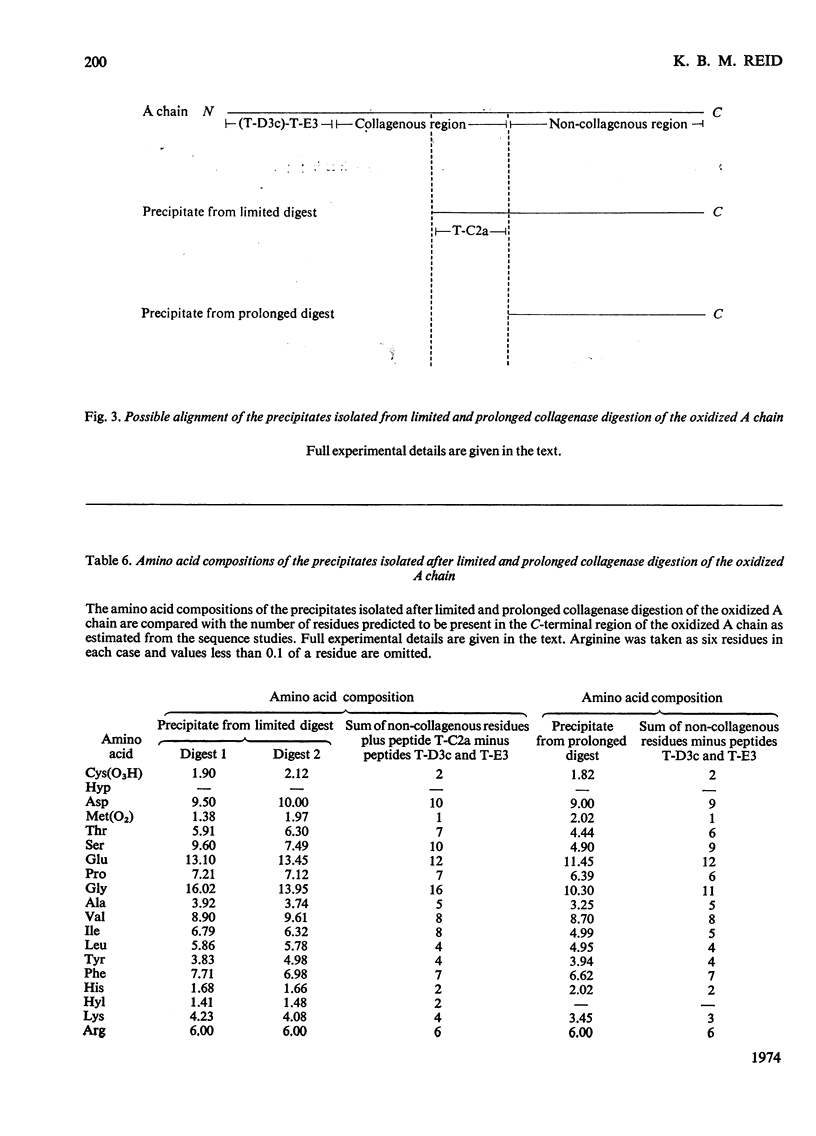
1. A partial amino acid sequence of 95 residues of the 191 residues in the oxidized A chain of human subcomponent C1q was determined. The partial nature of the sequence is because one overlapping peptide is missing in the proposed sequence, also the proof of some of the overlapping peptides depends partly on their amino acid composition and not on their complete sequence. 2. This region of the A chain contained a repeating sequence of glycine-X-Y (where X is often proline and Y is often hydroxyproline) for 78 residues. 3. The five hydroxylysine residues and the five hydroxyproline residues present in the oxidized A chain were all in these 78 residues and only in the Y position of the repeating sequence. 4. Prolonged collagenase digestion of the oxidized A chain yielded a large, apparently C-terminal, peptide which contained most of the non-collagenous sequences present in the chain. 5. It is concluded that there is a collagen-like region in the A chain of subcomponent C1q which constitutes most of the N-terminal half of the chain and that similar collagen-like regions will be found in the B and C chains.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balian G., Click E. M., Bornstein P. Structure of rat skin collagen 1-CB8. Amino acid sequence of the hydroxylamine-produced fragment HA1. Biochemistry. 1971 Nov 23;10(24):4470–4478. doi: 10.1021/bi00800a019. [DOI] [PubMed] [Google Scholar]
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Calcott M. A., Müller-Eberhard H. J. C1q protein of human complement. Biochemistry. 1972 Aug 29;11(18):3443–3450. doi: 10.1021/bi00768a018. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Wilkinson J. M. The immunological activity of some of the chymotryptic peptides of sperm-whale myoglobin. Biochem J. 1965 Mar;94(3):545–556. doi: 10.1042/bj0940545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide T. A., Jones W. M. Studies on the tryptophan residues in porcine pepsin. J Biol Chem. 1968 Jul 25;243(14):3906–3911. [PubMed] [Google Scholar]
- Grant M. E., Schofield J. D., Kefalides N. A., Prockop D. J. The biosynthesis of basement membrane collagen in embryonic chick lens. 3. Intracellular formation of the triple helix and the formation of aggregates through disulfide bonds. J Biol Chem. 1973 Nov 10;248(21):7432–7437. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isemura M., Zahn R. K., Schmid K. A new neuraminic acid derivative and three types of glycopeptides isolated from the Cuvierian tubules of the sea cucumber Holothuria forskali. Biochem J. 1973 Mar;131(3):509–521. doi: 10.1042/bj1310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pontz B. F., Müller P. K., Meigel W. N. A study on the conversion of procollagen. Release and recovery of procollagen peptides in the culture medium. J Biol Chem. 1973 Nov 10;248(21):7558–7564. [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton E., Yonemasu K., Stroud R. M. Ultrastructure of the human complement component, Clq (negative staining-glutamine synthetase-biologically active Clq). Proc Natl Acad Sci U S A. 1972 Jan;69(1):65–68. doi: 10.1073/pnas.69.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M., Niedermeier W., Butler W. T. Chemical studies of Clq; a modulator of immunoglobulin biology. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1388–1394. doi: 10.1016/s0006-291x(71)80028-1. [DOI] [PubMed] [Google Scholar]


