Abstract
1. A theory is developed to account for the kinetics of coupled-enzyme reactions without assuming that the second reaction follows first-order kinetics. 2. A simple procedure is described for applying the theory to the practical design of enzyme assays. 3. The validity of the theory is confirmed for the assay of glucokinase with glucose 6-phosphate dehydrogenase as coupling enzyme. 4. The possibility of extending the theory to three or more coupled reactions is discussed.
Full text
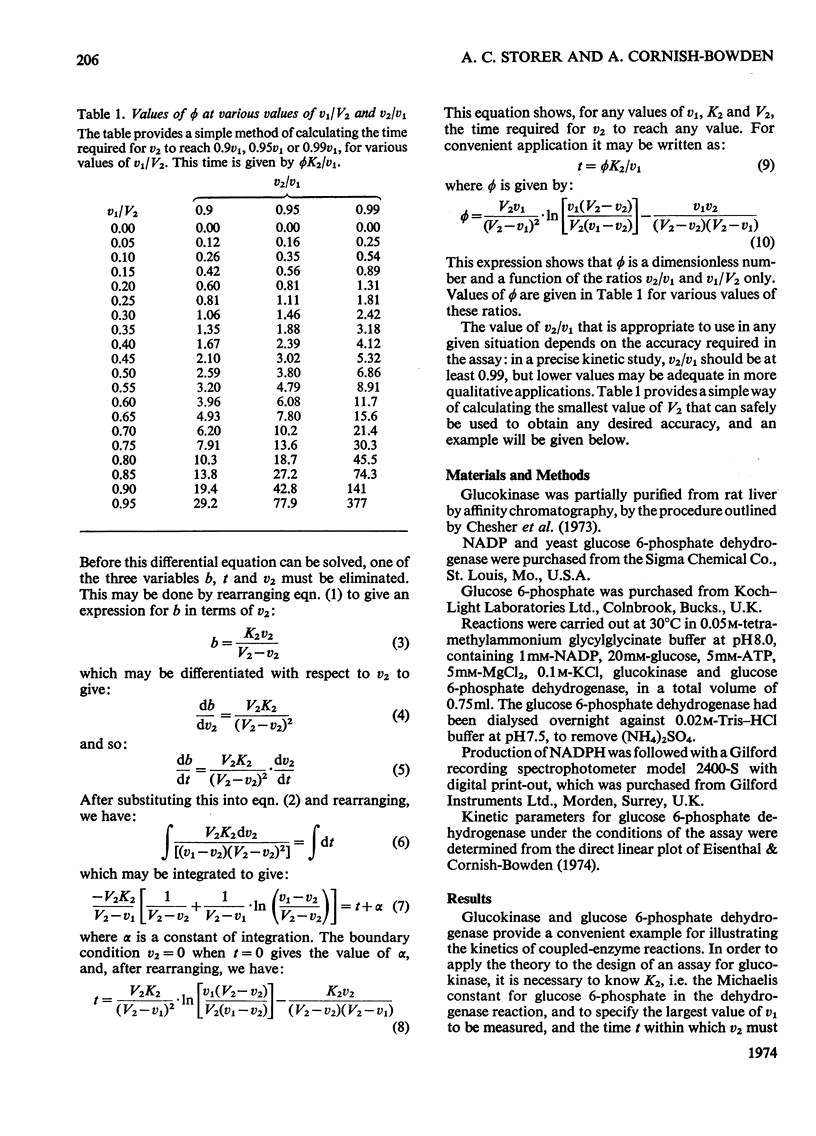
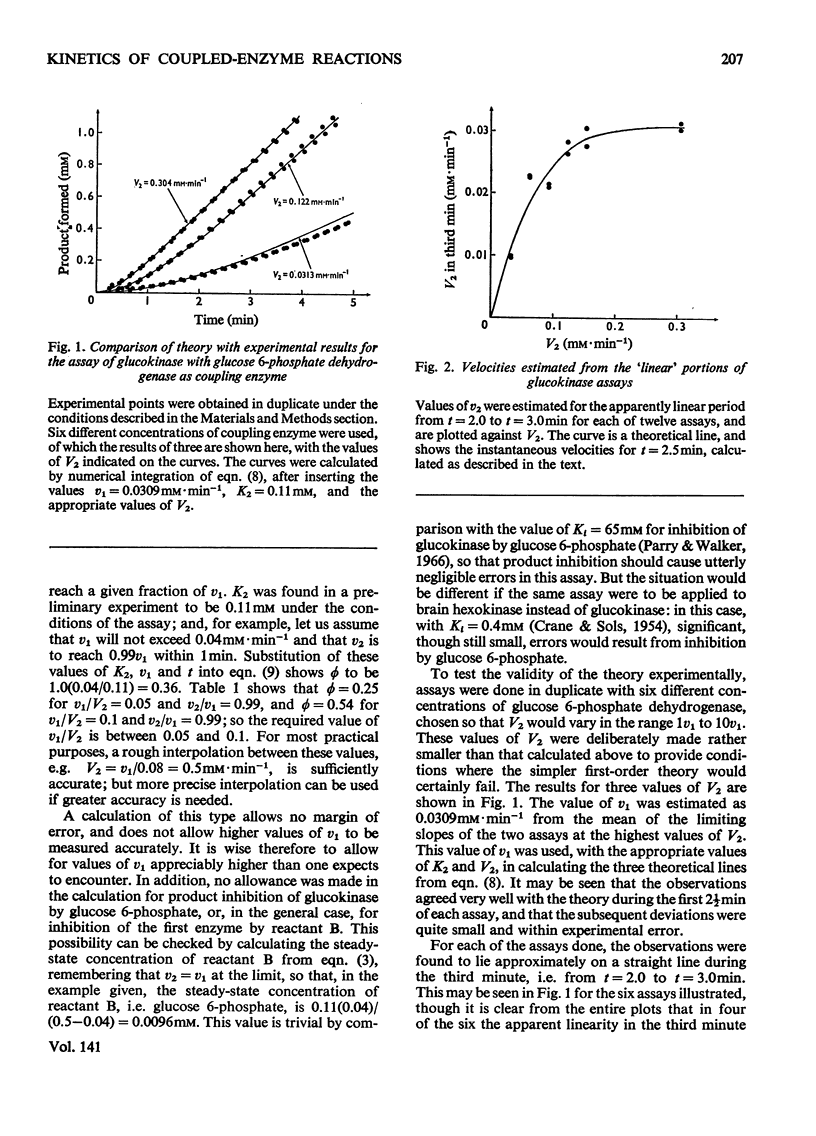
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigad G. Inhibition of glucose 6-phosphate dehydrogenase by adenosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1543–1547. doi: 10.1073/pnas.56.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMEYER H. U. Reaktionskinetische Untersuchungen zur Messung der Glykolyse. II. Die kinetische Analyse. Grundlagen. Biochem Z. 1953;324(6):408–432. [PubMed] [Google Scholar]
- Barwell C. J., Hess B. The transient time of the hexokinase-pyruvate kinase-lacae dehydrogenase system in vitro. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1531–1536. doi: 10.1515/bchm2.1970.351.2.1531. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- Easterby J. S. Coupled enzyme assays: a general expression for the transient. Biochim Biophys Acta. 1973 Feb 15;293(2):552–558. doi: 10.1016/0005-2744(73)90362-8. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Goldman R., Katchalski E. P. Kinetic behavior of a two-enzyme membrane carrying out a consecutive set of reactions. J Theor Biol. 1971 Aug;32(2):243–257. doi: 10.1016/0022-5193(71)90163-9. [DOI] [PubMed] [Google Scholar]
- Hart W. M., Jr A kinetic model of a cyclic system for the fluorometric microdetermination of adenosine triphosphatase activity. Mol Pharmacol. 1970 Jan;6(1):31–40. [PubMed] [Google Scholar]
- McClure W. R. A kinetic analysis of coupled enzyme assays. Biochemistry. 1969 Jul;8(7):2782–2786. doi: 10.1021/bi00835a014. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Sugden P. H. The apparent inhibition of phosphofrunctokinase by reduced nicotinamide-adenine dinucleotide: a problem of coupled-enzyme assays. Biochem J. 1970 Oct;119(4):787–789. doi: 10.1042/bj1190787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]


