Abstract
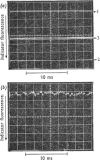
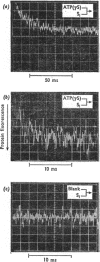
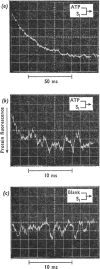
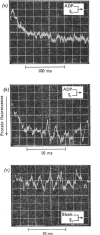
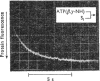
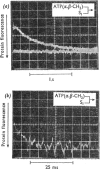
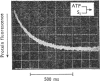
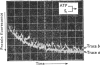
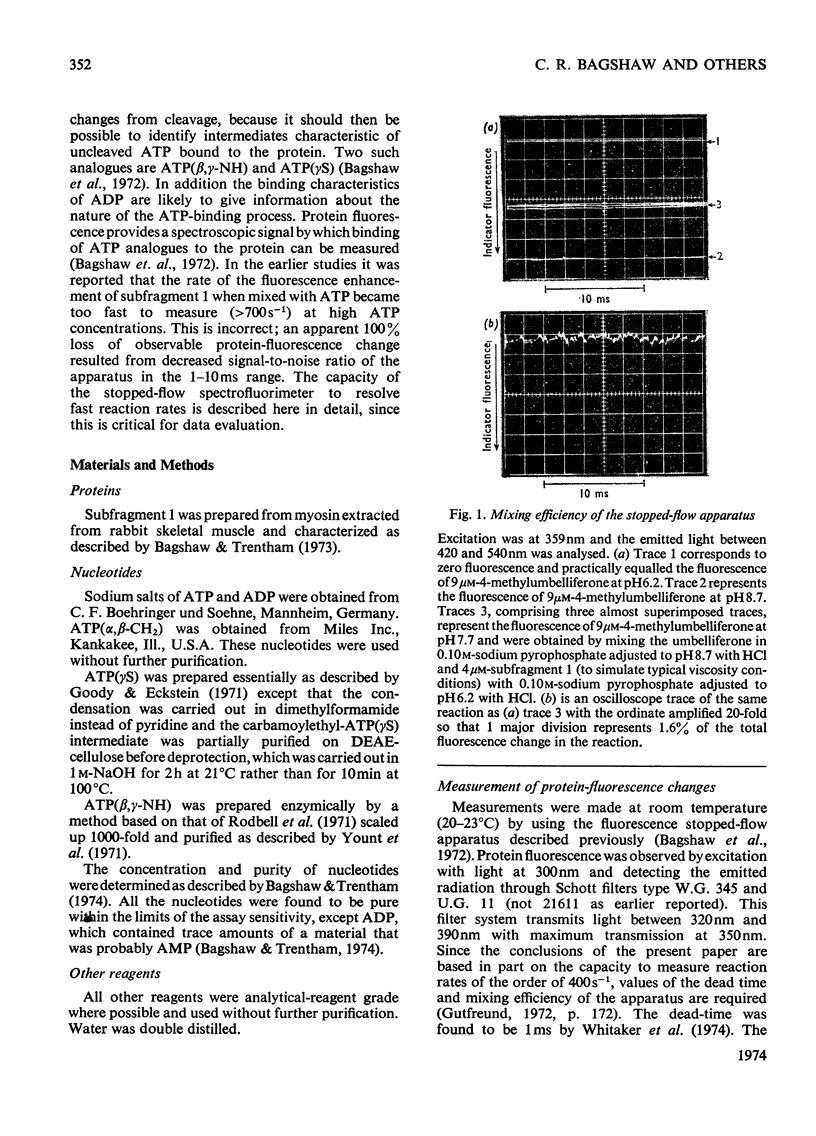
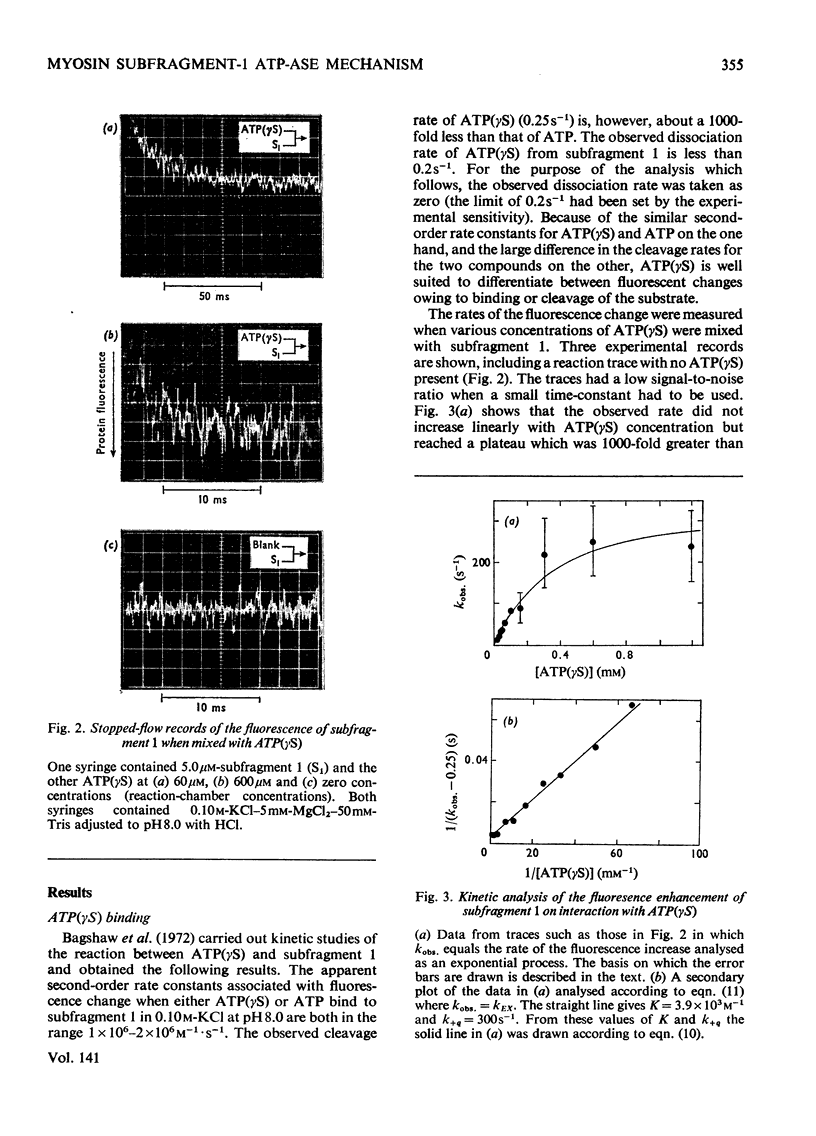
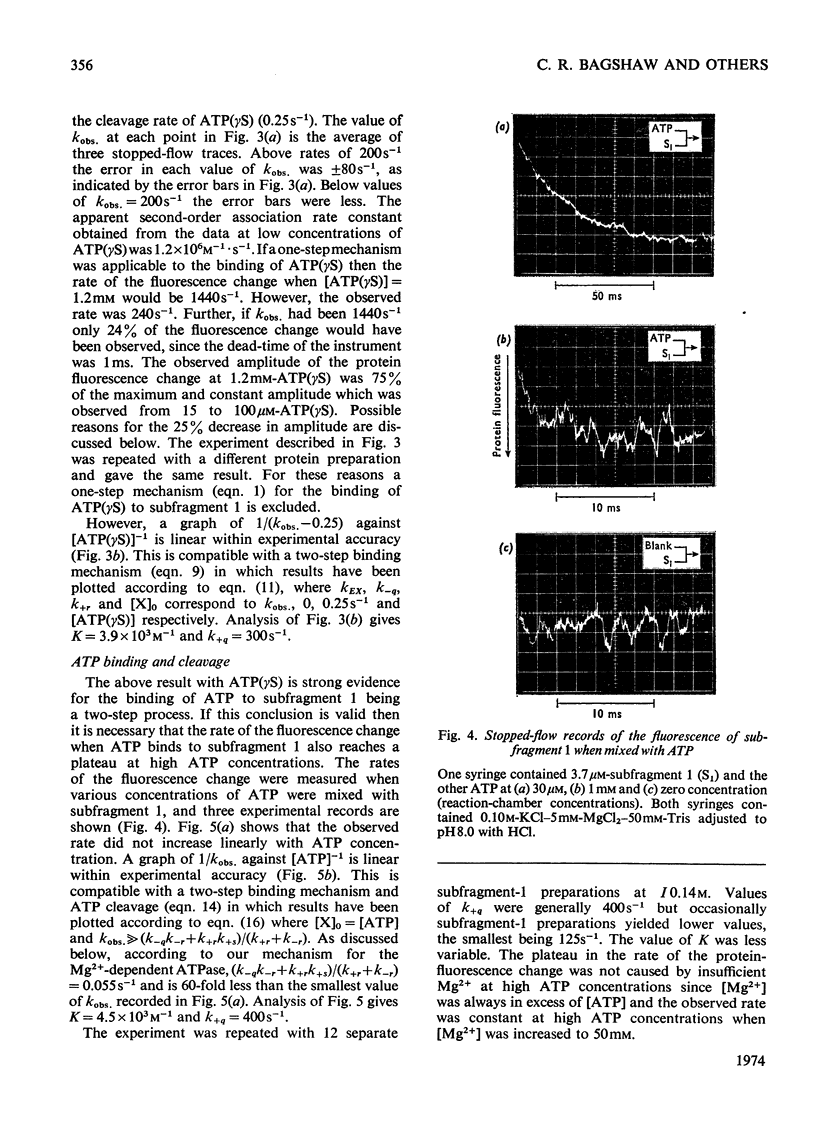
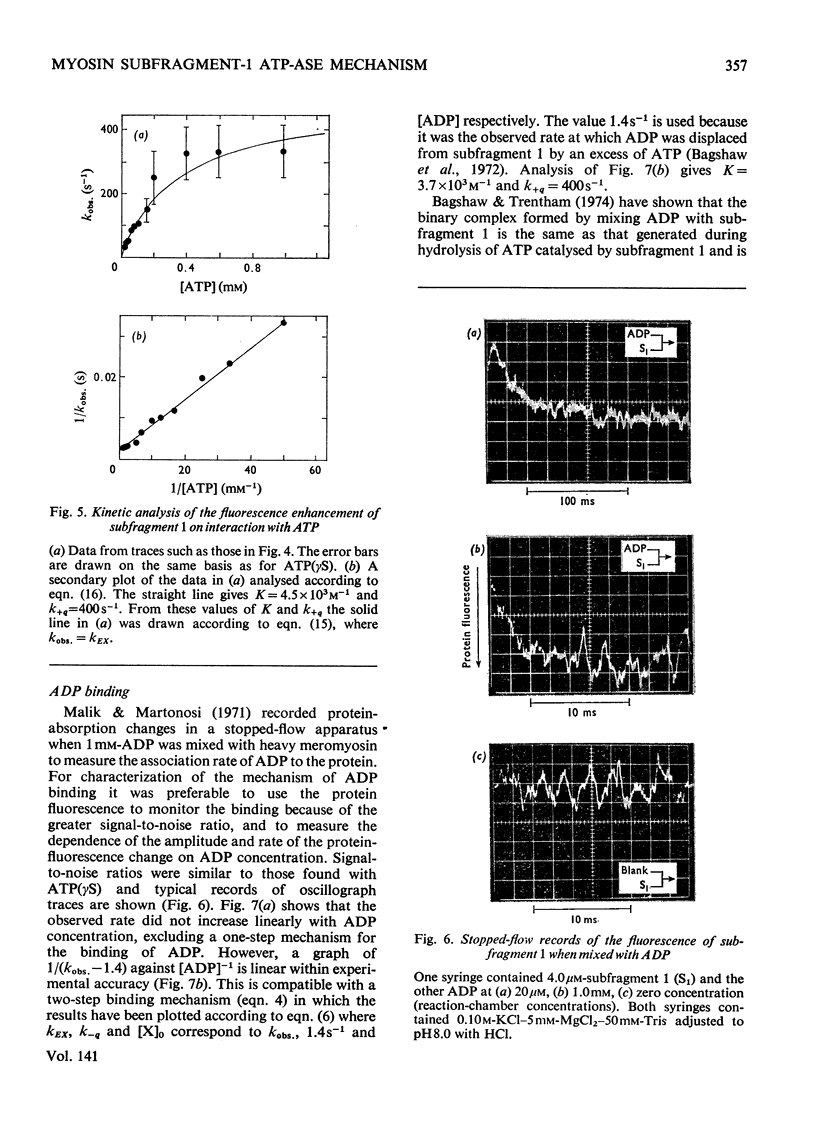
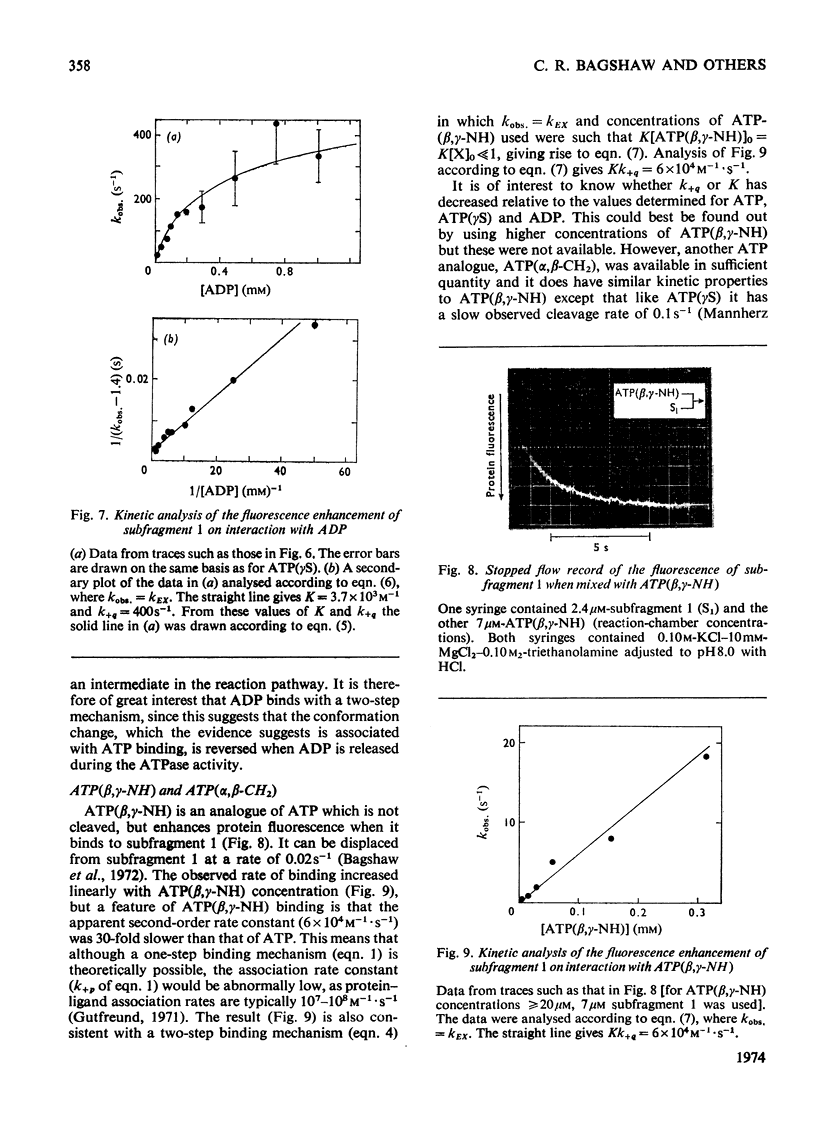
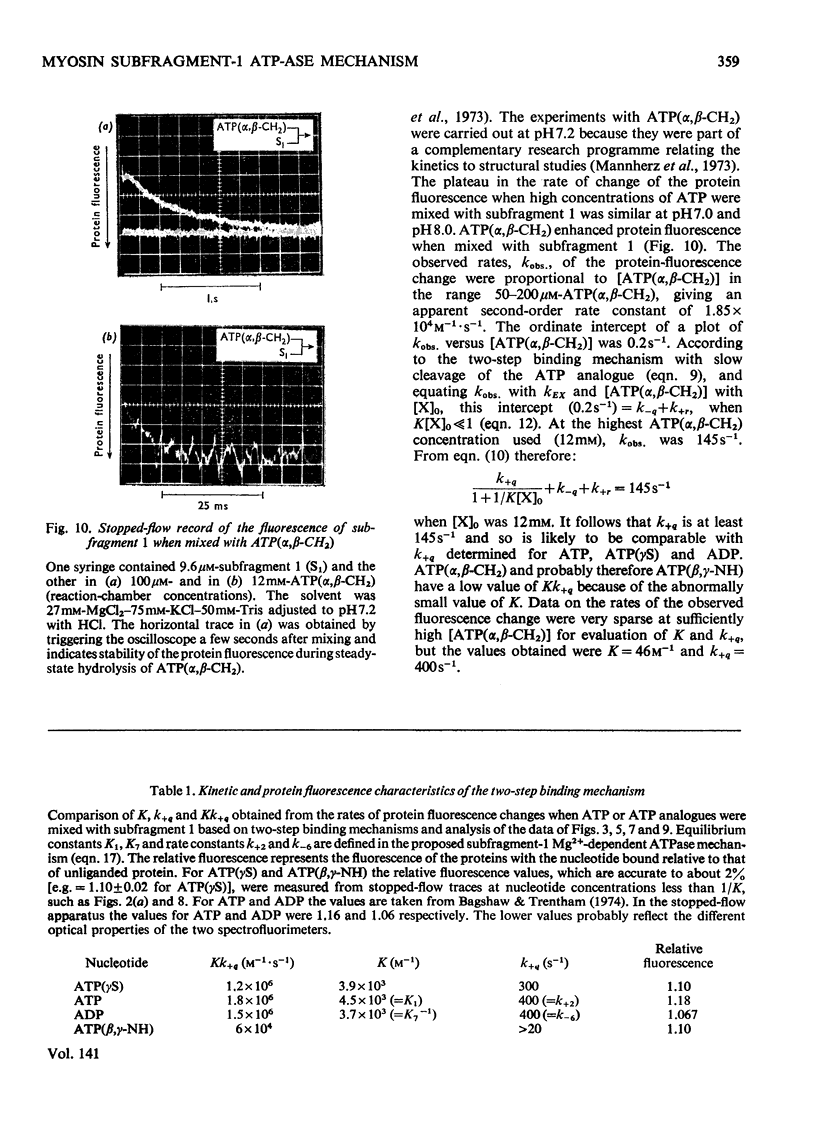
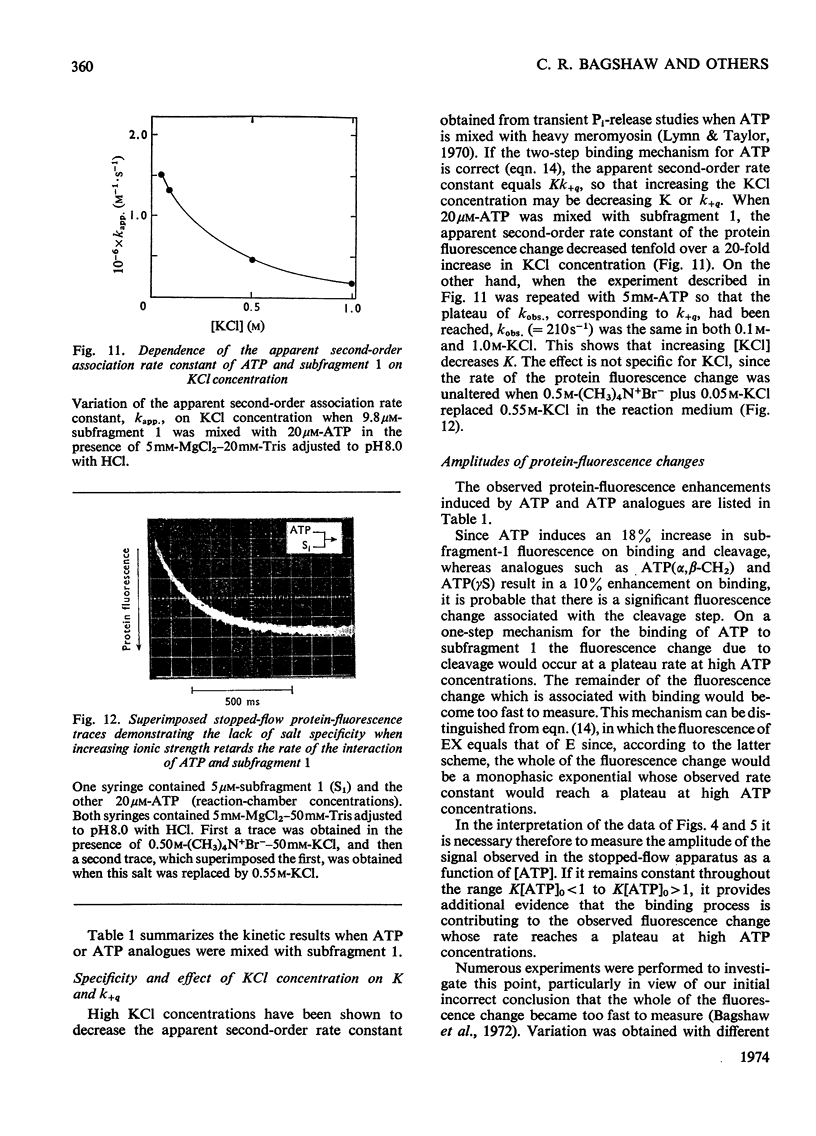
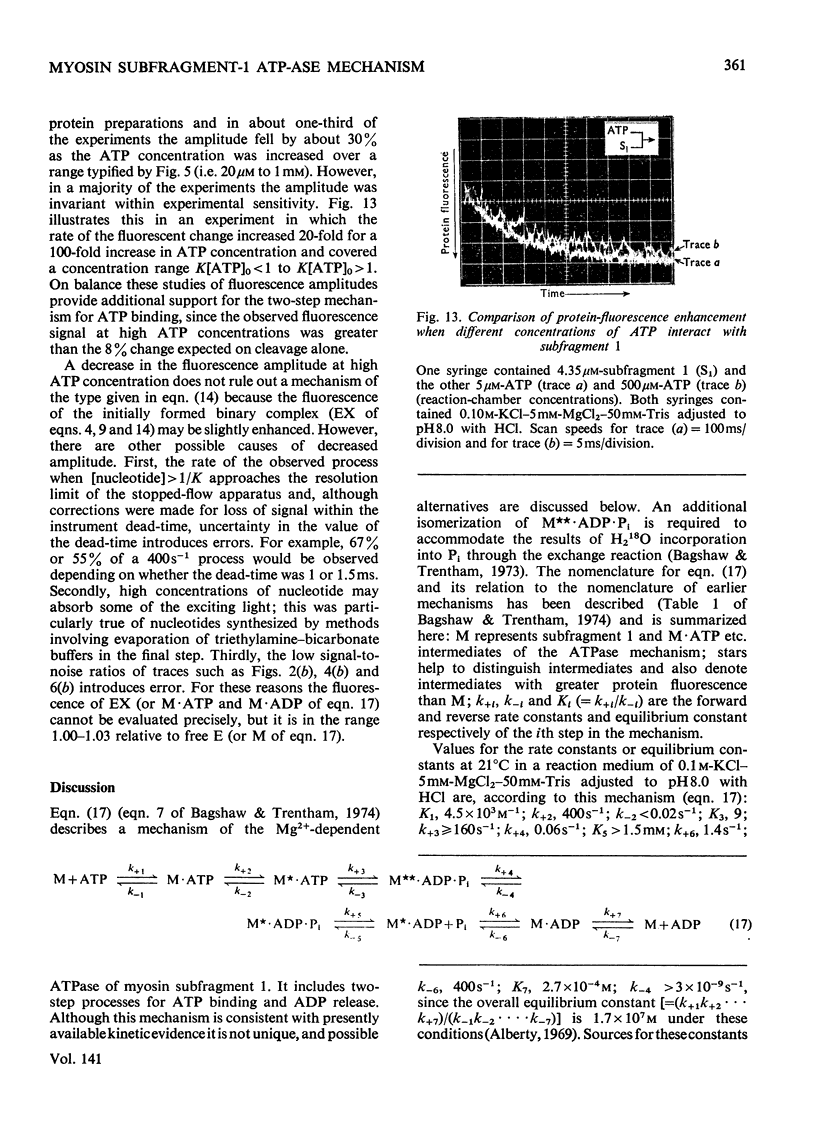
The kinetics of protein-fluorescence change when rabbit skeletal myosin subfragment 1 is mixed with ATP or adenosine 5′-(3-thiotriphosphate) in the presence of Mg2+ are incompatible with a simple bimolecular association process. A substrate-induced conformation change with ΔG0<−24kJ·mol−1 (i.e. ΔG0 could be more negative) at pH8 and 21°C is proposed as the additional step in the binding of ATP. The postulated binding mechanism is M+ATP⇌M·ATP⇌M*·ATP, where the association constant for the first step, K1, is 4.5×103m−1 at I 0.14m and the rate of isomerization is 400s−1. In the presence of Mg2+, ADP binds in a similar fashion to ATP, the rate of the conformation change also being 400s−1, but with ΔG0 for that process being −14kJ·mol−1. The effect of increasing ionic strength is to decrease K1, the kinetics of the conformation change being essentially unaltered. Alternative schemes involving a two-step binding process for ATP to subfragment 1 are possible. These are not excluded by the experimental results, although they are perhaps less likely because they imply uncharacteristically slow bimolecular association rate constants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. D., Cross R. L., Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H. C., Morales M. F. Studies of myosin conformation by fluorescent techniques. Biochemistry. 1969 May;8(5):2177–2182. doi: 10.1021/bi00833a059. [DOI] [PubMed] [Google Scholar]
- Dos Remedios C. G., Yount R. G., Morales M. F. Individual states in the cycle of muscle contraction. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2542–2546. doi: 10.1073/pnas.69.9.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Gutfreund H. Transients and relaxation kinetics of enzyme reactions. Annu Rev Biochem. 1971;40:315–344. doi: 10.1146/annurev.bi.40.070171.001531. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Bennett N. G., Trentham D. R., Gutfeund H. A substate-induced conformation change in the reaction of alkaline phosphatase from Escherichia coli. Biochem J. 1969 Sep;114(2):243–251. doi: 10.1042/bj1140243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E. Escherichia coli alkaline phosphatase. An analysis of transient kinetics. Biochem J. 1971 Nov;125(1):319–327. doi: 10.1042/bj1250319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E. Escherichia coli alkaline phosphatase. Relaxation spectra of ligand binding. Biochem J. 1972 Feb;126(3):727–738. doi: 10.1042/bj1260727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Transient state phosphate production in the hydrolysis of nucleoside triphosphates by myosin. Biochemistry. 1970 Jul 21;9(15):2975–2983. doi: 10.1021/bi00817a007. [DOI] [PubMed] [Google Scholar]
- Malik M. N., Martonosi A. Equilibrium and rapid kinetic studies of the effect of N-ethylmaleimide on the binding of ADP to myosin, and H-meromyosin. Arch Biochem Biophys. 1971 Jun;144(2):556–565. doi: 10.1016/0003-9861(71)90361-4. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Fluorimetric studies on the influence of metal ions and chelators on the interaction between myosin and ATP. FEBS Lett. 1973 Jul 1;33(2):161–166. doi: 10.1016/0014-5793(73)80183-8. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Leigh J. B., Holmes K. C., Rosenbaum G. Identification of the transitory complex myosin-ATP by the use of , -methylene-ATP. Nat New Biol. 1973 Feb 21;241(112):226–229. doi: 10.1038/newbio241226a0. [DOI] [PubMed] [Google Scholar]
- Morita F. Interaction of heavy meromyosin with substrate. I. Difference in ultraviolet absorption spectrum between heavy meromyosin and its Michaelis-Menten complex. J Biol Chem. 1967 Oct 10;242(19):4501–4506. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale R. O. Resolution of two simple protein-ligand binding schemes with kinetic measurements. J Theor Biol. 1971 Jun;31(3):501–507. doi: 10.1016/0022-5193(71)90025-7. [DOI] [PubMed] [Google Scholar]
- Watterson J. G., Schaub M. C. Conformational differences in Myosin, II. Evidence for differences in the conformation induced by bound or hydrolyzed adenosine triphosphate. Hoppe Seylers Z Physiol Chem. 1973 Dec;354(12):1619–1625. doi: 10.1515/bchm2.1973.354.2.1619. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- Werber M. M., Szent-Györgyi A. G., Fasman G. D. Fluorescence studies on heavy meromyosin-substrate interaction. Biochemistry. 1972 Jul 18;11(15):2872–2883. doi: 10.1021/bi00765a021. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Yates D. W., Bennett N. G., Holbrook J. J., Gutfreund H. The identification of intermediates in the reaction of pig heart lactate dehydrogenase with its substrates. Biochem J. 1974 Jun;139(3):677–697. doi: 10.1042/bj1390677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]