Abstract
In the era of biological treatments and small molecules, this study assessed therapeutic patient education (TPE) in managing adult atopic dermatitis (AD), focusing on disease severity, quality of life, and the use of systemic treatments. This multicentre study included 260 adult AD patients, with 184 undergoing a full TPE programme and 76 control patients. Evaluations included disease severity, quality of life (DLQI), and systemic treatment use. The primary goal was to measure AD improvement, with secondary goals assessing DLQI score changes and systemic treatment use. AD severity improved in 64.7% of TPE patients vs 45.7% of controls (p = 0.008). The mean DLQI score dropped by 5.7 points in the TPE group vs 2.4 points in controls (p = 0.006). Additionally, 69.8% of TPE patients had a DLQI score ≤ 4/30 compared with 50% of controls (p = 0.025). Regarding therapeutics, 83.6% of patients naive to systemic treatment at inclusion were maintained exclusively under topical treatment vs 21.7% in the control group. The likelihood of needing systemic treatment was 66% in controls vs 6% in the TPE group. TPE enhances AD severity and quality of life, ensures better disease control, and reduces systemic treatment use, highlighting its importance in managing adult AD.
Key words: atopic dermatitis, corticophobia, eczema, therapeutic patient education, quality of life
SIGNIFICANCE
This study examined the effect of therapeutic patient education among adults with atopic dermatitis. Patients who received a full therapeutic education programme were compared with a control group. The results showed that atopic dermatitis severity and quality of life improved more in patients who received therapeutic patient education. In addition, these patients were less likely to require systemic medication. In short, therapeutic patient education helps to better manage atopic dermatitis and improve patients’ lives.
Atopic dermatitis (AD) prevalence is estimated at 4.4% in adults in Europe (1, 2). Its pathophysiology is based on the association of genetic predisposition, alteration of the skin barrier, and environmental exposures generating a chronic skin inflammation associated with a skin dysbiosis (3, 4). Several scores are used to assess its severity, such as SCORing Atopic Dermatitis (SCORAD), Eczema Area and Severity Index (EASI), and Investigator Global Assessment (IGA) scores (5, 6). Its treatment was updated in 2022 in European recommendations (7, 8). With the development of new therapies, notably anti-Type 2 biologicals and Janus kinase inhibitors, the clinician has a therapeutic arsenal available when application of topical corticosteroids (TCS) does not control the disease (7–9). Nevertheless, therapeutic management is sometimes made difficult by poor adherence to treatments and a phobia of TCS, called corticophobia (10, 11).
AD has a considerable socioeconomic impact – on quality of life, sleep, and work – but also has a therapeutic cost (12–14). Optimal management relies on the patient’s understanding of the disease and its issues through the provision of educational tools. Therapeutic patient education (TPE) was defined in 1996 by the World Health Organization (WHO) and regular updated recommendations have been given by the different national regulatory agencies (15).
Multiple studies have been conducted to evaluate the impact of TPE on the disease severity and quality of life of AD patients. In children, in 2014, a Cochrane review and a review of the literature concluded that there was a trend toward a decrease in SCORAD and DLQI scores after intervention (16, 17). In adults, the literature is sparser, evaluating the impact of TPE on disease severity, quality of life, and psychological parameters but never on therapeutic use (18, 20). The first multicentre randomized controlled trial by Heratizadeh et al. in 2017 evaluated a 12-hour TPE programme in adult patients with moderate to severe AD followed for 1 year. This study found a decrease in SCORAD score and an improvement in coping and quality of life by decreasing the “Skindex 29” score but not the DLQI score (19). Thus, results of studies evaluating TPE in patients with AD are heterogeneous. Moreover, the number of studies in adults remains insufficient and these do not include an evaluation of therapeutics. Therefore, we undertook to evaluate the impact of TPE in adult patients with AD on the severity of their disease, their quality of life, and their use of systemic treatments and compare them with control patients who did not receive an educational programme.
MATERIALS AND METHODS
Study design
We conducted a multicentre retrospective study in 2 French university hospitals to evaluate the impact of a structured educational programme on disease severity, quality of life, and use of systemic treatments in adult patients with AD. In the intervention group (TPE group) patients participated in a complete education multi-professional programme. They were compared with a control group of adult patients with AD who had no access or declined to participate to the TPE programme.
Patients
In the TPE group, patients were recruited among adult patients with AD of any severity grade and enrolled in the TPE programme carried out at 1 of the 2 sites. They were included if they had completed the programme according to the French ARS (Agence Régionale de Santé) criteria, i.e., at least 1 initial and final visit and at least 1 educational session.
In the control group, patients were recruited among adult patients with AD of any severity who had not received therapeutic education sessions at the 2 sites, with at least 2 follow-up visits at 12-month intervals.
The following exclusion criteria were used: age under 18 years old, having therapeutic AD education sessions for the control group, an incomplete TPE programme for the TPE group, the presence of a comorbidity that could affect the quality of life at least as much as AD, a language barrier making the completion of scores impossible, and having AD affecting only the hands because of the possible differential diagnostic of an allergic contact eczema.
All patients were told via an information note by e-mail about the study and their inclusion. They could notify by return e-mail their opposition to the use of their data and could leave the study at any time. The study was approved by the ethics committee of the French Lyon South University (n°21_5633)
Intervention: patient educational programme
Organized in four stages, the educational programme begins with an initial visit or “educational diagnosis” to assess the patient’s knowledge and consequences of the disease, beliefs, motivations, and expectations. Educational objectives are then established. The first session, proposed “to better know my disease and my treatments”, includes a collective discussion with a maximum of 10 patients. Its aims are evaluating knowledge of the disease, how to distinguish contact eczemas and atopic dermatitis, then hygienic and dietetic advice and demonstrating use of local treatments. Session 2, “Living better, managing my disease better”, is based on games and advice. It enables patients to find alternatives to scratching and presents methods of stress management. Finally, the final visit or “somatic visit” is an evaluation of the patient’s achievements, evaluating whether the objectives of the programme have been reached. Patients could choose not to perform the sessions collectively; these were then transformed into individual visits with the same content. More specific workshops on psycho-social skills were also offered to patients. The total duration of the programme is between 4 and 8 h. Quality of life, severity, and corticophobia scores were performed at initial and final visits.
Study procedures
Study assessments were performed at the initial and final TPE programme visits for the intervention group and at baseline and at least 1 year after the initial visit (taking the closest available visit to 12 months for the final visit) for the control group. Details on mean follow-up times are available in Table I.
Table I.
Baseline characteristics
| Factor | TPE | Control | Total |
|---|---|---|---|
| Age, years, mean ± SD | 33.88 ± 12.40 | 37.54 ± 14.59 | 34.95 ± 13.15 |
| n = 184 | n = 76 | n = 260 | |
| Sex | |||
| Female | 117 (63.6%) | 36 (47.4%) | 153 (58.8%) |
| Male | 60 (36.34%) | 40 (52.6%) | 107 (41.2%) |
| n = 184 | n = 76 | n = 260 | |
| High school diploma | |||
| No | 60 (33.3%) | 12 (40%) | 72 (34.3%) |
| Yes | 120 (66.7%) | 18 (60%) | 138 (65.7%) |
| n = 180 | n = 30 | n = 210 | |
| Atopic comorbidity | |||
| No | 36 (19.6%) | 17 (22.4%) | 53 (20.4%) |
| Yes | 148 (80.4%) | 59 (77.6%) | 207 (79.6%) |
| Allergic rhinoconjunctivitis | 138 (75%) | 49 (64.5%) | 187 (71.9%) |
| Asthma | 89 (48.4%) | 51 (67.1%) | 140 (53.8%) |
| Food allergy | 50 (27.2%) | 8 (10.5%) | 58 (22.3%) |
| n = 184 | n = 76 | n = 260 | |
| Time of AD diagnosis | |||
| Childhood | 169 (91.8%) | 65 (85.5%) | 234 (90%) |
| After 18 years old | 15 (8.2%) | 11 (14/5%) | 26 (10%) |
| n = 184 | n = 76 | n = 260 | |
| Follow-up, months, mean ± SD | 7.84 ± 4.99 | 14.66 ± 5.26 | 9.83 ± 5.94 |
| n = 184 | n = 76 | n = 260 | |
| Scores (mean ± SD) | |||
| SCORAD | 41 ± 18.8 | 37.2 ± 10 | 39.3 ± 15.5 |
| n = 59 | n = 66 | n = 125 | |
| PO-SCORAD | 35.3 ± 20.1 | 35.3 ± 20.1 | |
| n = 184 | n = 0 | n = 184 | |
| IGA | 2.6 ± 1 | 2.5 ± 1.5 | 2.5 ± 1 |
| n = 108 | n = 43 | n = 151 | |
| EASI | 11.9 ± 11.3 | 8.4 ± 8.4 | 10.3 ± 8 |
| n = 58 | n = 74 | n = 132 | |
| Topical treatment | |||
| No | 0 (0%) | 0 (0%) | 0 (0%) |
| Yes | 184 (100%) | 76 (100%) | 260 (100%) |
| n = 184 | n = 76 | n = 260 | |
| Phototherapy | |||
| No | 173 (94%) | 32 (42.1%) | 205 (78.8%) |
| Yes | 11 (6%) | 44 (57.9%) | 55 (21.2%) |
| n = 184 | n = 76 | n = 260 | |
| Systemic treatment already received | |||
| No | 165 (89.7%) | 23 (30.3%) | 188 (72.3%) |
| Yes | 19 (10.3%) | 53 (69.7%) | 72 (27.7%) |
| Cyclosporine | 5 (2.7%) | 34 (44.7%) | 39 (15%) |
| Methotrexate | 7 (3.8%) | 30 (39.5%) | 37 (14.2%) |
| Dupilumab | 2 (1.1%) | 25 (32.3%) | 27 (10.4%) |
| Janus kinase inhibitors | 0 (0%) | 11 (14%) | 11 (4.2%) |
| Other | 1 (0.5%) | 6 (7.9%) | 7 (2.7%) |
| n = 184 | n = 76 | n = 260 |
EASI: Eczema Area and Severity Index; IGA: Investigator Global Assessment; PO-SCORAD: Patient Oriented-SCORAD; SCORAD: SCORing Atopic Dermatitis; SD: standard deviation; TPE: therapeutic patient education.
Assessment of sociodemographic data, atopic dermatitis severity, quality of life, therapeutic, and corticophobia
We collected for each patient their age, sex, age of onset of AD, socioeconomic level (high-school graduation or not) and atopic comorbidities (i.e., the presence of asthma, allergic rhinoconjunctivitis, or food allergy). Data are available in Table I. To retrospectively assess the AD severity, we performed a composite severity score using the validated SCORAD, IGA, EASI, and PO-SCORAD scores, classifying patients into 4 severity groups:
-
-
Absent AD: SCORAD = 0 and/or PO-SCORAD = 0 and/or EASI = 0 and/or IGA = 0
-
-
Mild AD: SCORAD < 25 and/or PO-SCORAD < 25 and/or EASI < 7 and/or IGA = 1 or 2
-
-
Moderate AD: SCORAD 25–50 and/or PO-SCORAD 25–50 and/or EASI 7–21 and/or IGA = 3
-
-
Severe AD: SCORAD > 50 and/or PO-SCORAD > 50 and/or EASI > 21 and/or IGA = 4
The highest score available was used to classify the patient.
Quality of life and corticophobia were estimated via the validated DLQI and TOPICOP scores.
We collected at each evaluation the presence of any topical treatment including emollients, topical corticosteroids, and calcineurin inhibitors, the use of phototherapy, and the use of systemic treatment including cyclosporine, methotrexate, dupilumab, tralokinumab, Janus kinase inhibitors, or any treatment other than those mentioned.
Primary outcome
Our primary endpoint was the proportion of patients improving their AD, meaning changing their initial severity group to a lower severity group. Patients with an initial AD classified as “absent” were excluded from the analysis evaluating improvement in AD severity.
Secondary endpoints
Our secondary endpoints aimed to assess changes in DLQI and TOPICOP scores between initial and final visit, percentage of patients achieving a DLQI score equal to or less than 4 out of 30 and the use of systemic treatments.
Statistical analysis
The quantitative variables were described by number of patients, mean, and standard deviation (SD), while the qualitative variables were described by number of patients and frequency of each modality. Regarding the primary outcome, the effect of being in the TPE group vs control on the probability of improving AD was modelled via a logistic regression adjusted by initial AD, age, sex, presence of atopic comorbidity status, and presence of initial systemic treatment. The interaction between the group effect and the adjustment factors was also investigated via 5 likelihood ratio tests (LRT). Interaction terms leading to an LRT with associated p-value less than 5% were included in the final model.
Regarding the secondary outcomes, the group effect on the absolute DLQI variation between the initial and final visit was modelled via linear regression adjusted by the same confounding factors as detailed above. The group effect on the probability of having a final DLQI less than or equal to 4 was modelled via logistic regression adjusted by the same confounding factors. The group effect on the probability of initiating systemic treatment during the study period, in patients who did not have an initial systemic treatment, was modelled via logistic regression adjusted by the same confounding factors.
RESULTS
Population and baseline characteristics
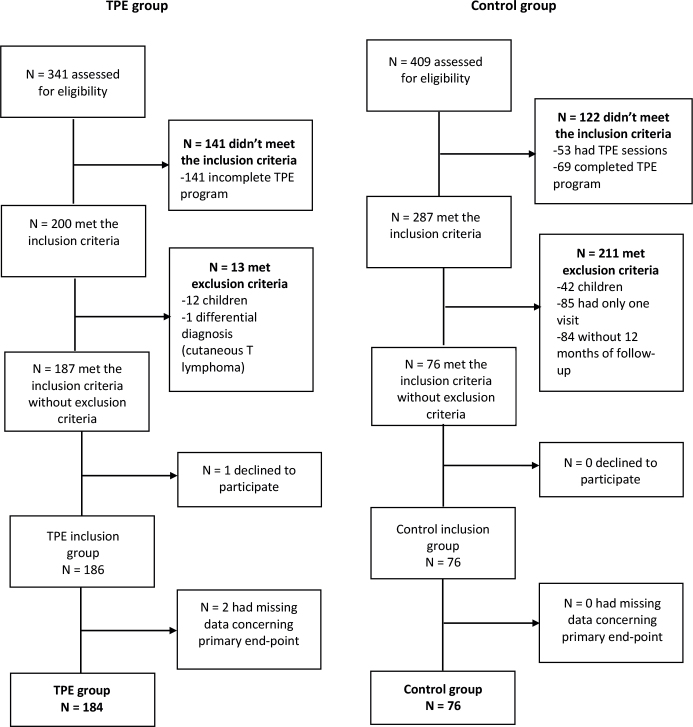
A total of 750 adult AD patients were assessed for eligibility between 1 January 2018 and 28 February 2022 (341 in the TPE group and 409 in the control group). After application of exclusion criteria and missing data, 184 patients were included in primary analysis in the TPE group and 76 in the control group. Details concerning number of study participants, exclusions, and missing data are given in the flowchart (Fig. 1). Baseline characteristics of patients, including AD history, atopic comorbidities, and therapeutic history are depicted in Table I.
Fig. 1.
Flowchart of study. TPE: therapeutic patient education.
Therapeutic patient education improves disease severity and quality of life in adult patients with atopic dermatitis
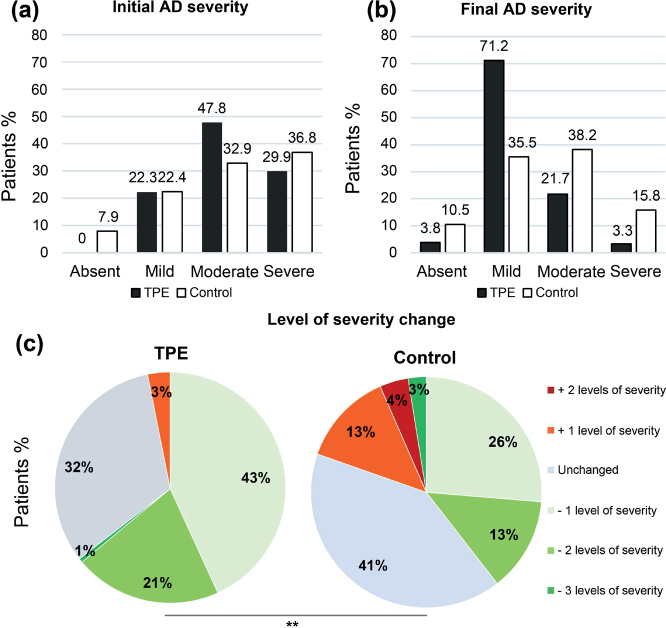
First, the AD severity course was evaluated, comparing TPE and control groups at baseline (initial AD severity) and at the end of the study (final AD severity). At baseline, the proportion of mild AD was similar in the TPE and control groups (22.3% [41/184] and 22.4% [17/76] respectively). At the end of the study a larger proportion (71.2%; 131/184) patients were classified as mild AD in the TPE group, while only 35.5% (27/76) of patients were mild in the control group (Fig. 2A, B). Regarding our primary endpoint, the change in severity was evaluated and revealed that 64.7% (119/184) of patients, who participated in the TPE programme improved their AD vs 45.7% (32/70) in the control group (p = 0.008), after exclusion of 6 patients (from the control group) classified as “absent AD” in initial evaluation (Fig. 2C).
Fig. 2.
Therapeutic patient education (TPE) improves disease severity and quality of life in patients with atopic dermatitis (AD). (A): Initial AD severity; (B): Final AD severity. Results are given in percentage of patients; black and white bars represent respectively the TPE and the control groups. (C): Level of AD severity changes. Results are depicted according to a colorimetric scale, related to the change in the composite severity score. Results are given in percentage of patients in the TPE and control groups. **p < 0.01.
Next, we assessed the changes in AD quality of life over the study period. Initial DLQI data were available for 183 patients in the TPE group and 47 patients in the control group respectively. Final DLQI score was also found in 169 patients and 42 patients. Considering the variation in DLQI, the mean of the initial DLQI score was not significantly different between the TPE and the control group (9.5 points vs 9.6 points, respectively). However, the mean of the final DLQI decreased more significantly in the TPE group than in the control group (4.0 vs 5.5, p = 0.031), indicating a higher improvement in the quality of life of AD adult patients after a TPE programme, compared with the control group (Fig. 3A). Interestingly, comparing the initial with the final DLQI score, the mean DLQI score decreased more in the TPE group than in the control group (5.7 vs 2.4 points, respectively, p = 0.006) (Fig. 3A). Moreover, 69.8% (118/169) of patients after the TPE programme achieved a DLQI score equal to or less than 4 vs 50.0% (21/42) in the control group (p = 0.025), showing that more than two-thirds of patients reported a very limited impact of their disease after a TPE programme (Fig. 3B).
Fig. 3.
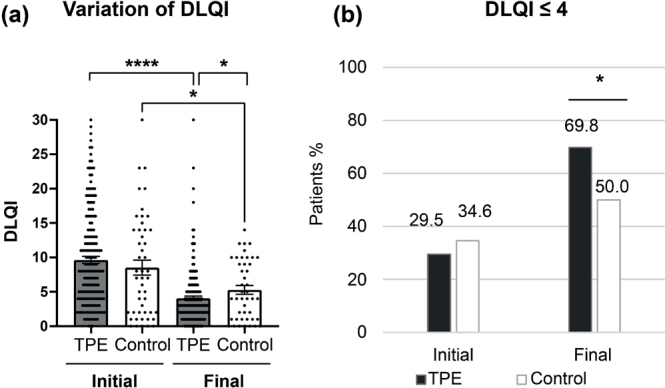
Therapeutic patient education (TPE) improves quality of life in patients with atopic dermatitis (AD). (A): Dermatology Life Quality Index score (DLQI) score. A dot represents 1 individual DLQI score and the mean DLQI is shown for TPE and control groups at initial and final evaluation. Results are expressed according to the absolute DLQI score. *p < 0.05. (B): Percentage of patients presenting a DLQI score ≤ 4/30 at initial and final evaluation. *p < 0.05.
After adjustment for initial AD severity, age, gender, initial treatment, and atopic comorbidities, the difference in mean absolute change in DLQI score was 2.66 points lower in the TPE group than in the control group (p = 0.06) and the TPE group has a DLQI odds ratio (OR) of less than or equal to 4 multiplied by 2.78 compared with the control group (OR = 2.78; CI [1.11–7.29]; p = 0.032). Thus, in the predictive model, for a 30-year-old woman with initially moderate AD on topical treatment alone, the probability of having a DLQI score less than or equal to 4 at the final assessment is 70% in the TPE group and 50% in the control group (Tables SI and SII).
Age and atopic comorbidities are predictive of a positive impact of therapeutic patient education
As TPE was shown to impact AD severity significantly, we looked for predictive factors for AD improvement in the TPE group compared with the control group. In multivariate analysis, after adjusting for age, gender, initial disease severity, having or not systemic treatment at inclusion, and the presence of atopic comorbidities, the TPE programme has a greater impact on the severity of AD in young adult patients with atopic comorbidity compared with control patients. This phenomenon of interaction comes from the fact that, in the control group, the evolution of the disease is more unfavourable in older patients as well as in patients without atopic comorbidities. Table II gives the ORs of improvement in AD patients by decade of age and atopic comorbidity status for patients in the TPE group compared with control patients. For example, the OR for improvement of AD in a 30-year-old patient is 0.30 in the absence of atopic comorbidity and becomes 7.43 with atopic comorbidity.
Table II.
Estimated odds ratios of atopic dermatitis improvement in the therapeutic patient education group compared with the control group according to age and presence of atopic comorbidity
| Age | Absence of atopic comorbidity | Presence of atopic comorbidity |
|---|---|---|
| 20 years old | 0.61 | 15.35 |
| 30 years old | 0.30 | 7.43 |
| 40 years old | 0.14 | 3.60 |
| 50 years old | 0.07 | 1.74 |
| 60 years old | 0.03 | 0.84 |
Details concerning estimated ORs of AD improvement are given in Table SIII.
Therapeutic patient education allows therapeutic maintenance and a reduction in the prescription of systemic treatments
Lastly, we looked for identifying effects of TPE on the therapeutic need in adults with AD. At baseline, 10.3% (19/184) of patients in the TPE group were treated or had used systemic therapy in the past vs 69.7% (53/76) in the control group (Fig. 4A). At the final evaluation, 22.3% (41/184) of the patients in the TPE group were treated or had used systemic therapy in the study vs 84.2% (64/76) in the control group (Fig. 4B). Among them, 2.7% (5/184) of patients in the TPE group received cyclosporine vs 18.4% (14/76) in the control group, 9.1% (17/184) vs 23.7% (18/76) methotrexate, 13.5% (25/184) vs 51.3% (39/76) dupilumab, 2.2% (4/184) vs 18.4% (14/76) a Janus kinase inhibitor, and 0.5% (1/184) vs 10.5% (8/76) another Type 2-targeted therapy. Only 1 patient in the control group had phototherapy initiated during the study.
Fig. 4.
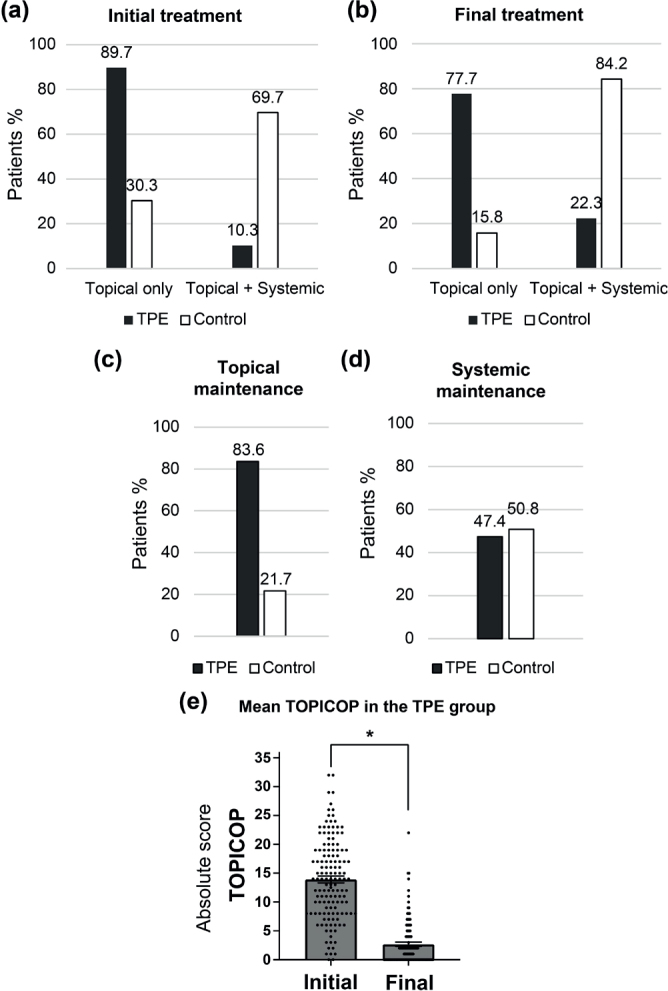
Impact of therapeutic patient education (TPE) on therapeutic use and maintenance. (A): Nature of the initial treatment, according to the use of only topical treatment or the association of topical and systemic treatments. Results are given in percentage of patients. (B): Nature of the final treatment. Results are given in percentage of patients under topical treatment only or topical and systemic treatments. (C): Topical maintenance. Results are given in percentage of patients who maintained the same topical treatment alone treatment during the study, in TPE and control groups. (D): Systemic maintenance. Results are given in percentage of patients who maintained the same systemic treatment during the study, in each of TPE and control groups. (E): Mean TOPIcal COrticosteroid Phobia score (TOPICOP) in the TPE group. Results are given in absolute points of TOPICOP score at initial and final evaluation in the TPE group. p < 0.05.
In term of therapeutic maintenance, 83.6% (138/165) vs 21.7% (5/23) of the patients (TPE group vs control group respectively) were able to maintain a topical treatment alone (Fig. 4C) and 47.4% (18/38) vs 50.8% (33/65) of the patients continued the same systemic treatment throughout the study (Fig. 4C, D). After adjustment for initial AD severity, age, gender, initial treatment, and atopic comorbidities, and for patients with topical treatment only at inclusion, the TPE group had a lower OR of needing systemic treatment compared with the control group (OR = 0.03 CI [0.01–0.12]; p < 0.001). Thus, in the predictive model, for a 30-year-old woman with initially moderate AD on topical treatment alone, the probability of having initiated systemic treatment is 6% in the TPE group vs 66% in the control group (Table SIV).
Finally, in the TPE group, the average TOPICOP score decreased significantly with a mean of 13.9 points at the initial assessment and a mean of 2.7 points at the final assessment, suggesting that the topical maintenance may be related to a lower corticophobia after the TPE programme and in fine a lower need to systemic therapy (Fig. 4E).
DISCUSSION
We found an improvement in AD severity, quality of life, and therapeutic maintenance after a complete TPE programme in adults with AD. Results are consistent with those presented by Herazitadeh and al. (19), although they did not find an impact on the DLQI score as we did. Our study has limitations, first because of its retrospective nature. Not all the severity and quality of life scores were available for all patients. We did not collect the SF-36 and WPAI scores, which would have enabled us to better assess the impact of AD on quality of life and work. The TOPICOP and PO-SCORAD scores were not performed in the control group. The 2 groups differ in some aspects: notably, patients in the control group used more systemic treatment at inclusion and the time between initial and final assessment could vary by a few months between patients, in part because the TPE programme is on average shorter than the 1-year follow-up imposed on patients in the control group. However, it also has some strengths: the educational programme was conducted by a multidisciplinary team trained in TPE and had the same reproducible content for each patient. Moreover, data concerning treatments during the study and their maintenance or change were available. Thus, our study found a significant proportion of patients who were able to maintain topical treatment alone in the TPE group. This can be explained by the reduction of the corticophobia score in this group. More globally, probability of initiating systemic treatment is decreased in the TPE group, which reinforces the postulate that a patient who adheres to care has better controlled disease over time, regardless of the treatment. In this respect, the ADCT (Atopic Dermatitis Control Tool) score is an interesting tool that allows this aspect to be addressed (21). Moreover, this also means that some patients do not need therapeutic escalation and TPE may allow a cost saving of care, especially for new AD therapies, which are often expensive. Concerning severity reduction, it can be explained, first, by advice given during therapeutic education, i.e., better use of local treatments and reduction in corticophobia, but also, second, by adopting measures of stress management, dietary balance, and reduction of triggering factors. We found that the impact of TPE was greater in younger patients and if they had atopic comorbidities. We can postulate that young adult populations benefit more from learning in TPE sessions and are more sensitive to the advice provided. Regarding atopic comorbidities, when added together they can potentialize alteration in patients’ quality of life and encourage them to understand and better manage their atopic pathology. Our study is encouraging and supports the benefits of TPE in an overall population adult patients of any AD severity, treated or not with systemic treatments, especially as the disease burden remains a major issue in AD (22).
Supplementary Material
ACKNOWLEDGEMENTS
IRB approval status
The study was approved by the ethics committee of the French Lyon South University (n°21_5633).
Conflict of interest disclosures
AN is an investigator for AbbVie, Eli Lilly, Incyte, LEO Pharma, Novartis, and Sanofi; a consultant for Pfizer Inc., AbbVie, Eli Lilly, Galderma, LEO Pharma, Novartis, and Sanofi; a speaker for AbbVie, Regeneron, and Sanofi; and is on advisory boards for Pfizer Inc., AbbVie, LEO Pharma, and Sanofi.
Funding Statement
Funding sources Groupe d’Education Thérapeutique en dermatologie gave support with the proofreading of this work.
REFERENCES
- 1.Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018; 73: 1284–1293. 10.1111/all.13401 [DOI] [PubMed] [Google Scholar]
- 2.Kowalska-Olędzka E, Czarnecka M, Baran A. Epidemiology of atopic dermatitis in Europe. J Drug Assess 2019; 8: 126–128. 10.1080/21556660.2019.1619570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360. 10.1016/S0140-6736(20)31286-1 [DOI] [PubMed] [Google Scholar]
- 4.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387: 1109–1122. 10.1016/S0140-6736(15)00149-X [DOI] [PubMed] [Google Scholar]
- 5.Chopra R, Vakharia PP, Sacotte R, Patel N, Immaneni S, White T, et al. Severity strata for Eczema Area and Severity Index ( EASI ), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol 2017; 177: 1316–1321. 10.1111/bjd.15641 [DOI] [PubMed] [Google Scholar]
- 6.Stalder J-F, Barbarot S, Wollenberg A, Holm EA, De Raeve L, Seidenari S, et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe: PO-SCORAD self-assessment scale validation. Allergy 2011; 66: 1114–1121. 10.1111/j.1398-9995.2011.02577.x [DOI] [PubMed] [Google Scholar]
- 7.Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema: part I – systemic therapy. J Eur Acad Dermatol Venereol 2022; 36: 1409–1431. 10.1111/jdv.18345 [DOI] [PubMed] [Google Scholar]
- 8.Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EUROGUIDERM) on atopic eczema – part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol 2022; 36: 1904–1926. 10.1111/jdv.18429 [DOI] [PubMed] [Google Scholar]
- 9.Arents BWM, van Zuuren EJ, Vermeulen S, Schoones JW, Fedorowicz Z. Global Guidelines in Dermatology Mapping Project (GUIDEMAP), a systematic review of atopic dermatitis clinical practice guidelines: are they clear, unbiased, trustworthy and evidence based (CUTE)? Br J Dermatol 2022; 186: 792–802. 10.1111/bjd.20972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin LJ, Wei TK, Choi E, Chandran NS. TOPICOP © scale for steroid phobia: difficulties and suggestions for application in clinical research. J Dermatol Treat 2020; 31: 624–625. 10.1080/09546634.2019.1657221 [DOI] [PubMed] [Google Scholar]
- 11.Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema: corticosteroid phobia in atopic eczema. Br J Dermatol 2000; 142: 931–936. 10.1046/j.1365-2133.2000.03473.x [DOI] [PubMed] [Google Scholar]
- 12.Langenbruch A, Radtke M, Franzke N, Ring J, Fölster-Holst R, Augustin M. Quality of health care of atopic eczema in Germany: results of the national health care study Atopic Health. J Eur Acad Dermatol Venereol 2014; 28: 719–726. 10.1111/jdv.12154 [DOI] [PubMed] [Google Scholar]
- 13.Thyssen JP, Hamann CR, Linneberg A, Dantoft TM, Skov L, Gislason GH, et al. Atopic dermatitis is associated with anxiety, depression, and suicidal ideation, but not with psychiatric hospitalization or suicide. Allergy 2018; 73: 214–220. 10.1111/all.13231 [DOI] [PubMed] [Google Scholar]
- 14.Weil C, Sugerman PB, Chodick G, Liang H, Wang H, Calimlim BM, et al. Epidemiology and economic burden of atopic dermatitis: real-world retrospective data from a large nationwide Israeli healthcare provider database. Adv Ther 2022; 39: 2502–2514. 10.1007/s12325-022-02120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therapeutic patient education: continuing education programmes for health care providers in the field of prevention of chronic diseases: report of a WHO working group. https://iris.who.int/bitstream/handle/10665/108151/E63674.pdf?sequence=1&isAllowed.
- 16.Ersser SJ, Cowdell F, Latter S, Gardiner E, Flohr C, Thompson AR, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev 2014(1): CD004054. 10.1002/14651858.CD004054.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbarot S, Stalder JF. Therapeutic patient education in atopic eczema. Br J Dermatol 2014; 170: 44–48. 10.1111/bjd.12932 [DOI] [PubMed] [Google Scholar]
- 18.Evers A, Duller P, Jong E, Otero ME, Verhaak CM, van der Valk PG, et al. Effectiveness of a multidisciplinary itch-coping training programme in adults with atopic dermatitis. Acta Derm Venereol 2009; 89: 57–63. 10.2340/00015555-0556 [DOI] [PubMed] [Google Scholar]
- 19.Heratizadeh A, Werfel T, Wollenberg A, Abraham S, Plank-Habibi S, Schnopp C, et al. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol 2017; 140: 845–853. 10.1016/j.jaci.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 20.Jaspers JPC, Span L, Molier L, Coenraads PJ. A Multimodal education and treatment program for young adults with atopic dermatitis: a randomized controlled trial. Dermatol Psychosom Dermatol Psychosom 2000; 1: 148–153. 10.1159/000057970 [DOI] [Google Scholar]
- 21.Pariser DM, Simpson EL, Gadkari A, Bieber T, Margolis DJ, Brown M, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr Med Res Opin 2020; 36: 367–376. 10.1080/03007995.2019.1699516 [DOI] [PubMed] [Google Scholar]
- 22.Kleyn CE, Barbarot S, Reed C, Losi S, von Arx LB, Robert C, et al. Burden of moderate to severe atopic dermatitis in adults from France, Italy, and the UK: patient-reported outcomes and treatment patterns. Dermatol Ther 2022; 12: 1947–1965. 10.1007/s13555-022-00777-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.