Abstract
Accurate and timely genetic material replication is essential for preserving genomic integrity. The replication process begins with chromatin licensing and DNA replication factor 1 (CDT1). It has been demonstrated that dysregulated CDT1 expression causes genomic instability, damages DNA, and may even cause cancer. Although this protein is overexpressed in several malignancies, its significance in gastric cancer is still unknown. This work aims to investigate the biological function and molecular mechanism of CDT1 in gastric cancer. Bioinformatics studies were carried out using TCGA database, Kaplan–Meier and GEPIA online data analysis software. The expression of CDT1 in stomach cancer tissues was determined by immunohistochemistry, and its relationship to clinicopathological features was examined. Using siRNA and cell function experiments, the impact of CDT1 expression on the capacity of gastric cancer cell lines to proliferate, migrate, and invade was investigated. In gastric cancer tissues, CDT1 is overexpressed and is linked to a dismal prognosis. Through the up-regulation of M-cyc, MCM5 (microchromosome maintenance protein 5), CyclinD1, N-cadherin, and the down-regulation of E-cadherin, CDT1 facilitated the proliferation and invasion of gastric cancer cells. The study’s findings shed insight on the possible molecular processes behind CDT1’s function in promoting the start and progression of gastric cancer and demonstrate the potential predictive and diagnostic use of CDT1 expression in gastric cancer.
Keywords: Gastric cancer, CDT1, Proliferation, Invasion, EMT
Subject terms: Cancer, Immunology
Introduction
Gastric cancer is the third most common cause of cancer-related death worldwide and the fifth most common type of cancer overall1. Among the main risk factors for gastric cancer are Helicobacter pylori infection, advanced age, excessive salt use, and poor dietary habits2. Due to infrequent routine screenings and mild symptoms in the early stages, the majority of individuals receive their diagnosis at an advanced level. Currently, perioperative chemotherapy is the standard treatment for resectable gastric cancer. Ongoing studies, however, are looking at the possible advantages of immunotherapy and targeted therapy in adjuvant and perioperative settings3. For example, AFF3 may influence immune checkpoint inhibitors (ICIs) by affecting TIICs and immune checkpoint (IC) expression in the tumor microenvironment (TME), which might be an excellent prospective marker for gastric cancer patients’ diagnosis and prognosis4. Furthermore, ZBTB20 stimulated NF-κB signaling in gastric cancer cells, causing phosphorylation of NF-κBp65 as well as stimulation of the synthesis and function of MMP-2 and MMP-9, hence facilitating the movement, infiltration, and multiplication of cells5.
The exact time of DNA replication is essential to maintaining genomic integrity. The replication starts, also known as the initial stage of eukaryotic DNA replication, takes place during G1 and involves the progressive loading of replication components at different genomic sites6. Pre-replication complexes (pre-RCs) are chromatin-bound macromolecular complexes that are assembled from core licensing factors. The pre-RCs comprise the origin recognition complex (ORC), cell division cycle 6 (Cdc6), minichromosome maintenance (MCM), and CDT1. DNA replication is restricted to one cycle per cell by CDT1, which loads the MCM2-7 deconjugated complex solely onto chromatin in the G1 phase of the cell cycle7. If an error in DNA replication or an endogenous or exogenous factor causes DNA damage, it must be sensed and repaired quickly. As a result, cellular proteins constantly scan the DNA to recognize the damaged site and recruit additional protein complexes to coordinate repair and signal to the cell cycle machinery that damage exists and therefore further cell cycle progression needs to be halted. This DNA damage response (DDR) must be closely coordinated with the cell cycle machinery to ensure that genome stability is maintained7. According to recent reports, CDT1 dysregulation may be responsible for certain aberrant DNA replication and uncontrollably moving through the cell cycle. For instance, CDT1 may be destroyed by ubiquitin-driven proteolysis facilitated by ubiquitin ligases or inhibited by geminin, a nuclear protein that interacts with CDT1, limiting its activity and blocking destruction8. In patients with hepatocellular carcinoma (HCC), overexpression of CDT1 is associated with a worse chance of survival9; in patients with breast cancer, it is a prognostic marker of cancer10. However, the importance and precise function of CDT1 in gastric cancer has not been established.
Here, we analyzed CDT1 expression in gastric cancer utilizing bioinformatics, clinical tissue specimens, and in vitro tests for function using CDT1 knockdown gastric cancer cells. The study provides insights into CDT1’s clinical significance, interacting networks, and connection to immune infiltration in gastric cancer. Additionally, CDT1 can be used as a potential marker for the diagnosis and determination of prognosis in gastric cancer.
Materials and methods
Cell lines and culture conditions
An immortalized gastric mucosal epithelial cell line (GES-1) and two human gastric cancer cell lines (AGS and MKN-45) were maintained at the Central Laboratory of the Affiliated Hospital of Binzhou Medical College, China. 10%fbs (Gibco, USA), 100 U/ml penicillin, and 100 μl/ml streptomycin (VivaCell, China) are added to RPMI 1640 medium used to cultivate the cells, in a sealed incubator at 37 °C with 5% CO2. Mycoplasma was regularly checked, with no detection in any of the cell lines during the experiment.
Patients and tissue samples
From January 2019 to May 2022, 76 cases of stomach cancer that were stored at the Department of Pathology at Binzhou Medical College provided tissue samples and clinical data. Twelve stomach cancer patients were among those from whom we collected fresh cancer and surrounding tissues. We then promptly froze the tumor tissues in liquid nitrogen to do protein blot analysis. Patients with stomach cancer were diagnosed but had not had immunotherapy, chemotherapy, or radiation. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research councils and the 1964 Declaration of Helsinki and its subsequent amendments or similar ethical standards. The study design was approved by the Ethics Committee of the Affiliated Hospital of Binzhou Medical College (approval number: 2024-KYLL-46). All patients provided informed consent. There was a total of 76 patients, with a median age of 65 years and a range of ages from 49 to 81 years, including 53 men and 23 women. There were 45 cases of high-moderate and 31 cases of low pathogenic differentiation, according to the degree of differentiation.17 instances were in stages 1–2 and 59 cases were in stages 3–4 of the T stage. There were 22 instances in the N0 stage and 54 cases in the N1–N3 stage, according to the N stage. 41 patients were in stages I–II and 35 instances were in stages III–IV according to postoperative pathologic TNM staging.
Analysis of the Cancer Genome Atlas (TCGA) database
The expression data of CDT1 from 207 normal and 375 tumor tissues were procured and analyzed from the TCGA database (https://portal.gdc.cancer.gov/). The mutation load statistics of the gastric cancer samples were obtained from the official website of the TCGA database. Perl software (10.0, CPAN) was used to process all TCGA gastric cancer mutation load data to obtain the tumor mutation burden (TMB) and mutation frequency data of gastric cancer samples, which were merged with the target gene expression data for mutation correlation analysis using a publicly accessible database used for gene expression analysis (GEPIA (Gene Expression Profiling Interactive Analysis) (cancer-pku.cn)). It has been utilized to investigate CDT1 expression in a variety of human malignancies.
Receiver operating characteristic (ROC) curves and survival analysis
GraphPad Prism software (GraphPad Prism 9) was employed to do the ROC curve analysis. The area under the curve (AUC) was subsequently utilized to determine the prognostic value for the expression level of CDT1 mRNA on gastric cancer. By examining the impacts of CDT1 on overall survival (OS), first progression (FP), and post-progression survival (PPS) among patients with gastric cancer using the Kaplan–Meier (KM) plotter website, the predictive influence of CDT1 mRNA on gastric cancer was ascertained (https://kmplot.com/analysis/)11.
Correlation of CDT1 with immunity
The Tumor Immune Dysfunction and Exclusion (TIDE) analysis is a new algorithm used to predict the efficacy of immunotherapeutic drugs received by patients by calculating TIDE scores12. The TIDE score was calculated after inputting the whole gene expression data from the database of gastric cancer samples, examining the association between CDT1 and TIDE scores as well as the variations in TIDE scores between the CDT1 high and low expression groups. The TIDE score predicts differences in immune escape potential and the efficacy of immunotherapy between subgroups with different biological statuses, and CIBERSORT quantifies the fraction of cells in gene expression profiles (GEPs) of a large number of tissues13. All gastric cancer samples were analyzed using the “CIBERSORT” function package in R V4.2.1 software, and the proportions of infiltration for 22 distinct classes of immune cells in the samples were calculated, and P < 0.05 was used as a criterion for screening eligible samples. The difference in the proportion of infiltrating immune cells between the high and low CDT1 expression groups was analyzed.
Correlation of CDT1 with mutations
The TMB was assessed for all samples using R V4.2.1, and mutation frequencies were calculated for every altered gene in the groups having high and low CDT1 expression. The research concentrated on the variations in TMB between the CDT1-expressed groups with high and low expression, along with the correlation between CDT1 mRNA and TMB. Mutation annotation format waterfall plots of the top 15 genes with mutation frequencies in the high and low expression groups were performed using R 4.2.1 software.
Immunohistochemistry
To repair antigens, tissue sections were heated with ethylenediaminetetraacetic acid (EDTA, pH = 9.0) after paraffin sections had been deparaffinized to water. To suppress endogenous peroxidase, the slices were immersed in a 3% hydrogen peroxide solution for 20 min. CDT1 antibody (Abcam, ab202067) was added dropwise at a dilution of 1:300, overnight at 4 °C, and horseradish peroxidase-labeled secondary antibody was added. The color was developed using DAB, and the mixture was washed in running water three times. And the slices were dried, cleared, sealed, stained again with hematoxylin, and photographed. The immunohistochemical staining results were categorized using the product of the staining intensity (score 0–3) and the percentage of positive cells (score 0–100%). Weak = 1, moderate = 2, strong = 3, and negative = 0. There were four categories for the proportion of favorably stained cells: < 25% = 1, 25–50% = 2, 50–75% = 3, and > 75% = 4. High expression was defined as > 6 and low expression as < 6. Two pathologists graded the staining intensity to eliminate bias in the interpretation of the clinical data.
Cell transfection
Following the manufacturer’s guidelines, the logarithmic phase of growth cells was inoculated into six-well plates and transfected using the transfection reagent GP transfect-Mate (Gene Pharma, China). After a duration of six hours, the culture media was changed. The cells were divided into blank, vector (transfection reagent), NC (nonspecific siRNA), and si-CDT1 groups (CDT1-siRNA). Primers were synthesized by Shanghai Gemma Pharmaceutical Technology Co, China.
si-CDT1: 5′-GCACCAGGAGGUCAGAUUATT-3′ 3′-UUGUGUGCUCCUGACAUGCTT-5′
si-NC: 5′-UUCUCCGAACGUGUCACGUTT-3′ 3′-ACGUGACACGUUCGGAGAATT-5′.
Western blotting assay
Proteins from gastric cancer tissues and cells were isolated using RIPA lysate (PhD, China) by lysis at 4 °C for 30 min. The proteins were measured using the BCA method, then boiled and denatured before being added to the sample buffer. Electrophoresis was conducted at 100 V until the proteins migrated to the bottom, followed by transfer to the PVDF membrane at a constant voltage of 110 V. The membranes were closed with 5% skimmed milk, then incubate the antibody for a whole night at 4 °C, which included anti-CDT1 antibody (1:1000, Abcam), MCM5 (1:1000, Abcam), Cyclin D1 (1:1000, PhD), C-myc (1:1000, PhD), E-cadherin (1:1000, Proteintech), and β-actin (1:5000, PhD). The next day, the cells were washed and then exposed to the suitable horseradish peroxidase-labeled secondary antibody (1:10,000, PhD) for about two hours at room temperature. The exposure was developed using electrochemiluminescence (ECL) and then photographed (Bio-Rad, USA).
Immunofluorescence
The cells underwent treatment with 4% paraformaldehyde for 20 min for fixation, followed by permeabilization using 0.1% Triton X-100 for 15 min to facilitate passage of substances across their membranes. Subsequently, the cells were incubated with goat serum for 30 min at room temperature. After removing the blocking solution, the cells were exposed to the primary antibody CDT1 (1:100, Abcam) and left to incubate at 4 °C overnight. The cells were exposed to FITC for a duration of one hour at the ambient temperature. Following a duration of five minutes, the cells were stained with DAPI and examined using a fluorescence microscope.
Colony formation assay
To assess the clonogenic ability of individual cells, experiments were conducted to observe colony formation. Gastric cancer cells transfected with si-CDT1 or si-NC were seeded in six-well plates with 800 cells per well. Following a week of incubation, fresh media was added every third day, and the cells were washed with PBS. Subsequently, the cells were fixed with 4% paraformaldehyde for 20 min and then stained with crystal violet for 10 min. Images were captured, and the number of cell colonies was quantified using the methodology described in previous studies14.
CCK8 assay
Cells are distributed on a 96-well plate at a density of 2500 cells per well, and then cultured for 5 days. Each time point consists of 5 replicate wells. After replacing the whole medium with 10% CCK8, the experiment was incubated for 2 h. After incubation, the absorbance at 450 nm was measured by a multifunctional enzyme marker and statistically analyzed.
Wound-healing assay
Transfected AGS and GES-1 cells were inoculated into 6-well plates and let to grow until 90% confluence was achieved. Discard the supernatant, ensure that the tip of the gun is vertical for scratching, and wash with PBS three times. Add serum-free medium into each well to continue incubation, observe the distribution of cells at 24-h intervals, and take pictures under the microscope.
Transwell assays
Before use, uniformly distribute 60 µl of matrix gel in the top chamber of an 8 mm Transwell. Cells that have been transfected for 24 h and their control cells should be seeded in serum-free media, numbered, then arranged at a density of 1 × 104 cells/well in the upper chamber. Simultaneously, it is necessary to add 500 µl of media containing 10% FBS to the lower chamber. The cells were cultured in an incubator for 24 h, then treated with 4% paraformaldehyde and stained with crystal violet. The proportion of cell movement was imaged and statistically assessed using an inverted microscope. Similar to the cell invasion experiment, the cell migration assay was carried out, excluding matrix gel spreading.
Statistical analysis
The experiment data were statistically evaluated using GraphPad Prism 9 software. Sample size n < 40 or theoretical frequency T < 5, using the continuous corrected test. Measurements are expressed as Χ ± S and tested using paired t-tests. One-way ANOVA was used to statistically evaluate data from three or more groups. Kaplan–Meier technique was used for survival analysis. A p-value of less than 0.05 was deemed statistically significant.
Results
CDT1 expression in gastric cancer tissues and associated prognosis in patients with gastric cancer
A public database was used to analyze CDT1 expression levels in different cancers (Fig. 1A). Compared to the corresponding non-tumor tissues, CDT1 was significantly expressed (P > 0.05) in adrenocortical cancer, breast cancer, cervical cancer, large B-cell lymphoma, head and neck squamous cell carcinoma, chromophobe renal papillary cell carcinoma, clear cell carcinoma of the kidney, squamous cell carcinoma of the lung, adenocarcinoma of the prostate, adenocarcinoma of the stomach, and carcinoma of the thyroid. By performing ROC curves of CDT1 mRNA expression in the data of integrated samples from the GTEx and TCGA databases, the AUC results suggested that CDT1 mRNA expression level has good diagnostic value for gastric cancer (AUC = 0.9526, P < 0.0001; Fig. 1B). Moreover, high CDT1 mRNA transcript levels were closely associated with poor OS, FP, and PPS in gastric cancer (P < 0.05, P = 0.00013, and P < 0.05, Fig. 1C–E).
Fig. 1.
CDT1 exhibits elevated expression in gastric cancer tissues and is correlated with poorer results in gastric cancer patients. (A) Transcriptional expression of CDT1 in 33 different forms of cancer from TCGA and GTEx datasets. (B) ROC curve of CDT1 expression. (C) Kaplan–Meier OS survival curves of CDT1 mRNA. (D) Kaplan–Meier FP survival curves of CDT1 mRNA. (E) Kaplan–Meier PPS survival curves of CDT1 mRNA. (F) Positive CDT1 expression in gastric cancer tissue. (G) Negative CDT1 expression in gastric cancer tissue. (H) GC: relative expression of CDT1 in gastric cancer tissues; GN: relative expression of CDT1 in paraneoplastic tissues. Comparative analysis using paraneoplastic tissues. The information is presented as the average ± standard deviation of three separate tests. **P < 0.01. Full-length blots/gels are presented in Supplementary Fig. 1.
Using immunohistochemistry, the expression of CDT1 in 76 gastric cancer samples was assessed to look into the effect of CDT1 on the development of gastric cancer (Fig. 1F,G). We discovered that CDT1 was overexpressed in gastric cancer samples and that in patients with gastric cancer, its high expression was strongly correlated with tumor stage, T stage, and nerve invasion (Table 1). To determine the expression of CDT1 in gastric cancer tissues, fresh gastric cancer specimens were collected. Compared to adjacent non-tumor tissues, CDT1 was expressed in gastric cancer tissues (Fig. 1H). In summary, the previously mentioned findings indicated that CDT1 is significantly overexpressed in gastric cancer and is linked to a poorer prognosis.
Table 1.
Relationship between CDT1 and clinical characteristics of gastric cancer patients (n = 76).
| Clinicopathologic features | n | CDT1 Immunohistochemistry | P | ||
|---|---|---|---|---|---|
| High expression | Low expression | ||||
| Gender | Male | 53 | 31 | 22 | 0.120 |
| Female | 23 | 9 | 14 | ||
| Age(year) | ≤ 65 years | 31 | 17 | 14 | 0.749 |
| > 65 years | 45 | 23 | 22 | ||
| Tumor diameter | ≤ 4.7 cm | 40 | 21 | 19 | 0.345 |
| > 4.7 cm | 36 | 15 | 21 | ||
| Differentiation | High–medium differentiation | 45 | 25 | 20 | 0.538 |
| Low differentiation | 31 | 15 | 16 | ||
| Stage | I–II | 41 | 17 | 24 | 0.035* |
| III–IV | 35 | 23 | 12 | ||
| T classification | T1–T2 | 17 | 3 | 14 | 0.001* |
| T3–T4 | 59 | 37 | 22 | ||
| N classification | N0 | 22 | 8 | 14 | 0.070 |
| N1–N3 | 54 | 32 | 22 | ||
| Nerve violation | Negative | 39 | 16 | 23 | 0.037* |
| Positive | 37 | 24 | 13 | ||
| Microsatellite status | MSS | 43 | 23 | 20 | 0.864 |
| MSI | 33 | 17 | 16 | ||
Analysis of CDT1 concerning immunity and mutations
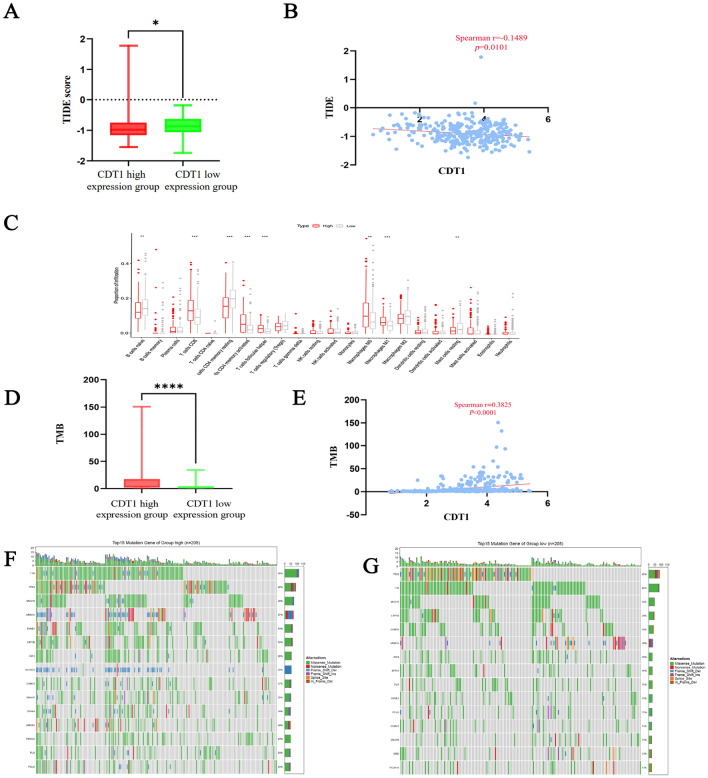
The low expression group had a much higher value than the high expression group, according to the TIDE score (P < 0.05; Fig. 2A). In the correlation analysis, the CDT1 mRNA expression level was significantly negatively to have a close connection with the TIDE score of gastric cancer (r = − 0.1489, P = 0.0101; Fig. 2B). Analysis of immune cell infiltration proportions revealed that B cells were much more numerous in the CDT1 low-expression group than the high-expression group, whereas T cells and the number of macrophages in the group with high CDT1 expression was considerably greater than in the group with low expression (P < 0.05; Fig. 2C). TMB analysis yielded considerably more findings in the high-expression group than in the CDT1 low-expression group (P < 0.0001; Fig. 2D). A correlation study showed a notable positive association between CDT1 mRNA expression and TMB in gastric cancer (r = 0.3825, P < 0.0001; Fig. 2E). The waterfall plots of the top 15 genes with mutation frequencies in the mutation annotation format showed that several genes such as titin (TTN), tumor protein P53 (TP53), and mucin 16 (MUC16) had different mutation frequencies with some differences between the CDT1 high- and low-expression groups, and the percentage of samples with mutated genes in the CDT1 high-expression group was significantly higher than that in the low-expression group. Of these, missense mutations are the most common form. (P < 0.05; Fig. 2F,G).
Fig. 2.
Analysis of CDT1 in relation to immunity and mutations. (A) Differences in TIDE scores between high and low CDT1 mRNA expression groups. (B) Correlation between CDT1 mRNA expression and TIDE scores. (C) Differences in immune cell infiltration between groups with high and low CDT1 mRNA expression. (D) Differences in TMB between high and low CDT1 mRNA expression groups. (E) Correlation between CDT1 mRNA expression and TMB. (F) Waterfall plot of mutation frequency of CDT1 mRNA high expression group. (G) Waterfall plot of mutation frequency of CDT1 mRNA low expression group.
siRNA interference technology on CDT1 expression
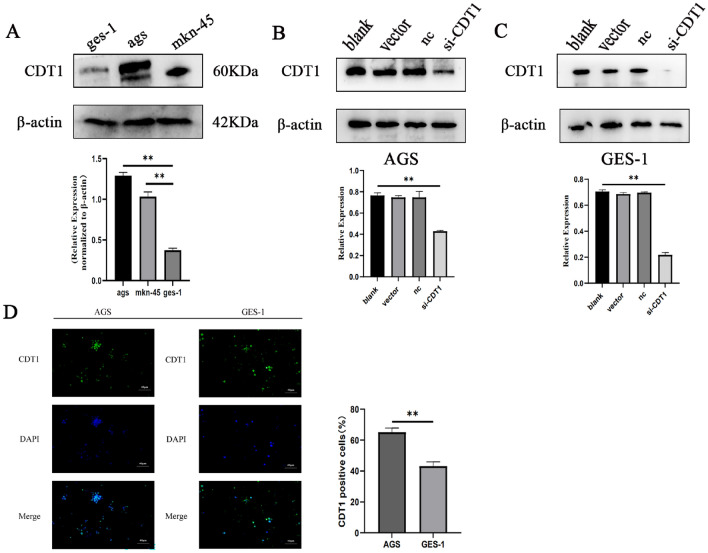
The initial step was assessing the protein expression of CDT1 in human gastric cancer cell lines (AGS and MKN-45) and an immortalized gastric mucosal epithelial cell line (GES-1). CDT1 expression was significantly elevated in gastric cancer cells compared to non-tumorigenic gastric cells, as seen in Fig. 3A. SiRNA was utilized to effectively suppress CDT1 expression in human gastric cancer cells and human normal gastric mucosa cells by inducing CDT1 knockdown in AGS and GES-1 cells. Both AGS and GES-1 cells were transfected with si-CDT1 or si-NC. To further analyze the knockdown efficiency, CDT1 protein expression levels were analyzed using western blotting. Figure 3B,C show that si-CDT1 interference dramatically (P < 0.05) reduced the protein expression of CDT1 in gastric cancer cells. In AGS and GES-1 cells transfected with si-CDT1, when comparing the CDT1 expression in case (si-CDT1) cells to blank cells, western blot analysis indicated an abrupt drop. The transfection procedure and reagents were found to be non-cytotoxic to AGS and GES-1 cells, as evidenced by the lack of substantial changes seen between blank and non-targeting control cells. Using immunofluorescence, the expression of CDT1 was analyzed in AGS and GES-1 cells, the cells were co-stained with antibodies to CDT1(green)and DAPI (blue) in Fig. 3D.
Fig. 3.
siRNA interference technology on CDT1 expression. (A) The basal protein expression of CDT1 in immortalized gastric cell lines (GES-1) and human gastric cancer cells (AGS and MKN-45). (B,C) Western blot analysis of CDT1 protein inhibited by si-CDT1. They employed β-actin as the internal control. The information is displayed as the average ± standard deviation of three separate tests. (D) Representative immunofluorescence imaging of CDT1 protein staining in AGS and GES-1 cells. The cells were stained with the anti-CDT1 antibody (green), and DAPI (blue)was used to identify the nuclei (40×). Fluorescence intensity of CDT1. The information is displayed as the average ± standard deviation of three separate tests. **P < 0.01. Full-length blots/gels are presented in Supplementary Fig. 2.
CDT1 in vitro growth inhibition of human gastric cancer cells
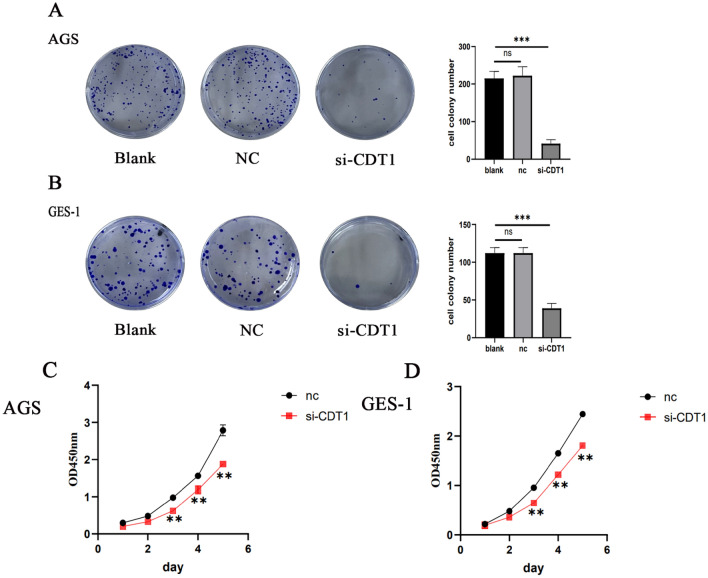
The assays used for assessing the impact of CDT1 knockdown on gastric cancer cell proliferation were colony formation and CCK8. The plate cloning experiment is capable of evaluating the interdependence of populations and the proliferative potential of gastric cancer cells, as seen in Fig. 4A,B. This assay might provide light on the connection between the proliferation of cell clones and cell population dependency. The CDT1 knockdown transfection group’s clones were much less in quantity and size than those of the negative control group, according to the data. These findings show that gastric cancer cells’ capacity for proliferation and colony formation was significantly suppressed by CDT1 knockdown. As seen in Fig. 4C,D, the CCK8 assay results indicated a decrease in cell proliferation in the si-CDT1 group compared to the si-NC group. AGS cells were suppressed by CDT1 knockdown starting from third day. Similar findings were noted in GES-1 cells.
Fig. 4.
CDT1 enhances cellular growth of human gastric cancer cells in vitro. (A,B) The results of the colony formation experiment showed that CDT1 knockdown reduced the capacity of gastric cancer cells to form colonies. (C) AGS cell proliferation was decreased by CDT1 knockdown. (D) CDT1 knockdown inhibited GES-1 cell proliferation. At 450 nm, the OD value was recorded. The information is displayed as the average ± standard deviation of three separate tests. **P < 0.01, ***P < 0.001.
Effect of CDT1 expression on the migration and invasion of AGS and GES-1 cells
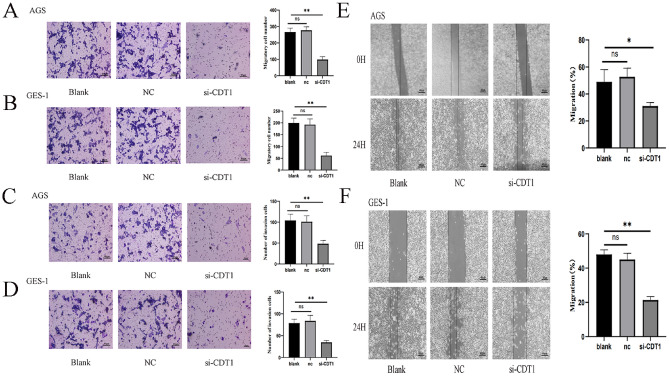
Migration assays and cell invasion experiments demonstrated that CDT1 silencing decreased the number of migrating (Fig. 5A,B) and invading (Fig. 5C,D) AGS and GES-1 cells, respectively. Moreover, the outcomes of the transwell assay and the wound-healing assay agreed with each other, in which CDT1 down-regulation significantly reduced the wound healing area (Fig. 5E,F). All of our findings showed that CDT1 facilitated gastric cancer cell line migration and invasion. AGS and GES-1 cell invasion and migration were significantly reduced by low CDT1 expression.
Fig. 5.
CDT1 enhances cellular migration and invasion in vitro. (A,B) The relationship between CDT1 and gastric cancer cell migration. (C,D) The relationship between CDT1 and gastric cancer cell invasion. (E,F) Using a wound healing test, the effects of CDT1 knockdown on cell migratory ability were measured. A minimum of three separate tests were used to choose representative photos. Photos were taken at 0 and 24 h. The information is displayed as the average ± standard deviation of three separate tests. *P < 0.05; **P < 0.01.
Effect of CDT1 on the expression of related proteins
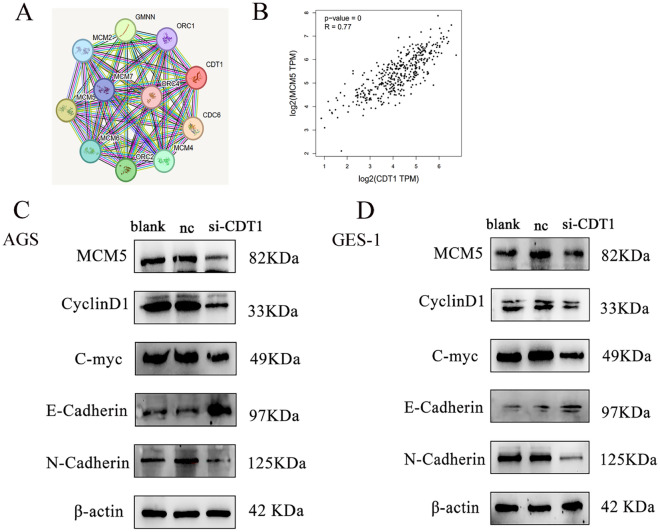
Genes with CDT1-like expression patterns in gastric cancer patients were found by GEPIA by identifying genes with comparable gene functions. To clarify possible relationships between CDT1 and genes with related activities, A network of protein–protein interactions (PPIs) was constructed (Fig. 6A). The MCM complex, helicase activity, nucleic acid binding, and DNA replication origin binding have all been linked to CDT1 and related genes, such as CDC6, Geminin DNA Replication Inhibitor (GMNN), MCMs, and Origin Recognition Complex Subunit 2 (ORC2). Using the correlation analysis function of the GEPIA database, CDT1 was found to be positively correlated with MCM5 expression and had the highest correlation (Fig. 6B). Consistent with the previously mentioned findings, the co-expression network of genes linked to CDT1 revealed the critical connections between the cell cycle and DNA replication.
Fig. 6.
Impact of CDT1 on the regulation of associated proteins. (A) Ten proteins most closely related to CDT1 were identified by string. (B) MCM5 has the highest correlation with CDT1. (C,D) Western blot experiment demonstrated that reducing CDT1 resulted in a reduction in the expression levels of MCM5, Cyclin D1, c-Myc, and N-cadherin while increasing the expression of E-Cadherin. Full-length blots/gels are presented in Supplementary Fig. 3.
Western blotting findings revealed that whereas E-cadherin expression was dramatically elevated, the levels of MCM5, Cyclin D1, c-Myc, and N-cadherin were significantly reduced in the si-CDT1 group compared to the control group (Fig. 6C,D).
Discussion
Stomach cancer is a common malignant tumor of the digestive system. China is one of the countries with high incidence of stomach cancer. Currently, surgical resection is the predominant treatment for individuals in the early stages of gastric cancer, whereas chemotherapy is the preferred option for those in the advanced stages15,16. However, the 5-year overall survival rate for GC patients is still below 30%. As a result, boosting the effectiveness of chemotherapy by finding novel targeted treatment targets is critical for improving the prognosis of gastric cancer.
CDT1 transcript levels were elevated in gastric cancer specimens compared to normal samples, which is relevant to other cancers determined by this and previous studies17. Increased chromosomal and genomic instability, DNA damage response, and microsatellite instability were all brought on by the abnormal expression of CDT18. Furthermore, the results suggested that CDT1 may play a role in promoting tumor development. According to the KM test, individuals with gastric cancer who had high CDT1 expression were at risk for more severe OS, FP, and PPS outcomes. However, the study of the ROC curve indicated that CDT1 expression is important for the diagnosis of gastric cancer. We observed an increased level of CDT1 in gastric cancer specimens, and its heightened presence demonstrated a significant link with tumor stage, T-grade, and nerve violation in gastric cancer patients. However, there was no statistically significant connection between CDT1 expression and other clinicopathological factors such as age, gender, or differentiation in the study. An essential pathological characteristic of gastric cancer is nerve infiltration (NI), which is linked to a worse prognosis and tumor recurrence18.
In immunotherapy research, TMB has become an emerging biomarker for tumor immunotherapy19. Therefore, TMB is the total amount of base substitutions, insertions, deletions, and somatic gene-coding mistakes found per million bases20. In general, immunotherapy can achieve better outcomes in patients with high TMB levels21. CDT1 mRNA expression exhibited a positive correlation with TMB in gastric cancer as indicated by the results of the TMB research. The TIDE score calculations revealed that samples with high CDT1 expression had low TIDE scores, indicating that samples with low CDT1 expression were more likely to exhibit immune escape and could potentially have unfavorable outcomes with immune-related therapy. This is consistent with the immunotherapy results of TMB analysis.
A growing amount of data points to the theory that immune cell infiltration impacts cancer development and progression, which has a negative impact on immunotherapy efficacy and clinical prognosis22. Tumor-infiltrating lymphocytes are positively correlated with patient survival and are now considered predictive biomarkers of responses to immunotherapy and chemotherapy23,24. CD4+ T lymphocytes have a significant impact on the local immune microenvironment and immune response mechanisms of malignant tumors, suggesting that CDT1 might play a role in the pathogenesis of gastric cancer by regulating the recruitment of immune cells25.
It has been revealed that the MCM protein family, which includes MCM2–10, controls DNA replication and the cell cycle in eukaryotes26. Additionally, it has been previously demonstrated that several human malignancies overexpress CDT1 and MCMs27,28. Analysis of the protein–protein interaction network unveiled a critical correlation between the gene expression of the MCM family and CDT1 in gastric cancer. Recently, it was discovered that CDT1 is a replication initiation factor required for MCM loading29. When combined, these findings point to a tight link between MCMs and CDT1, pointing to a possible mechanism by which the two may work together to encourage the onset and progression of gastric cancer. Relevant to the results of this work, Cai et al.'s recent discovery that the MCM family regulates CDT1 expression and that overexpression of CDT1 stimulates the proliferation of cancer cells found that CDT1 silencing decreased the expression of MCM5 in gastric cancer cells. Additionally, MCM5 is an important member of the small chromosome maintenance protein family. They are involved in the initiation and elongation processes of DNA replication and transcription.
The human Myc transcription factor network controls numerous genes involved in essential cellular processes such as growth, proliferation, differentiation, metabolism, and apoptosis30,31. MYC acts as a transcription factor for many DDR genes, including WRN deconjugases involved in aberrant replication intermediates and stop-replication fork repair. MYC also indirectly threatens genome stability32. It has been shown that the Myc-Max complex targets CDT1 transcriptionally and that dysregulated Myc expression causes the essential DNA replication factor to be expressed more highly in transformed cells. Through late mitosis and the G1 phase, the transcriptional activation of CDT1 is caused by Myc expression. Moreover, human tumor cells with increased expression of c-myc showed elevated levels of CDT1 expression30. In a study on the pathogenesis of gastric cancer, the oncogene c-Myc was found to directly interact with CDT1 to promote the growth of gastric cancer cells. This suggests that CDT1 promotes the progression of gastric cancer.
The CCK8 experiment demonstrated that, CDT1 down-regulation significantly reduced AGS and GES-1 cell growth. Furthermore, the inhibition of colony formation by CDT1 downregulation was further validated by plate cloning tests. Thus, the specific mechanism by action of CDT1 low expression causing the reduction of cell proliferation was further investigated. Research has shown that cell cycle protein D1 is a component of the cell cycle protein family, involved in regulating cell cycle advancement and facilitating the transition from G1 to S phase. Its expression is elevated in several types of cancerous tumors33. As of the current investigation, we found that the modulation of CDT1 expression could affect Cyclin D1 expression, suggesting that CDT1 affects the biological behavior of gastric cancer in vitro by regulating Cyclin D1 protein expression.
The process of carcinogenesis known as the epithelial-mesenchymal transition (EMT) is crucial to the early phases of cancer spread. According to studies, EMT controls vascular permeability and is thought to be a crucial process in the spread of cancer. There are also some correlations between EMT and DDR. EMT has been identified as a strong regulator of the pathways involved in DSB repair and DDR signaling34. It is often recognized that in patients with advanced stomach cancer, metastasis and recurrence are the primary causes of mortality. We looked at how CDT1 down-regulation could impact gastric cancer cells’ migration and metastasis. Using a wound healing experiment, we discovered that the si-CDT1 group’s scratch area was substantially lower than the control groups. Similar findings were obtained from the Transwell assay, with the si-CDT1 group exhibiting fewer moving cells than the control group. Our research illustrated that CDT1 knockdown inhibited cell migration and invasion, along with the EMT procedure. Nonetheless, additional analysis is necessary to clarify the exact function of CDT1 in the advancement of gastric cancer.
In conclusion, we have presented, for the first time, the role of CDT1 in gastric cancer and its plausible molecular mechanism. Through bioinformatics analysis, it was observed that CDT1 exhibited a heightened expression in gastric cancer and was linked to an unfavorable prognosis. Immunohistochemistry findings indicated that increased CDT1 levels were connected with nerve violation, T stage, and TNM stage. Experimental data further confirmed that CDT1 boosts the proliferation, migration, invasion, and EMT of gastric cancer cells. CDT1 might serve as a potential oncoprotein in gastric cancer and could serve as a molecular indicator for the early detection of gastric cancer.
Supplementary Information
Author contributions
S. W. conducted data analysis, prepared the original draft. M.C. conceptualized and designed the methodology. N.Z. and M.T. used software and validated the data. Funding and crucial discourse by C.Z. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MH243) and the National Natural Science Foundation of China (grant number 81772637).
Data availability
The data supporting the conclusions of this research may be obtained from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hong Li, Email: lihongtianshi@163.com.
Zhang Cao, Email: caoning1997@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81977-9.
References
- 1.Kim, D. J. et al. Accuracy of preoperative clinical staging for locally advanced gastric cancer in KLASS-02 randomized clinical trial. Front. Surg.9, 1001245 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet396, 635 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Guan, W. L., He, Y. & Xu, R. H. Gastric cancer treatment: recent progress and future perspectives. J. Hematol. Oncol.16, 57 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng, Y., Zhang, X., Li, F., Wang, Y. & Wei, M. AFF3 is a novel prognostic biomarker and a potential target for immunotherapy in gastric cancer. J. Clin. Lab. Anal36, e24437 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang, Y. et al. ZBTB20 promotes cell migration and invasion of gastric cancer by inhibiting IkappaBalpha to induce NF-kappaB activation. Artif. Cell Nanomed. B47, 3862 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Mechali, M. Eukaryotic DNA replication origins: many choices for appropriate answers. Nat. Rev. Mol. Cell Biol.11, 728 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Kanellou, A., Giakoumakis, N. N., Panagopoulos, A., Tsaniras, S. C. & Lygerou, Z. The licensing factor cdt1 links cell cycle progression to the DNA damage response. Anticancer Res.40, 2449 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Petropoulos, M. et al. Cdt1 overexpression drives colorectal carcinogenesis through origin overlicensing and DNA damage. J. Pathol.259, 10 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Karavias, D. et al. Overexpression of CDT1 is a predictor of poor survival in patients with hepatocellular carcinoma. J. Gastrointest. Surg.20, 568 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Mahadevappa, R. et al. The prognostic significance of Cdc6 and Cdt1 in breast cancer. Sci. Rep.-UK7, 985 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanczky, A. & Gyorffy, B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J. Med. Internet Res.23, e27633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med.24, 1550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods12, 453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, Y. et al. S100A10 accelerates aerobic glycolysis and malignant growth by activating mTOR-signaling pathway in gastric cancer. Front. Cell Dev. Biol.8, 559486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan, Z. Recent advances in the surgical treatment of advanced gastric cancer: a review. Med. Sci. Monit.25, 3537 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonelli, P. et al. Precision medicine in gastric cancer. World J. Gastro Oncol.11, 804 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, W. et al. Intermittent hypoxia-induced downregulation of microRNA-320b promotes lung cancer tumorigenesis by increasing CDT1 via USP37. Mol. Ther. Nucl. Acids24, 528 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng, J. et al. Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS One9, e88907 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cyriac, G. & Gandhi, L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin. Cancer Biol.52, 269 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Addeo, A., Friedlaender, A., Banna, G. L. & Weiss, G. J. TMB or not TMB as a biomarker: That is the question. Crit. Rev. Oncol. Hematol.163, 103374 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Chan, T. A. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol.30, 44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao, X. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer20, 131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, W. et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell165, 1092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paijens, S. T., Vledder, A., de Bruyn, M. & Nijman, H. W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol.18, 842 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, F. et al. CD4/CD8 + T cells, DC subsets, Foxp3, and IDO expression are predictive indictors of gastric cancer prognosis. Cancer Med.-US8, 7330 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu, S. et al. MCMs in cancer: prognostic potential and mechanisms. Anal. Cell Pathol.2020, 3750294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, B. & Xi, S. Bioinformatics analysis of the transcriptional expression of minichromosome maintenance proteins as potential indicators of survival in patients with cervical cancer. BMC Cancer21, 928 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai, C. et al. CDT1 is a novel prognostic and predictive biomarkers for hepatocellular carcinoma. Front. Oncol.11, 721644 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohlschlegel, J. A. et al. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science290, 2309 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Valovka, T. et al. Transcriptional control of DNA replication licensing by Myc. Sci. Rep.-UK3, 3444 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang, C. V. MYC on the path to cancer. Cell149, 22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kciuk, M., Gielecinska, A., Kolat, D., Kaluzinska, Z. & Kontek, R. Cancer-associated transcription factors in DNA damage response. BBA-Rev. Cancer1877, 188757 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Montalto, F. I. & De Amicis, F. Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells-Basel9 (2020). [DOI] [PMC free article] [PubMed]
- 34.Moyret-Lalle, C. et al. Role of EMT in the DNA damage response, double-strand break repair pathway choice and its implications in cancer treatment. Cancer Sci.113, 2214 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this research may be obtained from the corresponding author upon request.