Abstract
Drug addiction is a multifactorial syndrome in which genetic predispositions and exposure to environmental stressors constitute major risk factors for the early onset, escalation, and relapse of addictive behaviors. While it is well known that stress plays a key role in drug addiction, the genetic factors that make certain individuals particularly sensitive to stress and, thereby, more vulnerable to becoming addicted are unknown. In an effort to test a complex set of gene x environment interactions—specifically gene x chronic stress—here we leveraged a systems genetics resource: BXD recombinant inbred mice (BXD5, BXD8, BXD14, BXD22, BXD29, and BXD32) and their parental mouse lines, C57BL/6J and DBA/2J. Utilizing the chronic social defeat stress (CSDS) and chronic variable stress (CVS) paradigms, we first showed sexual dimorphism in social and exploratory behaviors between the mouse strains. Further, we observed an interaction between genetic background and vulnerability to prolonged exposure to non-social stressors. Finally, we found that DBA/2J and C57BL/6J mice pre-exposed to stress displayed differences in morphine sensitivity. Our results support the hypothesis that genetic variation influences chronic stress-induced behavioral outcomes such as social and approach-avoidance behaviors, reward responses, as well as morphine sensitivity, and is likely to modulate the development of drug addiction.
Subject terms: Addiction, Neuroscience, Emotion, Stress and resilience
Introduction
Drug addiction is a partly heritable, polygenic disorder determined by a complex interaction between multiple genes and the environment1. Familial, twin, and adoption studies have consistently reported that genetic factors contribute to aspects of addiction primarily through interactions with environmental factors and exposure2,3. Converging lines of evidence from both clinical and preclinical investigations support the view that environmental stress is a major risk factor for developing drug addiction4–6. Indeed, clinical studies strongly suggest that cumulative or prolonged stress is a reliable predictor of drug addiction5, and preclinical studies in rodents have confirmed that chronic exposure to stress increases initiation of drug use, induces more robust drug-induced conditioned place preference (CPP), and escalates drug self-administration5,7,8.
Although genetic studies have shown addiction to be roughly 50% heritable9–13, opioid addiction is accelerating in the US, and we are faced with a worldwide problem that accounts for tremendous morbidity and mortality, thereby posing collateral damage to social and political systems14–17. Still, the DNA variants that contribute to the behavioral relationship between stress and addiction remain elusive. To this end, the development of expanded families of fully isogenic replicable cohorts18–20, in particular, the extended BXD family of recombinant inbred mouse strains generated from a cross between C57BL/6J females and DBA/2J males, have enabled large research efforts in systems genetics and have been used widely to investigate genetic factors underlying complex heritable phenotypes observed in metabolic21–23 and psychiatric disorders24–26. Each of the ~ 120 extant BXD strains are essentially immortal allowing for reliable and reproducible genetic investigations. The BXD family provides a unique system to map the complex set of gene x environment (GxE) interactions, such as those between genes and stress that are implicated in the vulnerability to drug addiction26–31.
In the present study, we first exposed male and female C57BL/6J, DBA/2J, BXD5, BXD8, BXD14, BXD22, BXD29 and BXD32 mice to the chronic social defeat stress paradigm (CSDS)32,33. We identified social interaction and exploratory behavior variabilities amongst BXD strains and sexes after CSDS. To investigate the interaction between genetics and vulnerability to non-social stressors, we also exposed C57BL/6J, DBA/2J, BXD8, BXD22, and BXD29 male and female mice to the chronic variable stress paradigm (CVS)34. We established that novelty-suppressed feeding, sucrose preference and exploratory behaviors after CVS differ among BXD progeny and by sex. Finally, female DBA/2J and C57BL/6J mice pre-exposed to CSDS displayed differences in morphine sensitivity. Characterization of the genetic, social, and environmental factors mediating addiction risk will fundamentally improve our understanding of individual variations in response to stress and drug abuse and provide highly useful information for developing new treatments for psychiatric disorders.
Results
C57BL/6J, DBA/2J, and BXD mice have distinct behavioral performances following chronic social stress
Drug addiction, including morphine-related behaviors, has been linked to several factors such as sex, age, social environment, and stress experience prior to drug exposure5. Similar to humans, mice exposed to chronic social stress exhibit a higher propensity to develop addictive-like behaviors. In our study, we first aimed to determine the effect of genetics, including sex divergence, on chronic social stress-induced behavioral outcomes. Here we used CSDS, a well-establish mouse model for social stress-induced behavioral alterations32,35.
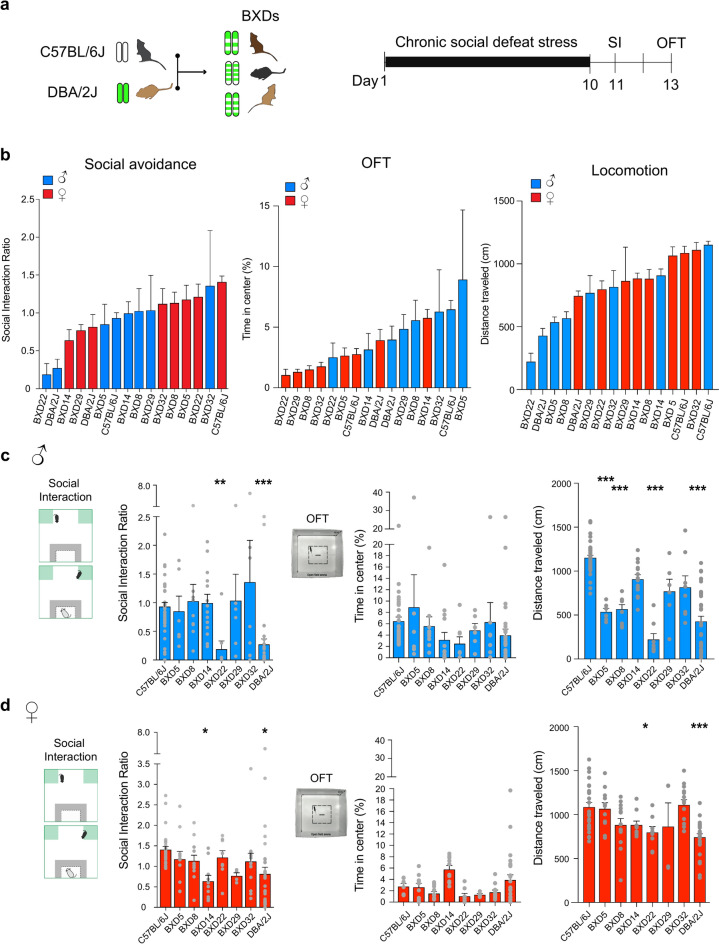
We exposed adult male and female mice from the BXD founders, C57BL/6J and DBA/2J, and the BXD5, BXD8, BXD14, BXD22, BXD29, and BXD32 strains to CSDS (Fig. 1a). We assessed their social interaction behavior determined by the social interaction ratio (see detailed information in the methods section). We first tested the null hypothesis that behavioral performance was independent of the mice’s genetic profile, including sex chromosomes as genetic divergence. We observed a large range of social interaction ratios across mouse lines and sex (Fig. 1b; One-Way ANOVA, F(15,223) = 3.805, p < 0.001; see Supplementary Table S1 for details). We further observed an interaction between sex and genetic background (Two-Way ANOVA, F(1,225) = 5.573, p < 0.001). Following social interaction, we tested exploratory behaviors in an open-field test (OFT). We observed a large range of stress-induced anxiety-like behaviors across the mouse lines (Fig. 1b; One-Way ANOVA, F(15,198) = 2.262, p < 0.001). We also observed an interaction between sex and genetic background (Two-Way ANOVA, F(1,198) = 2.018, p < 0.05). We then calculated the distance traveled in the open-field chamber and further observed a range of locomotor activity across mouse lines and sex (ANOVA, One-Way ANOVA, F(15,224) = 19.38, p < 0.001; Two-Way ANOVA, interaction F(7,224) = 6.482, p < 0.0001).
Fig. 1.
C57BL/6J, DBA/2J, and BXD mice have distinct behaviors following chronic social defeat stress. (a) Experimental timeline illustrating the CSDS paradigm followed by the social interaction test (SI) and the open-field test (OFT). (b) Behavioral assessment showing the heterogeneous impact of CSDS on SI, OFT, and locomotion by genetic background in male (blue) and female (red) stressed mice. (c) CSDS-exposed male mice display distinct SI ratio, percentage of time spent in the center of the OFT, and distance traveled in the OFT in a “no-target” context amongst C57BL/6J, BXD: 5, 8, 14, 22, 29, and 32, and DBA/2J male mice following CSDS (n = 40, 6, 9, 14, 8, 6, 8, 35). (d) Same as c in CSDS-exposed female mice, C57BL/6J, BXD: 5, 8, 14, 22, 29, and 32, and DBA/2J (n = 28, 11, 12, 10, 8, 4, 14, 26). Bars represent mean ± SEM. ANOVA or Kruskal–Wallis tests followed by post-hoc comparison to C57BL/6J mice with Bonferroni correction; see Supplementary Table S1; *P < 0.05, **P < 0.01, and ***P < 0.01.
Observing sex differences in response to CSDS amongst the different BXD lines tested, we examined the behavioral responses to CSDS of male mice independently from female mice (Fig. 1c,d). We observed that male DBA/2J and BXD22 were more susceptible to CSDS-induced social avoidance than the male founder C57BL/6J line (Kruskal–Wallis, K(7,126) = 48.05; Dunns’ corrected z = 3.517, p = 0.003; z = 5.983, p < 0.001). We also observed that male BXD5, 8, 22 and DBA/2J mice had lower distance traveled than the male founder C57BL/6J line (Kruskal–Wallis, K(7,126) = 82.07; Dunns’ corrected z = 3.805, z = 4.317, z = 5.737, z = 7.827, p < 0.001).
Confirming the higher sensitivity of the DBA/2J mice to CSDS-induced social avoidance, female DBA/2J mice had a lower social interaction ratio than C57BL/6J female mice following CSDS (Fig. 1d, One-Way ANOVA, F(7,105)=2.213; Bonferroni corrected t = 3.156, p = 0.01). While BXD14 male mice presented a similar degree of social interaction as male C57BL/6J line, female BXD14 had a lower social interaction ratio than female C57BL/6J mice (One-Way ANOVA, F(7,105) = 2.213; Bonferroni corrected t-tests, t = 2.983, p = 0.02). Additionally, we also observed that female DBA/2J and BXD22 (Kruskal–Wallis, K(7,114) = 33.78; Dunns’ corrected z = 4.510, p < 0.001, z = 2.771, p = 0.04) lines had lower distances traveled than the female founder C57BL/6J line. Together, these results suggest that CSDS-induced behavioral outcomes are impacted by sex and genetic background.
C57BL/6J, DBA/2J, and BXD strains have distinct behavioral performance following non-social chronic stress
Adverse events are highly heterogeneous, and behavioral and physiological responses to stress vary with the nature of stressors. To capture the impact of genetics and sex on anxiety-like and exploratory behavior following chronic non-social stress exposure, we tested behavioral responses of BXD and founder C57BL/6J and DBA/2J mouse lines to the CVS paradigm. We and others have established that exposure to this non-social chronic stress disrupts exploratory behaviors, reward responses, and motivated behaviors34,36.
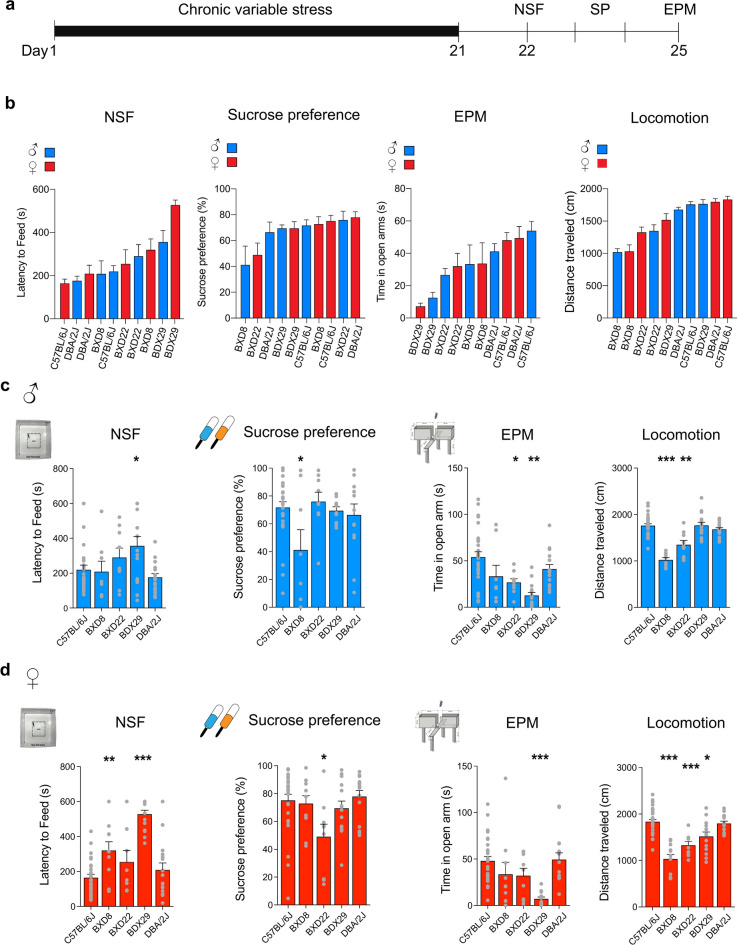
We first exposed male and female mice from the BXD founder lines C57BL/6J and DBA/2J and the BXD8, BXD22, and BXD29 lines to the CVS paradigm (Fig. 2a). We then assessed anxiogenic behaviors in the novelty-suppressed feeding (NSF) task and observed a large range of behaviors across mouse lines and sexes (Fig. 2b; One-Way ANOVA, F(9,141) = 9.474, p < 0.001; see Supplementary Table S1 for details). Our results also showed distinct latency to feed between mouse lines and sex (Fig. 2b; Two-Way ANOVA, F(4,141) = 17.18, p < 0.001; Interaction F(4,141) = 3.366, p = 0.0072). We then tested the extent of CVS-induced anhedonia by measuring the preference for sucrose solution over water (One-Way ANOVA, F(9,133) = 2.74, p = 0.006). We observed an interacting effect of sex and genetics on sucrose preference (Two-Way ANOVA, F(4,141) = 3.998, p = 0.004). Following these measurements, we tested exploratory behavior in an elevated plus maze (EPM, Fig. 2b; One-Way ANOVA, F(9,140) = 6.244, p < 0.001). Further confirming the variable sensitivity to stressful stimuli, we observed a large range of locomotor behaviors across mouse lines and sex (Fig. 2b; One-Way ANOVA, F(9,142) = 18.88, p < 0.001; Two-Way ANOVA, gene F(4,142) = 39.61, p < 0.0001, interaction F(4,142) = 2.368, p = 0.0475).
Fig. 2.
C57BL/6J, DBA/2J, and BXD mice have distinct sensitivity to non-social chronic stress. (a) Experimental timeline illustrating the chronic variable stress (CVS) paradigm followed by the novelty suppress feeding test (NSF), the sucrose preference test (SP), the elevated plus-maze test (EPM), and locomotion in an open field. (b) Behavioral assessment showing the heterogeneous impact of CVS on NSF, SP, EPM, and locomotion by genetic background. Bars represent mean ± SEM for n = 6–40 mice. (c) CVS-exposed male mice display distinct latency to feed during the NSF task, SP ratio (%), time spent as percentage in open arms of the EPM, and distance traveled in an open field in the “no-target” context amongst C57BL/6J, BXD: 8, 22, and 29 and DBA/2J male mice (n = 27, 8, 9, 15, 16). (d) CVS-exposed female mice display distinct latency to feed times during the NSF task, SP ratio (%), time spent in EPM open arms, and distance traveled in an open field in “no-target” context amongst C57BL/6J, BXD: 8, 22, and 29 and DBA/2J (n = 27, 10, 9, 15, 15). Bars represent mean ± SEM. ANOVA followed by post hoc comparison to C57BL/6 J mice with Bonferroni correction; see Supplementary Table S1; *P < 0.05, **P < 0.01, and ***P < 0.01.
We observe that BXD29 had lower performances than the male founder C57BL/6J line on NSF (Fig. 2c; One-Way ANOVA, F(4,70) = 3.304, t = 2.783, p = 0.03) and CVS exposed BXD8 mice had a blunted sucrose preference (One-Way ANOVA, F(4,62) = 2.749, t = 3.07, p = 0.01). Additionally, BXD22 and BXD29 males had higher anxiety levels when compared to the male founder C57BL/6J line (One-Way ANOVA, F(4,70) = 7.596, t-tests, t = 2.915, p = 0.02; t = 5.272, p = 0.001; Fig. 2c). We also observed that the male BXD 8 and BXD22 (One-Way ANOVA, F(4,70) = 23.19; t-tests, t = 8.39, t = 4.87, p < 0.001) lines had lower distances traveled than the male founder C57BL/6J line (Fig. 2c).
Confirming the heterogeneous stress-induced behavioral responses between sex and strain, we observed that female BXD8 and BXD29 mice displayed a higher latency to feed when compared to C57BL/6J female mice (Fig. 2d, One-Way ANOVA, F(4,71) = 20.18, t = 3.191, t = 8.660, p = 0.008, p < 0.001). While sucrose preference in BXD22 males was similar to C57BL/6J males, BXD22 females had a lower sucrose preference ratio relative to C57BL/6J females (One-Way ANOVA, F(4,71) = 3.155, t-tests, t = 3.224, p = 0.008). In addition to high NSF, BXD29 females displayed higher CVS-induced anxiety-like levels when compared to C57BL/6J females (One-Way ANOVA, F(4,70) = 6.534, t-tests, t = 4.677, p < 0.001). We also observed that BXD8, BXD22, and BXD29 (One-Way ANOVA, F(4,72) = 20.09, t-tests, t = 7.987, t = 4.673, t = 3.359, p < 0.001, p = 0.005) females had lower distances traveled relative to C57BL/6J females. Together these results confirm that sex and strain have a relevant influence on chronic social and non-social stress behavioral responses.
Distinct morphine-induced motor effects between C57BL/6J and DBA/2J mouse lines following chronic stress exposure
Variability in response to psychoactive drugs in mice is known to depend in part on genetic differences37–40. To test if genetics and sex contribute to the impact of chronic stress on morphine sensitivity, we measured morphine-induced motor activity in stressed male and female C57BL/6 J and DBA/2 J mice.
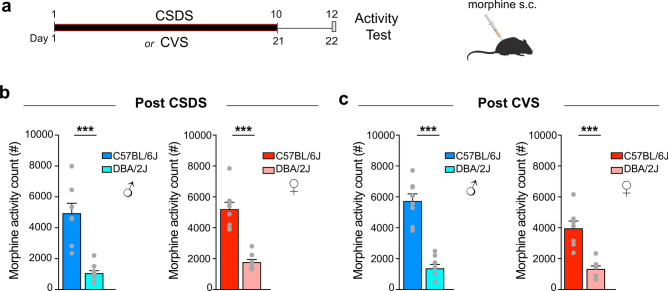
Following the CSDS or CVS paradigm, male and female C57BL/6J and DBA/2J mice were injected with 7.5 mg/kg of morphine (intraperitoneal; Fig. 3a). This dose was selected because it consistently and reproducibly produces a sub-maximal degree of behavioral responses in C57BL/6J mice (males and females), making it possible to detect manipulations that either increase or decrease sensitivity to the place conditioning effects of the drug. Fifteen minutes after the morphine injection, mice were placed in a large cage allowing for tracking of their locomotor activity. We observed that male and female C57BL/6J mice exposed to CSDS had higher locomotor activity when compared to DBA/2J mice (Fig. 3b; t-tests, t = 6.452, p < 0.001; t = 6.960, p < 0.001). Recapitulating these data, male and female C57BL/6 J mice exposed to CVS had higher locomotor activity when compared to DBA/2J mice (Fig. 3c; t-tests, t = 8.080, p < 0.001; t = 5.301, p < 0.001). We observed that sex had an interacting effect with the mice’s genotype on morphine-induced locomotor activity (Two-Way ANOVA, sex F(1,27) = 6.025, p = 0.02; interaction F(1,27) = 5.517, p = 0.02; gene effect F(1,27) = 90.17, p < 0.001; see Supplementary Table S1). However, we did not detect distinct behavioral responses of the C57BL/6j and DBA2J mice due to the different nature of the stress (Two-Way ANOVA, interaction F(1,61) = 0.059, p = 0.807; stress F(1,61) = 0.174, p = 0.736; gene effect F(1,61) = 158.5, p < 0.001; see Supplementary Table S1). Together, our results confirmed that the interaction between chronic stress and behavioral responses to morphine vary between genetic backgrounds.
Fig. 3.
Parental C57BL/6J and DBA/2J mice have distinct locomotor responses to acute morphine after chronic stress exposure. (a) Experimental timeline illustrating the two procedures performed, i.e. CSDS or CVS, followed by locomotor activity monitoring for 60 min in a locomotor apparatus 15 min after the animals were given an intraperitoneal injection of 7.5 mg/kg morphine. (b) Locomotor activity counts in C57BL/6J and DBA/2J male (left) and female (right) mice (n = 8) exposed to CSDS. (c) Locomotor activity counts in C57BL/6J and DBA/2J male (left) and female (right) mice (n = 8) exposed to CVS. Bars represent mean ± SEM. Unpaired t-test; see Supplementary Table S1; *P < 0.05, **P < 0.01, and ***P < 0.01.
Sex-specific impact on morphine’s rewarding effects in stressed C57BL/6J and DBA/2J mice
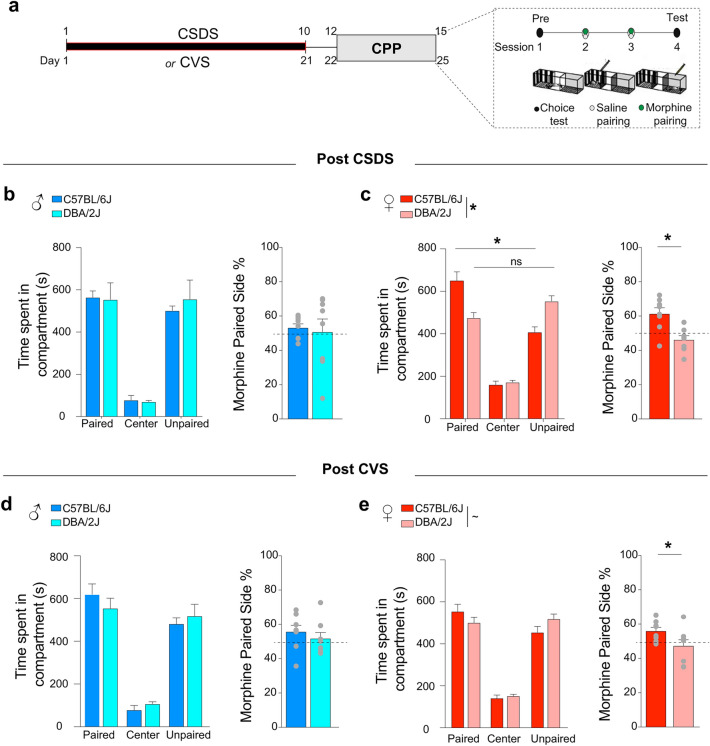
To test the hypothesis that the rewarding properties of morphine differ between stressed C57BL/6J and DBA/2J male and female mice, we employed the CPP paradigm (Fig. 4a). Following CSDS or CVS, male and female C57BL/6J and DBA/2J mice were conditioned with once-daily alternating injections of saline and morphine (7.5 mg/kg, s.c.) over a period of 2 days. Side preference was assessed one day prior to morphine conditioning (pretest), and an unbiased CPP approach was utilized. One day after conditioning, the preference for the morphine-paired compartment was assessed.
Fig. 4.
Parental C57BL/6J and DBA/2J mice have distinct place conditioning responses to morphine after chronic stress exposure. (a) Experimental timeline illustrating the two procedures performed, i.e. CSDS or CVS, prior to performing morphine conditioned place preference (CPP). Morphine CPP was performed using an unbiased approach: one compartment was paired with saline (0.3 mL, s.c.) and the other paired with morphine (7.5 mg/kg, s.c.). Sessions were 20 min long. (b) Time spent in each compartment of the three-chamber apparatus after paired-conditioning with morphine (vs. saline) in CSDS-exposed C57BL/6J and DBA/2J male mice (n = 8) and respective percentage in the morphine-paired chamber. (c) Same as (b), in CSDS-exposed C57BL/6J and DBA/2J female mice (n = 8). (d) Time spent in each compartment of the three-chamber apparatus after paired-conditioning with morphine (vs. saline) in CVS-exposed C57BL/6J and DBA/2J male mice (n = 8) and respective percentage in the morphine-paired chamber. (e) Same as (b), in CVS-exposed C57BL/6J and DBA/2J female mice (n = 8). Bars represent mean ± SEM. Two-way ANOVA followed by post-hoc comparison to C57BL/6J mice with Bonferroni correction and non-paired t-test; see Supplementary Table S1; *P < 0.05, **P < 0.01, and ***P < 0.01.
We observed that CPP scores and preferences did not show significant differences between male CSDS-exposed C57BL/6J and DBA/2J mice (Fig. 4b; Two-Way ANOVA, F(2,42) = 0.038, p = 0.84; t-test, t = 0.315, p = 0.75). Two-Way ANOVA comparison showed that, while CSDS-exposed female C57BL/6J mice established morphine CPP, CSDS-exposed female DBA/2J mice did not establish a preference for the morphine-paired compartment (Fig. 4b; Two-Way ANOVA, interaction F(2.42) = 18.09, p < 0.0001; paired-side t = 4.886 p < 0.001, unpaired-side t = 4.047 p = 0.007). We further observed significant differences in preference for the morphine-paired compartment between CSDS female C57BL/6J and DBA/2J mice (Fig. 4c; t-test, t = 3.677, p = 0.002). We defined that sex had an effect on morphine CPP (Two-Way ANOVA, sex F(1,28) = 3.995, p = 0.049; see Supplementary Table S1). Recapitulating these results, CVS-exposed female C57BL/6J and DBA/2J mice showed trending differing CPP profiles (Fig. 4d; Two-Way ANOVA, interaction F(2,42) = 3.72, p = 0.059), whereas CVS-exposed male C57BL/6J and DBA/2J mice did not (Fig. 4e; Two-Way ANOVA, interaction F(2,42) = 1.079, p = 0.35). Additionally, CVS-exposed male C57BL/6J and DBA/2J mice did not show a significant difference in preference for the morphine-paired side (Fig. 4d; t-test, t = 0.791, p = 0.44), and CVS-exposed female C57BL/6J mice established CPP, while DBA/2J females did not (Fig. 4e; t-test, t = 2.677, p = 0.047). We did not detect distinct behavioral responses of the C57BL/6j and DBA2J mice due to the different nature of the stress (Two-Way ANOVA, interaction F(1,60) = 0.651, p = 0.42; stress F(1,60) = 0.004, p = 0.94; gene effect F(1,60) = 2.06, p = 0.15; see Supplementary Table S1). Our results establish a strain- and sex-specific impact on the rewarding properties of morphine in stressed C57BL/6J and DBA/2J mice.
Discussion
While stress is a well-known risk factor in the development of drug addiction, the genetic factors that make certain individuals particularly susceptible or resilient to stress and, thereby, more or less vulnerable to becoming addicted, remain elusive. In this study, we tested if genetic components map onto the behavioral and physiological mechanisms underlying (1) behavioral deficits following chronic stress exposure and (2) the ability of prior stress experience to influence drug responses. The BXD family of recombinant inbred strains derived from crossing two inbred parental mouse lines—C57BL/6J and DBA/2J—have been extensively used for almost 50 years in fields such as neuropharmacology26, immunology41, and addiction18,25,38 to answer important biological questions. Combining the use of the BXD mouse lines and their founder lines with models of chronic stress exposure and morphine sensitivity, we first showed that CSDS-induced behavioral outcomes are impacted by sex and strain. Further, we established that vulnerability to chronic non-social stress (i.e. CVS) also varies depending on sex and genetics. We revealed that DBA/2J and BXD22 male and female mice are more susceptible to chronic social stress than C57BL/6J mice, evidenced by stronger social avoidance and anxiety-like behaviors. We observed sexual dimorphism in responses to CSDS amongst the BXD5, BXD8, BXD14, and BXD32 lines. To investigate the interaction between genetics and vulnerability to prolonged exposure to non-social stressors, we exposed C57BL/6J, DBA/2J, BXD8, BXD22, and BXD29 male and female mice to CVS and observed that DBA/2J female mice are more sensitive to CVS when compared to C57BL/6J female mice (i.e. a heightened decrease in sucrose preference). Confirming the stress vulnerability of BXD22 mice observed after CSDS, CVS-exposed male and female BXD22 mice displayed higher levels of anxiety-like measures than C57BL/6J mice. Interestingly, while BXD29 mice behaved like C57BL/6J after CSDS, both BXD29 female and male mice developed a higher anxiety profile following CVS when compared to C57BL/6J mice. Finally, we identified that DBA/2J and C57BL/6J mice pre-exposed to CSDS displayed differences in morphine sensitivity.
Here, we aimed to define the impact of genetics in the expression of the behavioral outcomes of chronic stress exposure, including social and exploratory behaviors, measures of anxiety-like endpoints, as well as morphine sensitivity. While our study highlights the difference in response to stress-eliciting stimuli, it is important to consider that pre-existing behavioral differences may also contribute to the observed behavioral divergence across mouse lines41. For example, in the absence of stress exposure, DBA mice show decreased reciprocal social behavior in the resident intruder task and the three-chamber sociability test42. Further, previous work has shown that social approach/sniff, ultrasonic vocalizations, partner sniffing, anxiety-like, and aggressive behavior all vary between various BXD strains43–45, which would also impact stress vulnerability. These strain-specific behaviors are reflected in differences in brain morphology as well. A stereological assessment of lateral septum (LS) volume revealed wide variability among the BXD strains and their parental lines, which, given the role of the LS in social and drug reward, may mediate GxE impacts on stress- and morphine-sensitivity46,47. We observed that DBA/2J mice exhibit stronger social avoidance behaviors when compared to C57BL/6J mice after CSDS. Interestingly, following CVS exposure, DBA/2J and C57BL/6J mice exhibited similar behavioral responses. Conversely, while BXD29 mice behaved like C57BL/6J mice after CSDS, the BXD29 mice developed a heightened anxiety profile following CVS compared to C57BL/6J mice. These results suggest distinct genetic contributions in the mechanisms underlying these two stress-induced behavioral outcomes. In line with these observations, several studies have reported distinct and even opposite modifications of neural circuits involved in social and non-social stress exposure, including within the dopamine system32,48,49. In particular, it has been shown that CSDS induces a hyper-dopaminergic state in mice expressing social avoidance and decreased sucrose preference. Oppositely, chronic non-social stress reduces the activity of midbrain dopaminergic neurons in mice exhibiting depressive-like behaviors such as a lower sucrose preference ratio. Together, these results emphasize that opposite neuronal modifications, supported by distinct genetic mechanisms, may converge to similar pathological behavioral responses to distinct types of stressors.
In line with evidence in humans, we observed sexual dimorphism across the parental and BXD mouse lines in behavioral outcomes after chronic stress exposure. For example, we observed that, while male BXD22 mice developed stronger social avoidance behaviors compared to male C57BL/6J mice, female BXD22 mice did not and maintained social behaviors similar to those of female C57BL/6J mice exposed to CSDS. Notably, we observed that CSDS-exposed female mice had lower exploratory behaviors in the center of the open-field when compared to their male counterparts. Interestingly, as a result of prolonged exposure to non-social stressors, we observed similar behavioral alterations between male and female mice within the mouse lines. Both male and female BXD29 mice exhibited the strongest stress-induced behavioral deficits compared to C57BL/6J mice. These results indicate stress-specific sex differences in the mechanisms underlying behavioral responses to these two types of stress. In a broader assessment of fear-related behavior in the absence of stress, one study showed that anxiety-like behavior and fear-conditioning tendencies were independent of sensory and motor functions suggesting that these genetically divergent characteristics could also contribute to innate behavioral differences precipitated by chronic stress exposure50.
Similar to the behavioral changes after chronic stress exposure51, sex is a key factor that may play a role in the development of addiction. Women tend to progress more rapidly than men through the stages of substance use disorder despite initiating drug use at a later age52. Women are more likely to report misusing prescription opioids to cope with negative affect, while also showing the characteristic rapid progression from first drug experience to developing substance use disorder53,54. To allow for the detection of subtle but robust sex differences in behavioral responses to morphine, we utilized a threshold dose of morphine (7.5 mg/kg), below the dose known to induce CPP or somatic withdrawal in stress-naïve mice55. We observed a robust strain effect on morphine-induced locomotor activity between the C57BL/6J and DBA/2J mice. Additionally, female C57BL/6J mice showed greater CPP when compared to female DBA/2J mice, while male DBA/2J and C57BL/6J mice did not establish morphine CPP at the dose used. We found that strain and sex contributed to stress-induced sensitivity to morphine reward, independently from the nature of the chronic stressor, i.e., social or non-social. Further genetic analyses seeking to identify the specific genetic loci involved in the impact of stress on morphine sensitivity will help identify novel mechanisms in gene x environment interactions in neuropsychiatric disorders.
To conclude, our results support the hypothesis that genetic variations contribute to stress responses, such as social avoidance and anxiety-like behaviors, and influence sensitivity to morphine and, as a result, presumably modulate the risk of psychiatric disorders and addiction. Our previous studies performed in isogenic mouse lines have allowed the identification of physiological processes and epigenetic mechanisms contributing to stress-susceptibility and drug responses on a single genetic background56–58. In parallel, important genetic mapping research efforts using recombinant inbred mouse models have identified the high degree of genetic homology between humans and mice59. This homology has allowed for impactful cross-species studies that will define the genes interacting in the regulation of behavioral stress responses and addictive behaviors. Characterization of the genetic, neurobiological, social, and environmental factors that mediate addiction risk will fundamentally improve our understanding of individual variations in psychiatric disorders and responses to drugs of abuse and provide highly useful information for the development of new treatment strategies and, eventually, prevention measures.
Material and methods
Mice
The study is reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (https://arriveguidelines.org). C57BL/6J, DBA2J, and BXD female and male mice (7–12 weeks old; Jackson Laboratory, Bar Harbor) were used for all experiments and habituated to the animal facility for one week before experimental manipulations. Mice were housed in groups of five at a constant ambient temperature (24 ± 1 °C) under a 12-h light/dark cycle (lights on from 7:00 A.M.) with ad libitum access to water and food, except when otherwise specified for behavioral testing. Following experiments, mice were euthanized with lethal doses of anesthesia (ketamine 300 mg/kg/xylazine 150 mg/kg) followed by cervical dislocation. All efforts were made to minimize the number of animals used in the experiments and to limit their distress and suffering. Experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Mount Sinai. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Mount Sinai.
Chronic social defeat stress (CSDS) paradigm
Pre-screened CD-1 retired male breeder mice were singled-housed in one compartment of a large mouse cage partitioned by a perforated Plexiglass divider vertically placed through the cage, which has a metal top to hold food and water on both sides. This is used as the resident cage and is the permanent home for the CD-1 mouse. The CSDS paradigm begins when an experimental mouse is introduced into the CD-1 home cage and the ensuing fighting is allowed to occur for 10 min. Following the interaction, mice are individually confined to one compartment for the rest of the day, allowing for continued sensory exposure. This is repeated for 10 days, and the effect of the 10-day defeat is measured on day 11 through a social interaction test32. Similar to the male CSDS paradigm, female mice are placed, as an intruder, in the home cage of an Esr1-Cre mouse. The Esr1-Cre mouse is injected with CNO, an exogenous drug targeting the DREADD expressed in the ventrolateral subdivision of the ventromedial hypothalamus, to induce aggressive behavior toward the female experimental mice. Following 5 min of physical contact, the female mice are placed back into their home-cage, where they are grouped housed. Similar to the male CSDS paradigm, this schedule is repeated for 10 days33.
Social interaction test (SI test)
Mice were placed in an OFT box containing a wire mesh cage on one side under red-light conditions (< 15 lux)32. The open field arena and wire-mesh enclosures were thoroughly cleaned between mice with an odorless 5% ethanol solution. The CSDS-exposed mice were individually placed in the open field box and allowed to explore for 2.5 min (without a social target present, known as the “no target phase”). The experimental mouse was then removed, the open field was cleaned, and an unfamiliar aggressor (CD-1/Esr1-Cre) mouse was placed into the mesh cage (known as the “target phase”). The experimental mouse was then allowed to explore for another 2.5 min. Time spent interacting with the social target and locomotion were measured using an automated video tracking system (Ethovision). Experimental mice were then placed back into their home cage (single- housed). The social interaction ratio was calculated as [time in social interaction zone with “target”]/[time in social interaction zone with “no target”], as described previously32,60. The distance traveled was analyzed during the “no target” phase to avoid potential biases due to the novel social target being present.
Chronic variable stress (CVS)
CVS consists of three different stressors over 30 days34. Environmental stressors are presented on an alternating schedule to prevent habituation. Stressors are administered in the following order: 100 random mild foot shocks at 0.45 mA for one hour (10 mice to a chamber), a tail suspension stress for one hour, and restraint stress (placed inside a 50 mL falcon tube) for one hour. The three stressors are then repeated in the same cycle for the remainder of the stress protocol.
Open field test (OFT)
Mice were placed in the open field arena (44 × 44 cm) for 5 min to compare the distance traveled and time spent in the peripheral zone compared to the center zone (10 × 10 cm). Testing conditions occurred under red-light conditions (< 10 lux) in a room isolated from external sound sources. The apparatus was thoroughly cleaned between mice with an odorless 5% ethanol solution. The mouse’s time spent in specific open field areas—was video-tracked and scored with Ethovision software32.
Elevated plus maze test (EPM)
The EPM was designed in black Plexiglass (L/W/D: 70/5/20 cm) and fitted with white surfaces to provide contrast. Testing conditions occurred under red-light conditions (< 10 lux) in a room isolated from external sound sources. The EPM apparatus was thoroughly cleaned between mice with an odorless 5% ethanol solution. Mice were positioned in the center of the maze, and behavior was video-tracked for 5 min32. Time in EPM compartments was measured using a video-tracking system (Ethovision) set to focus on the mouse center-point at the commencement of each trial.
Sucrose preference (SP)
Mice were habituated to having access to two water bottles (50-ml tubes with fitted ball-point sipper tubes) for one day, and were then given free access to both water and a 1% sucrose solution, for two consecutive days32. Bottles were weighed daily and interchanged (left to right, right to left) to avoid biases from a potential side preference. Sucrose preference scores were calculated as ([sucrose solution consumed]/[sucrose + water solutions consumed]) × 100.
Novelty suppressed feeding (NSF)
The NSF test elicits conflicting motivations in mice: the drive to eat vs. the fear of moving to the center of a novel, open arena49. Mice were food-restricted for 24 h prior to testing34. Under red-light conditions, mice were placed in the corner of a clear plastic testing chamber (50 × 50 × 20 cm) lined with corncob bedding. A single food pellet was present in the center of the box. The latency to begin consumption in a 6-min test was measured.
Morphine conditioned place preference (CPP)
Morphine CPP was conducted as previously described58. We used an unbiased, three-compartment apparatus. First day 0, mice were allowed to freely explore the entire apparatus for 30 min to obtain baseline preference to any of the three compartments; we know that mice show no inherent group biases for a given chamber across multiple strains52. Mice were then given conditioning trials (two per day) on two consecutive days. For both trials, mice received saline (0.3 mL, subcutaneous; s.c.) and were confined to one of the compartments of the apparatus. After 4 h, mice received morphine (7.5 mg/kg in 0.3 mL, s.c.) and were confined to the opposite compartment. The drug-paired chamber is randomized across mice. On test day (day 4), mice are again allowed to freely explore the entire apparatus for 30 min. CPP scores are calculated as the time spent in the morphine-paired chamber minus the time spent in the saline-paired chamber. The time spent in each compartment was determined using an automated system and CPP scores were calculated as the percent of time spent in the morphine-paired compartment.
Locomotor sensitization
Locomotor activity was individually monitored in specialized locomotor chambers with no bedding, 15 min after the animals were given an intraperitoneal injection of 7.5 mg/kg morphine. Locomotor activity was monitored for 60 min.
Data analyses and statistics
All behaviors were monitored and scored using automated and unbiased Ethovision software32,51. Experimenters analyzing the dataset were blinded to the experimental conditions. The statistical analyses were performed using Graphpad Prism (version 8, La Jolla, CA, USA) and R (version 3.3.3) software and collected in Supplementary Table S1. The normality of the distributions was assessed using Kolmogorov–Smirnov tests. The statistical analyses were performed considering the sample size, normality, and homoscedasticity of the distributions. The data fitting assumptions of the general linear model were subjected to two-sided Student’s t-tests, or multiple comparisons using a one-way, two-way, or repeated measures (RM) ANOVA followed by post hoc two-sided t-tests with Bonferroni correction for multiple comparisons (t-test, p-values). Non-parametric Kruskal–Wallis followed by post hoc Dunn’s corrected comparisons and Mann–Whitney two-sided statistical analyses were performed for datasets that did not follow a normal distribution. The statistical significance threshold was set at 0.05.
Supplementary Information
Acknowledgements
This work was supported by National Institute of Mental Health grants R01MH051399 (EJN) and R01MH120514 (CM, SJR) and by National Institute on Drug Abuse grant P01DA047233 (EJN), National Key R&D Program of China 2021ZD0202900 & 2021ZD0202902 (MHH), Research Fund for International Senior Scientists T2250710685 (MHH), Shenzhen Natural Science Foundation J20220127 (MHH) Shenzhen Medical Research Fund SMRF B2303012 (MHH), Shenzhen Key Laboratory of Precision Diagnosis and Treatment of Depression ZDSYS20220606100606014 (MHH), Science and Technology Research and Development Foundation of Shenzhen (High-level Talent Innovation and Entrepreneurship Plan of Shenzhen Team Funding) KQTD20221101093608028 (MHH). This study was also supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation 29699 (CM) and 31194 (LFP), Leon Levy Foundation (LFP), the Alzheimer’s Association (Grant No. 24AARG-NTF-1198746 to CM), and the Hope for Depression Research Foundation (EJN).
Author contributions
C.M., L.P., Y.V.Z., O.I., M.C., K.C., A.V.A., A.B., performed the behavioral assessments and with the assistance of C.B., C.M., L.P. Y.V.Z., M.C., O.I., analyzed the results. C.M., L.P., S.J.R., E.J.N., and M.H.H. designed the experiments with the assistance of R.W.W. M.K.M. C.M., L.P., O.I., S.J.R., E.J.N., and M.H.H. interpreted the results and wrote the paper, which was edited by all authors.
Data availability
All data generated and analyzed during this study are included in this article and available in Supplementary Table S2.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Carole Morel, Lyonna F. Parise and Yentl Van der Zee.
Contributor Information
Scott J. Russo, Email: scott.russo@mssm.edu
Eric J. Nestler, Email: eric.nestler@mssm.edu
Ming-Hu Han, Email: ming-hu.han@mssm.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80767-7.
References
- 1.Shurtleff, D., Sasek, C. & Kautz, M. Sponsor’s foreword: NIDA at forty. Neuropharmacology76(Pt B), 195–197 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Kendler, K. S., Karkowski, L. M., Neale, M. C. & Prescott, C. A. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch. Gen. Psychiatry57, 261–269 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Tsuang, M. T., Bar, J. L., Harley, R. M. & Lyons, M. J. The harvard twin study of substance abuse: what we have learned. Harv. Rev. Psychiatry9, 267–279 (2001). [PubMed] [Google Scholar]
- 4.Carlezon, W. A. J., Duman, R. S. & Nestler, E. J. The many faces of CREB. Trends Neurosci.28, 436–445 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Sinha, R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci.1141, 105–130 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khibnik, L. A. et al. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol. Psychiatry79, 898–905 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morel, C. et al. Nicotinic receptors mediate stress-nicotine detrimental interplay via dopamine cells’ activity. Mol. Psychiatry23, 1597–1605 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Andersen, S. L. Stress, sensitive periods, and substance abuse. Neurobiol. Stress10, 100140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabb, D. W., Edenberg, H. J., Bosron, W. F. & Li, T. K. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant. J. Clin. Investig.83, 314–316 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton, P. R., Tobin, M. D. & Hopper, J. L. Key concepts in genetic epidemiology. Lancet (London, England)366, 941–951 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Kreek, M. J., Nielsen, D. A. & LaForge, K. S. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromol. Med.5, 85–108 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Deak, J. D. et al. Genome-wide association study in individuals of European and African ancestry and multi-trait analysis of opioid use disorder identifies 19 independent genome-wide significant risk loci. Mol. Psychiatry27, 3970–3979 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatoum, A. S. et al. Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nat. Ment. Health1, 210–223 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strain, E. C. Assessment and treatment of comorbid psychiatric disorders in opioid-dependent patients. Clin. J. Pain18, S14-27 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Conway, K. P., Compton, W., Stinson, F. S. & Grant, B. F. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry67, 247–257 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Hser, Y.-I. et al. Long-term follow-up assessment of opioid use outcomes among individuals with comorbid mental disorders and opioid use disorder treated with buprenorphine or methadone in a randomized clinical trial. Addiction117, 151–161 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenk, S., Eisenbarth, H. & Dixon, L. Treating opioid use disorders in the criminal justice system with pharmacotherapy. Forensic Sci. Int. Mind Law1, 100009 (2020). [Google Scholar]
- 18.Ashbrook, D. G. et al. A platform for experimental precision medicine: The extended BXD mouse family. Cell Syst.12, 235-247.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams, R. W., Strom, R. C., Rice, D. S. & Goldowitz, D. Genetic and environmental control of variation in retinal ganglion cell number in mice. J. Neurosci. Off. J. Soc. Neurosci.16, 7193–7205 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams, R. W., Gu, J., Qi, S. & Lu, L. The genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Genome Biol.2, RESEARCH0046 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreux, P. A. et al. Systems genetics of metabolism: the use of the BXD murine reference panel for multiscalar integration of traits. Cell150, 1287–1299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKnite, A. M. et al. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS One7, e39191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmanov, A. A. et al. Genetics of sweet taste preferences. Flavour Fragr. J.26, 286–294 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashbrook, D. G., Williams, R. W., Lu, L. & Hager, R. A cross-species genetic analysis identifies candidate genes for mouse anxiety and human bipolar disorder. Front. Behav. Neurosci.9, 171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chunduri, A., Watson, P. M. & Ashbrook, D. G. New insights on gene by environmental effects of drugs of abuse in animal models using GeneNetwork. Genes (Basel)13, 614 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grisel, J. E. et al. Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J. Neurosci. Off. J. Soc. Neurosci.17, 745–754 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabbe, J. C., Kosobud, A., Young, E. R. & Janowsky, J. S. Polygenic and single-gene determination of responses to ethanol in BXD/Ty recombinant inbred mouse strains. Neurobehav. Toxicol. Teratol.5, 181–187 (1983). [PubMed] [Google Scholar]
- 28.Tolliver, B. K., Belknap, J. K., Woods, W. E. & Carney, J. M. Genetic analysis of sensitization and tolerance to cocaine. J. Pharmacol. Exp. Ther.270, 1230–1238 (1994). [PubMed] [Google Scholar]
- 29.Wahlström, A., Hammar, L., Lundin, L. G. & Rane, A. Morphine metabolism in mouse brain. NIDA Res. Monogr.75, 603–606 (1986). [PubMed] [Google Scholar]
- 30.Phillips, T. J., Belknap, J. K. & Crabbe, J. C. Use of recombinant inbred strains to assess vulnerability to drug abuse at the genetic level. J. Addict. Dis.10, 73–87 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Badea, A., Johnson, G. A. & Williams, R. W. Genetic dissection of the mouse CNS using magnetic resonance microscopy. Curr. Opin. Neurol.22, 379–386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morel, C. et al. Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors. Nat. Commun.13, 1532 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golden, S. A., Covington, H. E., Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc.6, 1183–1191 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodes, G. E. et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci.35, 16362–16376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barik, J. et al. Chronic stress triggers social aversion via glucococorticoid receptor in dopaminoceptive neurons. Science339, 332–335 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Sinha, R. How does stress increase risk of drug abuse and relapse?. Psychopharmacology (Berl.)158, 343–359 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Crabbe, J. C. & Belknap, J. K. Behavior genetic analyses of drug withdrawal. Alcohol Alcohol Suppl.2, 477–482 (1993). [PubMed] [Google Scholar]
- 38.Baker, J. A., Brettin, J. T., Mulligan, M. K. & Hamre, K. M. Effects of genetics and sex on acute gene expression changes in the hippocampus following neonatal ethanol exposure in BXD recombinant inbred mouse strains. Brain Sci.12, 1634 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitahama, K. & Valatx, J. L. Strain differences in amphetamine sensitivity in mice. II. Overcompensation of paradoxical sleep after deprivation in two C57 strains. Psychopharmacology (Berl.)66, 291–295 (1979). [DOI] [PubMed] [Google Scholar]
- 40.Belknap, J. K. et al. Localization to chromosome 10 of a locus influencing morphine analgesia in crosses derived from C57BL/6 and DBA/2 strains. Life Sci.57, PL117-24 (1995). [DOI] [PubMed] [Google Scholar]
- 41.Grizzle, W. E. et al. BXD recombinant inbred mice represent a novel T cell-mediated immune response tumor model. Int. J. Cancer101, 270–279 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Mulligan, M. K., Mozhui, K., Prins, P. & Williams, R. W. GeneNetwork: A toolbox for systems genetics. Methods Mol. Biol.1488, 75–120. 10.1007/978-1-4939-6427-7_4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma, L., Piirainen, S., Kulesskaya, N., Rauvala, H. & Tian, L. Association of brain immune genes with social behavior of inbred mouse strains. J. Neuroinflamm.18(12), 75. 10.1186/s12974-015-0297-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knoll, A. T., Jiang, K. & Levitt, P. Quantitative trait locus mapping and analysis of heritable variation in affiliative social behavior and co-occurring traits. Genes Brain Behav.17(5), e12431. 10.1111/gbb.12431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delprato, A. et al. A quantitative trait locus on chromosome 1 modulates intermale aggression in mice. Genes Brain Behav.17(7), e12469. 10.1111/gbb.12469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talishinsky, A. & Rosen, G. D. Systems genetics of the lateral septal nucleus in mouse: heritability, genetic control, and covariation with behavioral and morphological traits. PLoS One7(8), e44236. 10.1371/journal.pone.0044236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ZióŁkowska, B., Gieryk, A., Solecki, W. & PrzewŁocki, R. Temporal and anatomic patterns of immediate-early gene expression in the forebrain of C57BL/6 and DBA/2 mice after morphine administration. Neuroscience22(284), 107–124. 10.1016/j.neuroscience.2014.09.069 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Friedman, A. K. et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science10.1126/science.1249240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, S. et al. Sex Differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience10.1016/j.neuroscience.2018.02.021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brigman, J. L., Mathur, P., Lu, L., Williams, R. W. & Holmes, A. Genetic relationship between anxiety-related and fear-related behaviors in BXD recombinant inbred mice. Behav. Pharmacol.20(2), 204–209. 10.1097/FBP.0b013e32830c368c (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li, L. et al. Social trauma engages lateral septum circuitry to occlude social reward. Nature613, 696–703 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McHugh, R. K., Votaw, V. R., Sugarman, D. E. & Greenfield, S. F. Sex and gender differences in substance use disorders. Clin. Psychol. Rev.66, 12–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jamison, R. N., Butler, S. F., Budman, S. H., Edwards, R. R. & Wasan, A. D. Gender differences in risk factors for aberrant prescription opioid use. J. Pain11, 312–320 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis, B., Hoffman, L. A. & Nixon, S. J. Sex differences in drug use among polysubstance users. Drug Alcohol Depend.145, 127–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papaleo, F. & Contarino, A. Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav. Brain Res.170, 110–118 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Kiraly, D. D. et al. Alterations of the host microbiome affect behavioral responses to cocaine. Sci. Rep.6, 35455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Issler, O. et al. The long noncoding RNA FEDORA is a cell type- and sex-specific regulator of depression. Sci. Adv.8, eabn9494 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calipari, E. S. et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun.8, 13877 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emes, R. D., Goodstadt, L., Winter, E. E. & Ponting, C. P. Comparison of the genomes of human and mouse lays the foundation of genome zoology. Hum. Mol. Genet.12, 701–709 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Takahashi, A. et al. Establishment of a repeated social defeat stress model in female mice. Sci. Rep.7, 12838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are included in this article and available in Supplementary Table S2.