Abstract
The current manuscript presents a mathematical model of dengue fever transmission with an asymptomatic compartment to capture infection dynamics in the presence of uncertainty. The model is fuzzified using triangular fuzzy numbers (TFNs) approach. The obtained fuzzy-fractional dengue model is then solved and analyzed through fuzzy extension of modified residual power series algorithm, which utilizes residual power series along with Laplace transform. Numerical analysis has also been performed in this study and obtained results are shown as solutions and residual errors for each compartment to ensure the validity. Graphical analysis depict the model’s behavior under varying parameters, illustrating contrasting trends for different values of  and examining the impacts of transmission and recovery rates on dengue model in uncertain environment. The current findings highlighted the effectiveness of proposed uncertainty in epidemic system dynamics, offering new insights with potential applications in other areas of engineering, science and medicine.
and examining the impacts of transmission and recovery rates on dengue model in uncertain environment. The current findings highlighted the effectiveness of proposed uncertainty in epidemic system dynamics, offering new insights with potential applications in other areas of engineering, science and medicine.
Keywords: Fuzzy-fractional model, Caputo fractional derivative, Triangular fuzzy numbers, Residual power series
Subject terms: Infectious diseases, Applied mathematics
Introduction
Dengue is one of the rapidly growing disease, transmitted by mosquitoes. Dengue cases have been increasing throughout the world. Approximately 3.9 billion people of 128 countries1 are living in the dengue risk area. Globally, 390 million new cases of dengue infection are reported each year2. Currently, there are 90 countries experiencing active dengue transmission in 2024. Still there is no approved vaccine or antiviral medication for this disease. According to world health organization, approximately 2.5 percent of dengue patients died each year1. Dengue fever spread in an endemic area due to one of dengue viruses of different serotypes DENV-i, where i=1,2,3,4. Dengue viruses belongs to single-stranded RNA viruses and can spread through different type of mosquitoes including Asian Tiger mosquito, Aedes Albopictus, and day-feeding mosquito3.
Mathematical models are useful for understanding the dynamics of disease transmission to identify the variables that influence disease spread which ultimately helps in proposing disease control strategies4,5. Mathematical modeling for investigating dynamics of infectious diseases has a long history. Kermack and McKendrick initially proposed SIR model for mathematical research on infectious diseases6. Esteva and Vargas reformulated this model in the dengue vector host dynamics for calculating variable and constant human populations7,8. Since then, dengue disease transmission dynamics have been studied by many researchers. Gakkhar et al. studies dengue infection with and without awareness effect in Ref.9. Phaijoo and Gurung analyzed mobility effect on the spread of dengue in Ref.10. Agarwal and Verma have made significant contributions to epidemiological modeling by addressing various infectious diseases11–13. Different scholars have studied various epidemic diseases for better control and prediction purposes14–18.
It is observed that most of the work present in literature is analyzed using deterministic approach in modeling by considering fixed constant values of parameters. They assume that every individual could spread the disease and heal from it at a constant rate. However, such an assumption is in conflict with actual epidemic. There is always uncertainty in the parameters like transmission and recovery rates. In biological models, Zadeh was to first one to introduce uncertainty19 by developing fuzzy set theory. Mondal et al.20 modified SIS epidemic model by considering disease transmission and treatment control parameters with uncertainties. De Barros et al. considered interactivity along with fuzziness in SI model of disease transmission21–23.
A fractional derivative is generalization of integer order derivative. Fractional derivatives also play vital role in modeling and analysis of differential systems and provide better insights with memory effects24. A fractional derivative approach is applied to many physical models, such as Fisher reaction-diffusion model25, Lotka-Volterra population model26, Schrodinger model27, fuid flow model28, cancer-tumor models29,30, fuzzy fisher models31,32 and Wu-Zhang model33. Chatterjee and his co-authors have worked on developing epidemic models for measles and COVID-19 using fractional order derivatives34–37. Ullah and his co-authors have explored innovative applications of fractional-order models to epidemic dynamics and policy interventions38–42. Fuzzy-fractional differential equations (FFDEs) allow us to model and examine real-world problems including fractional order derivatives and uncertainty. Chellamani et al. applied fuzzy-fractional order approach to epidemic model for COVID-1943. Qayyum and Tahir made a important contribution to the field by exploring a mathematical model for cancer tumor dynamics using multiple fuzzification approaches within a fractional environment44. Qayyum et al. employed the extended He-Mohand algorithm within a fuzzy-Caputo framework to capture the dynamics of the system under uncertainty by modeling and analyzing a fuzzy-fractional chaotic financial system45.
Motivated from above literature, a new model in fuzzy-fractional environment using triangular fuzzy numbers along with Caputo fractional derivative has been proposed for the analysis of epidemic dengue transmission. We apply this new idea to dengue transmission model and investigates some new insights into the dengue dynamics. According to the author’s knowledge, no one had previously used the concept of a fuzzy fractional to dengue dynamics, so this work is unique. For researchers working on epidemic models, the use of asymptomatic carriers and its analysis in fuzzy fractional environment will open up new doors of investigation. The remaining paper is structured as follows: in Section 2, the preliminaries are discussed. Section 3 focuses on the transmission dynamics of the dengue model. Section 4 provides a stability analysis for the reproduction number using the next-generation matrix method. Section 5 presents the fuzzy fractional modeling of the dengue virus. In Section 6, the application of the extended Residual Power Series Algorithm to fuzzy fractional dengue model is explored. Section 7 presents the numerical results and discussion of findings, and finally, Section 8 concludes the paper.
Preliminaries
Definition 1
Reference45 The Caputo fractional derivative  of a function
of a function  is defined by:
is defined by:
 |
1 |
Definition 2
Reference45 Let  be a piece-wise continuous function on the interval
be a piece-wise continuous function on the interval  of exponential order
of exponential order  , the Laplace transform of
, the Laplace transform of  ,
,  is given by
is given by
 |
2 |
and the inverse Laplace transform of  is given by
is given by
 |
3 |
The necessary properties of the Laplace transform and its inverse are summarize in the following Lemma.
Lemma 1
Reference46 Let  ,
,  be a piecewise continuous function on the interval
be a piecewise continuous function on the interval  . If
. If
 ,
,  and
and  then
then
 .
. .
. .
. for
for  .
. ,
,  .
.
Definition 3
Reference45 Let  be a real set. Then, a fuzzy set
be a real set. Then, a fuzzy set  in
in  can be characterized by a membership function
can be characterized by a membership function  , where,
, where, :
:  An r-level set of
An r-level set of  is
is  =
= for
for 
There are following conditions for a fuzzy set  to be a fuzzy number:
to be a fuzzy number:
 is normal, that is, for
is normal, that is, for  we have
we have  .
. is convex,that is
is convex,that is  min
min  for all
for all  and
and 
 is semi-continuous.
is semi-continuous.Te set
 is compact.
is compact.
Definition 4
Reference45 A fuzzy number  is categorized as a triangular fuzzy number (TFN) if it is defined by three distinct values
is categorized as a triangular fuzzy number (TFN) if it is defined by three distinct values  where
where  , forming a triangular shape. The membership function for this TFN is expressed as follows:
, forming a triangular shape. The membership function for this TFN is expressed as follows:
 |
The interval form of TFN can be expressed as follows by using the r-Cut notation
 |
4 |
where  and
and  represent the upper and lower bounds, respectively, for
represent the upper and lower bounds, respectively, for 
Definition 5
Reference45 A fuzzy number  can also be expressed as
can also be expressed as  , satisfying the following condition:
, satisfying the following condition:
 is a left continuous bounded monotonic increasing function.
is a left continuous bounded monotonic increasing function. is a left continuous bounded monotonic decreasing function.
is a left continuous bounded monotonic decreasing function. for
for 
Dengue model transmission
In an existing host-vector model for dengue transmission, human populations were traditionally divided into five compartments: exposed  , susceptible
, susceptible  , symptomatic
, symptomatic  (class of individuals who have a dengue fever and showing the symptoms), hospitalized
(class of individuals who have a dengue fever and showing the symptoms), hospitalized  , and recovered
, and recovered  , and three mosquito populations including exposed
, and three mosquito populations including exposed  , susceptible
, susceptible  , and infectious
, and infectious  .
.  . The sum of total human individual population defined as:
. The sum of total human individual population defined as:
 |
However, in the present work, we have extended this model by adding a new compartment for asymptomatic individuals  . This new compartment represents individuals who are infected with dengue but do not show symptoms. Despite being asymptomatic, these individuals contribute to the spread of the disease and can infect others.
. This new compartment represents individuals who are infected with dengue but do not show symptoms. Despite being asymptomatic, these individuals contribute to the spread of the disease and can infect others.
By incorporating the asymptomatic class into the model, we aim to more accurately capture the dynamics of dengue transmission, as asymptomatic individuals play a significant role in the overall infection process. With the above discussion, we formulated a modified dengue model which is given as follows:
 |
5 |
with
 |
6 |
The biological description and values of parameters of model 5 are given in Table 1.
Table 1.
Biological description and values of parameters for dengue model.
| Parameters | Description | Values | References |
|---|---|---|---|

|
Mosquito’s recruitment rate | 3839.9 
|
47 |

|
Biting rate per mosquito per person | 1.1971 
|
47 |

|
Transmission rate from infected human to susceptible mosquito | 0.8541 | 47 |

|
Mosquito’s natural death rate | 0.0244 
|
47 |

|
Incubation rate of mosquito | 0.7186 
|
47 |

|
Human’s recruitment rate | 1525.1426 
|
47 |

|
Transmission rate from infected mosquito to susceptible human | 0.6794 | 47 |

|
Incubation rate of human | 0.5550 
|
47 |

|
Human’s natural death rate |


|
47 |

|
Proportion of exposed to asymptomatic class | 0.4450 
|
Assumed |

|
Natural recovery rate of infected human | 0.0154 
|
47 |

|
Recovery rate of hospitalized infected human | 0.0840 
|
47 |

|
Recovery rate of asymptomatic humans | 0.9846 
|
Assumed |

|
Hospitalization rate of infected human | 0.0904 
|
47 |

|
Death rate of human from disease | 0.0969 
|
47 |
Consider the feasible region
 |
with
 |
7 |
Lemma 2
Reference47 The region given by  is positively invariant for model (5) with the non-negative initial conditions (6).
is positively invariant for model (5) with the non-negative initial conditions (6).
Proof
The dengue model 5 leads to the following form:
 |
and
 |
Hence,  , if
, if  and
and  , if
, if  . So,
. So,
 |
and
 |
Thus, the region defined by  is positively invariant. Furthermore, if
is positively invariant. Furthermore, if  and
and  , then either the solutions will enter
, then either the solutions will enter  within a finite time, or
within a finite time, or  will asymptotically approach
will asymptotically approach  and
and  will asymptotically approach
will asymptotically approach  . Consequently, the regions defined by
. Consequently, the regions defined by  attract all solutions in
attract all solutions in  .
.
Stability analysis
This section investigates the stability of dengue model for disease-free equilibrium ( ). By setting right side of (5) equal to zero, following expressions is obtained:
). By setting right side of (5) equal to zero, following expressions is obtained:
 |
Reproduction number  using the next generation matrix method we find reproduction number
using the next generation matrix method we find reproduction number  for dengue model (5). Considering the infected compartments in (5), specifically
for dengue model (5). Considering the infected compartments in (5), specifically  ,
,  ,
,  ,
,  ,
,  , and
, and  and following the guidelines outlined in Ref.48, we derive the following matrices:
and following the guidelines outlined in Ref.48, we derive the following matrices:
 |
and
 |
Let  ,
,  ,
,  , and
, and  . The essential basic reproduction number for the model is determined through the spectral radius of the matrix
. The essential basic reproduction number for the model is determined through the spectral radius of the matrix  , as defined by the following equation:
, as defined by the following equation:
 |
 represents the average number of secondary infections occurring in both mosquitoes and human hosts due to one invective individual throughout their infectivity period. This parameter serves as an indicator of the potential spread of an emerging infectious disease within a community or population, and it helps determine the proportion of the population that should be vaccinated to achieve disease eradication. In biological models,
represents the average number of secondary infections occurring in both mosquitoes and human hosts due to one invective individual throughout their infectivity period. This parameter serves as an indicator of the potential spread of an emerging infectious disease within a community or population, and it helps determine the proportion of the population that should be vaccinated to achieve disease eradication. In biological models,  , means infection will be uncontrollably spread in the population and hence will be more challenging to control epidemic. In the following lines, local stability of disease-free equilibrium (DFE)
, means infection will be uncontrollably spread in the population and hence will be more challenging to control epidemic. In the following lines, local stability of disease-free equilibrium (DFE)  for the model (5).
for the model (5).
Theorem 3
 remains locally asymptotically stable for system (5) whenever
remains locally asymptotically stable for system (5) whenever  .
.
Proof
The Jacobian matrix by evaluating the model (5) at the disease-free equilibrium  , is:
, is:
 |
From the matrix  provided above, it can observed that the eigenvalues
provided above, it can observed that the eigenvalues  ,
,  ,
,  ,
,  and
and  are clearly negative. The remaining four eigenvalues with negative real parts can be determined using the following equations:
are clearly negative. The remaining four eigenvalues with negative real parts can be determined using the following equations:
 |
where
 |
The coefficients represented by  for
for  are positive for
are positive for  ,
,  , and
, and  , whereas
, whereas  may assume positive or negative depending on the value of
may assume positive or negative depending on the value of  . In the case of the disease-free equilibrium (DFE), where the value of the basic reproduction number should be less than 1, the last coefficient becomes positive when
. In the case of the disease-free equilibrium (DFE), where the value of the basic reproduction number should be less than 1, the last coefficient becomes positive when  . Consequently, all coefficients
. Consequently, all coefficients  for
for  are positive, and they must conform the Routh-Hurwitz criteria. This criteria can be readily satisfied under the provided conditions
are positive, and they must conform the Routh-Hurwitz criteria. This criteria can be readily satisfied under the provided conditions  , where
, where  for all
for all  . This condition ensures that
. This condition ensures that  .
.
Here
 |
Therefore, the satisfaction of the Routh-Hurwitz criteria guarantees the local asymptotic stability of the dengue model described in (5) at the disease-free equilibrium  .
. 
Fuzzy-fractional modeling of dengue virus
This section focuses on modeling of fuzzy fractional dengue system. The specific modified dengue system (5-6) were examined.
The system (5) is modeled in fractional form by utilizing Definition 3, which is presented in the following equations. By using the definition 1, the given modified dengue system modeled in fractional form is given as
 |
8 |
where  is fractional parameter in a Caputo sense. By using Definitions 3-5, we have incorporated triangular fuzzy numbers (TFNs) approach in initia conditions
is fractional parameter in a Caputo sense. By using Definitions 3-5, we have incorporated triangular fuzzy numbers (TFNs) approach in initia conditions  to introduce uncertainty in the system. In parametric form, which can be written as:
to introduce uncertainty in the system. In parametric form, which can be written as:
 |
9 |
Thus, the fuzzy-fractional modified dengue system as follows
 |
10 |
with fuzzified conditions
 |
11 |
Application of extended residual power series algorithm to fuzzy-fractional dengue model
In this section, we write in details the steps of applying the LRPS to solve fuzzy fractional modified dengue system (10,11)
where  =
=  .
.  represents the upper bound solution and
represents the upper bound solution and  represents the lower bound solution.
represents the lower bound solution.
After applying the Laplace transform the system (10) and (11) we get the following
 |
12 |
Let we assume  fractional truncated series of
fractional truncated series of  and
and  as follows
as follows
 |
13 |
Where  are the unknown coefficients
are the unknown coefficients
 Laplace residual functions are
Laplace residual functions are
 |
14 |
To determine 
 let we consider
let we consider  in (14) as follows
in (14) as follows
 |
15 |
Since  we obtain
we obtain
 |
16 |
Multiply (16) by  . Then we solve the following system
. Then we solve the following system
 |
17 |
to obtain the following outputs
 |
18 |
In a same way unknown  can be found.
can be found.
with the fuzzified initial conditions
 |
19 |
Results and discussion
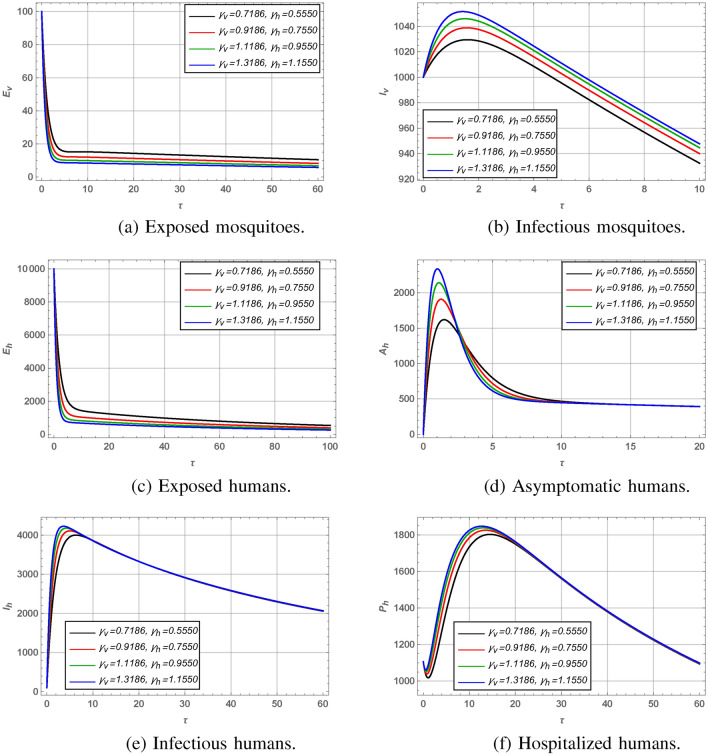
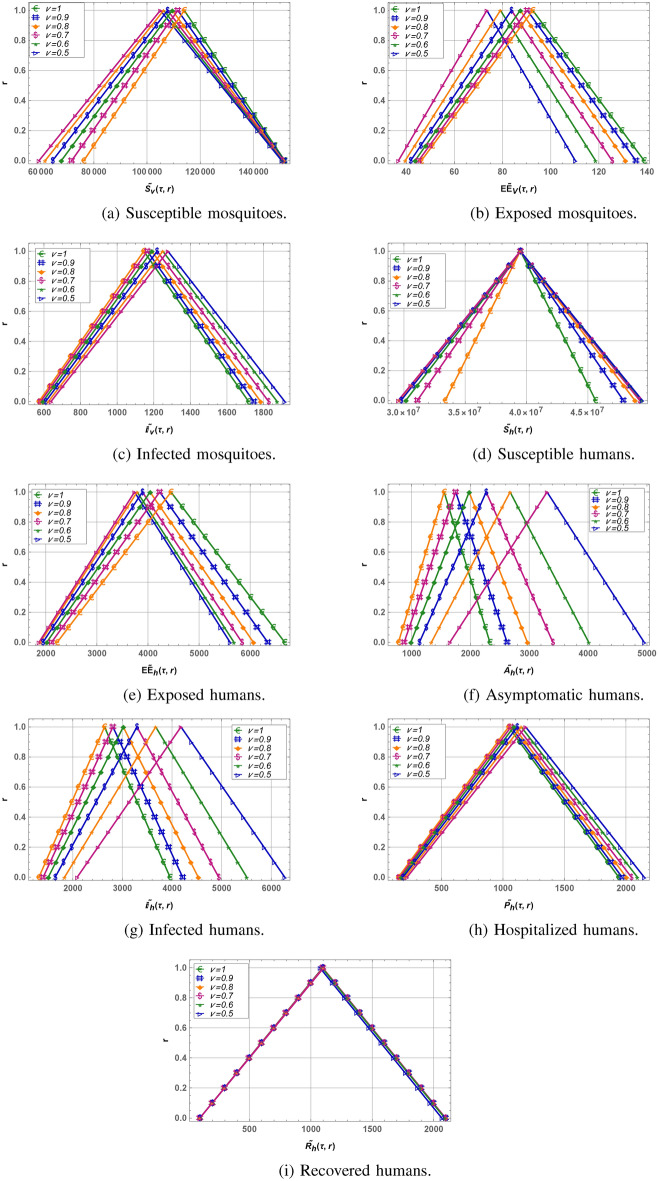
We modified an existing dengue model by adding an asymptomatic compartment to better represent individuals who are infected but do not show symptoms, yet still contribute to transmission. After this, we extended the model to a fuzzy-fractional framework to handle uncertainties and memory effects, using triangular fuzzy numbers to capture real-world variability. For the numerical analysis of the dengue fever model, we employed the Residual Power Series Method (RPSM) to derive approximate solutions and evaluate residual errors for various model compartments. Tables 2, 3 and 4 detail these approximate solutions and corresponding residual errors for the susceptible and infected classes of mosquitoes and humans, as well as for other compartments. Graphical analysis, presented in Figs. 1 and 2, explored the impacts of transmission and recovery rates on different model compartments, including exposed and infectious mosquito classes and exposed, infectious, asymptomatic, and hospitalized human classes. Figure 3 illustrates the lower and upper bounds of the fuzzy-fractional model for various values of the fractional parameter  , showing the range of outcomes under uncertainty represented by triangular fuzzy numbers. These analyses provide valuable insights into dengue transmission dynamics and assess the robustness of model predictions under uncertain conditions.
, showing the range of outcomes under uncertainty represented by triangular fuzzy numbers. These analyses provide valuable insights into dengue transmission dynamics and assess the robustness of model predictions under uncertain conditions.
Table 2.
RPS solution along with residual error for susceptible and infectious classes of mosquitoes and humans.
 |
 |
 |
 |
 |
||||
|---|---|---|---|---|---|---|---|---|
| RPS solution | Residual error | RPS solution | Residual error | RPS solution | Residual error | RPS solution | Residual error | |
| 0. | 100000 | 0 | 1000 | 0 |  |
0 | 100 | 0 |
| 0.1 | 100140 | 0 | 1004.48 |  |
 |
 |
338.898 | 0 |
| 0.2 | 100279 |  |
1008.46 |  |
 |
0 | 564.278 | 0 |
| 0.3 | 100418 | 0 | 1011.98 |  |
 |
 |
776.883 |  |
| 0.4 | 100557 |  |
1015.08 |  |
 |
 |
977.416 |  |
| 0.5 | 100695 |  |
1017.79 |  |
 |
 |
1166.54 |  |
| 0.6 | 100833 |  |
1020.15 |  |
 |
 |
1344.89 |  |
| 0.7 | 100970 |  |
1022.17 |  |
 |
 |
1513.04 |  |
| 0.8 | 101107 |  |
1023.9 |  |
 |
 |
1671.57 |  |
| 0.9 | 101244 |  |
1025.35 |  |
 |
 |
1821.01 |  |
| 1 | 101380 |  |
1026.54 |  |
 |
 |
1961.84 |  |
Table 3.
RPS solution along with residual error for Asymptomatic , Hospitalized and Recovered classes of humans.
 |
 |
 |
 |
|||
|---|---|---|---|---|---|---|
| RPS solution | Residual error | RPS solution | Residual error | RPS solution | Residual error | |
| 0. | 0 |  |
1106 |  |
1095 | 0 |
| 0.1 | 286.415 |  |
1088.15 |  |
1217.78 |  |
| 0.2 | 532.84 |  |
1072.69 | 0 | 1365.47 |  |
| 0.3 | 743.741 |  |
1059.48 |  |
1534.57 |  |
| 0.4 | 923.129 |  |
1048.35 |  |
1721.97 |  |
| 0.5 | 1074.6 |  |
1039.17 |  |
1924.93 |  |
| 0.6 | 1201.38 |  |
1031.79 |  |
2141.04 |  |
| 0.7 | 1306.35 |  |
1026.1 |  |
2368.15 |  |
| 0.8 | 1392.1 |  |
1021.98 |  |
2604.4 |  |
| 0.9 | 1460.93 |  |
1019.31 |  |
2848.15 |  |
| 1 | 1514.93 |  |
1017.98 |  |
3097.95 |  |
Table 4.
RPS solution along with residual error for Exposed classes of mosquitoes and humans.

|

|

|
||
|---|---|---|---|---|
| RPS solution | Residual error | RPS solution | Residual error | |
| 0 | 100 | 0 | 10000 | 0 |
| 0.1 | 92.8948 | 0 | 9539.36 |

|
| 0.2 | 86.3563 |

|
9103.92 |

|
| 0.3 | 80.3411 |

|
8692.29 |

|
| 0.4 | 74.8085 |

|
8303.14 |

|
| 0.5 | 69.7213 |

|
7935.23 |

|
| 0.6 | 65.0449 |

|
7587.39 |

|
| 0.7 | 60.7473 |

|
7258.5 |

|
| 0.8 | 56.7991 |

|
6947.51 |

|
| 0.9 | 53.173 |

|
6653.45 |

|
| 1 | 49.8438 |

|
6375.36 |

|
Fig. 1.
Effect of biting rate per person per mosquito on different classes of dengue model.
Fig. 2.
Effect of incubation rates of mosquitoes and humans on different classes of dengue model.
Fig. 3.
Fuzzy lower and upper bound solutions of fuzzy-fractional dengue model at different fractional points.
Conclusion
The objective of this research is modeling and simulation of fuzzy-fractional dengue virus by considering asymptomatic class of individuals. In current study, we combine fuzzy logic with fractional calculus to incorporate uncertainty along with memory effects in the dengue model. The Caputo sense of time-fractional derivative is employed for capturing memory effect while triangular fuzzy numbers (TFNs) approach is used for incorporating uncertainty in the dengue model. To solve and analyze obtained model, residual power series algorithm is hybridized with Laplace transform, and error analysis is illustrated through numerical tables. It is observed that errors are ranging from  to
to  , and
, and  to
to  , for mosquito and human classes respectively. From this, it can be observed that the proposed methodology is providing convergent solutions for dengue model. The impact of fractional parameter on the dengue system profile concerning the r-cut are analyzed. As the r-cut approaches to 1, the system solution becomes less-fuzzified, and eventually transitioning into a crisp form at
, for mosquito and human classes respectively. From this, it can be observed that the proposed methodology is providing convergent solutions for dengue model. The impact of fractional parameter on the dengue system profile concerning the r-cut are analyzed. As the r-cut approaches to 1, the system solution becomes less-fuzzified, and eventually transitioning into a crisp form at  . Moreover, the behavior of transmission and recovery rates are examined across various classes of mosquitoes and humans in the study. In conclusion, the modeled fuzzy-fractional dengue system offers deeper insights for better understanding, predictions and control assessments of dengue transmission dynamics. Furthermore, proposed methodology holds promise for addressing various research areas in enhancing our understanding of infectious diseases, guiding public health responses, and ultimately saving lives by preventing and controlling outbreaks.
. Moreover, the behavior of transmission and recovery rates are examined across various classes of mosquitoes and humans in the study. In conclusion, the modeled fuzzy-fractional dengue system offers deeper insights for better understanding, predictions and control assessments of dengue transmission dynamics. Furthermore, proposed methodology holds promise for addressing various research areas in enhancing our understanding of infectious diseases, guiding public health responses, and ultimately saving lives by preventing and controlling outbreaks.
Author contributions
M. Q. Conceptualization; Methodology; Validation; Writing, Review and Editing; Supervision. Q.F. Code Writing; Visualization; Validation; Writing Original Draft. A.A. Analysis; validation; Writing, review and editing of manuscript; Software. M.K.H. Analysis; validation; Writing, review and editing of manuscript; Software.
Data availibility
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ajlan, Balgees A. et al. Assessment of the new world health organization’s dengue classification for predicting severity of illness and level of healthcare required. PLoS Negl. Trop. Dis.13(8), e0007144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt, S. et al. The global distribution and burden of dengue. Nature496(7446), 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Roche, Rosmari & Gould, Ernest A. Understanding the dengue viruses and progress towards their control. Biomed. Res. Int.1–20, 2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atangana, Abdon & Khan, Muhammad Altaf. Analysis of fractional global differential equations with power law. AIMS Math.8(10), 24699–24725 (2023). [Google Scholar]
- 5.Khan, Muhammad Altaf & Atangana, Abdon. Numerical Methods for Fractal-Fractional Differential Equations and Engineering: Simulations and Modeling (CRC Press, 2023). [Google Scholar]
- 6.Kermack, W. O. & McKendrick, A. G. Contributions to the mathematical theory of epidemics-I. Bull. Math. Biol.53(1–2), 33–55 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Esteva, Lourdes & Vargas, Cristobal. A model for dengue disease with variable human population. J. Math. Biol.38(3), 220–240 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Esteva, Lourdes & Vargas, Cristobal. Analysis of a dengue disease transmission model. Math. Biosci.150(2), 131–151 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Gakkhar, Sunita & Chavda, Nareshkumar C. Impact of awareness on the spread of dengue infection in human population. Appl. Math.04(08), 142–147 (2013). [Google Scholar]
- 10.Phaijoo, G. R. & Gurung, D. B. Sensitivity analysis of seir-sei model of dengue disease. GAMS J. Math. Math. Biosci. (2018).
- 11.Agarwal, Manju & Verma, Vinay. A mathematical model for dengue disease with saturation and bilinear incidence. Int. J. Ecol. Econ. Stat. (2012).
- 12.Agarwal, M. & Verma, V. Stability analysis of an seirs model for the spread of malaria. Int. J. Appl. Math. Comput. J. (2012).
- 13.Agarwal, M. & Verma, V. The impact of media on the spreading and control of Japanese encephalitis. Int. J. Math. Sci. Comput (2012).
- 14.Khan, M. A. & Atangana, A. Mathematical modeling and analysis of covid-19: A study of new variant omicron. Phys. A Stat. Mech. Appl.599, 127452 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan, M. A. & Atangana, A. Mathematical analysis of an eco-epidemiological model with different competition factors in its fractional-stochastic form. Phys. Scr.96(10), 104015 (2021). [Google Scholar]
- 16.Atangana, A. & Araz, S. I. A novel covid-19 model with fractional differential operators with singular and non-singular kernels: Analysis and numerical scheme based on newton polynomial. Alex. Eng. J.60(4), 3781–3806 (2021). [Google Scholar]
- 17.Atangana, A. & Araz, S. I. Mathematical model of covid-19 spread in Turkey and South Africa: Theory, methods and applications (2020). [DOI] [PMC free article] [PubMed]
- 18.Solís-Pérez, J. E., Gómez-Aguilar, J. F. & Atangana, A. A fractional mathematical model of breast cancer competition model. Chaos Solitons Fractals127, 38–54 (2019). [Google Scholar]
- 19.Zadeh, L. A. Fuzzy sets. Inf. Control8(3), 338–353 (1965). [Google Scholar]
- 20.Mondal, P. K. et al. Dynamical behavior of an epidemic model in a fuzzy transmission. Int. J. Uncertainty Fuzziness Knowl. Based Syst.23(05), 651–665 (2015). [Google Scholar]
- 21.Barros, L. C., Ferreira Leite, M. B. & Bassanezi, R. C. The si epidemiological models with a fuzzy transmission parameter. Comput. Math. Appl.45(10–11), 1619–1628 (2003). [Google Scholar]
- 22.Kimbi, Y. H., Djam, X. Y., Wajiga, G. M. & Blamah, N. V. Afuzzy expert system for the management of malaria. Int. J. Pure Appl. Sci. Technol.5(2), 84–108 (2011). [Google Scholar]
- 23.Emokhare, B.O. & Igbape, E.M. Fuzzy logic base approachto early diagnosis of ebola hemorrhogic fever. In Proc. of the World Congress on Engineering and Computer Science (2015).
- 24.Atangana, Abdon & Owolabi, Kolade M. New numerical approach for fractional differential equations. Math. Model. Nat. Phenomena13(1), 3 (2018). [Google Scholar]
- 25.Atangana, Abdon. On the new fractional derivative and application to nonlinear fisher’s reaction–diffusion equation. Appl. Math. Comput.273, 948–956 (2016). [Google Scholar]
- 26.Kumar, Sunil, Kumar, Ranbir, Agarwal, Ravi P. & Samet, Bessem. A study of fractional Lotka-volterra population model using Haar wavelet and Adams-Bashforth-Moulton methods. Math. Methods Appl. Sci.43(8), 5564–5578 (2020). [Google Scholar]
- 27.Qayyum, Mubashir, Ahmad, Efaza, Ahmad, Hijaz & Almohsen, Bandar. New solutions of time-space fractional coupled schrödinger systems. AIMS Math.8(11), 27033–27051 (2023). [Google Scholar]
- 28.Farnaz, Q. et al. Homotopic fractional analysis of thin film flow of oldroyd 6-constant fluid. Alex. Eng. J.60(6), 5311–5322 (2021). [Google Scholar]
- 29.Ghanbari, B., Kumar, S. & Kumar, R. A study of behaviour for immune and tumor cells in immunogenetic tumour model with non-singular fractional derivative. Chaos Solitons Fractals133, 109619 (2020). [Google Scholar]
- 30.Qayyum, Mubashir, Ahmad, Efaza & Ali, Mohamed R. New solutions of time-fractional cancer tumor models using modified he-laplace algorithm. Heliyon10(14), e34160 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qayyum, Mubashir, Tahir, Aneeza & Acharya, Saraswati. New solutions of fuzzy-fractional fisher models via optimal he–laplace algorithm. Int. J. Intell. Syst.2023, 1–21 (2023). [Google Scholar]
- 32.Qayyum, M., Tahir, A., Saeed, S. T. & Akgül, A. Series-form solutions of generalized fractional-fisher models with uncertainties using hybrid approach in caputo sense. Chaos Solitons Fractals172, 113502 (2023). [Google Scholar]
- 33.Qayyum, M. et al. Homotopy perturbation method-based soliton solutions of the time-fractional (2+1)-dimensional wu–zhang system describing long dispersive gravity water waves in the ocean. Front. Phys., 11 (2023).
- 34.Chatterjee, A. N. et al. A compartmental approach to modeling the measles disease: a fractional order optimal control model. Fractal Fract.8(8), 446 (2024). [Google Scholar]
- 35.Wang, B., Mondal, J., Samui, P., Chatterjee, A. N. & Yusuf, A. Effect of an antiviral drug control and its variable order fractional network in host covid-19 kinetics. Eur. Phys. J. Spec. Top.231(10), 1915–1929 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee, A. N., Basir, F. A., Ahmad, B. & Alsaedi, A. A fractional order compartmental model of vaccination for covid 19 with the fear factor. Mathematics10(9), 1451 (2022). [Google Scholar]
- 37.Chatterjee, A. N. & Ahmad, B. A fractional order differential equation model of covid-19 infection of epithelial cells. Chaos Solitons Fractals147, 110952 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurunnahar, M. A. et al. An innovative fractional-order evolutionary game theoretical study of personal protection, quarantine, and isolation policies for combating epidemic diseases. Sci. Rep., 14(1) (2024). [DOI] [PMC free article] [PubMed]
- 39.Ullah, M. S. & Ariful Kabir, K. M. Behavioral game of quarantine during the monkeypox epidemic: Analysis of deterministic and fractional order approach. Heliyon10(5), e26998 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullah, M. S., Kamrujjaman, Md. & Ariful Kabir, K. M. Understanding the relationship between stay-at-home measures and vaccine shortages: a conventional, heterogeneous, and fractional dynamic approach. J. Health Popul. Nutr., 43(1) (2024). [DOI] [PMC free article] [PubMed]
- 41.Ullah, M. S., Ariful Kabir, K. M. & Khan, Md. A. H. A non-singular fractional-order logistic growth model with multi-scaling effects to analyze and forecast population growth in bangladesh. Sci. Rep., 13(1) (2023). [DOI] [PMC free article] [PubMed]
- 42.Ullah, M. S., Higazy, M. & Ariful Kabir, K. M. Dynamic analysis of mean-field and fractional-order epidemic vaccination strategies by evolutionary game approach. Chaos Solitons Fractals162, 112431 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chellamani, P., Julietraja, K., Alsinai, A. & Ahmed, H. A fuzzy fractional order approach to sidarthe epidemic model for covid-19. Complexity, 2022(1) (2022).
- 44.Qayyum, Mubashir & Tahir, Aneeza. Mathematical Modeling of Cancer Tumor Dynamics with Multiple Fuzzification Approaches in Fractional Environment (Springer International Publishing, 2023). [Google Scholar]
- 45.Qayyum, Mubashir, Ahmad, Efaza, Tahir, Aneeza & Acharya, Saraswati. Modeling and analysis of the fuzzy-fractional chaotic financial system using the extended He–Mohand algorithm in a fuzzy-caputo sense. Int. J. Intell. Syst.2023, 1–15 (2023). [Google Scholar]
- 46.Eriqat, T. et al. A new attractive analytic approach for solutions of linear and nonlinear neutral fractional pantograph equations. Chaos Solitons Fractals138, 109957 (2020). [Google Scholar]
- 47.Khan, M. A. Dengue infection modeling and its optimal control analysis in East Java, Indonesia. Heliyon7(1), e06023 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Driessche, P. & Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci.180(1–2), 29–48 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.