Abstract
In order to elucidate novel actions of degalactosylated whey protein (D-WP) in comparison with intact whey protein (WP), the effects of oral intake of D-WP on peripheral blood telomere length and telomerase were examined in young and aged mice. In young mice, peripheral blood telomere length was significantly elongated following oral intake of D-WP for 4 weeks. mRNA expression of both telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC) was significantly increased in the peripheral blood following oral intake of D-WP for 4 weeks. In aged mice, peripheral blood telomere length was significantly decreased as compared with that of young mice, and significantly restored to the level of young mice drinking water by the oral intake of D-WP for 4 weeks. The mRNA expression of peripheral blood TERT and TERC mRNA in aged mice significantly decreased as compared with the level in young mice drinking water, and was significantly restored to the level of expression of young mice drinking water by oral intake of D-WP for 4 weeks. These results suggest that D-WP, but not WP, potently increases peripheral blood telomere length accompanied by increased mRNA expression of TERT and TERC in both young and aged mice.
Keywords: Whey protein, Degalactosylation, Peripheral blood telomere length, Telomerase, Aging, Mice
Subject terms: Drug discovery, Health occupations
Introduction
Whey protein, the liquid remaining after precipitation and removal of milk casein curd during the production of cheese, comprises beta-lactoglobulin, alpha-lactalbumin, bovine serum albumin, lactoferrin, immunoglobulins, lactoperoxidase enzymes, glycomacropeptides, lactose, and minerals1. Whey protein has immunoregulatory, antioxidant, antihypertensive, antitumor, antiviral, hypolipidemic, and antibacterial effects2–10 and is thus now recognized as a functional food with nutritional applications and health benefits1,2. Protein glycosylation is involved in the induction of multiple biologic activities of peptide hormones, antibodies, lectins, membrane-bound proteins, collagen, and fibronectin11–19. On the other hand, deglycosylation reportedly converts biologically inactive proteins to biologically active proteins20. We recently demonstrated in vivo and in vitro that degalactosylated whey protein (D-WP), but not intact whey protein (WP), potently prevents lipopolysaccharide-induced inflammatory activity in mice, suggesting that deglycosylation enhances the functions of whey protein promotes novel biologic effects of the protein21.
Aging organs accumulate senescence cells induced by DNA damage, which results in induction of organismal aging and multiple age-related dysfunction22. DNA damage is considered to be mainly attributed to shorten of telomere length owing to incomplete lagging-strand DNA synthesis, oxidative damage, exonucleolytic processing events and other factors23, because telomeres are essential for chromosome-end integrity (telomere capping) and chromosomal stability24. Telomere length is maintained by telomerase, a DNA polymerase that consists of an RNA component (TERC) and a catalytic subunit, telomerase reverse transcriptase (TERT), and adds six-base DNA repeats (TTAGGG) to the telomeric ends of chromosomes25,26. Telomerase activity is detected in peripheral blood cells and highly proliferative organs such as the gut, liver, skin, and testis27–29, although it is suppressed or absent in most adult somatic tissues27,30,31. Critically short telomeres can trigger a persistent DNA damage response, leading to cellular senescence and/or apoptosis32. Recent clinical data suggest that parameters of telomere biology in circulating mononuclear cells are associated with cardiovascular morbidity and can be used as indicators for the effects of therapeutic interventions33–35. Moreover, telomere dysfunction in peripheral leukocytes is described in psychiatric conditions36. Accelerated telomere shortening and decreased telomerase activity are reported in chronically stressed individuals37, mood disorders38, and schizophrenia39,40. Interactions of TERT and TERC with shelterin and dyskerin complexes also have important modulatory effects on telomere maintenance and elongation23,41. Shelterin complex facilitates the formation of the t-loop to shield the exposed chromosome ends of telomeric DNA from DNA damage machinery23,41. The combination of shelterin components and a telomere of sufficiently long tract length is essential to protect a chromosome end from eliciting DNA damage responses23,41. A telomerase accessory component, dyskerin complex binds to TERC is essential for TERC stability and telomerase function23,41. Shelterin complex components are made up of six component proteins, such as telomere repeat-binding factor 1 (TRF1), TRF2, repressor/activator protein 1 (RAP1), TRF1-interacting nuclear protein 2 (TIN2), TIN2-interacting protein 1 (TPP1), and protection of telomeres 1 (POT1); and dyskerin complex components comprise dyskerin, NHP2, GAR1, and NOP1023,41.
In order to elucidate novel actions of D-WP in comparison with WP, the effects of oral intake of D-WP on peripheral blood telomere length and telomerase were examined in young and aged mice. We also examined the effects of D-WP on the mRNA expression of telomerase (TERT and TERC) and the shelterin and dyskerin complex components in young mice. Our findings revealed that D-WP, but not WP, significantly increases telomere elongation and the mRNA expression of TERT and TERC in the peripheral blood of both young and aged mice.
Results
Effects of oral intake of D-WP on peripheral blood telomere length in young mice
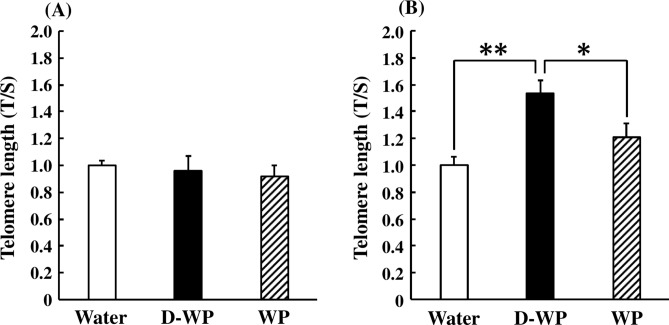
Oral intake of D-WP and WP for 2 weeks did not change peripheral blood telomere length in young mice [F(2, 33) = 0.25, p > 0.05] (Fig. 1A). Peripheral blood telomere length in young mice was significantly elongated following oral intake of D-WP for 4 weeks to 154% of that in the water group, but not after oral intake of WP (121% of that in the water group) [F(2, 36) = 6.19, p < 0.01] (Fig. 1B).
Fig. 1.
Effects of oral intake of D-WP and WP on peripheral blood telomere length in young mice. Telomere length was examined following oral intake of D-WP and WP for 2 weeks (A) and 4 weeks (B). Results are expressed as mean ± SE for 10–12 mice. *p < 0.05, **p < 0.01.
Effects of oral intake of D-WP on the mRNA expression of telomerase and their modulatory factors in the peripheral blood of young mice
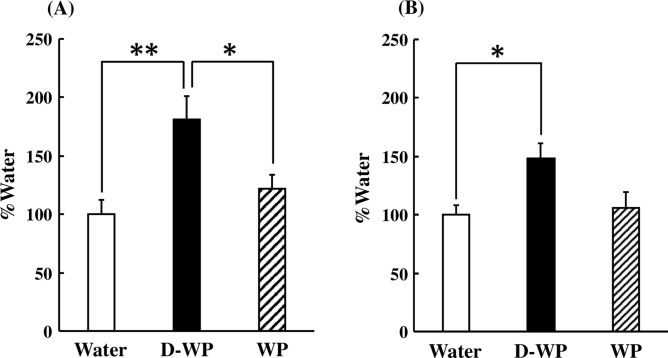
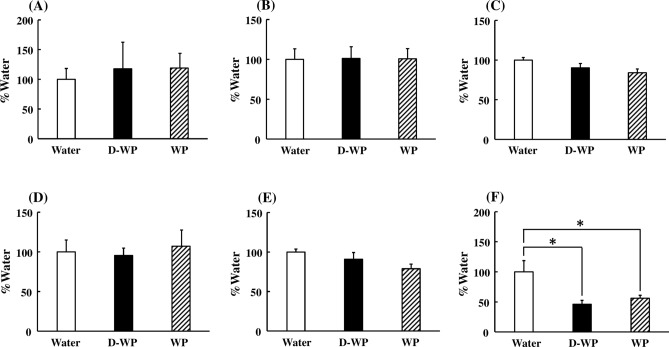
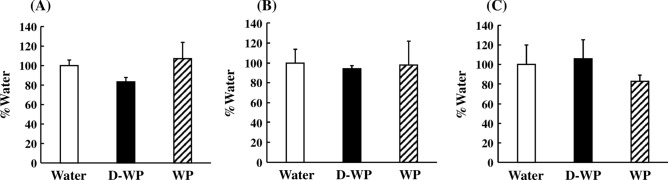
To ascertain the effects of D-WP on peripheral blood telomere elongation, mRNA expression of TERT, TERC, and their modulatory factors in peripheral blood was examined in young mice. mRNA expression of TERT was significantly increased following oral intake of D-WP for 4 weeks to 180% of that in the water group, but not by oral intake of WP (122% of that in water group) [F(2, 25) = 7.39, p < 0.01] (Fig. 2A). In addition, the mRNA expression of TERC following oral intake of D-WP for 4 weeks was significantly increased to 148% of that in the water group, but not affected by oral intake of WP compared with that in the water group [F(2, 21) = 5.40, p < 0.05] (Fig. 2B). Moreover, we examined the effects of D-WP on the mRNA expression of shelterin and dyskerin complex components. The mRNA expression of shelterin complex components, such as TERF2IP, TINF2, TPP1, TRF1, and TRF2, was not changed following the oral intake of either D-WP or WP for 4 weeks (Fig. 3A–E). On the other hand, the mRNA expression of POT1, a shelterin complex component, was significantly decreased following oral intake of both D-WP and WP for 4 weeks to 46% and 56%, respectively, of that in the water group [F(2, 17) = 5.65, p < 0.05] (Fig. 3F). mRNA expression of dyskerin complex components, such as DKC1, GAR1 and NHP2, was not changed by oral intake of D-WP or WP in mice (Fig. 4A–C).
Fig. 2.
Effects of oral intake of D-WP and WP on the mRNA expression of TERT and TERC in the peripheral blood of young mice. mRNA expression of TERT (A) and TERC (B) was examined following oral intake of D-WP and WP for 4 weeks. Results were presented as the percentage of the group of water intake (Water). Results are expressed as mean ± SE for 6–10 mice. *p < 0.05, **p < 0.01.
Fig. 3.
Effects of oral intake of D-WP and WP on the mRNA expression of shelterin complex components in the peripheral blood of young mice. mRNA expression of TERF2IP (A), TINF2 (B), TPP1 (C), TRF1 (D), TRF2 (E) and POT1 (F) was examined following oral intake of D-WP and WP for 4 weeks. Results were presented as the percentage of the group of water intake (Water). Results are expressed as mean ± SE for 5–7 mice. *p < 0.05.
Fig. 4.
Effects of oral intake of D-WP and WP on the mRNA expression of dyskerin complex components in the peripheral blood of young mice. mRNA expression of DKC1 (A), GAR1 (B), and NHP2 (C) was examined following oral intake of D-WP and WP for 4 weeks. Results were presented as the percentage of the group of water intake (Water). Results are expressed as mean ± SE for 5–7 mice.
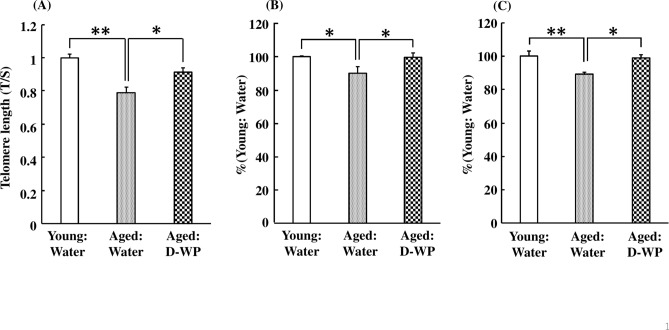
Effects of oral intake of D-WP on peripheral blood telomere length and the mRNA expression of TERT and TERC in aged mice
The peripheral blood telomere length in aged mice was significantly decreased to 79% of that of young mice ingesting water [F(2, 24) = 15.04, p < 0.01] (Fig. 5A). The oral intake of D-WP for 4 weeks significantly elongated the telomere length in aged mice to the same level as in young mice ingesting water (Fig. 5A). Moreover, the mRNA expression of peripheral blood TERT in aged mice was significantly decreased to 90% of that of young mice ingesting water, and was significantly restored to the level of young mice by oral intake of D-WP for 4 weeks [F(2, 22) = 5.34, p < 0.05] (Fig. 5B). Similarly, the mRNA expression of peripheral blood TERC in aged mice was also significantly decreased to 89% of that in young mice ingesting water, and was significantly restored to the level of young mice by oral intake of D-WP for 4 weeks [F(2, 26) = 7.32, p < 0.01] (Fig. 5C).
Fig. 5.
Effects of oral intake of D-WP on peripheral blood telomere length and the mRNA expression of TERT and TERC in aged mice. Telomere length (A) and the mRNA expression of TERT (B) and TERC (C) were examined following oral intake of D-WP for 4 weeks. Results were presented as the percentage of the group of water intake in young mice (Young: Water). Results are expressed as mean ± SE for 8–10 mice. *p < 0.05, **p < 0.01.
Discussion
Whey protein is considered a functional food with nutritional applications and health benefits. In the present study, oral intake of D-WP for 4 weeks by both young and aged mice led to significantly elongated peripheral blood telomere length accompanied by a significant increase in the mRNA expression of TERT and TERC. While intake of WP also tended to increase the telomere length and the mRNA expression of TERT in the peripheral blood of young mice, the increase was not statistically significant. These findings suggest that D-WP is a potent functional food/supplement for maintaining and promoting healthy aging.
Telomeres protect chromosome ends from degradation and DNA repair activities and are essential for chromosome-end integrity and chromosomal stability24. Telomere maintenance and elongation are regulated by telomerase, TERT, and TERC25,26. Recent evidence indicates that telomere biology is a central regulator of the aging process on the cellular level42. Short telomeres can trigger a persistent DNA damage response, which leads to cellular senescence and/or apoptosis32,43. Mice show significant telomere attrition with age, as reflected by a significant decrease in the mean telomere length and a significant increase in the percentage of short telomeres, demonstrating that mice undergo telomere shortening in association with the aging process44. The present findings demonstrated that D-WP led to significant elongation of peripheral blood shortened telomere length and increased the mRNA expression of TERT and TERC in aged mice, indicating that D-WP may have beneficial actions on aging processes. On the other hand, in the present study, WP tended, but not significantly, to increase telomere length in young mice, which did not indicate that WP has no action on telomere elongation but rather indicates the possibility that WP may elongate telomere length for over 4 weeks of oral intake of WP.
Interestingly, telomere dysfunction in peripheral leukocytes is described in psychiatric conditions36. Accelerated telomere shortening and decreased telomerase activity are reported in chronically stressed individuals, mood disorders, and schizophrenia37–40. TERT-deficient mice exhibit telomere shortening over successive generations45, and a significantly altered anxiety-like behaviors46. Thus, telomere length and telomerase activity may be involved in the regulation of several central nervous system functions36. Furthermore, clinical data suggest that parameters of telomere biology in circulating mononuclear cells are associated with cardiovascular morbidity33–35. In particular, a recent study demonstrated that leukocyte telomere length shortening is associated with cardiovascular diseases such as the progression of atherosclerosis47,48. Moreover, increasing evidence indicates that telomerase itself is involved in several disorders independent of the regulation of telomere length and maintenance. Telomerase regulates endothelial cell growth and survival, and acts as an antiapoptotic factor49. Because aging is a predominant and independent risk factor for the development of atherosclerotic diseases, the decrease in telomerase associated with aging may be related to the development of atherosclerotic diseases42. These findings suggest that telomerase has additional functions beyond regulation of telomere length and maintenance50. Taken together, it is possible that D-WP may have another beneficial action on telomerase-related disorders.
Shelterin complex facilitates the formation of the t-loop to shield the exposed chromosome ends of telomeric DNA from DNA damage machinery23,41. The combination of shelterin components and a telomere of sufficiently long tract length is essential to protect a chromosome end from eliciting DNA damage responses23,41. A telomerase accessory component, dyskerin complex binds to TERC is essential for TERC stability and telomerase function23,41. In the present study, the mRNA expression of shelterin and dyskerin complex components was not distinctively changed by oral intake of D-WP for 4 weeks, demonstrating that neither shelterin nor dyskerin complexes are involved in the elongation of telomere length induced by D-WP. However, in the present study mRNA expression of POT1 of the shelterin components was significantly decreased by treatment of both WP and D-WP in young mice. Although POT1 was demonstrated to be critical to telomere maintenance and telomerase processivity51,52, the effects of D-WP on telomere length elongation are different from those of WP. Therefore, further studies will be needed to clarify the reason for decreased mRNA expression of POT1 induced by both WP and D-WP.
Telomere elongation induced by oral intake of D-WP may occur via various mechanisms. D-WP may act on hematopoietic stem cells to elongate telomere length, and old peripheral blood cells with a short telomere length may be supplanted by newly prepared peripheral blood cells with a normal or long telomere length over the long-term. Physical exercise for 1–6 months significantly increases telomere length and telomerase gene expression, indicating that a long period of time is necessary for telomere length elongation induced by physical exercise53,54. Similarly, in the present study, oral intake of D-WP for 4 weeks significantly increased telomere length and telomerase mRNA expression. D-WP may directly act on current blood cells with a short telomere length to elongate telomere length. In this regard, in mouse thymus cell cultures, telomere length was significantly decreased within 3 h after dexamethasone application, and returned to normal telomere length with increased telomerase expression 18 h after dexamethasone application55.
The findings of the present study demonstrated that oral intake of D-WP potently induces telomere elongation and increases mRNA expression of telomerase genes in both young and aged mice. Although little is known about the mechanisms underlying the regulatory effects of D-WP on telomere length and telomerase, these findings suggest that D-WP may enhance chromosome-end integrity and chromosomal stability.
Materials and methods
Experimental animals
Male C57BL/6J mice (2 months old) were obtained from CLEA Japan, Inc. (Tokyo, Japan) and housed in plastic cages under a 12:12 h light/dark cycle (lights on at 7 am) at room temperature (23 ± 1 °C), with free access to water and food (CE-2; CLEA Japan, Inc.). The experiments were started at 3 months and 20–21 months of age as young and aged mice, respectively. Mice were randomly divided into three groups of young and aged mice, respectively; young mice; water intake (n = 12), D-WP intake (n = 12), and WP intake (n = 12): aged mice; water intake (n = 10), D-WP intake (n = 10), and WP intake (n = 10). All animal experiments complied with the ARRIVE guidelines and were performed in accordance with the guidelines established by the Institute of Laboratory Animal Science Research Support Center at Kagoshima University and approved by the Kagoshima University Institutional Animal Care and Use Committee, and in accordance with the guidelines established by the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised in 1996). Every effort was made to minimize the number of animals used and to optimize their comfort.
Preparation of D-WP
Whey protein was obtained from Yotsuba Milk Products Co., Ltd. (Sapporo, Japan). The D-WP was prepared by Saisei Pharma (Moriguchi, Japan) as described previously56. Briefly, 1 mg of whey protein was dissolved in 1 ml of 50 mM sodium phosphate buffer (pH 7.0) and incubated with 65 mU of β-d-galactosidase (from Escherichia coli; WAKO Pure Chemical Industries, Ltd., Osaka, Japan) at 37 °C for 1 h. The reaction mixture was heated at 60˚C for 10 min to inactivate the enzyme. The protein concentrations were determined using a Pierce® BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Experimental schedule
Whey protein mixed in drinking water at a concentration of 60 μg/ml was given to mice for 2 and 4 weeks. Water was used as the control. Whey protein-mixed water was freshly prepared and changed every 3 days. At the end of the experiments, blood samples were collected from the retroorbital vein under isoflurane anesthesia using anesthesia apparatus for small animals (MK-AT210D, Muromachi Kikai Co. Ltd., Osaka, Japan) and immediately transferred to tubes containing ethylenediaminetetraacetic acid (EDTA; 50 μl of 0.2 M EDTA/tube)57. Immediately after blood collection, mice were killed by cervical spine fracture dislocation. Blood samples for DNA extraction were immediately frozen in liquid nitrogen and stored at -80 °C until assayed. Blood samples for RNA extraction were immediately transferred to tubes containing EDTA (10 ml of 0.2 M EDTA/tube) and aprotinin (0.1 mg/tube, Merck KGaA, Darmstadt, Germany), and were centrifuged at 3000×g for 5 min at 4 °C. The plasma was separated and stored at − 80 °C until assayed.
DNA extraction
The blood DNA was extracted using a PureLink® Genomic DNA Mini Kit (Themo Fisher Scientific Inc., Waltham, MA, USA) following the manufacturer’s protocol. DNA samples were diluted to 20 ng/μl with double distilled water for the telomere length test.
Telomere length test
Telomere length (TL) was assessed using real-time quantitative polymerase chain reaction (qPCR) according to the protocol established by Cawthon39,58. The ratio of telomere repeats length (T) to copy number for a single-copy gene (S) was used to indicate TL in each sample. The qPCR for both the telomere and single-copy gene was performed separately in duplicate using FastStart SYBR Green Master (Roche Applied Science, Basal, Switzerland) on a thermal Cycler Dice Real Time System (TAKARA BIO INC., Shiga, Japan). Primer sequences are shown in Table 1. The T and S reactions for each sample were precisely located in the same well position. Then, 20 ng of template DNA was added to each reaction well. To amplify telomeres, 600 nM telo1 primer and 600 nM telo2 primer were used in each reaction. After activating the polymerase at 95 °C for 10 min, the cycling parameters for telomere PCR included 30 cycles of denaturation at 95 °C for 5 s, annealing at 59 °C for 60 s, and extension at 72 °C for 90 s. The acidic ribosomal phosphoprotein PO gene (36B4) was used as a single-copy control. To amplify 36B4, 1000 nM 36B4 forward primer and 600 nM 36B4 reverse primer were used in each reaction. The cycling conditions for 36B4 included initial denaturation at 95 °C for 10 min, followed by 30 cycles of denaturation at 95 °C for 5 s, annealing at 58 °C for 15 s, and extension at 72 °C for 15 s. Variations between places were controlled by a standard curve method prepared by triple serial dilutions of a reference genomic DNA sample ranging from 1.85 to 150 ng (1.85, 5.56, 16.7, 50, 150 ng, referred to as STD1 to STD5, respectively). The mean of the correlation coefficient of the standard curves was > 0.99 for both the T and S reactions. The inter-plate coefficients of variance of the Ct value for the calibrator sample were 2.83% for T and 1.96% for S. DNA samples with a CV > 0.05 between triplicate tests or a Ct value of > 36 were excluded from further analysis (n = 2). The T/S ratio was calculated according to the comparative 2−ΔΔCt method, where ΔΔCt = ΔCtsample − ΔCtcalibrator sample, ΔCtsample = CtT − CtS, and Ctcalibrator sample = (CtSTD2 + CtSTD3)/259,60.
Table 1.
Primers used to determine telomere length.
| Primer name | Sequence (5ʹ–3ʹ) |
|---|---|
| Telo1 | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT |
| Telo2 | GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT |
| m36B4 forward | ACTGGTCTAGGACCCGAGAAG |
| m36B4 reverse | TCAATGGTGCCTCTGGAGATT |
Quantitative real-time reverse transcription-PCR
Although it is recognized that examination of protein levels and enzyme activity assays is appropriate for conformation of expression of TERT, TERC and its related other regulatory factors, several previous reports have been demonstrated that telomere length is correlated with mRNA expression of TERT, TERC and its related other regulatory factors61,62. Therefore, mRNA expression was examined in this study to evaluate correlation between telomere length and these factors. The mRNA levels were measured by quantitative real-time reverse transcription-PCR57. The blood RNA was extracted using NucleoSpin® RNA Blood (TAKARA BIO INC.), and cDNA was synthesized from total RNA samples using a Verso cDNA Synthesis Kit (Thermo Fisher Scientific Inc.). The PCR was performed in duplicate using FastStart SYBR Green Master (Roche Applied Science) on a thermal Cycler Dice Real Time System (TAKARA BIO INC.). Primer sequences are shown in Table 2. All gene-specific mRNA expression values were normalized against the internal housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The reaction conditions for all primers were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 30 s. As shown in Supplementary Fig. 1, the threshold cycle (Ct) value of GAPDH mRNA in each PCR experiment was not different between treated groups.
Table 2.
PCR primers used to determine mRNA expression.
| Primer name | Sequence (5ʹ–3ʹ) | |
|---|---|---|
| Telomerase | ||
| TERT | F | TGGGTCTCCCCTGTACCAAAT |
| R | GGCCTGTAACTAGCGGACACA | |
| TERC | F | CTGTTTTTCTCGCTGACTTCCA |
| R | GAGCTCCTGCGCTGACGTTTGT | |
| Shelterin complex | ||
| TERF2IP | F | TGCCTTGTGGAAAGCGATG |
| R | TGTTCTGTGGCTCTCCGCTAT | |
| TINF2 | F | TCGGTTGCTTTGCACCAGTAT |
| R | GCTTAGCTTTAGGCAGAGGAC | |
| TPP1 | F | GAGTCTCACTTTTGCGCTGAA |
| R | CTCCAGGGTTAGGTACTTTCCA | |
| TRF1 | F | CCGAGGACTTTCGTCGTACTC |
| R | CTTTCCAGATGCAACTCTTGTCA | |
| TRF2 | F | TCTAAGGACCCCACAACTCAG |
| R | TCTCTAGGAAACGCAGCATC | |
| POT1 | F | GGTTTCAACAGCTCCCTATAC |
| R | AGGGCTTCATAGTTTCCACT | |
| Dyskerin complex | ||
| DKC1 | F | AAAGACCGGAAGCCATTACAAG |
| R | GCCACTGAGAAGTGTCTAATTGA | |
| GAR1 | F | GAACGTGTCGTCTTGTTAGGAG |
| R | AGTAAACAGGGGCGTTGAAGT | |
| NHP2 | F | CATTGCCGATTGAGGTGTACT |
| R | GTCCGTCTTAGAGGGGATGTAG | |
| House-keeping gene | ||
| GAPDH | F | TGCACCACCAACTGCTTAGC |
| R | GGATGCAGGGATGATGTTCTG | |
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis of the data was performed by ANOVA followed by the Tukey–Kramer test. Statistical significance was defined as P < 0.05.
Supplementary Information
Author contributions
T.I., N.K., K.K. and G.K. performed experiments, contributed to discussions, and wrote the manuscript. M.Y., H.Y., N. S-A., and Y.O. contributed to discussions, and reviewed and edited the manuscript. N.K. and G.K. are the guarantors of this work and, as such, had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Toshio Inui, Email: toshio.inui1003@gmail.com.
Goro Katsuura, Email: gorokatsuura48@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81597-3.
References
- 1.Walzem, R. L., Dillard, C. J. & German, J. B. Whey components: millennia of evolution create functionalities for mammalian nutrition: what we know and what we may be overlooking. Crit. Rev. Food Sci. Nutr.42, 353–375 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Marshall, K. Therapeutic applications of whey protein. Altern. Med. Rev.9, 136–156 (2004). [PubMed] [Google Scholar]
- 3.Hakansson, A. et al. Multimeric alpha-lactalbumin from human milk induces apoptosis through a direct effect on cell nuclei. Exp. Cell Res.246, 451–460 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu, J., Dupont, C. & Lemieux, P. Whey proteins and peptides: beneficial effects on immune health. Therapy3, 1–10 (2006). [Google Scholar]
- 5.Rusu, D., Drouin, R., Pouliot, Y., Gauthier, S. & Poubelle, P. E. A bovine whey protein extract stimulates human neutrophils to generate bioactive IL-1Ra through a NF-kappaB- and MAPK-dependent mechanism. J. Nutr.140, 382–391 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Castro, G. A., Maria, D. A., Bouhallab, S. & Sgarbieri, V. C. In vitro impact of a whey protein isolate (WPI) and collagen hydrolysates (CHs) on B16F10 melanoma cells proliferation. J. Dermatol. Sci.56, 51–57 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Ebaid, H., Salem, A., Sayed, A. & Metwalli, A. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis.10, 235–244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kume, H., Okazaki, K. & Sasaki, H. Hepatoprotective effects of whey protein on D-galactosamine-induced hepatitis and liver fibrosis in rats. Biosci. Biotechnol. Biochem.70, 1281–1285 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi, T. et al. A new enteral diet, MHN-02, which contains abundant antioxidants and whey peptide, protects against carbon tetrachloride-induced hepatitis. J. Parenter. Enteral. Nutr.35, 516–522 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Badr, G., Ebaid, H., Mohany, M. & Abuelsaad, A. S. Modulation of immune cell proliferation and chemotaxis towards CC chemokine ligand (CCL)-21 and CXC chemokine ligand (CXCL)-12 in undenatured whey protein treated mice. J. Nutr. Biochem.23, 1640–1646 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Lin, B., Qing, X., Liao, J. & Zhuo, K. Role of protein glycosylation in host-pathogen interaction. Cells9, 1022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagano, M. R., Mendieta, J. R., Munoz, F. F., Daleo, G. R. & Guevara, M. G. Roles of glycosylation on the antifungal activity and apoplast accumulation of StAPs (Solanum tuberosum aspartic proteases). Int. J. Biol. Macromol.41, 512–520 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Yan, Q. J., Qi, X. W., Jiang, Z. Q., Yang, S. Q. & Han, L. J. Characterization of a pathogenesis-related class 10 protein (PR-10) from Astragalus mongholicus with ribonuclease activity. Plant Physiol. Biochem.46, 93–99 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Park, J. I., Semyonov, J., Chang, C. L. & Hsu, S. Y. T. Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans. Endocrine26, 267–276 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem.278, 3466–3473 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Su, Y. P. et al. Glycosylation influences the lectin activities of the macrophage mannose receptor. J. Biol. Chem.280, 32811–32820 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Tytgat, H. L. P. & de Vos, W. M. Sugar coating the envelope: Glycoconjugates for microbe-host crosstalk. Trends Microbiol.24, 853–861 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Bann, J. G., Peyton, D. H. & Bachinger, H. P. Sweet is stable: Glycosylation stabilizes collagen. FEBS Lett.473, 237–240 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Sano, K. et al. Glycosylation and ligand-binding activities of rat plasma fibronectin during liver regeneration after partial hepatectomy. Carbohydr. Res.343, 2329–2335 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Innamorati, G., Sadeghi, H. & Birnbaumer, M. A fully active nonglycosylated V2 vasopressin receptor. Mol. Pharmacol.50, 467–473 (1996). [PubMed] [Google Scholar]
- 21.Inui, T., Kawamura, N., Nakama, R., Inui, A. & Katsuura, G. Degalactosylated whey protein suppresses inflammatory responses induced by lipopolysaccharide in mice. Front. Nutr.9, 852355 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossiello, F., Jurk, D., Passos, J. F. & di Fagagna, F. D. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol.24, 135–147 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shay, J. W. & Wright, W. E. Telomeres and telomerase: three decades of progress. Nat. Rev. Genet.20, 299–309 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Chan, S. W. L. & Blackburn, E. H. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene21, 553–563 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Blackburn, E. H. Switching and signaling at the telomere. Cell106, 661–673 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Feng, J. et al. The RNA component of human telomerase. Science269, 1236–1241 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Greenberg, R. A., Allsopp, R. C., Chin, L., Morin, G. B. & DePinho, R. A. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene16, 1723–1730 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Martín-Rivera, L., Herrera, E., Albar, J. P. & Blasco, M. A. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc. Natl. Acad. Sci. U. S. A.95, 10471–10476 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi, Y. et al. Change in telomerase activity of rat organs during growth and aging. Exp. Cell Res.242, 120–127 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Kim, N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science266, 2011–2015 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Burger, A. M., Bibby, M. C. & Double, J. A. Telomerase activity in normal and malignant mammalian tissues: feasibility of telomerase as a target for cancer chemotherapy. Br. J. Cancer75, 516–522 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell130, 223–233 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Brouilette, S. W. et al. West of Scotland Coronary Prevention Study Group. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet369, 107–114 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Wong, L. S. M., de Boer, R. A., Samani, N. J., van Veldhuisen, D. J. & van der Harst, P. Telomere biology in heart failure. Eur. J. Heart Fail.10, 1049–1056 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Farzaneh-Far, R. et al. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler. Thromb. Vasc. Biol.28, 1379–1384 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou, Q.-G. et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J. Neurosci.31, 12258–12269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA101, 17312–17315 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon, N. M. et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry60, 432–435 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Kao, H.-T. et al. Rapid telomere erosion in schizophrenia. Mol. Psychiatry13, 118–119 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Porton, B. et al. Telomerase levels in schizophrenia: a preliminary study. Schizophr. Res.106, 242–247 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armanios, M. & Blackburn, E. H. The telomere syndromes. Nat. Rev. Genet.13, 693–704 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werner, C. et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation120, 2438–2447 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature345, 458–460 (1990). [DOI] [PubMed] [Google Scholar]
- 44.Vera, E., de Jesus, B. B., Foronda, M., Flores, J. M. & Blasco, M. A. The rate of increase of short telomeres predicts longevity in mammals. Cell Rep.2, 732–737 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Yuan, X. et al. Presence of telomeric G-strand tails in the telomerase catalytic subunit TERT knockout mice. Genes Cells4, 563–572 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Lee, J. et al. Telomerase deficiency affects normal brain functions in mice. Neurochem. Res.35, 211–218 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Yin, H. & Pickering, J. G. Telomere Length: Implications for Atherogenesis. Curr. Atheroscler. Rep.25, 95–103 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagris, M., Theofilis, P., Antonopoulos, A. S., Tsioufis, K. & Tousoulis, D. Telomere length: a cardiovascular biomarker and a novel therapeutic target. Int. J. Mol. Sci.23, 16010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haendeler, J. et al. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res.94, 768–775 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Bennaceur, K. et al. Atorvastatin induces T cell proliferation by a telomerase reverse transcriptase (TERT) mediated mechanism. Atherosclerosis236, 312–320 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Liu, D. et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol.6, 673–680 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature423, 1013–1018 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Denham, J. & Sellami, M. Exercise training increases telomerase reverse transcriptase gene expression and telomerase activity: A systematic review and meta-analysis. Ageing Res. Rev.70, 101411 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Sánchez-González, J. L., Sánchez-Rodríguez, J. L., Martín-Vallejo, J., Martel-Martel, A. & González-Sarmiento, R. Effects of physical exercise on cognition and telomere length in healthy older women. Brain Sci.27, 1417 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichiyoshi, H., Kiyozuka, Y., Kishimoto, Y., Fukuhara, S. & Tsubura, A. Massive telomere loss and telomerase RNA expression in dexamethasone-induced apoptosis in mouse thymocytes. Exp. Mol. Pathol.75, 178–186 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Uto, Y. et al. Degalactosylated/desialylated bovine colostrum induces macrophage phagocytic activity independently of inflammatory cytokine production. Anticancer Res.35, 4487–4492 (2015). [PubMed] [Google Scholar]
- 57.Kawamura, N. et al. Impaired brain fractalkine-CX3CR1 signaling is implicated in cognitive dysfunction in diet-induced obese mice. BMJ Open Diab. Res. Care9, e001492 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res.30, e47 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martisson, I. et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Trans. Psychiatry.3, e261 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao, S., Ye, N., Hu, H., Shen, Y. & Xu, Q. Variants in TERT influencing telomere length are associated with paranoid schizophrenia risk. Am. J. Med. Genet. B Neuropsychiatr. Genet.171B, 317 (2016) [DOI] [PubMed] [Google Scholar]
- 61.Zang, M.-W. et al. Tissue iron is negatively correlated with TERC or TERT mRNA expression: a heterochronic parabiosis study in mice. Aging-US 10, 3834 (2018) [DOI] [PMC free article] [PubMed]
- 62.Liu, Z. et al. Inverse changes in telomere length between the blood and brin in depressive-like mice. J. Affect. Disord.273, 453 (2020) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.