Abstract
The production of lipopolysaccharide (LPS)-free recombinant proteins from culture supernatants is of great interest to biomedical research and industry. Due to the LPS-free cell wall structure and the well-defined secretion factor B (SecB)-dependent secretion pathway, Gram-positive bacteria are a superior alternative to Escherichia coli expression systems. However, the lack of inducible expression systems for high yields has been a bottleneck. To address this, we developed the pKS81 plasmid, featuring the uhpT (glucose-6-phosphate [G6P] transporter) promoter for high expression of recombinant proteins induced by extracellular G6P via a three-component hexose phosphate transport regulatory system (HptARS), the N-terminal SecB-dependent signal peptide sequence for recombinant protein secretion, and the C-terminal 8 × histidine tag for purification by nickel affinity chromatography. We also generated an expression host strain, Staphylococcus aureus LAC9, lacks the uhpT gene and harmful superantigen and leukotoxin genes, allowing for constitutive HptARS activation by extracellular G6P and increased safety, respectively. Using the pKS81 plasmid, we successfully achieved high yields of prokaryotic (staphylococcal leukotoxin E) and eukaryotic (human annexin A2 protein tagged with mouse IgG1) recombinant proteins, up to 900 mg/L. Our newly established inducible and secretory expression system provides for efficient production and easy purification of LPS-free recombinant proteins, making it valuable for biomedical research and industrial applications.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81001-0.
Keywords: Hexose phosphate transport system, Inducible and secretory protein expression system, Extracellular production
Subject terms: Genetic engineering, Expression systems, Bacterial techniques and applications
Introduction
A large-scale production of recombinant proteins is important for biopharmaceutical companies and other industries. Escherichia coli (E. coli) has been the most widely used recombinant protein expression agent due to the ease of genetic manipulation and the availability of various inducible expression vectors that reproducibly produce large protein yields. The main challenges with protein expression and purification in E. coli systems are a lack of an efficient secretory system that requires complicated purification processes and results in contamination by lipopolysaccharides (LPS) which is highly toxic to humans1,2. In E. coli, proteins are synthesized in the cytoplasm and excreted by the secretion factor B (SecB)-dependent type II secretion system by which pre-proteins are carried to the inner membrane and transferred to the periplasmic space where they are folded and excreted by non-specific periplasmic leakage3,4. However, many recombinant proteins are trapped in the periplasm5, which requires enzymatic and/or mechanical disruption for protein purification. During these processes, recombinant proteins are mechanically or enzymatically damaged resulting in functional loss or poor yield6. Furthermore, recombinant proteins purified from E. coli are heavily contaminated with LPS which can cause secretion of proinflammatory cytokines, inhibition of cell growth, and hyperactivation of immune cells, resulting in endotoxic shock or even death7,8. Therefore, LPS must be removed from FDA-approved biologics which requires extensive and costly downstream procedures9,10.
Gram-positive bacteria possess a single layer of cytoplasmic membrane free of LPS and the presence of defined SecB-dependent secretory system which allows the translocation of recombinant proteins into the culture supernatants without LPS contamination, making it an ideal system to produce recombinant proteins for biomedical uses11,12. Several Gram-positive bacteria including Bacillus, Lactococcus, and Streptomyces have been used in industry to produce a variety of recombinant proteins13–16. However, large-scale production of recombinant protein has been challenging due to the lack of efficient inducible expression systems.
Recently, we characterized the hexose phosphate transport (Hpt) system in Staphylococcus aureus17. The Hpt system is composed of the three-component regulatory system (HptARS) and the UhpT hexose phosphate transporter. HptA senses extracellular glucose-6-phosphate (G6P) which induces phosphorylation of the HptS histidine kinase, followed by phosphorylation of the HptR transcriptional factor in the HptRS regulatory system. The phosphorylated HptR binds to the uhpT gene promoter region (GTTCAGTATTTTGGATAATTTAATAATTTT) and induces uhpT transcription at several hundred-fold to facilitate uptake G6P from the media17,18.
In this study, we developed an inducible secretory expression vector system by generating a pKS81 plasmid, containing the uhpT gene promoter and the SecB-dependent signal peptide sequence, in a S. aureus UhpT deletion mutant expression host strain. Using this system, we demonstrated successful expression of prokaryotic and eukaryotic proteins at yields of up to 900 mg/L. These results indicate that the inducible secretory expression vector system established in this study provides for efficient production and easy purification of LPS-free recombinant protein which can be broadly used for biomedical research and industry.
Materials and methods
Bacterial strains, plasmids, DNA constructs, and peptide sequences
The E. coli-S. aureus shuttle vector, pOS1, was obtained from Dr. Taeok Bae (Indiana University). The DNA sequences of the uhpT promoter, the signal peptide sequence of Hlb (UniProtKB: P09978), LukE (UniProtKB: O54081), AnxA2 (UniProtKB: P07355), and mouse IgGFc (UniProtKB: P01868) were chemically synthesized (GenScript Biotech). A bioluminescent reporter plasmid, pLuxABCDE (Caliper) was used to assess the transcriptional activity of the uhpT promoter.
Construction of inducible secretory expression vector system, pKS81 and S. aureus expression host strain
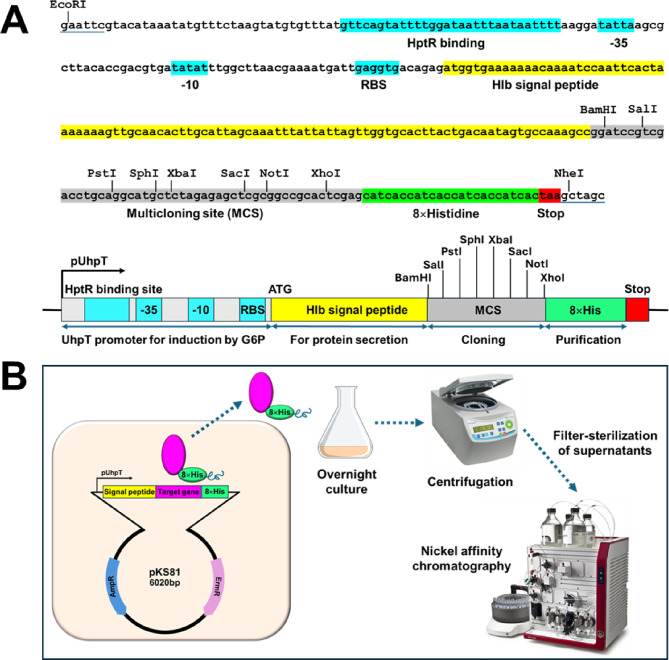
The synthetic oligonucleotides containing the uhpT gene promoter, a signal peptide sequence of Hlb, multiple cloning sites, and the C-terminal 8 × histidine residues were synthesized (GenScript Biotech) and cloned into the EcoRI/NheI restriction sites in the pOS1 plasmid, resulting in a pKS81 plasmid (Fig. 1A).
Fig. 1.
Construction of an inducible secretory expression system, pKS81. (A) The sequence of the HptR binding site, uhpT promoter (-35 and -10), ribosome binding site, and N-terminal signal peptide sequence of the Hlb, multicloning sites, and 8 × histidine tag cloned into the pOS1 plasmid. (B) A schematic illustration of recombinant protein expression, secretion, and purification process.
A modified pMAD-CM-GFPuv, a temperature-sensitive shuttle vector system, was used to generate markerless deletion of the uhpT, superantigen and leukotoxin genes from S. aureus LAC resulting in S. aureus LAC9 (ΔuhpT, ΔhlgABC, ΔlukFS, Δhla, ΔlukGH, Δpsm, ΔlukDE, Δselq, Δselk, Δselx) as described previously17. Briefly, the upstream and downstream of the target gene fragments were amplified by PCR and cloned into pMAD-CM-GFPuv. The resulting plasmid was electroporated into S. aureus LAC using an ECM 630 exponential decay wave electroporation system (BTX®, Harvard Apparatus). The transformed strain was cultured in CY (1% casamino acids, 1% yeast extract, 100 mM NaCl) agar plate with chloramphenicol (10 µg/mL) at 43 °C using Isotemp™ incubator (Fisher Scientific), which is a non-permissive temperature for the plasmid replication, to promote the first homologous recombination. A single colony was transferred to CY agar plate with chloramphenicol and cultured at 37 °C to promote the second recombination, resulting in a deletion of the target gene. The deletion of the uhpT, superantigen, and leukotoxin genes was confirmed by sequencing using a nanopore sequencing kit (Nanopore technologies, UK).
Measuring transcriptional activity of the uhpT promoter by bioluminescent signal
To measure the induction of target gene expression by G6P under the control of the uhpT promoter, a synthetic DNA fragment containing the uhpT gene promoter region was cloned into a promoterless bioluminescent plasmid (pLuxABCDE)19. The resulting plasmid was transformed into E. coli DH5α, transferred into S. aureus RN4220, and subsequently electroporated into S. aureus LAC wild type or LAC9. S. aureus wild type or LAC9 strains, harboring the constructed plasmid, were cultured in CY broth without or with 2% G6P at 37 oC and bioluminescence was measured using Cytation 5 cell imaging multimode reader (BioTek Instrument). To determine the optimal G6P concentration for inducing maximal target gene expression, LAC9 harboring the constructed plasmid were cultured in CY broth supplemented with varying G6P concentrations (0, 0.5, 1, 2 or 3% w/v), corresponding to approximately 19.2 mM, 38.4 mM, 76.8 mM, and 115.3 mM, respectively, at 37 oC and bioluminescence was measured using the same method.
Expression and purification of staphylococcal leukotoxin E (LukE) and human annexin A2 fused with mouse IgG1 heavy chain (AnxA2-mIgG1) using pKS81 and S. aureus expression host strain
Synthetic DNA encoding LukE or AnxA2-mIgG1 was cloned between the BamHI and XhoI sites in the pKS81. The resulting plasmids were transformed into S. aureus LAC9 by electroporation. To determine the optimal G6P concentration for maximal LukE expression, the transformed strains harboring pKS81 LukE were cultured in CY broth containing various concentrations of G6P (0, 0.5, 1, 2, or 3% w/v) at 37 oC for 18 h with shaking at 200 rpm using MaxQ™ 4450 Benchtop Orbital Shaker (ThermoFisher scientific). To verify the protein expression in culture supernatants, the samples were centrifuged at 13,500 × g using a microcentrifuge (Eppendorf), supernatant proteins were then concentrated by precipitation with trichloroacetic acid (10%, w/v). The proteins were analyzed by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 125 V for 1.5 h using a XCell SureLock™ Mini-Cell electrophoresis system (Invitrogen) and visualized using Coomassie Blue staining. The image of the stained gel was digitized by the ChemiDoc™ MP imaging system (Bio-Rad Laboratories, Inc) using Image Lab™ Touch Software, version 2.4.0.03, with optimal auto-exposure setting.
For protein purification, LAC9 strains harboring the constructed plasmids were cultured in 50 mL of CY broth containing 2% G6P at 37 oC for 18 h with shaking at 200 rpm. Generally, OD600 of overnight culture ranged around 1.8. The culture supernatants were collected by centrifugation at 18,000 × g for 10 min using Sorvall RC6 + superspeed centrifuge, filter-sterilized using 0.45 μm polyethersulfone syringe filter (Corning), and the His-tagged proteins were purified on the Hisprep™ Fast flow 16/10 column (Cytiva) by ÄKTA pure™ chromatography systems (Cytiva) with Unicorn 6.4 software, as suggested by the manufacturer. Purification was conducted at a flow rate of 5 mL/min, 22 oC, with detection at 280 nm and the fraction volume of 5 mL. The elution buffer was exchanged with PBS using Amicon® Ultra Centrifugal filter tube (10 kDa MWCO, Millipore Sigma), and the final volume was adjusted to 3 mL. Protein concentrations were quantified by measuring the absorbance at 280 nm using a Nanodrop Spectrophotometer (Thermo Fisher Scientific). Purified proteins were analyzed by 12.5% SDS-PAGE and visualized using Coomassie Blue staining, with digitized gel images captured as described above.
Cytotoxicity assay with human leukocytes
A cytotoxicity assay with human leukocytes was performed to verify the biological activity of recombinant LukE expressed in our pKS81 vector system. Blood was obtained by venipuncture from healthy volunteers and informed consent was obtained from the volunteers in accordance with the protocol (18–283), which was reviewed and approved by the Institutional Review Board at Mississippi State University. Human leukocytes were purified by lysing red blood cells with ammonium-chloride-potassium lysing buffer (Invitrogen). Purified human leukocytes were adjusted to 1 × 106/mL in serum free RPMI media. Cells were co-incubated with purified LukE (1 µg), LukD (1 µg, Abcam), or both LukD and LukE for 30 min and then propidium iodine solution (1µM) was added. The fluorescent intensity as an indication of membrane damage was measured using Cytation 5 cell imaging multimode reader (BioTek Instrument).
Statistical analysis
The statistical significance of data for cytotoxicity assay was analyzed by Student t-test using GraphPad Prism version 9.4.1 (GraphPad).
Results
Construction of an inducible and secretory expression vector system and a compatible S. Aureus host strain
Synthetic DNA containing the HptR binding sites, the promoter region of the uhpT gene (-35 and -10 sites), a ribosome binding site, and the N-terminal secretory signal peptide sequence of the β-hemolysin (Hlb), the multicloning sites, and C-terminal 8 × histidine residues was chemically synthesized and cloned into the pOS1 E.coli-S. aureus shuttle vector, resulting in an inducible and secretory expression vector system, pKS81 (Fig. 1A). The resulting pKS81 vector allows for induced expression of the target gene by extracellular G6P and secretion of the expressed protein into the media due to the SecB-dependent Hlb signal peptide sequence. The C-terminal 8 × histidine tag allows for simplified purification of the expressed protein from the supernatant using nickel affinity column chromatography (Fig. 1B).
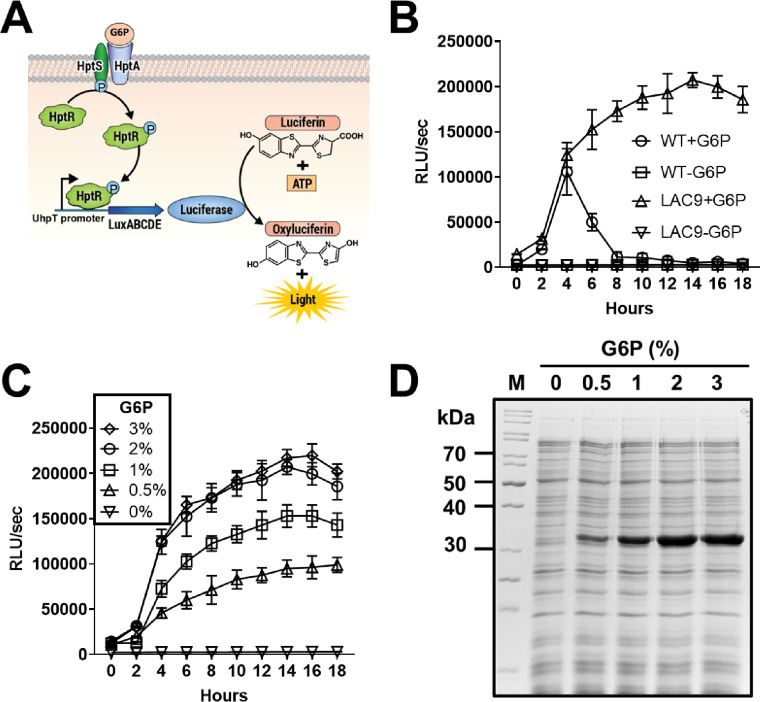
Since G6P is a highly metabolizable sugar quickly depleted by bacterial metabolism, the transcriptional activation of the uhpT promoter by G6P could be temporal, lasting only as long as G6P remains in the culture media. However, since UhpT is the only G6P transporter in S. aureus17, we speculated that a deletion of the uhpT gene would conserve G6P in the culture media which could constitutively activate the HptARS system and sustain transcriptional activation from the uhpT promoter. In addition, S. aureus LAC strain produces superantigens and leukotoxins and the contamination of these toxins could be harmful. To ensure high yield production of recombinant protein and increased safety, we removed the uhpT, superantigen, and leukotoxin genes from S. aureus LAC wild type strain using a markerless deletion system17, resulting in S. aureus LAC9 strain (ΔuhpT, ΔhlgABC, ΔlukFS, Δhla, ΔlukGH, Δpsm, ΔlukDE, Δselq, Δselk, Δselx). To demonstrate constitutive transcriptional activation of the uhpT promoter by extracellular G6P, the uhpT promoter was cloned into a promoterless bioluminescent pLuxABCDE plasmid, which was transformed into S. aureus LAC wild type and LAC9 strain (Fig. 2A). When S. aureus wild type strain was cultured in the CY broth supplemented with G6P, the bioluminescent signal rapidly increased, peaked at 4 h, and then decreased thereafter (Fig. 2B). By contrast, the bioluminescent signal from S. aureus LAC9 was sustained throughout a whole culture period. These results indicated that a deletion of the uhpT gene resulted in the sustained presence of G6P in the culture media, which constitutively activated the HptARS system to induce continuous transcriptional activation of the uhpT promoter. It is noted that no bioluminescent signal was observed from both S. aureus wildtype and ΔuhpT strains cultured in the CY broth without G6P supplementation. These results indicated that transcriptional activation of the uhpT promoter is strictly dependent on the presence of G6P in the culture media.
Fig. 2.
Analyzing transcriptional activation of the uhpT promoter and protein expression by G6P. (A) A schematic illustration of bioluminescent signal induced by extracellular G6P via HptARS, three-component regulatory system and the uhpT promoter cloned into the promoterless pLuxABCDE plasmid. (B) S. aureus LAC wild type and LAC9 strains harboring a bioluminescent reporter plasmid pLuxABCDE were cultured in CY broth supplemented without or with 2% G6P. Activation of the uhpT gene promoter, indicated by induction of bioluminescent signal, was measured using Cytation 5 cell imaging multimode reader (BioTek). Data shown are the mean ± SEM combined from three independent experiments. (C) S. aureus LAC9 harboring a bioluminescent reporter plasmid pLuxABCDE were cultured in CY broth supplemented with various concentrations of G6P (0, 0.5, 1, 2, or 3% w/v). Activation of the uhpT gene promoter, indicated by induction of bioluminescent signal, was measured using Cytation 5 cell imaging multimode reader (BioTek). Data shown are the mean ± SEM combined from three independent experiments. (D) A synthetic DNA fragment encoding LukE was cloned into BamHI and XhoI sites in the pKS81 plasmid which was transformed into S. aureus LAC9 strain. Transformed strain was cultured in CY broth supplemented with various concentrations of G6P (0, 0.5, 1, 2, or 3% w/v) for 18 h at 37 oC with shaking at 200 rpm. The proteins in culture supernatant were collected and protein expression was analyzed by SDS-PAGE followed by Coomassie Blue staining. M: Protein marker, lane 1: culture supernatant from CY broth without G6P, lane 2: with 0.5% G6P, lane 3: with 1% G6P, lane 4: with 2% G6P, lane 5: with 3% G6P.
In addition, to determine the optimal conditions for maximal the uhpT promoter activity, LAC9 was cultured with various G6P concentrations (0–3% w/v). The bioluminescence intensity exhibited a dose-dependent response, with 2% and 3% G6P yielding robust and sustained levels of bioluminescent signals (Fig. 2C). This result suggests that 2% and 3% G6P supplementation would be suitable for maximal expression of target protein.
Expression of prokaryotic proteins using an inducible and secretory protein expression vector system
To further confirm the optimal G6P concentration for protein expression, the staphylococcal leukotoxin E (lukE) gene was amplified and cloned into the pKS81 plasmid which was transformed into S. aureus LAC9. When cultured in CY media with varying G6P concentrations (0–3% w/v), the strain showed a dose-dependent increase in LukE protein expression levels (Fig. 2D), consistent with the dose-dependent uhpT promoter activity observed in Fig. 2C. The bioluminescence signals and protein expression levels were similar at 2% and 3% G6P, indicating that 2% G6P was fully sufficient for maximal transcriptional activity and protein expression. Therefore, 2% G6P was selected as the optimal concentration for target protein expression and purifications in the subsequent experiments.
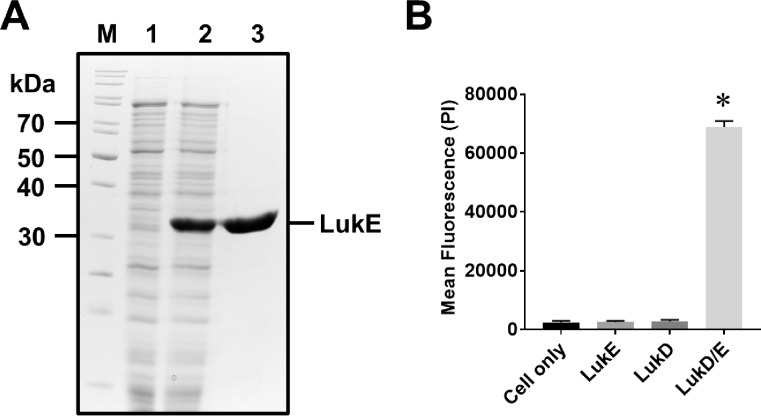
To demonstrate that the inducible and secretory expression system established in this study can produce a large quantity of prokaryotic protein LukE, the supernatant from the strain cultured in the CY media supplemented with 2% G6P (w/v) showed high level of LukE expression at the expected molecular weight of 32.7 kDa which was absent in the culture supernatant from the CY media without G6P (Fig. 3A). A highly pure LukE protein was obtained by nickel affinity chromatography, a yield of up to 15 mg/50 mL of culture.
Fig. 3.
Biological activity of recombinant LukE expressed and purified from an inducible and secretory protein expression vector system, pKS81. (A) A synthetic DNA fragment encoding LukE was cloned into BamHI and XhoI sites in the pKS81 plasmid which was transformed into S. aureus LAC9 strain. Transformed strain was cultured in CY broth without or with supplementation of G6P (2%, w/v) for 18 h at 37 oC with shaking at 200 rpm. The culture supernatant was collected and recombinant LukE protein was purified by the nickel affinity chromatography. Protein expression and purification were analyzed by the SDS-PAGE with Coomassie Blue staining. M: Protein marker, lane 1: culture supernatant from CY broth without G6P, lane 2: culture supernatant from CY broth with G6P, lane 3: purified LukE. (B) Human leukocytes (1 × 106 cell/mL) were incubated with purified LukE (1 µg), LukD (1 µg, Abcam), or both LukE/LukD (1 µg each) for 30 min at 37o C. Cytotoxicity as indicated by incorporation of propidium iodine (PI) to the cellular DNA was measured using Cytation 5 cell imaging multimode reader (BioTek). Data shown are the mean ± SEM combined from three independent experiments. Asterisk indicates statistical significance in Student t-test, compared to the results from cell only (p < 0.001).
To verify the bioactivity of the purified LukE, we performed a cytotoxicity assay using human leukocytes. The LukE is an S component of the bi-component leukotoxin, which is not cytotoxic by itself but requires an F-component of the bi-component leukotoxin D (LukD) for cytotoxicity20. As shown in Fig. 3B, incubation of human leukocytes with LukE alone did not induce any cytotoxicity. By contrast, incubation of human leukocytes with both LukE and LukD (obtained from Abcam) induced a strong cytotoxicity, as indicated by the propidium iodine signal. These results indicated that the LukE expressed and purified from our study was highly pure and biologically active. It also indicates that the expression host S. aureus LAC9 strain did not produce any other harmful bi-component leukotoxins.
Expression of eukaryotic protein using an inducible and secretory protein expression vector system
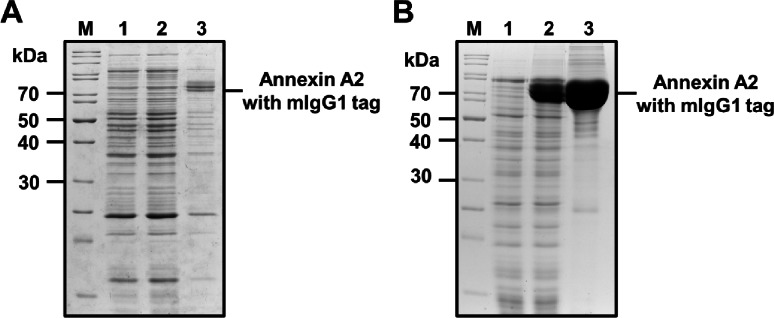
Initially, we attempted to express AnxA2-mIgG1 using a conventional isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible pET expression system in E. coli BL21(DE3) pLysS. However, we were unable to obtain soluble AnxA2-mIgG1; instead, we produced a low quantity of insoluble protein, rendering it unsuitable for bioassays (Fig. 4A). Additionally, the expression levels were inconsistent across trials, with some experiments yielding no detectable expression in the E. coli expression system. We then cloned the gene encoding the human annexin A2 fused with mouse IgG1 Fc (AnxA2-mIgG1) into the pKS81 plasmid which was transformed into S. aureus LAC9. The supernatant from the strain cultured in the CY media supplemented with 2% G6P (w/v) showed a high level of AnxA2-mIgG1 expression at the expected molecular weight of 69.1 kDa, while no expression was observed in the culture supernatant from the CY media without G6P (Fig. 4B). A large quantity of pure AnxA2-mIgG1 protein was obtained by nickel affinity chromatography, with a yield of up to 45 mg/50 mL of culture.
Fig. 4.
Enhanced expression and purification of human annexin A2 tagged with mIgG1 by an inducible and secretory protein expression vector system, pKS81. (A) Expression of AnxA2-mIgG1in pET expression system using a pET28 vector in E. coli BL21 (DE3) pLysS strains with IPTG induction. Protein expression was analyzed by the SDS-PAGE with Coomassie Blue staining. M: Protein marker, lane 1: Uninduced, lane 2: IPTG induced soluble fraction, lane 3: IPTG induced insoluble fraction. (B) Expression of AnxA2-mIgG1 using a pKS81vector in S. aureus LAC9. Transformed S. aureus LAC9 was cultured in the CY broth with or without G6P (2%, w/v) for 18 h at 37o C with shaking at 200 rpm. The culture supernatant was collected and AnxA2-mIgG1 was purified by the nickel affinity chromatography. Protein expression and purification were analyzed by the SDS-PAGE with Coomassie Blue staining. M: Protein marker, lane 1: culture supernatant from CY broth without G6P, lane 2: culture supernatant from CY broth with G6P, lane 3: purified human annexin A2 with mIgG1 tag.
Discussion
A lack of LPS and efficient secretion of recombinant proteins are great advantages of protein production in Gram-positive bacteria12,16,21. However, the lack of efficient inducible expression systems has been a challenge22. In this study, we leveraged the HptARS system in S. aureus, a bacterial three-component regulatory system which responds to extracellular G6P and induces transcriptional activation of the uhpT promoter by several thousand folds17. We also utilized S. aureus LAC9 strain lacking the uhpT gene as an expression host so that extracellular G6P would not be metabolized by S. aureus, and rather, constitutively activate the HptARS system for high-yield production of recombinant protein by the uhpT promoter. It is noteworthy that transcriptional activation of the uhpT promoter is tightly regulated by G6P as indicated by no bioluminescent signal from S. aureus LAC9 cultured in CY media without supplementation of G6P (Fig. 2B). This is because G6P is the only signal molecule that activates the HptARS system. In addition, varying concentrations (0–3%) of G6P significantly influence the activation of the uhpT promoter (Fig. 2C) and the yield of LukE proteins (Fig. 2D) in a dose-dependent manner, with 2% G6P showing optimal induction for high yields. This tight regulation is advantageous for producing toxic proteins or proteins that tend to form inclusion bodies when expressed at high concentrations.
For simple expression and purification of target proteins, we designed an expression vector system to clone the target gene in-frame, fused between the N-terminal signal peptide sequence of Hlb gene and a C-terminal 8 × histidine residue sequence. Secretion of proteins in Gram-positive bacteria is mostly mediated by the Sec-dependent pathway in which the N-terminal signal peptide of target protein is recognized by the signal recognition particle (SRP), which transfers the target protein to the cytoplasmic membrane22–24. The target protein is then translocated across the cytoplasmic membrane by the Sec translocase and cleaved by the signal peptidase (SPase) at the alanine-X-alanine motif in the signal peptide sequence, resulting in the release of target protein into the culture media22,24,25. This provides the added benefit that recombinant proteins retain their native conformation, thereby maintaining their functional properties. Moreover, the concentration of G6P used in the culture has been shown to directly affect the yield of recombinant proteins, underscoring the importance of optimizing this parameter for efficient protein production.
As proof of principle, we expressed both a prokaryotic (LukE) and a eukaryotic protein (AnxA2-mIgG tag) using a pKS81 and demonstrated yields of 15 mg and 45 mg, respectively, from a 50 mL culture supplemented with 2% G6P. Typically, the yield of these recombinant LukE and AnxA2-mIgG in E. coli with IPTG inducible system was 5 mg and < 1 mg, respectively. Eukaryotic protein expressions in E. coli are often challenging due to factors such as their tendency to form insoluble aggregates withing the cytoplasm, making recovery of functional proteins difficult and inefficient26–28. For instance, in our experience, AnxA2-mIgG expressed in E. coli primarily partitioned into the insoluble fraction, yielding either a small amount of protein or none at all in different experiments (Fig. 4A). High expression levels can also induce stress responses in the cells, which further reduce protein yields. A study by Sivashanmugam et al.29 optimized conditions to achieve high yields of recombinant proteins in E. coli using high cell density methods, reaching a very high cell density of OD600 10–20 and resulting in 14–34 mg from a 50 mL culture for seven different eukaryotic proteins. In contrast, our expression vector system and the S. aureus host strain established in this study have demonstrated higher efficiency in producing both prokaryotic and eukaryotic recombinant proteins, using a cell density of only OD600 1.8. This suggests that our expression system could provide an effective tool for expressing challenging proteins, such as AnxA2-mIgG.
To evaluate the purity and bioactivity of recombinant proteins expressed from the pKS81 system, we tested the cytotoxicity of LukE against human leukocytes. Our results showed that LukE alone did not result in cytotoxicity against human leukocytes but was highly cytotoxic in the presence of LukD, the F component of the bi-component leukotoxin. This indicates that the LukE purified using our system is both highly pure and functionally active.
In conclusion, our newly established inducible and secretory expression system underscores the versability and high efficiency in generating a large amount of highly pure and LPS-free recombinant proteins, both prokaryotic and eukaryotic, from Gram-positive bacteria, suitable and useful for production of FDA-approved biologics. This system is a robust tool for generating and purifying biologically active proteins, with the added benefit that adjusting G6P concentrations can optimize protein yields, thereby enhancing its applicability in biomedical research and biotechnology.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
S.Y., K.S.S., and J.Y.P conceived the studies, designed experiments, and wrote the manuscript. S.Y., C.K., N.P., and P.D. performed experiments and data analyses. J.A.T. wrote and edited the manuscript.
Funding
This work was supported by grants from Center for Biomedical Research Excellence in Pathogen- Host interactions, National Institute of General Medical Sciences (NIGMS), NIH (2P20GM103646-01A1) and the Mississippi Center for Clinical and Translational Research, NIGMS, NIH (5U54GM115428).
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosano, G. L. & Ceccarelli, E. A. Recombinant protein expression in Escherichia coli: advances and challenges. Front. Microbiol.5, 172. 10.3389/fmicb.2014.00172 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.İncir, İ. & Kaplan, Ö. Escherichia coli as a versatile cell factory: advances and challenges in recombinant protein production. Protein Expr Purif.219, 106463. 10.1016/j.pep.2024.106463 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Mergulhão, F. J., Summers, D. K. & Monteiro, G. A. Recombinant protein secretion in Escherichia coli. Biotechnol. Adv.23, 177–202. 10.1016/j.biotechadv.2004.11.003 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Johnson, T. L., Abendroth, J., Hol, W. G. & Sandkvist, M. Type II secretion: from structure to function. FEMS Microbiol. Lett.255, 175–186. 10.1111/j.1574-6968.2006.00102.x (2006). [DOI] [PubMed] [Google Scholar]
- 5.Chen, S., Liu, Z., Chen, J. & Wu, J. Study on improvement of extracellular production of recombinant Thermobifida fusca cutinase by Escherichia coli. Appl. Biochem. Biotechnol.165, 666–675. 10.1007/s12010-011-9286-z (2011). [DOI] [PubMed] [Google Scholar]
- 6.Slouka, C., Kopp, J., Spadiut, O. & Herwig, C. Perspectives of inclusion bodies for bio-based products: curse or blessing? Appl. Microbiol. Biotechnol.103, 1143–1153. 10.1007/s00253-018-9569-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heine, H., Rietschel, E. T. & Ulmer, A. J. The biology of endotoxin. Mol. Biotechnol.19, 279–296. 10.1385/mb:19:3:279 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Bonhomme, D., Cavaillon, J. M. & Werts, C. The dangerous liaisons in innate immunity involving recombinant proteins and endotoxins: examples from the literature and the Leptospira field. J. Biol. Chem.300, 105506. 10.1016/j.jbc.2023.105506 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magalhães, P. O. et al. Methods of endotoxin removal from biological preparations: a review. J. Pharm. Pharm. Sci.10, 388–404 (2007). [PubMed] [Google Scholar]
- 10.Mamat, U. et al. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell. Fact.14, 57. 10.1186/s12934-015-0241-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreillon, P. & Majcherczyk, P. A. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis.35, 632–641. 10.1080/00365540310016259 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Yamane, K., Bunai, K. & Kakeshita, H. Protein traffic for secretion and related machinery of Bacillus subtilis. Biosci. Biotechnol. Biochem.68, 2007–2023. 10.1271/bbb.68.2007 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Song, A. A., In, L. L. A., Lim, S. H. E. & Rahim, R. A. A review on Lactococcus lactis: from food to factory. Microb. Cell. Fact.16, 55. 10.1186/s12934-017-0669-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souza, C. C., Guimarães, J. M., Pereira, S. D. S. & Mariúba, L. A. M. The multifunctionality of expression systems in Bacillus subtilis: emerging devices for the production of recombinant proteins. Exp. Biol. Med. (Maywood)246, 2443–2453. 10.1177/15353702211030189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anné, J., Maldonado, B., Van Impe, J., Van Mellaert, L. & Bernaerts, K. Recombinant protein production and streptomycetes. J. Biotechnol.158, 159–167. 10.1016/j.jbiotec.2011.06.028 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Anné, J., Economou, A. & Bernaerts, K. Protein secretion in Gram-positive Bacteria: from multiple pathways to Biotechnology. Curr. Top. Microbiol. Immunol.404, 267–308. 10.1007/82_2016_49 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Park, J. Y. et al. Characterization of a novel two-component regulatory system, HptRS, the regulator for the hexose phosphate transport system in Staphylococcus aureus. 83, 1620–1628. 10.1128/iai.03109-14 (2015). [DOI] [PMC free article] [PubMed]
- 18.Yang, Y. et al. Regulatory mechanism of the three-component system HptRSA in glucose-6-phosphate uptake in Staphylococcus aureus. Med. Microbiol. Immunol.205, 241–253. 10.1007/s00430-015-0446-6 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Francis, K. P. et al. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect. Immun.68, 3594–3600. 10.1128/iai.68.6.3594-3600.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonzo, F. 3, Torres, V. J. & rd & The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev.78, 199–230. 10.1128/mmbr.00055-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan, C. et al. Construction of a highly active secretory expression system via an engineered dual promoter and a highly efficient signal peptide in Bacillus subtilis. N Biotechnol.33, 372–379. 10.1016/j.nbt.2016.01.005 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Peng, C. et al. Factors influencing recombinant protein secretion efficiency in Gram-positive Bacteria: signal peptide and Beyond. Front. Bioeng. Biotechnol.7, 139. 10.3389/fbioe.2019.00139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneewind, O. & Missiakas, D. Sec-secretion and sortase-mediated anchoring of proteins in Gram-positive bacteria. Biochim. Biophys. Acta1843, 1687–1697. 10.1016/j.bbamcr.2013.11.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freudl, R. Signal peptides for recombinant protein secretion in bacterial expression systems. Microb. Cell. Fact.17, 52. 10.1186/s12934-018-0901-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zalucki, Y. M. & Jennings, M. P. Signal peptidase I processed secretory signal sequences: selection for and against specific amino acids at the second position of mature protein. Biochem. Biophys. Res. Commun.483, 972–977. 10.1016/j.bbrc.2017.01.044 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Frenzel, A., Hust, M. & Schirrmann, T. Expression of recombinant antibodies. Front. Immunol.4, 217. 10.3389/fimmu.2013.00217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis, D. M. & Page, R. Strategies to optimize protein expression in E. Coli. Curr. Protoc. Protein Sci. Chap510.1002/0471140864.ps0524s61 (2010). [DOI] [PMC free article] [PubMed]
- 28.Huleani, S., Roberts, M. R., Beales, L. & Papaioannou, E. H. Escherichia coli as an antibody expression host for the production of diagnostic proteins: significance and expression. Crit. Rev. Biotechnol.42, 756–773. 10.1080/07388551.2021.1967871 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Sivashanmugam, A. et al. Practical protocols for production of very high yields of recombinant proteins using Escherichia coli. Protein Sci.18, 936–948. 10.1002/pro.102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information file.