Abstract
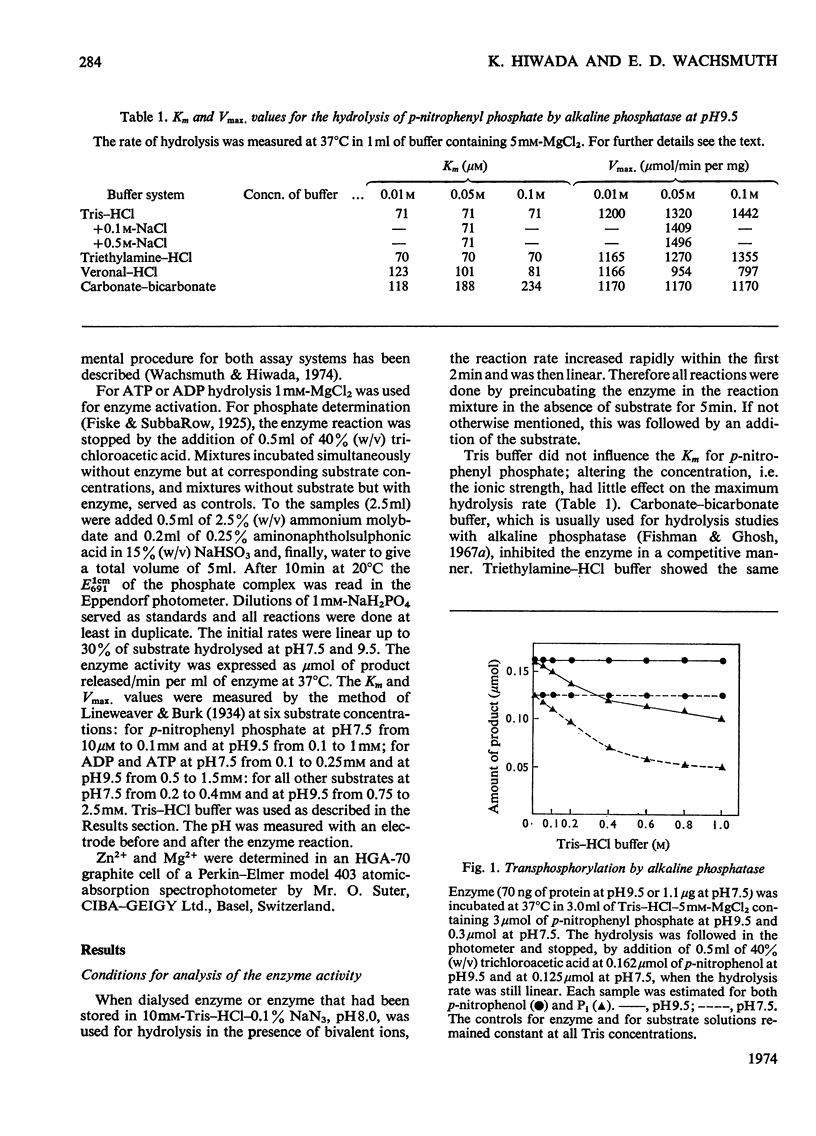
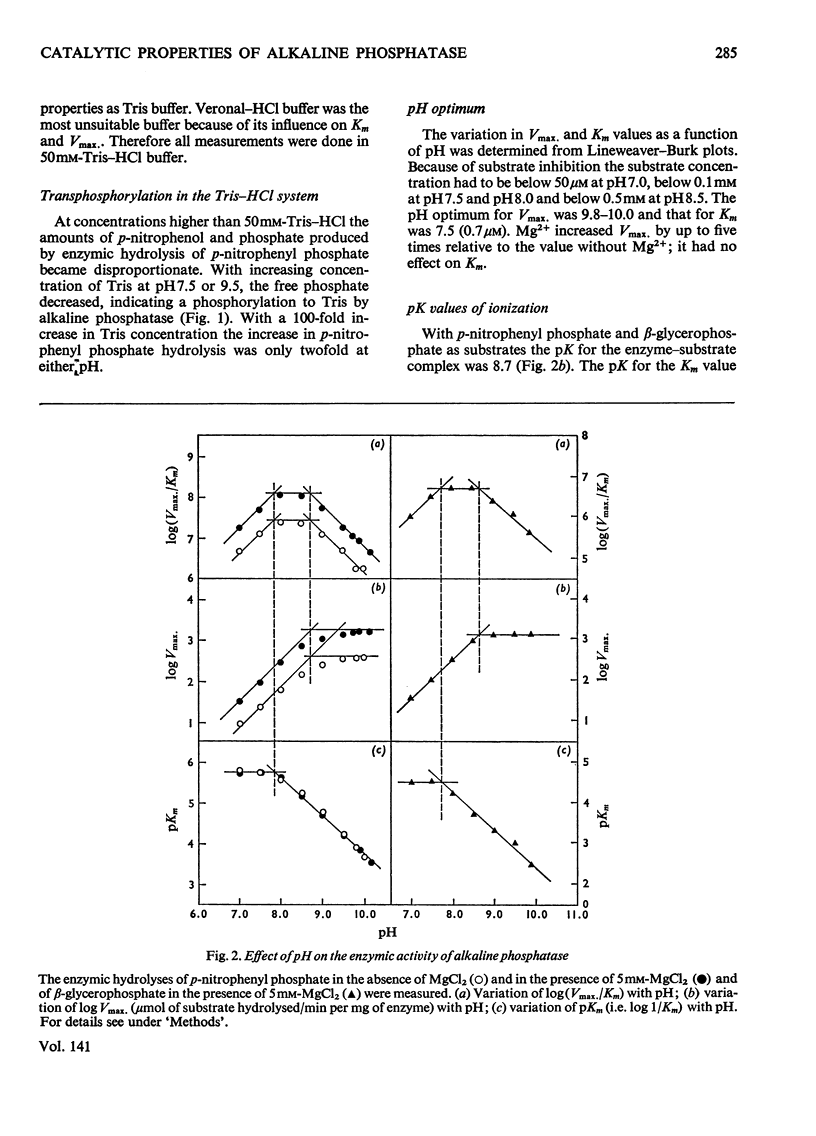
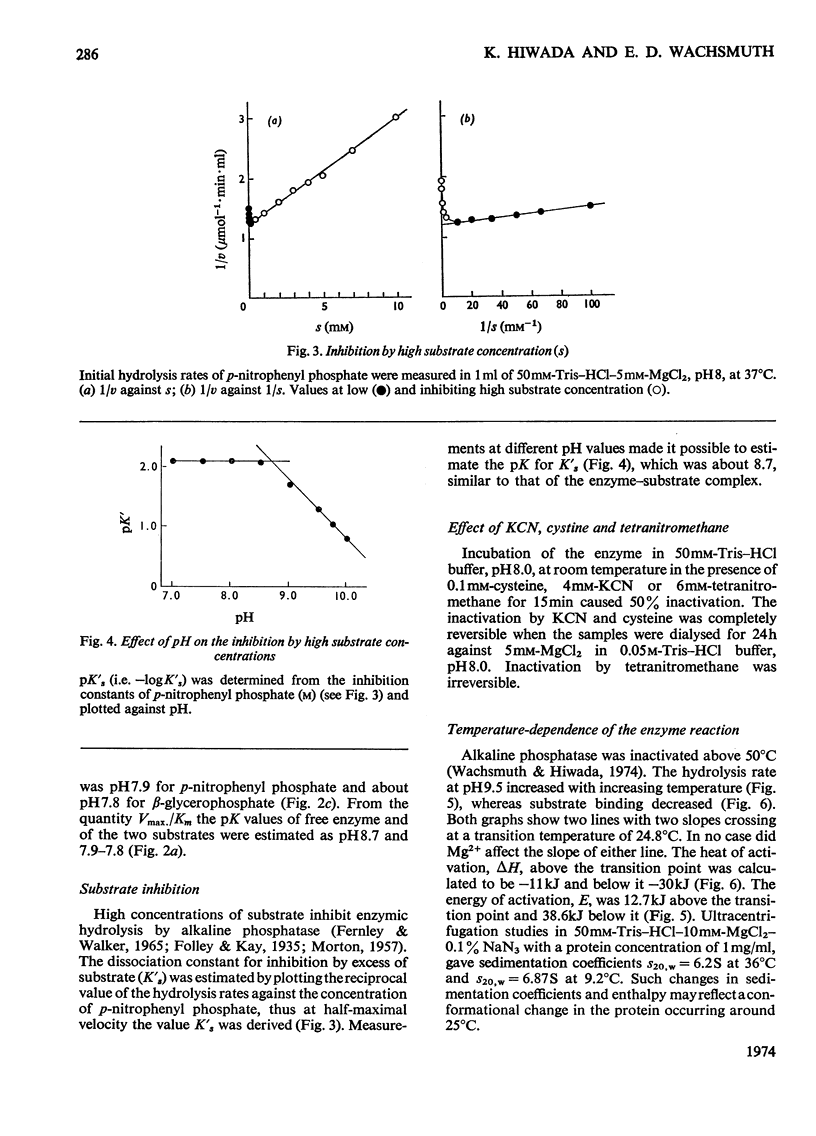
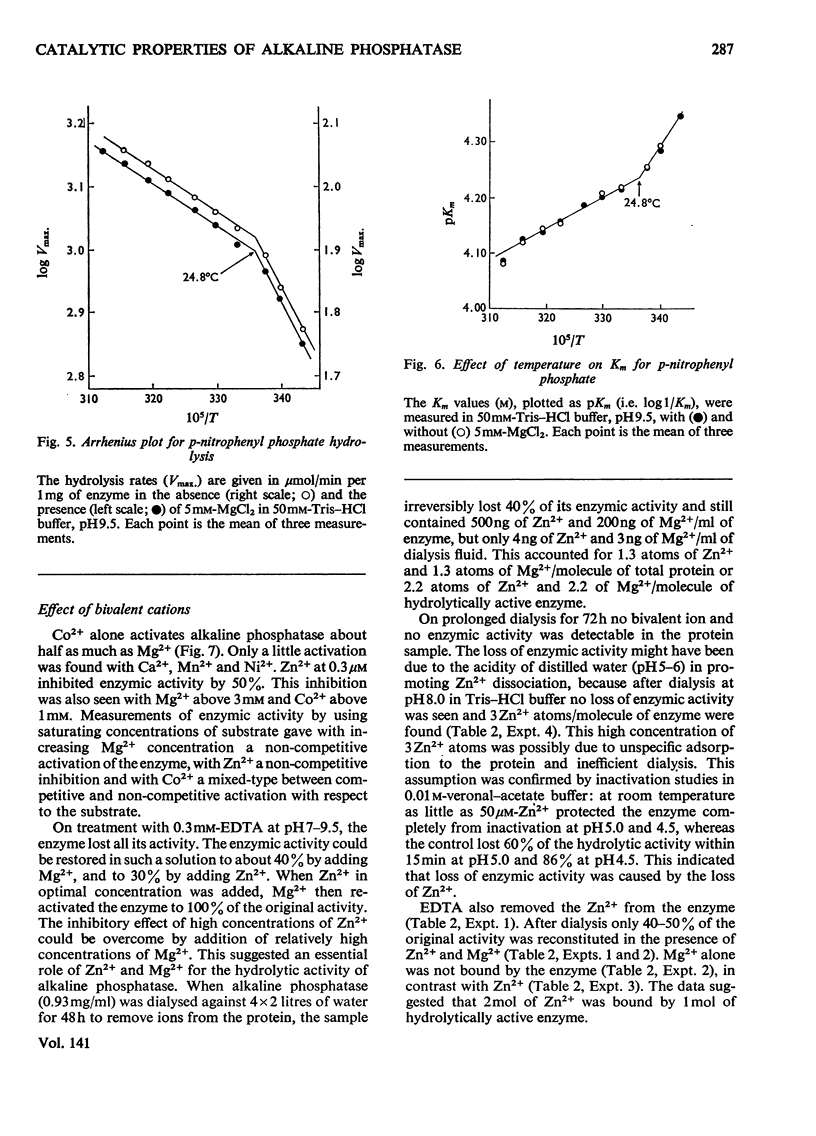
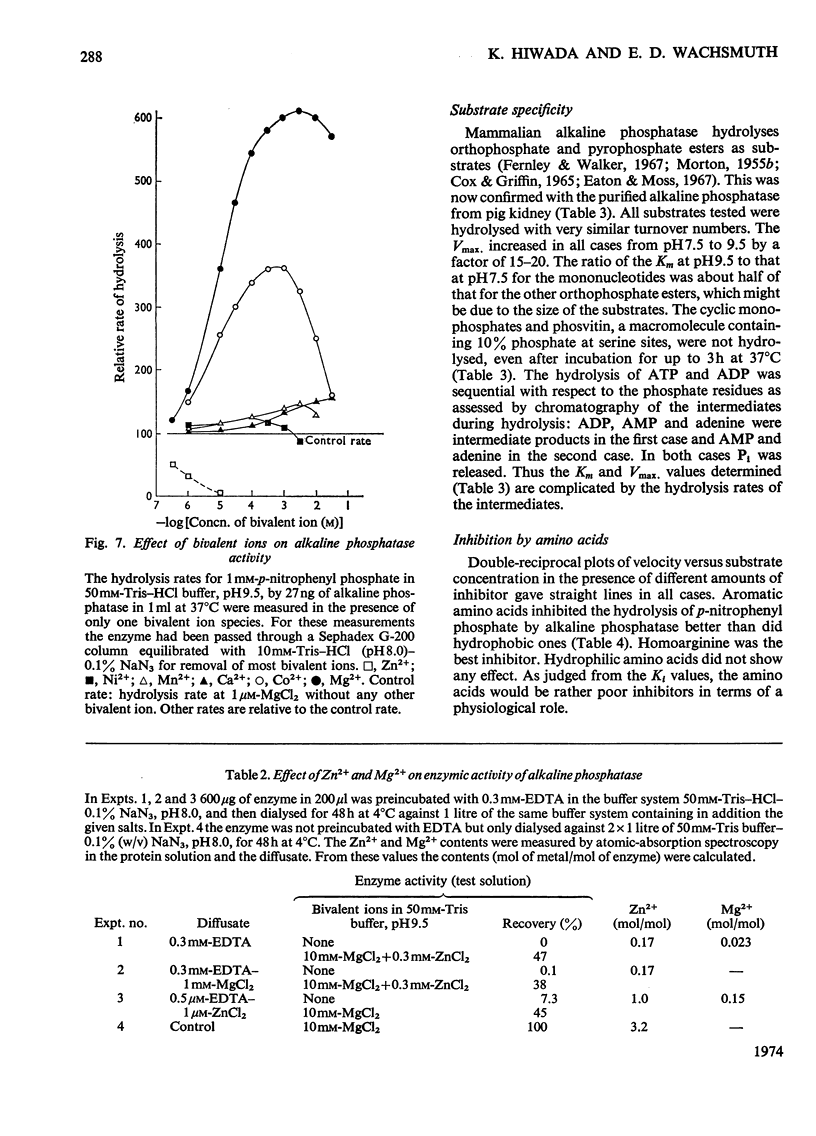
The enzymic properties of alkaline phosphatase (EC 3.1.3.1) from pig kidney brush-border membranes were studied. 1. It hydrolyses ortho- and pyro-phosphate esters, the rate limiting step (Vmax.) being independent of the substrate. It transphosphorylates to Tris at concentrations above 0.1m-Tris. 2. The pH optimum for hydrolysis was between 9.8 and 10. The pK of the enzyme–substrate complex is 8.7 for p-nitrophenyl phosphate and β-glycerophosphate. Excess of substrate inhibits the enzymic activity with decreasing pH. The pK of the substrate-inhibited enzyme–substrate complex, 8.7, is very similar to that for the enzyme–substrate complex. The pK values of the free enzyme appear to be 8.7 and 7.9. 3. Inactivation studies suggest that there is an essential tyrosine residue at the active centre of the enzyme. 4. The energy of activation (E) and the heat of activation (ΔH) at pH9.5 showed a transition at 24.8°C that was unaffected by Mg2+. 5. Kinetic and atomic-absorption analysis indicated the essential role of two Zn2+ ions/tetrameric enzyme for an ordered association of the monomers. Zn2+ in excess and other bivalent ions compete for a second site with Mg2+. Mg2+ enhances only the rate-limiting step of substrate hydrolysis. 6. Amino acid inhibition studies classified the pig kidney enzyme as an intermediate type of previously described alkaline phosphatases. It has more similarity with the enzyme from liver and bone than with that from placenta.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED Z., KING E. J. Kinetics of placental alkaline phosphatase. Biochim Biophys Acta. 1960 Dec 18;45:581–592. doi: 10.1016/0006-3002(60)91497-9. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Pike G. Z. Membrane marker enzymes: isolation, purification, and properties of 5'-nucleotidase from rat cerebellum. Biochim Biophys Acta. 1971 Feb 10;227(2):402–412. doi: 10.1016/0005-2744(71)90071-4. [DOI] [PubMed] [Google Scholar]
- CLARK B., PORTEOUS J. W. THE METAL ION ACTIVATION OF THE ALKALINE BETA-GLYCEROPHOSPHATASE OF RABBIT SMALL INTESTINE. Biochem J. 1965 May;95:475–482. doi: 10.1042/bj0950475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M. J., Butterworth P. J. The action of cyanate on human and pig kidney alkaline phosphatases. Biochem J. 1969 Mar;111(5):745–748. doi: 10.1042/bj1110745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delory G. E., King E. J. The rate of enzymic hydrolysis of phosphoric esters: 2. Relation of structure to dissociation constant, Michaelis constant, and rate of hydrolysis. Biochem J. 1943;37(5):547–550. doi: 10.1042/bj0370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGSTROM L. Studies on calf-intestinal alkaline phosphatase. II. Incorporation of inorganic phosphate into a highly purified enzyme preparation. Biochim Biophys Acta. 1961 Sep 2;52:49–59. doi: 10.1016/0006-3002(61)90902-7. [DOI] [PubMed] [Google Scholar]
- Eaton R. H., Moss D. W. Inhibition of the orthophosphatase and pyrophosphatase activities of human alkaline-phosphatase preparations. Biochem J. 1967 Mar;102(3):917–921. doi: 10.1042/bj1020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley H. N., Walker P. G. Kinetic behaviour of calf-intestinal alkaline phosphatase with 4-methylumbelliferyl phosphate. Biochem J. 1965 Oct;97(1):95–103. doi: 10.1042/bj0970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley H. N., Walker P. G. Studies on alkaline phosphatase. Inhibition by phosphate derivatives and the substrate specificity. Biochem J. 1967 Sep;104(3):1011–1018. doi: 10.1042/bj1041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Ghosh N. K. Influence of reagents reacting with metal, thiol and amino sites of catalytic activity and l-phenylalanine inhibition of rat intestinal alkaline phosphatase. Biochem J. 1967 Dec;105(3):1163–1170. doi: 10.1042/bj1051163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Kay H. D. The alkaline phosphomonoesterase of the mammary gland. Biochem J. 1935 Aug;29(8):1837–1850. doi: 10.1042/bj0291837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. On the mechanism of inhibition of intestinal alkaline phosphatase by L-phenylalanine. I. Kinetic studies. J Biol Chem. 1966 Jun 10;241(11):2516–2522. [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. Purification and properties of molecular-weight variants of human placental alkaline phosphatase. Biochem J. 1968 Aug;108(5):779–792. doi: 10.1042/bj1080779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N. K. Purification and molecular properties of placental and intestinal alkaline phosphatases. Ann N Y Acad Sci. 1969 Oct 14;166(2):604–640. doi: 10.1111/j.1749-6632.1969.tb46423.x. [DOI] [PubMed] [Google Scholar]
- Harkness D. R. Studies on human placental alkaline phosphatase. II. Kinetic properties and studies on the apoenzyme. Arch Biochem Biophys. 1968 Aug;126(2):513–523. doi: 10.1016/0003-9861(68)90436-0. [DOI] [PubMed] [Google Scholar]
- Harkness E. R. Studies on human placental alkaline phosphatase. I. Purification and crystallization. Arch Biochem Biophys. 1968 Aug;126(2):503–512. doi: 10.1016/0003-9861(68)90435-9. [DOI] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- Lin C. W., Fishman W. H. L-Homoarginine. An organ-specific, uncompetitive inhibitor of human liver and bone alkaline phosphohydrolases. J Biol Chem. 1972 May 25;247(10):3082–3087. [PubMed] [Google Scholar]
- MORTON R. K. Some properties of alkaline phosphatase of cow's milk and calf intestinal mucosa. Biochem J. 1955 Aug;60(4):573–582. doi: 10.1042/bj0600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The kinetics of hydrolysis of phenyl phosphate by alkaline phosphatases. Biochem J. 1957 Apr;65(4):674–682. doi: 10.1042/bj0650674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The purification of aklaline phosphatases of animal tissues. Biochem J. 1954 Aug;57(4):595–603. doi: 10.1042/bj0570595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The substrate specificity and inhibition of alkaline phosphatases of cow's milk and calf intestinal mucosa. Biochem J. 1955 Oct;61(2):232–240. doi: 10.1042/bj0610232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. Transferase activity of hydrolytic enzymes. Nature. 1953 Jul 11;172(4367):65–68. doi: 10.1038/172065a0. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Gutfreund H. The kinetics of the reaction of nitrophenyl phosphates with alkaline phosphatase from Escherichia coli. Biochem J. 1968 Jan;106(2):455–460. doi: 10.1042/bj1060455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmuth E. D., Hiwada K. Alkaline phosphatase from pig kidney. Method of purification and molecular properties. Biochem J. 1974 Jul;141(1):273–282. doi: 10.1042/bj1410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]


