ABSTRACT
Bone, cartilage, and soft tissue regeneration is a complex process involving many cellular activities across various cell types. Autografts remain the “gold standard” for the regeneration of these tissues. However, the use of autografts is associated with many disadvantages, including donor scarcity, the requirement of multiple surgeries, and the risk of infection. The development of tissue engineering techniques opens new avenues for enhanced tissue regeneration. Nowadays, the expectations of tissue engineering scaffolds have gone beyond merely providing physical support for cell attachment. Ideal scaffolds should also provide biological cues to actively boost tissue regeneration. As a new type of injectable biomaterial, hydrogel microspheres have been increasingly recognised as promising therapeutic carriers for the local delivery of cells and drugs to enhance tissue regeneration. Compared to traditional tissue engineering scaffolds and bulk hydrogel, hydrogel microspheres possess distinct advantages, including less invasive delivery, larger surface area, higher transparency for visualisation, and greater flexibility for functionalisation. Herein, we review the materials characteristics of hydrogel microspheres and compare their fabrication approaches, including microfluidics, batch emulsion, electrohydrodynamic spraying, lithography, and mechanical fragmentation. Additionally, based on the different requirements for bone, cartilage, nerve, skin, and muscle tissue regeneration, we summarize the applications of hydrogel microspheres as cell and drug delivery carriers for the regeneration of these tissues. Overall, hydrogel microspheres are regarded as effective therapeutic delivery carriers to enhance tissue regeneration in regenerative medicine. However, significant effort is required before hydrogel microspheres become widely accepted as commercial products for clinical use.
Keywords: drug delivery, fabrication techniques, hydrogel microspheres, microgels, tissue regeneration
Introduction
Bone, cartilage, and soft tissue regeneration is a balanced and dynamic process that involves various cellular activities, including cell metabolism, proliferation, differentiation, migration, and cell-cell and cell-material interactions across different cell types.1, 2 Tissue engineering strategies aim to provide an appropriate microenvironment that is conductive to these cellular activities: a scaffold serves as a temporary and artificial extracellular matrix (ECM) for cell growth and tissue regeneration. With the evolution of regenerative medicine, the expectations for tissue engineering scaffolds have gone beyond barely providing mechanical support and physical guidance for the ingrowth of host cells. Instead, ideal scaffolds should also possess bio-functionality to actively promote tissue regeneration by regulating the above-mentioned cellular activities. A common strategy for enhancing the bio-functionality of tissue engineering scaffolds is to provide biological cues by loading them with drugs or donor cells.3, 4
Direct injection of cells into defect areas can be problematic as the shear force generated during the injection process may damage the cells. Directly injected cells also tend to rapidly disperse due to a lack of local adhesion.5, 6 On the other hand, directly injecting drugs or growth factors (GFs) can significantly shorten their half-life because they are quickly exposed to the immune system, leading to rapid degradation or clearance from the body.7 Loading cells and drugs into tissue engineering scaffolds can be an effective and safe alternative. Therefore, future tissue engineering scaffolds for bone, cartilage, and soft tissue regeneration should simultaneously provide structural support for cell growth and supply biological cues via sustained and controlled release of cells and drugs.
Hydrogels behave appealingly like both solids and liquids as they are composed of hydrophilic polymer networks infiltrated with abundant water.8 Their hydrophilicity and similarity to the native ECM make hydrogels an excellent microenvironment for cell ingrowth.8, 9 The crosslinked polymer network provides deformability, swelling, degradability, and sufficient mechanical support, which are beneficial for cell/drug loading and release.9-11 Since the emergence of the first synthetic hydrogel in 1960,12 they have been applied as drug delivery systems in many fields, including would healing, cancer treatment, diabetes, immunology, and tissue engineering.11, 13, 14 Specifically, tissue engineering hydrogels with substantially improved properties have been developed. For example, Lin et al.15 synthesised hydrogels that continuously exude small concentrations of lipids to create a slippery layer that reduces friction and closely mimics the characteristics of natural articular cartilage that in part uses a lipid lubricant layer naturally. Lee et al.16 developed an advanced three dimensional printing platform to build complex collagen-based hydrogels for a wide range of organ systems, including the human heart. Jin et al.17 designed an injectable tissue prosthesis composed of biocompatible hydrogels with instantaneous bidirectional electrical conduction, demonstrating accelerated tissue repair in the early stage of severe muscle injury in rats. Despite these exciting and encouraging advances in recent years, bulk hydrogels may not be always available or suitable, especially in scenarios requiring smaller sizes or localised and systemic injection.18, 19 Moreover, as a bulk material, the rate of cell migration and drug release from hydrogels is often governed by degradation and diffusion, which can be slow and inefficient.
The unique colloidal behaviour of micro-sized materials allows microparticles to form stable colloidal dispersions.20 Appropriately sized microparticles can also maximally evade from or delay their clearance by macrophages.21 Hydrogel microspheres, also known as microgels, leverage the advantages of both hydrogels and microparticles, making them widely used in many biomedical research.22-24 Compared to bulk hydrogels, microgels possess distinctive advantages such as a larger specific area for more efficient cell/drug loading, more controllable cell/ drug release, higher optical/acoustic transparency for easier visualisation of loaded cells and drugs, smaller size for less invasive injection, unique stable dispersion for high protection of loaded cells from the shear stress-induced damage during injection, and greater flexibility and tunability for different purposes, as multiple microgels with different size, composition, and biological loadings can be easily mixed and delivered simultaneously.18, 25, 26 As a result, hydrogel microspheres have been increasingly recognised as promising delivery carriers for drugs and cells in regenerative medicine. Their excellent biocompatibility, high tunability, and efficient drug/cell delivery capabilities make them ideal candidates for applications where precise control and targeted delivery are essential.22, 27
With the rapid progress in hydrogel materials science, advanced hydrogel microspheres have been developed to satisfy the onerous and growing demands for more efficient and safer cell and drug delivery in tissue engineering. In this review, we first introduce the material requirements and compare the five different methods used to manufacture hydrogel microspheres. We then focus on the applications of hydrogel microspheres in regenerative medicine, specifically for bone, cartilage, and soft tissue regeneration. Finally, we discuss some of the current challenges and future opportunities in the field, aiming to offer guidance for the better design and fabrication of hydrogel microspheres as cell and drug delivery carriers for tissue regeneration.
Literature Search
Articles on hydrogel microspheres for tissue regeneration were searched using the search terms: “hydrogel microspheres fabrication methods”, or “hydrogel microspheres” combined with “bone regeneration”, “cartilage regeneration”, “nerve regeneration”, “skin regeneration”, and “muscle regeneration”. These searches were conducted on Google Scholar and Web of Science between May and June 2024, and articles published after 2019 received particular interest. After careful screening, 175 articles are included in this review.
Materials and Processing Routes of Hydrogel Microspheres
Hydrogel microspheres can be made from synthetic and natural polymers, or a combination of both. Essential properties for making hydrogel microspheres include hydrophilicity, biocompatibility, biodegradability, gelation ability, mechanical properties, non-toxicity, and functionality. While processing routes can be briefly classified into five main categories: microfluidics, batch emulsion, electrohydrodynamic spraying, lithography, and mechanical fragmentation. Among these, microfluidics and batch emulsion are “bottom-up” techniques, while the latter three are considered “top-down” approaches.28 These five technologies vary in terms of productivity, consistency of particle size, and complexity of particle shape. Table 1 summarises a list of materials with their chemically modified option and crosslinking method for each processing route.6, 23, 29-70
Table 1. Various methods and materials used in producing hydrogel microspheres.
| Fabrication technique | Material | Chemical modification | Crosslinking method |
|---|---|---|---|
| Microfluidic emulsion | Alginate | Unmodified29-34 | Ionic crosslinking with Calcium ions |
| Microfluidic emulsion | Gelatine | Methacrylamide6, 23, 35-43 | UV crosslinking |
| Microfluidic emulsion | Hyaluronic acid | Methacrylate,44, 45 methacrylate + tetrazole,46 norbornene,45 pentenoate45 | UV crosslinking |
| Microfluidic emulsion | PEG | Vinyl sulfone,47 vinyl sulfone + thiol,48, 49 maleimide50, 51 | Michael addition |
| Batch emulsion | Hyaluronic acid | Glycidyl methacrylate,52 norbornene,45 pentenoate,45 methacrylate45 | UV crosslinking |
| Batch emulsion | Hyaluronic acid | Aldehyde + hydrazide53 | Inverse emulsion crosslinking |
| Batch emulsion | PEG | Diacrylate54 | Light-induced crosslinking |
| Batch emulsion | Silk fibroin | Norbornene55 | Thiol-ene photo-click reaction |
| Electrohydrodynamic spraying | Alginate | Unmodified,56 arginine–glycine–aspartic acid57 | Ionic crosslinking with Calcium ions |
| Electrohydrodynamic spraying | PEG | Norbornene58 | Thiol-ene reaction |
| Electrohydrodynamic spraying | PEG | Acrylate + toluene & mercaptopropionic acid59 | Michael addition |
| Electrohydrodynamic spraying | Chitosan | Unmodified79 | Electrostatic interactions |
| Lithography | Gelatine | Methacrylate60 | UV crosslinking |
| Lithography | Hyaluronic acid | Methacrylate,61 tyramine62 | UV crosslinking |
| Lithography | Hyaluronic acid | Vinyl ester63 | Thiol-ene reaction |
| Lithography | PEG | Diacrylate64-68 | UV crosslinking |
| Mechanical fragmentation | Hyaluronic acid | Norbornene,45 pentenoate,45 methacrylate45 | UV crosslinking |
| Mechanical fragmentation | PEG | Maleimide69 | Thiol-ene reaction |
| Mechanical fragmentation | Carboxybetaine acrylamide | Unmodified70 | UV crosslinking |
Note: PEG: poly(ethylene glycol); UV: ultraviolet.
Materials
Materials used for the fabrication of hydrogel microspheres are mainly derived from four commonly used polymers: natural alginate, gelatine, and hyaluronic acid (HA), and synthetic poly(ethylene glycol) (PEG). Generally, polymers with good hydrophilicity, characterised by surface functional groups capable of forming stable hydrogen bonds with water molecules, are essential for maintaining structural integrity. Appropriate biodegradability, suitable mechanical properties, and good biocompatibility are also important. Biodegradability has the advantage of eliminating the need for additional surgical removal, and it allows the hydrogel microspheres to control drug release through degradation. In this regard, the degradation rate of hydrogel microspheres targeted to different tissues should be well considered as the regeneration duration of different tissues is different. For example, bone fractures typically take a few weeks to months to heal, peripheral nerve injuries take months, while the recovery of cartilage could take up to several years.71-73 Ideally, the degradation rate of biomaterials should accommodate the rate of tissue regeneration to maintain a sustained drug release over the course of recovery. The mechanical properties of hydrogel microspheres are important considerations. For instance, the injection of hydrogel microspheres into the joint cavity will change the pressure distribution on the surface of articular cartilage and thereby impact regeneration effectiveness.74 Materials for nerve tissue regeneration should then have suitable mechanical properties that match their surrounding tissues to avoid complications such as compression syndrome.73 Overall, compared to bone tissues, which mainly consist of apatite mineralised collagen, microgels for cartilage regeneration often require a lower elastic modulus due to a lack of minerals in native cartilage tissue.3, 75 Microgels for soft tissues such as nerve and skin would require a higher elasticity. In addition, the biocompatibility and the toxicity of hydrogel microspheres and their leaching and degradation products need to be considered, as the application process involves direct contact with tissues and cells after injection. According to international standard ISO 10993-5, the toxicity of biomaterials can be evaluated by three types of in vitro tests: extract test, direct contact test, and indirect contact test.
Another important factor is the gelation capacity, which depends on both the inherent properties of the materials and the crosslinking methods used. Alginate-based biomaterials can be ionically crosslinked to form a strong and stable hydrogel in the presence of divalent cations, such as calcium,29-34, 56, 57 magnesium,30 and barium ions.30, 34 This process can be accomplished by collecting alginate droplets in a calcium bath, and the gelation process initiates when the alginate droplets come into contact with calcium ions.30 Gelatine-, HA-, and PEG-based materials (such as methacrylate-gelatine, thio-alkenes-HA, and PEG diacrylate) can be used to form hydrogels when exposed to UV light, a process called photopolymerisation.76, 77 This method is more flexible as the size and shape of the hydrogel microspheres can be controlled by using a mask with a desired shape to block the UV light or by varying the density of the UV light so that it crosslinks only in the desired area, providing a precise spatial control and therefore enabling the creation of complex microstructures. Alternatively, PEG-based hydrogels can be crosslinked using the Michael addition. This process is operated under mild conditions and, therefore, maintains the activity of sensitive biomolecules; it also provides good flexibility for functionalisation, allowing functional groups to enter the hydrogel network.48
The last is the functionality, which relates to the further applications to construct biomimetic microstructures, deliver drugs, cells, or proteins through injection, scaffolding, or three dimensional printing. For example, theuse of bioinks composed of gelatine methacrylate (GelMA) hydrogel microspheres and chitosan microspheres (GC-MSs) in three dimensional-printed biomimetic scaffolds provides a good simulation of neural network systems in both micro- and macro-environments.36 In another study, pH-responsive alginate/calcium carbonate (CaCO3) composite hydrogel microparticles with sustained dual release function of antibiotic and GFs, rifamycin and basic fibroblast growth factor (bFGF) have been shown to promote wound healing.32 The crosslinking method is an essential factor for cell-loaded hydrogel microspheres as the light intensity, type of photoinitiator, and photoinitiator concentration all affect the viability of the cells.61
Microfluidics
Microfluidics is the most widely used technology for fabricating microgels because it provides better control of the synthesis process and produces highly consistent particle sizes.25 However, microfluidics has lower productivity compared to batch emulsion techniques and has difficulties in the production of specially shaped particles. Microfluidics can be further categorised into two approaches: microfluidic chips and microcapillary devices. Using microfluidic chips provides superior control over the shape and size of hydrogel microspheres, but this process often comes at the cost of a more complex and costly fabrication process.
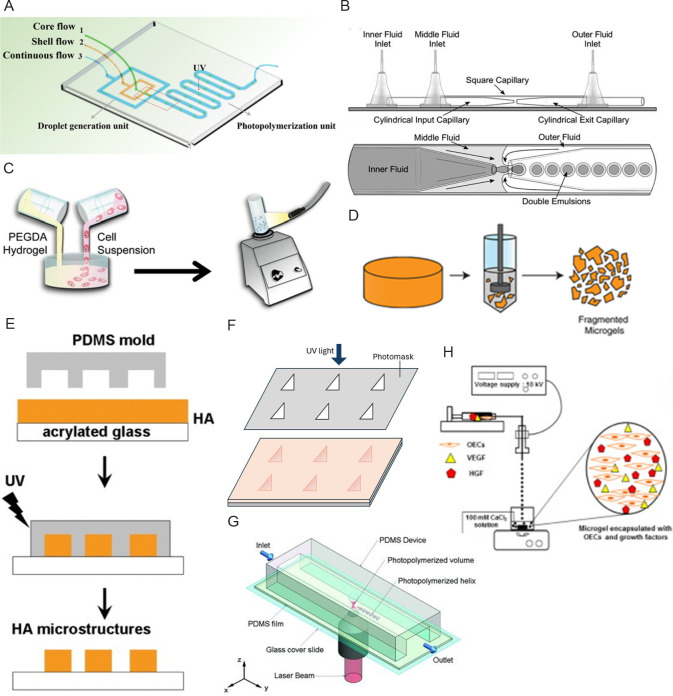
A microfluidic chip includes a channel with one or more inlets and an outlet on the chip (Figure 1A). The water and oil phases enter the inlets separately to form microspheres, and crosslinking of the materials can be done while exiting the outlet.30, 31, 33-36, 39, 40, 46, 48-51 The microfluidic device can be fabricated by standard photolithography and soft lithography. Typically, polydimethylsiloxane (PDMS) chips are degassed and cured on negative SU-8 moulds, and bonded to glass substrates to form microchannels using surface oxygen plasma treatment.30, 31, 33, 34, 40, 48, 49 The PDMS replicas can then be baked at 80°C30 or treated with AquapelTM solution to ensure the hydrophobicity of the microfluidic channels.31, 34, 50
Figure 1. Schematic diagrams of hydrogel microsphere processing routes. (A, B) Microfluidic: (A) The microfluidic chip has three chips to form a shell-like microsphere in a continuous oil flow. Reprinted from Wang et al.40 Copyright 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (B) A microcapillary device with a magnified image of the part where droplets formed. Reprinted from Martinez et al.29 Copyright 2012 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (C) Batch emulsion: Cell encapsulated hydrogel microsphere made by mixing PEGDA hydrogel precursor solution and allogeneic skin fibroblasts in mineral oil. Reprinted from Sonnet et al.54 Copyright 2013 Orthopaedic Research Society. (D) Mechanical fragmentation: Fragmented microgels can be obtained by applying forces to bulk hydrogels using a fragmenting device like the tissue homogeniser. Reprinted from Widener et al.69 (E–G) Lithography: (E) Imprint lithography places PDMS moulds on a layer of hyaluronic acid and crosslinks the material by exposing it to UV light. Reprinted from Khademhosseini et al.61 Copyright 2006 Wiley Periodicals, Inc. (F) Photolithography uses a photomask to screen the UV light and crosslink the materials exposed to the UV light. (G) The flow lithography technique allows a continuous stream of material to pass through a region of UV light with specific shape. Reprinted from Laza et al.68 Copyright 2012 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (H) Electrohydrodynamic spraying: A syringe pump sprays the hydrogel and cell precursor solution into the oil bath through a needle tip connected to a high-pressure source. Reprinted from Kim et al.57 HA: hyaluronic acid; HGF: hepatocyte growth factor; OECs: outgrowth endothelial cells; PDMS: polydimethylsiloxane; PEGDA: poly(ethylene glycol) diacrylate; UV: ultraviolet; VEGF: vascular endothelial growth factors.
The microcapillary device has a similar concept to the microfluidic chip in producing microspheres (Figure 1B). But it uses capillary action to manipulate fluids. As opposed to channels on a microfluidic chip, microcapillary device features multiple concentrically arranged capillaries, offering better flexibility in channel design and higher resolution.6, 23, 29, 32, 37, 38, 41-44, 47 The most common method is to use two different-sized syringe needles or capillaries, and insert one coaxially into the other.6, 23, 37, 41-44 The oil phase is used as a continuous phase on the outer needle, while the internal water phase is injected through the inner needle. The ratio of internal over continuous phase was found to act as an essential factor influencing the particle size of microgels.6 An alternative method is to inject the material by inserting a needle into a capillary tube supplied with a constantly flowing oil phase.32
Batch emulsion
The batch emulsification technique produces hydrogel microspheres in a simpler and more efficient way. The general process involves pouring the material solution into an oil phase, followed by stirring or homogenisation, after which the material is crosslinked to form microspheres (Figure 1C).53, 54 Additionally, this method has fewer restrictions on equipment and instrumentation, making it the most versatile process. As a mixture of two material solutions plus a sufficient amount of initiator can be crosslinked by UV light to form hydrogel microspheres, the oil base is not necessary for all procedures.52 However, a drawback of this technique is the lack of control over the size and shape of the particles compared to microfluidics. The diameters of microspheres made by batch emulsion have a coefficient of variation of 30% to 40% (diameters ranging from 20 to 200 μm), while those by microfluidic have only 10% to 15%.45
Mechanical fragmentation
Mechanical fragmentation involves mechanically breaking bulk hydrogels into microscale particles (Figure 1D). Similar to the batch emulsion, it is a relatively simple and straightforward process with the potential to be easily scaled up for industrial production. The bulk hydrogels can be minced with a tissue homogeniser and the fragmented particles can then be collected through centrifugation.69 Alternatively, microparticles can be obtained by extruding the bulk hydrogels from a micronic steel mesh, with the mesh size adjustable to modify the particle size.70 Another method involves placing the hydrogel precursor solution into a syringe, crosslinking the materials with UV light, and extruding the particles through syringe needles.45 However, like batch emulsion and electrohydrodynamic spraying, mechanical fragmentation has difficulty in controlling the particle size, shape, and size distribution. Compared with microspheres made by batch emulsion, the obtained microspheres by mechanical fragmentation are larger in size and different in shape, making them less effective in encapsulating cells or drugs within the interstitial spaces.45, 69 On the other hand, the irregular shape also makes the microsphere made by mechanical fragmentation a higher viscosity.45
Lithography
Lithography is the second most common technique because it is capable of producing hydrogel microspheres of customised size and shape with great precision. Lithography offers more flexibility to create microgels with customised internal and external architectures, which cannot be achieved with “bottom-up” processes.18, 28 Since oil phase or surfactants are not required to induce microgel formation in lithography, this process is more compatible with cell encapsulation.60, 64 The processing routes of lithography can be further categorised into three approaches: imprint lithography, photolithography, and flow lithography.
Imprint lithography places a layer of hydrogel precursor on PDMS moulds with premade patterns and then cures the material with a specific crosslinking method (Figure 1E). The size and shape of the resulting hydrogel microspheres are determined by the prefabricated patterns on the PDMS moulds. Typically, silicon masters are made to ensure the consistency of the PDMS moulds and to prevent the formation of inconsistent hydrogel microspheres.61 Therefore, imprint lithography provides higher resolution and controllability for customising the internal and surface structures of microspheres.
Photolithography has mostly opted to use a photomask to screen and cure specific regions of material (Figure 1F).60, 62, 63 However, the use of a confocal laser scanning microscope as a replacement for the photomask is a good alternative as it provides more detail on the produced three dimensional microspheres.63 The control of the photon density allows a more accurate determination of the size and shape of the microspheres. However, the process requires more time to cure the microspheres than the general method.
Flow lithography involves a stream of pre-polymer solution that continuously passes through PDMS channels, followed by polymerisation with pulsed UV light in selected areas guided by photomasks (Figure 1G).64-68 The structure of the hydrogel microspheres is defined by two views: the top view is controlled by the pattern on the digital micromirror device, and the side view is controlled by the cross-sectional shape of the channel.65 It is capable of producing hydrogel microspheres that encapsulate different cells at the same time.68 Moreover, flow-lithography combines lithography with microfluidics, making it a relatively high-throughput method for fabricating hydrogel microspheres compared to the other two lithography routes.67, 78
As a high-precision method, however, lithography overall results in the lowest productivity among the five techniques for microgel fabrication. Moreover, the hydrogel precursors used in lithographic processing are typically limited to acrylic-functionalised polymers such as acrylates and methacrylates.18, 28 Advancing lithography techniques to achieve high efficiency and accommodate a wider range of materials is needed.
Electrohydrodynamic spraying
Electrohydrodynamic spray is a type of technique that extrudes material into small droplets while applying a voltage to the nozzle (Figure 1H). The parameters that affect particle sizefrom most to least are nozzle voltage, material flow rate, and nozzle diameter.59 Nozzle voltage is inversely proportional to the yield particle size, while material flow rate and nozzle diameter are directly proportional to the particle size.56, 59 In addition, the biological properties such as cell viability and cell dispersion within the microspheres produced by electrohydrodynamic spraying can be tuned by adjusting the spraying parameters. For example, large microspheres with uniform cell dispersion can be achieved while a low nozzle voltage is applied.59 However, this technique does have difficulty controlling the polydispersity of the microspheres. The coefficient of variation has a range from 6.8 ± 1.3% to 21.4 ± 8.2% with the variations of fabrication parameters (cell/drug encapsulated, nozzle voltage, material flow rate, and nozzle diameter). Also, for microspheres over 200 μm or an applied voltage over 10 kV, a decrease in the cell viability might occur due to diffusion limitations and hypoxia. As a result, electrohydrodynamic spraying is an effective tool for manufacturing microspheres for a variety of biological applications, such as injectable cell delivery,59 generation of in vitro multicellular spheroid models,59 and tissue culture applications that require temporal control of substrate properties.55 The microsphere made by electrohydrodynamic spraying has been proven to be a potential treatment for diseases like intervertebral disc degeneration56 and hindlimb ischaemia.57
Application in Tissue Engineering
As injectable biomaterials, the application of hydrogel microspheres in tissue engineering creates less invasive trauma during the transplantation process, causing minimum pain to the patients, making them advantageous for tissue engineering applications compared to bulk scaffolds. The microscale size provides versatility for multifunctional application as multiple particles with different properties and functions can be easily co-injected.27 The adjustable size, shape, and some of the customised internal and external architectures can be used to fill irregular defects.80 Moreover, the large surface area of microgels offers more efficient loading and controllable delivery of cells/drugs. In this section, we discuss the application of hydrogel microspheres as cell and drug delivery systems for bone, cartilage, and soft tissue regeneration.
Bone regeneration
Bone defects resulting from trauma, infection, tumour, and other conditions pose challenges to orthopaedic physicians.81 The regeneration of bone is an intricate process orchestrated by different cell types and their signalling pathways.2, 82 Hydrogel microspheres offer significant advantages in bone regeneration that other treatments do not possess. Although bone tissue has the capability to self-repair, it can significantly be disrupted depending on the type and severity of the injury or malformation.81 Therefore, bone grafts are turned to for clinical treatment as they successfully provide sufficient support for regeneration.83, 84 However, bone grafts induce risks to the patient due to their invasive process and challenges associated with the immune response. In comparison, hydrogel microspheres provide advantages that can overcome these challenges as they require limited invasive procedures while facilitating cell growth and adhesion.
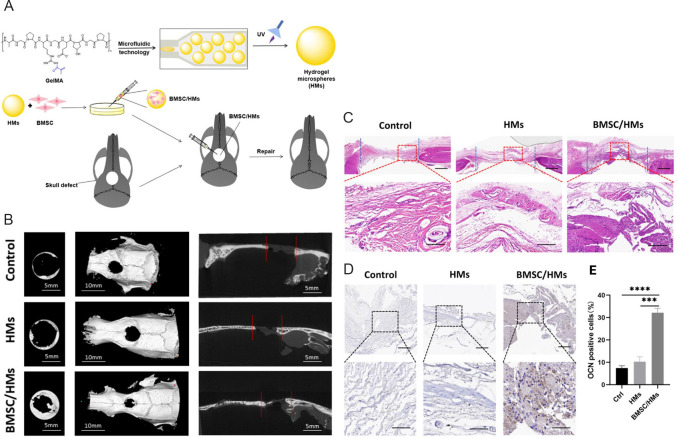
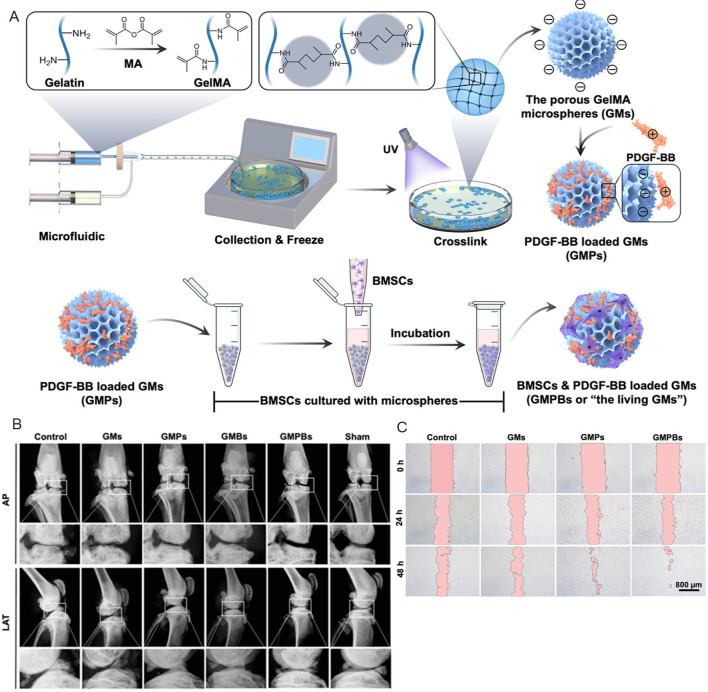
Hydrogel microspheres loaded with stem cells such as bone marrow mesenchymal stem cells (BMSCs) and dental pulp stem cells have been proven to be an effective and minimally invasive method for bone regeneration.6, 83, 85-87 Teng et al.83 synthesised hydrogel microspheres using a microfluidic system based on light-induced gelatine of GelMA (Figure 2A). To enhance bone repair, BMSCs were loaded on the surface of the hydrogel microspheres. Due to their appropriate mechanical strength, with the diameter of hydrogel microspheres being about 282.92 ± 3.82 qμm and the mechanical properties being about 2.28 kPa, such designed hydrogel microspheres could support bone regeneration and withstand the increase in intracranial pressure. Overall, the BMSC-loaded GelMA hydrogel microspheres demonstrated excellent osteogenic potential in vitro and accelerated bone regeneration in a rat cranial bone defect model in vivo (Figure 2B–E). In another attempt, Wu and colleagues6 developed a novel microfluidic technology which could be used to prepare homogenous cell-loaded porous microgels. The prepared microspheres not only exhibited an attractive ability to adsorb BMSCs, but these BMSC-loaded microgels also showed appreciable osteogenic potential in vitro and bone remodelling in vivo, as demonstrated in a mouse femur bone defect model.
Figure 2. Schematic diagram of the fabrication process of BMSCs-loaded GelMA HMs and their results of micromorphometric and histological analysis. (A) HMs produced from microfluidic methods are then crosslinked by UV light, seeded with BMSCs, and transplanted to the skull defect. (B) Micromorphometric analysis of the skull defect 8 weeks after transplantation. Images are superficial, three-dimensional, and sagittal views of microcomputed tomography images. Scale bars: 5 mm (left and right), 10 mm (middle). (C) HE staining in the skull defect area of control, HMs and BMSC/HMs groups at 8 weeks after transplantation. Scale bars: 50 μm. (D) Immunohistochemical staining of OCN-positive cells in the skull defect area 8 weeks after transplantation of HMs and BMSC/HMs. Scale bars: 50 μm (upper), 20 μm (lower). (E) Semi-quantitative analysis of the relative number of OCN-positive cells in the control, HMs and BMSC/HMs groups. Reprinted from Teng et al.83 BMSCs: bone marrow mesenchymal stem cells; GelMA: gelatin methacrylate; HE: hematoxylin-eosin; HMs: hydrogel microspheres; OCN: osteocalcin; UV: ultraviolet.
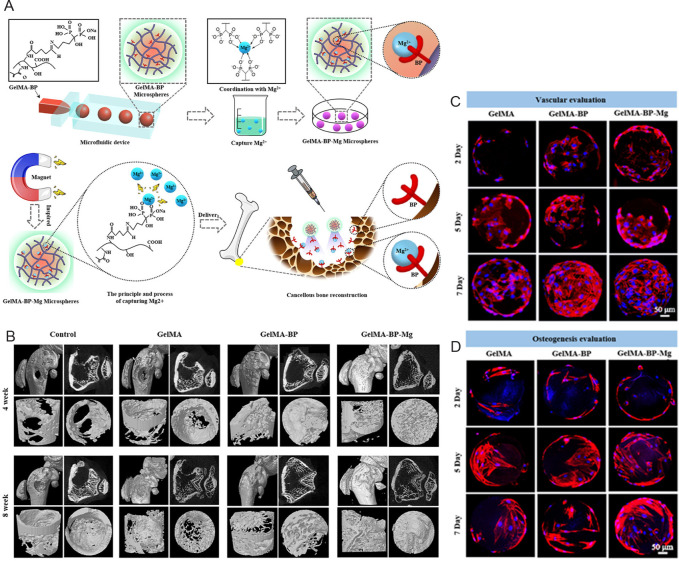
Except for stem cells delivery, successful bone repair often requires scaffolds to sustainedly deliver biological molecules such as GFs and bone-related trace elements. GFs such as bone morphologic proteins (BMPs),54, 88 transforming growth factor-β (TGF-β),89 fibroblast growth factors (FGFs),90, 91 insulin-like growth factors,88 and trace elements including Mg2+,92, 93 Mn2+,94 Fe2+,84 Zn2+,95 Cu2+,96 and Sr2+,95 etc. have been demonstrated to promote bone regeneration by activating related signalling pathways or regulating other processes such as bone metabolism. Using a gas-assisted microfluidic technique, Dai et al.97 fabricated double crosslinked hydrogel microspheres based on ionic crosslinking of alginate with Cu2+ and photo-crosslinking of GelMA. Moreover, vascular endothelial growth factors (VEGFs) and BMP-2 were loaded into the hydrogel microspheres. The sustained release of Cu2+, VEGFs, and BMP-2 synergistically promoted bone healing in a rat femoral defect model by showing multifunctional antibacterial, angiogenetic, and osteogenic capabilities. In another study, microfluidic GelMA-bisphosphonate hydrogel microspheres exhibited powerful Mg2+ capture ability (Figure 3A).92 The captured Mg2+ in the GelMA-bisphosphonate hydrogel microspheres showed a slow and sustained release and, therefore, promoted cancellous bone repair by activating osteoblasts and endothelial cells while limiting the activation of osteoclasts, simultaneously facilitating bone and vascular regeneration (Figure 3B–D).
Figure 3. Schematic diagram of the fabrication process of GelMA-BP-Mg microspheres and their results of micromorphometric analysis and biocompatibility. (A) GelMA-BP microspheres were prepared by a microfluidic device and Mg was captured by Schiff alkali reactivity. GelMA-BP-Mg microspheres were then constructed by metal ion coordination ligands and delivered by injection. (B) Regeneration efficacy of the distal femur of rats with osteoporotic bone defects at 4 and 8 weeks after injection. Microcomputed tomography images show the results for control, GelMA, GelMA-BP, and GelMA-BP-Mg groups. (C, D) Proliferation of BMSCs on GelMA, GelMA-BP, and GelMA-BP-Mg microspheres after 2, 5, and 7 days. Scale bars: 50 μm. Red shows the skeleton; blue shows the nucleus. Reprinted with permission from Zhao et al.92 Copyright 2021, American Chemical Society. BMSC: bone marrow mesenchymal stem cells; BP: bisphosphonate; GelMA: gelatin methacrylate.
Cartilage regeneration
Articular cartilage is mainly composed of collagen and proteoglycans. Mature cartilage has a low cell density and lacks sufficient nerve, lymphatic, and vascular tissues, leading to low self-repair capabilities.74 Additionally, due to the limited understanding and complexity of the cartilage environment, cartilage regeneration remains a significant challenge.98 Joint replacement is eventually required if no early intervention is taken after the initiation of articular cartilage injury, as the two opposing bones continuously rubbing against each other will cause damage to the subchondral bone.3 Current practice in treating cartilage defects mainly relies on surgical approaches and oral administration or joint cavity injection of drugs such as glucosamine sulfate and chondroitin sulfate.99-101 However, these treatments can only yield short-term relief of symptoms and delay the progression of disease.3, 80 It is agreed that the key to effectively treating cartilage injury is to stimulate the self-repair activity of the defected tissue.74 The use of tissue engineering scaffolds is such a strategy. As a microscale carrier, hydrogel microspheres especially provide a functional solution for cartilage regeneration as they combat the limitations of traditional large scaffolds by increasing the indwelling time of loaded drugs and cells and strengthening their fluidity, leading to more favourable injections.74 Furthermore, hydrogel microspheres are capable of filling more complex structures to expand functionality and have a porous network structure, allowing for the capturing and sustained release of cells, drugs, and other bioactive molecules. For example, Yao et al.44 designed an adhesive hydrogel microsphere which has a positively charged nanosized secondary structure that allows it to penetrate inside cartilage with the aid of charge guidance, making it possible to conveniently deliver drugs to the deep layer of cartilage tissue and repair cartilage injury.
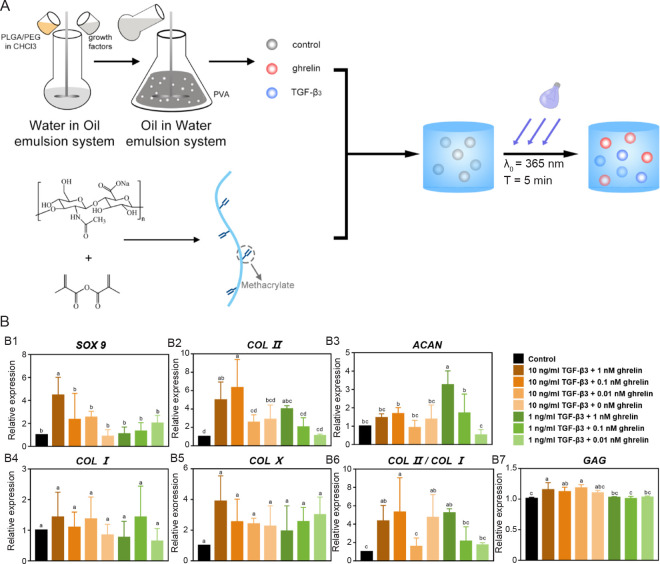
GFs such as TGF-β,102, 103 insulin-like growth factors,104 FGFs,105 VEGFs,106, 107 and platelet-derived growth factors (PDGFs),108 and synthetic molecules such as bevacizumab,109 dexamethasone (DEX),110 and kartogenin44, 111 have shown beneficial applications in cartilage regeneration by creating a suitable microenvironment to facilitate cellular growth of chondrocytes and chondrogenic differentiation of stem cells and chondroprogenitor cells.112 It is worth noting that the GFs used for bone and cartilage regeneration are typically different species even in the same superfamily. For instance, BMP-2 has been a common GF for bone regeneration, while evidence has been found that BMP-6 is beneficial for inducing cartilage regeneration.88, 113 The use of vascular-related GFs for bone and cartilage regeneration is also different. On the one hand, angiogenesis is a prerequisite for osteogenesis as insufficient vascularisation in bone defects results in hypoxia and cellular necrosis which are detrimental to bone formation.114 On the other hand, cartilage is an avascular tissue, and vascularisation in cartilage leads to cartilage mineralisation and thus ultimately causing pain and structural damage in cartilage.109, 115 Therefore, pro-angiogenetic GFs such as VEGFs and placental growth factor are typically used for bone regeneration while anti-angiogenetic factors such as angiostatin and thrombospondins are commonly used to treat cartilage lesions.116-118 Dehghan-Baniani et al.119 reported an injectable chitosan-based nanocomposite hydrogel microspheres system to simultaneously delivery two agents, kartogenin and diclofenac sodium, to promote chondrogenesis of stem cells and suppress inflammation, respectively. The hydrogel microspheres system showed a sustained linear drug release for over a month. Lin et al.103 developed a dual-delivery microsphere/hydrogel system encapsulated with TGF-β3 and ghrelin (Figure 4). TGF-β3 promotes the formation of cartilage tissue but has side effects when used alone,120 whereas growth hormone-releasing peptide has been shown to promote cartilage differentiation when combined with TGF-β3 significantly, thereby the authors encapsulated TGF-β3 and ghrelin, a growth hormone-releasing peptide, in a microspheres/hydrogel system. TGF-β3 and ghrelin were encapsulated into poly (lactic-co-glycolic acid) (PLGA)/ PEG microspheres with a double emulsion solvent extraction technology (water-in-oil-in-water) and then the drug loading microspheres were further encapsulated in a methacrylated HA hydrogel. Such a microspheres/hydrogel system demonstrated sustained release of the dual drugs and therefore exhibited enhanced chondrogenic differentiation ability of stem cells.103
Figure 4. Schematic diagram of the fabrication process producing hydrogel microspheres with growth factor and chondrogenic differentiation results of hMSCs used for cartilage regeneration. (A) Fabrication of the PEG/PLGA microspheres containing TGF-β3 or ghrelin. (B) Results of the chondrogenic differentiation results of hMSCs with different concentrations of TGF-β3 and ghrelin after 21 days. (B1–7) The qRT-PCR analyses are done for SOX9, COL II, ACAN, COL I, COL X, COL II/COL I, and GAG. Reprinted from Lin et al.103 ACAN: aggrecan; COL I: type I collagen; COL II: type II collagen; COL X: type X collagen; GAG: glycosaminoglycan; hMSCs: human bone marrow mesenchymal stem cells; PEG: poly(ethylene glycol); PLGA: poly(lactic-co-glycolic acid); PVA: poly(vinyl alcohol); qRT-PCR: quantitative reserve transcription-polymerase chain reaction; SOX 9: Sry-type high-mobility-group box 9; TGF: transforming growth factor).
Cell-loaded hydrogel microspheres have also been proven to be a suitable treatment for promoting cartilage regeneration.74 A microsphere culture technique was used to develop artificial cartilage particles by culturing collagen hydrogel microspheres with allogenic chondrocytes.121 The optimised artificial cartilage particles demonstrated better cartilage repair and integration with the surrounding host tissue in a rabbit osteochondral defects model. In another study, injectable individual cell-loaded hydrogel microspheres were crosslinked into a three dimensional construct using a 4-arm PEG-N-hydroxysuccinimide.122 This process largely preserved the viability and cellular functions of encapsulated BMSCs and therefore increased the chondrogenic markers in both gene and glycosaminoglycan expression levels.122 PDGF-BB-loaded GelMA microspheres and BMSCs + PDGF-BB-loaded GelMA microspheres produced by Li et al.123 also achieved significant results in in vitro analyses and in vivo tests in Sprague-Dawley rats (Figure 5).123 Overall, culturing cells with microgels not only provides higher mechanical integrity compared to direct injection of cells, which protected the cells from damage during injection, but also increases cell-cell interactions compared to culturing in bulk scaffolds, thereby enhancing cartilage regeneration.
Figure 5. Schematic diagram of the fabrication process producing GMPs/GMPBs and results of in vivo and in vitro tests. (A) Microspheres were prepared by microfluidic method and loaded with PDGF-BB and BMSCs. (B) X-ray images of the knee joints of rats in the control group, GMs group, GMPs group, GMBs group, GMPBs group and Sham group at AP and LAT angles. (C) Cell migration images of control, GMs, GMPs, GMPBs groups at 0, 24 and 48 hours. Scale bars: 800 μm. Reprinted from Li et al.123 Copyright 2023 Wiley‐VCH GmbH. AP: anteroposterior; BMSCs: bone marrow mesenchymal stem cells; GelMA: gelatin methacrylate; GMs: GelMA microspheres; GMBs: BMSCs loaded GelMA microspheres; GMPs: PDGF-BB-loaded GelMA microspheres; GMPBs: BMSCs+PDGF-BB-loaded GelMA microspheres; LAT: lateral; MA: methacrylate; PDGF-BB: platelet-derived growth factors-BB; UV: ultraviolet.
Soft tissue regeneration
Nerve
The nervous system is classified into central and peripheral nervous systems. Central nervous system typically do not spontaneously regenerate after injury.124 Peripheral nerves are capable of self-repairing, however, such a capacity is poor, especially when the defect is large (> 5 mm) or the types of the injury are severe, like axonotmesis (loss of axonal continuity) and neurotmesis (loss of continuity of both axonal and surrounding connective tissues).125 Existing nerve repair methods include drug treatment, physical therapy, and surgical approaches such as neurorrhaphy and the use of “gold standard”, autograft. However, these methods are associated with some limitations. Oral and intravenous administration of drugs results in low efficiency due to a lack of targeted delivery. Physical therapy mainly focuses on short-term relief of symptoms and their long-term effectiveness is not yet proven.126 Neurorrhaphy only works for short nerve gaps in the clinic, while the use of autograft is associated with drawbacks include the need of multiple surgeries, donor shortage, and the potential neuroma and loss of function at the donor site.127-129 The regeneration of nerve tissue requires Schwann cells to form a highly ordered arrangement, called the “bands of Büngner”, to guide axons to regrow along the tubular structure of nerve fibres from the proximal end to the distal end and eventually bridge the nerve defect.130 Therefore, nerve regeneration using tissue engineering strategies, such as the application of drug- or cell-loaded nerve guidance conduits to facilitate the formation of “bands of Büngner” and guide axonal elongation, provides an effective alternative. Specifically, the use of hydrogel microspheres as the cell/drug delivery carriers has several benefits in tissue engineering based nerve regeneration. Hydrogel microspheres as scaffolds or embedded within other polymeric nerve guidance conduits have the advantage of controlling the desired microstructure and are effective in guiding and protecting axonal growth. In injection therapy, where force and pressure can lead to cell death and drug failure, microspheres also protect the encapsulated cells and drugs, resulting in improved cell survival and better release of functional drugs.
Neurotrophic factors such as nerve growth factor (NGF),36, 131, 132 brain-derived neurotrophic factor (BDNF),133 and glial cell derived neurotrophic factor,132 and other molecules such as tacrolimus,134 insulin,135 and folate136 have been shown to improve central and peripheral nerve regeneration in vitro and in vivo. NGF-loaded multi-scale composite scaffolds made of GelMA/GelMA hydrogel and chitosan microspheres by a microfluidic method provided a favourable microenvironment for the growth of Schwann cells and PC12 cells by sustained release of NGF.36 The composite scaffold was as biocompatible as the treated polystyrene tissue culture plate. The hydrogel microspheres not only provided sufficient space for the growth of PC12 and RSC96 cells, but also allowed for the sustained release of NGF, which induced neurite growth and elongation. An injectable fibrin hydrogel combining topical tacrolimus and mesenchymal stem cells delivery also showed enhanced nerve regeneration.134 Tacrolimus encapsulated in PLGA microspheres can be continuously released through the surface erosion of the PLGA microspheres. Insulin loaded biomimetic hydrogel microspheres composed of phenol-substituted HA and collagen were fabricated using a microfluidic device with a water-in-oil emulsion system, and crosslinked by a laccase-mediated crosslinking.135 The biomimetic hydrogel microspheres exhibited sustained and prolonged release of insulin which has been proved to significantly increase axonal regeneration and remyelination, and enhance functional recovery of nerve in a rat sciatic nerve defect model.135
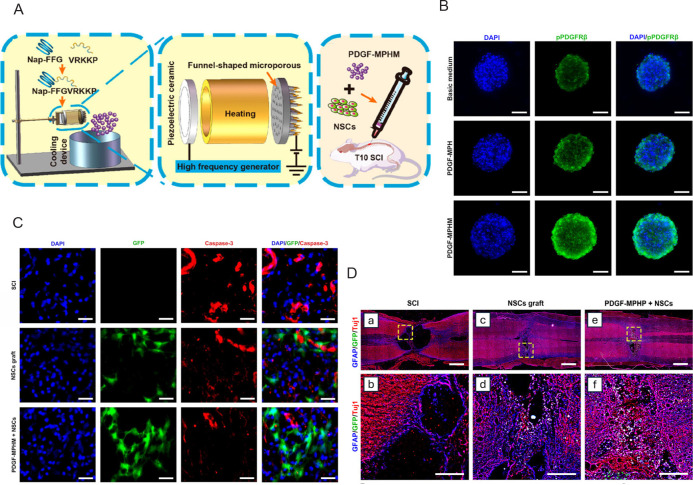
Neural stem cells (NSCs) are considered a potential replacement for spinal cord injury (SCI) that causes loss of neural cells, while unfavourable microenvironments have a strong impact on the survival and differentiation of NSCs.137, 138 Hydrogel microspheres also played an important role in regenerating central nervous system. Injectable PDGF mimetic peptide hydrogel microspheres (PDGF-MPHM), with an average diameter of 9 μm have been shown to effectively activate PDGF receptor β of NSCs to provide a higher survival rate of transplanted NSCs (Figure 6A, and B).137 The animal tests showed significant improvement in motor function recovery in SCI rats compared with the NSCs transplantation group. In vitro, under the presence of myelin extract, PDGF-MPHM showed strong neuroprotective effects by maintaining the proliferation of NSCs and inhibiting their apoptosis; in vivo, PDGF-MPHM significantly promoted the survival and neuronal differentiation of loaded NSCs and the NSC-loaded PDGF-MPHM reduced the size of the defect by stimulating the regeneration of axons, synapse formation, and angiogenesis (Figure 6C, and D). In another study, BDNF-encapsulated, tannic acid (TA) modified PLGA microspheres were suspended in an injectable and conductive polysaccharide-based hydrogel, which was composed of oxidised Dex and HA. The results indicated that the BDNF@TA-PLGA/Dex-HA allowed for sustained release of BDNF for the repair of SCI.138 The cross-section of the BDNF@TA-PLGA/Dex-HA hydrogel demonstrated a porous structure, which can enhance nutrient transport, cell adhesion and proliferation; mixing TA in the hydrogel mixture could improve the stability of Dex-HA hydrogel and the biological activity of BDNF, and prolong the release time of BDNF, which is beneficial to the differentiation of NSCs and the formation of new nerve tissues. Compared with other hydrogels, BDNF@TA-PLGA/DEX-HA showed better cell proliferation activity and viability, promoted the differentiation of NSCs into neurons, and inhibited stellate cell differentiation.
Figure 6. Schematic diagram of the fabrication process of hydrogel microspheres with NSCs and results of using it on SCI. (A) PDGF-MPHM was formed using an electrospray device and implanted into the T10 SCI site of rats 1 day after SCI. (B) Representative fluorescence images of stained NSC cultured with basic medium, PDGF-MPH, and PDGF-MPHM. Green shows the phosphorylated platelet-derived growth factor receptor beta, and blue shows the nucleus. Scale bars: 50 μm. (C) Representative immunofluorescence images of cross-sections of the spinal cord of rats in the SCI group, NSCs grafting group, and PDGF-MPHM + NSCs group. Red shows the apoptosis cells, blue shows the nucleus, and green shows the grafted cells. Scale bars: 250 μm. (D) Representative immunofluorescence images of the SCI, NSCs graft, and PDGF-MPHM + NSCs groups 8 weeks after SCI. Scale bars: 1 mm (upper), 250 μm (lower). Reprinted with the permission from Wu et al.137 Copyright 2023, American Chemical Society. DAPI: 4′6-diamidino-2-phenylindole; GFAP: glial fibrillary acidic protein; GFP: green fluorescent protein; Nap-FFG: naphthalene acetic acid-phenylalanine-phenylalanine-glycine; NSCs: neural stem cells; PDGF-MPH: platelet-derived growth factor mimetic peptide hydrogel; PDGF-MPHM: platelet-derived growth factor mimetic peptide hydrogel microspheres; PDGFRβ: platelet-derived growth factor receptor β; SCI: spinal cord injury; Tuj1: beta tubulin III.
Skin
Skin is the largest organ of the human body. It is composed of the epidermis and dermis. The epidermis acts as a barrier between the internal and external environment, while the dermis provides support and nutrition to the epidermis.139 Skin wounds resulted from trauma and pathophysiological conditions represents a major public health issue.140 The healing process of skin wounds consists of a few continuous and overlapping processes include haemostasis, inflammation, proliferation and ECM remodelling.141 It is agreed that grafting is needed for treatment of skin wounds with loss of full-thickness skin more than 4 cm in diameter.142, 143 However, traditional surgical procedures involving the use of autograft is often associated with donor shortage, while the use of allograft or xenograft brings concerns of pathogen infection and immune rejection. Hence, tissue engineered skin substitutes have been gaining momentum. The materials requirements for skin regeneration of tissue engineering approaches include biocompatibility, biodegradability, mechanical properties, protectivity of loaded drugs and cells, and the ability to keep humidity. Hydrogel microspheres are composed primarily of water molecules, which is beneficial to keep a moisture environment in the skin defect area, promoting better healing and skin tissue regeneration. Moreover, the microscale size allows them to fill into the lesions with irregular shape and depth. The large surface area and degradation behaviour also facilitate efficient drug loading and controlled release.
Scaffolds made of catechol functionalised chitosan, β-sodium glycerophosphate, and oyster peptide microspheres contain a porous network structure that is beneficial for water retention.144 The release of the oyster peptides from the scaffolds exhibited three distinct processes: burst release, continuous release, and sustained release. The burst release occurred within 2 hours, the continuous release within 2-12 hours, and the sustained release after 12 hours up to a few days. The catechol functionalised chitosan/oyster peptide microsphere/β-sodium glycerophosphate hydrogel showed excellent wound closure in vivo, and promoted protein synthesis in granulation tissues and accelerated wound healing by showing a higher density of neovascularisation, denser tissues, and more orderly arranged fibroblasts. The major issues in skin regeneration are insufficient angiogenesis and poor dermal self-regeneration, whereas bFGF has beneficial effects in promoting angiogenesis and accelerating wound healing.145, 146 Shamloo et al.145 designed a poly(vinyl alcohol)/chitosan/gelatine composite hydrogel containing bFGF-loaded polycaprolactone microspheres. The composite hydrogel exhibited a highly porous microstructure with a porosity of 54% and an average pore size of 35 ± 7 μm, which is within the ideal range for the regeneration of the dermis. The bFGF remained bioactivity for up to 2 weeks within the composite, which is an important time period for skin regeneration. Moreover, the use of chitosan significantly enhanced the antimicrobial properties of the scaffolds. As a result, the sustained release of bFGF promoted wound healing and angiogenesis, resulting in a wound closure rate of 50% 4 days post-grafting. In a similar study, scaffolds made from bFGF-loaded alginate microspheres and carboxymethyl chitosan-poly(vinyl alcohol) have also shown potential for dermal tissue regeneration owing to a sustained release of highly biologically active bFGF over 2 weeks.146
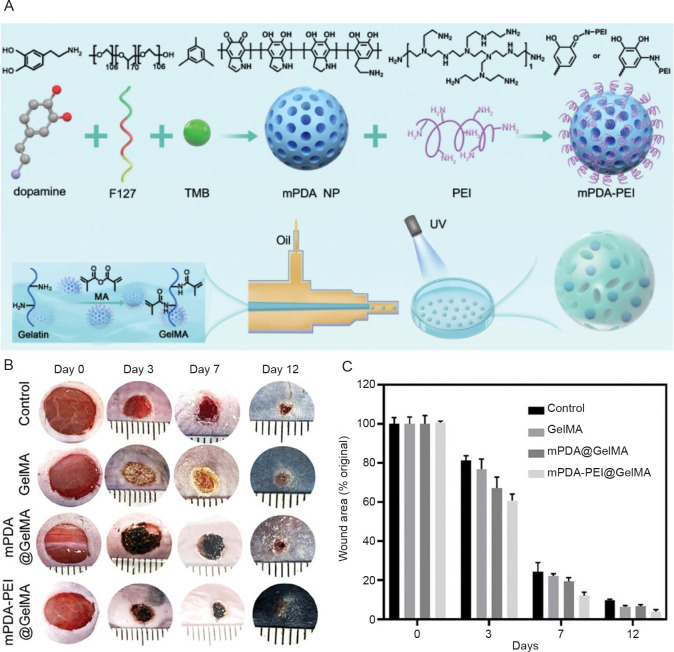
The high water retention capability of hydrogels and hydrogel microspheres has been shown to be beneficial to skin wound healing. For example, Kong et al.147 demonstrated that HA in a hydrogel composed of type I and III collagens and HA was critical to the long-term retention of moisture, facilitating rapid and scar-free healing of skin wounds. In another study, GelMA hydrogel microspheres, which exhibit excellent water absorption and expansion properties, were combined with cationic polyethyleneimine functionalised mesoporous polydopamine (Figure 7A).148 In vivo tests on diabetic mice showed that the combination of GelMA hydrogel microspheres and polyethyleneimine-functionalised polydopamine significantly alleviated pro-inflammatory responses associated with diabetic wounds by serving as a neutrophil extracellular trap scavenger and therefore enhanced the diabetic skin wound heling process (Figure 7B, and C).
Figure 7. Schematic diagram of the synthesis process of mPDA-PEI@GelMA and wound healing results in diabetic mice in vivo. (A) The mixture of mPDA-PEI and GelMA was crosslinked under UV light after exiting the microfluidic device. (B) Representative images of wound healing in control, GelMA, mPDA@GelMA, and mPDA-PEI@GelMA groups. (C) Wound healing rate in four treatment groups on days 0, 3, 7 and 12. Reprinted from Xiao et al.148 GelMA: gelatin methacrylate; MA: methacrylate; mPDA: mesoporous polydopamine; mPDA NP: mesoporous polydopamine nanoparticle; PEI: polyethyleneimine; UV: ultraviolet.
Muscle
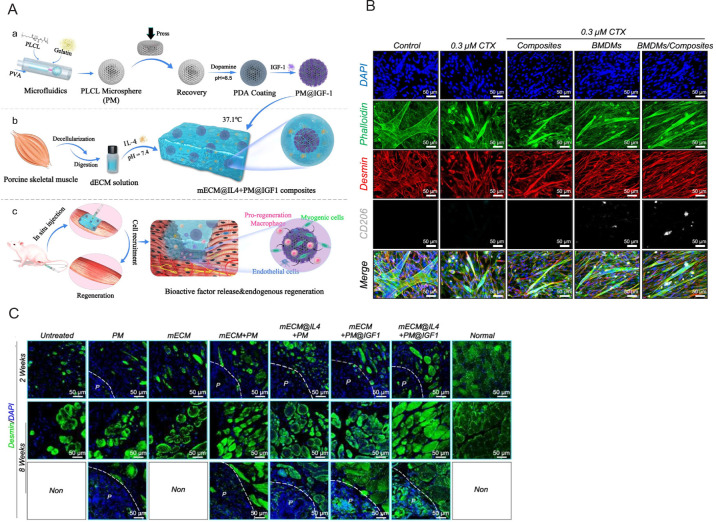
The repair of muscle tissue after traumatic injury or muscle disease often presents a challenging clinical scenario, i.e., if significant tissue loss occurs, the natural regenerative potential of skeletal muscle will not be able to grow sufficiently to cover the defect.149 Tissue engineering applications using cell- or drug-load hydrogels are potential alternatives for repairing large volumetric muscle loss.150 For example, an injectable three dimensional arginine-glycine-aspartic acid tripeptide-coupled alginate hydrogel with multiple GFs delivery capacity was developed to encapsulate gingival mesenchymal stem cells for muscle regeneration. Results indicated that the gingival mesenchymal stem cells within the composite hydrogel system showed increased capillary density and improved neovascularisation and local angiogenesis, demonstrating better muscle tissue regeneration.149 The addition of microspheres provides better mechanical strength to the ECM hydrogel and compensates for the lack of stabilised drug release due to its rapid degradation.151 Injectable composites composed of elastic porous poly(l-lactide-co-ε-caprolactone) microspheres mixed with ECM hydrogel, which can release the two encapsulated drugs, interleukin-4 and insulin-like growth factor-1, have shown good injectability and biocompatibility (Figure 8A). Intramuscular injection of the composites regulated the behaviour of macrophages and tissue-specific cells (Figure 8B). In vivo tests on a rat model with volumetric muscle loss showed new muscle formation, vascularisation, and neuralisation, indicating enhanced muscle regeneration (Figure 8C).
Figure 8. Schematic diagram of the fabrication process of mECM@IL-4 + PM@IGF-1 composites and their muscle regeneration potential. (A) PLCL microspheres fabricated by microfluidics were modified with PDA-conjugated IGF-1 and complexed with mECM and IL-4 to form a composite material injected into the damaged area. (B) The representative images show the differentiation-promoting effects of control, composite, BMDMs and BMDMs/composite groups on injured muscle satellite cells. Green shows phalloidin staining area, red shows desmin staining area, grey shows the CD206 cells, and blue shows the nucleus. (C) Immunofluorescence images showing muscle regeneration at 2 and 8 weeks in a rat VML model. Green shows phalloidin staining area, and blue shows the nucleus. Scale bars: 50 μm. P represents the microspheres, and the white dashed line represents the border between the microspheres and the tissue. Reprinted from Li et al.151 BMDMs: bone marrow-derived macrophages; CTX: cardiotoxin; DAPI: 4′6-diamidino-2-phenylindole; dECM: decellularised extracellular matrix; IGF-1: growth factor-1; IL-4: interleukin-4; mECM: muscle-derived extracellular matrix; PDA: polydopamine; PLCL: poly(l-lactide-caprolactone); PVA: polyvinyl alcohol; PM: PLCL microsphere; VML: volumetric muscle loss.
The use of hydrogel microspheres also allows for efficient encapsulation of GFs that can enhance the effectiveness of stem cell therapy for muscle regeneration. For instance, FGF19, a myogenic cytokine, was encapsulated into GelMA microspheres using a microfluidic technology.152 Adipose-derived stem cells (ADSCs) were then adsorbed into the porous structure of the microgels. As a result, the loading efficiency and viability of the ADSCs were significantly enhanced. The continuous delivery of pro-myogenic FGF19 at the lesion site significantly promoted myoblast recruitment, myogenic differentiation, and myofibril growth. ADSCs were evenly distributed on the surface of the microspheres and exhibited well-organised F-actin and; they survived on the microspheres for at least 21 days, indicating that GelMA microgels as an ADSC carrier provided a sufficient surface area for cell expansion and survival. Moreover, the increased co-delivery efficiency of FGF19 and ADSCs resulted in the production of more ECM and angiogenic factors, leading to more efficient cell regeneration and blood perfusion. In vivo results from a mouse ischaemic hindlimb model demonstrated that the GelMA hydrogel microsphere, functioning as a co-delivery system for myogenic cytokine and ADSCs, exhibited rapid presence of blood reperfusion, minimal fibrosis, and a high level of skeletal muscle restoration in the ischaemic regions.
Conclusion and Future Perspectives
Hydrogel microspheres have played an important role as effective cell/drug delivery systems in tissue engineering applications due to their excellent water retention capacity, injectability, large surface area, low physical barrier, similarity to ECM, and great flexibility. While we strive to provide a comprehensive summary, we acknowledge that the broad scope of this review, covering the application of microgels for the regeneration of various tissues, each with its own distinct complexities, may have led to some omissions and oversights. We apologise for any suchshortcomings we have made. Overall, in this review, we have covered the material requirements and compared the common techniques used to manufacture hydrogel microspheres. Based on the characteristics and requirements for various types of tissue regeneration, we have summarised the application of hydrogel microspheres for bone, cartilage, central and peripheral nerves, skin, and muscle regeneration. By sustainedly delivering GFs, essential trace elements, and other molecules in a minimally invasive manner, hydrogel microspheres have shown promise in repairing these tissues by tuning the microenvironment for cell attachment, proliferation, migration, differentiation, and cell-cell and cell-material interactions. Moreover, due to their microscale size and unique stable dispersion, the use of hydrogel microspheres as cell delivery carriers significantly enhanced the retention and protection of cells in stem cell therapy, by providing a suitable local environment for cell growth and absorbing the shear stress applied to the loaded cells during injection. Despite these many advantages of hydrogel microspheres, the lack of clinical trials and commercial products highlights the need for further advances in the field. The translation from bench to bedside remains challenging, and progress across all discussed areas is expected to realise the broad application of microgels in clinical settings.
Most hydrogel microspheres are based only on a few material systems, including gelatin, HA, alginate, and PEG, owing to their good water retention capacity and rapid gelation capability with the use of chemical, photo, and ionic crosslinking approaches.97, 153 That said, many biomaterials with biomimetic features and bio-functionalities have not yet been adapted to make hydrogel microspheres, mainly due to their lack of sufficient gelation capability or rapid crosslinking approach. This could possibly be addressed by chemically modifying synthetic and natural polymers or combining materials currently available for making bulk hydrogels, such as collagen, chitosan, cellulose, poly(sebacic acid), poly(vinyl alcohol), poly(vinyl pyrrolidone) and others. For example, the inventions of GelMA154 and PEG diacrylate155 are goodexamples providing gelatine and PEG with rapid photopolymerisation capabilities at room temperature, making the two material systems widely used in drug delivery, tissue engineering and biofabrication. Alternatively, this can be improved by developing versatile rapid crosslinking techniques. Recently, Bao et al.156 reported a universal rapid photo-crosslinking strategy, which was used to prepare biomimetic hydrogels across a series of material systems with excellent mechanical properties (tensile strength = 15.31 MPa) within seconds. This technique could potentially be used to expand the selection of biomaterials or improve the current fabrication approaches for making hydrogel microspheres.
The bio-functionality of the current material systems available for the fabrication of hydrogel microspheres can be further improved by being chemically or physically loaded with functional molecules, rather than drugs or GFs, which are limited by their short half-life and high cost. For instance, citric acid has been recognised as a functional small molecule that regulates tissue regeneration through energy metabolism, metabonegenesis, angiogenesis, and immunomodulation.157-161 Kartogenin has rapidly been regarded as an effective small molecule that can be conjugated with many biomaterials for cartilage repair.162, 163 Folic acid has been shown to have the potential to enhance both central and peripheral nerve regeneration through epigenetic regulation.136, 164 TA can also be incorporated into various biomaterials to provide biofunctions, including anti-inflammatory, antimicrobial, wound healing, and antioxidant properties, and therefore accelerate tissue regeneration.165-168 Additionally, many studies have recently revealed the role of extracellular vesicles secreted from different types of cells in regenerative medicine. Extracellular vesicles including microvesicles and exosomes carry important intracellular components such as proteins, lipids, and nucleic acids, which are key mediators to facilitate cell recruitment, proliferation, differentiation, macrophage polarisation, and cell-cell interactions and therefore beneficial to tissue regeneration.169-175 Hydrogel microspheres with abundant water molecules and stable fluidic dispersion are ideal carriers to load, protect, and sustainedly deliver extracellular vesicles to boost tissue regeneration.
Lastly, there is a need for continued improvement in the current fabrication approaches for hydrogel microspheres to optimize their performance and scalability for clinical use. The product safety, stability, uniformity, industrial-scale productivity, and production costs of fabricating hydrogel microspheres are all significant considerations for practical commercialisation. As the most widely used technique for manufacturing hydrogel microspheres, the basic principle of microfluidics is based on the shear force produced at the interface of oil and water surfactants in the junction of the cross flow.25 As a type of emulsion polymerisation, which is a subclass of free radical polymerisation, the microfluidic approach relies on the use of surfactants to form homogeneous suspensions between the oil and water phases and the addition of initiators to start the polymerisation. Therefore, eliminating residual oil, surfactants and initiators, which are typically detrimental to cells, is expected to lead to a better control of the biocompatibility of hydrogel microspheres. Moreover, most microfluidic devices are developed in laboratories, how to scale-up these devices with high-throughput but low cost for commercialisation application and clinical translation is necessary. Additionally, many microfluidic devices face challenges in reuse due to the microscale channels being easily clogged by the highly viscous fluids, which significantly increases production costs. Other methods used for synthesising hydrogel microspheres are also associated with a series of drawbacks. For example, the batch emulsion method relies on emulsion polymerisation, which involves the use of oil, surfactants and crosslinking agents. Hydrogel microspheres made using “top-down” approaches, including batch emulsion, mechanical fragmentation, and electrohydrodynamic spraying, often exhibit a broad range of size distribution. The shapes of these microgels also lack mono-dispersity. The productivity of lithography technology is low, making it difficult for large-scale production. Therefore, future research should be devoted to advancing the current techniques and developing new technologies for the fabrication of hydrogel microspheres with good quality, functionality, and productivity.
Footnotes
Author contributions: LY and CL conceptualised the review; CL, JS, and LY drafted the manuscript; CL, LY, WS, MX, SY, and JY revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Financial support: This work was partially supported by Nationals Institute of Health grants (Nos. R01NS123433, and R01HL158204).
Acknowledgement: None.
Conflicts of interests statement: Dr. Jian Yang and The Pennsylvania State University have a financial interest in Acuitive Technologies, Inc. and Aleo BME, Inc. These interests have been reviewed and managed by the University’s Institutional and Individual Conflict of Interest Committees. Other authors declare no competing financial interest.
References
- 1.Ye J., Xie C., Wang C., Huang J., Yin Z., Heng B. C., Chen X., Shen W. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact Mater. 2021;6:4096–4109. doi: 10.1016/j.bioactmat.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong Y., Mi B. B., Lin Z., Hu Y. Q., Yu L., Zha K. K., Panayi A. C., Yu T., Chen L., Liu Z. P., Patel A., Feng Q., Zhou S. H., Liu G. H. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9:65. doi: 10.1186/s40779-022-00426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu L., Cavelier S., Hannon B., Wei M. Recent development in multizonal scaffolds for osteochondral regeneration. Bioact Mater. 2023;25:122–159. doi: 10.1016/j.bioactmat.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair A., Thevenot P., Dey J., Shen J., Sun M. W., Yang J., Tang L. Novel polymeric scaffolds using protein microbubbles as porogen and growth factor carriers. Tissue Eng Part C Methods. 2010;16:23–32. doi: 10.1089/ten.tec.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Liu W., Liu F., Zeng Y., Zuo S., Feng S., Qi C., Wang B., Yan X., Khademhosseini A., Bai J., Du Y. Primed 3D injectable microniches enabling low-dosage cell therapy for critical limb ischemia. Proc Natl Acad Sci U S A. 2014;111:13511–13516. doi: 10.1073/pnas.1411295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Li G., Ye T., Lu G., Li R., Deng L., Wang L., Cai M., Cui W. Stem cell-laden injectable hydrogel microspheres for cancellous bone regeneration. Chem Eng J. 2020;393:124715. [Google Scholar]
- 7.Zhang G., Suggs L. J. Matrices and scaffolds for drug delivery in vascular tissue engineering. Adv Drug Deliv Rev. 2007;59:360–373. doi: 10.1016/j.addr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Liu J., Lin S., Zhao X. Hydrogel machines. Mater Today. 2020;36:102–124. [Google Scholar]
- 9.Zhang Y. S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356:eaaf3627. doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong W., Zhao X., Zhou J., Suo Z. A theory of coupled diffusion and large deformation in polymeric gels. J Mech Phys Solids. 2008;56:1779–1793. [Google Scholar]
- 11.Li J., Mooney D. J. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichterle O., LÍM D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. [Google Scholar]
- 13.Fuchs S., Ernst A. U., Wang L. H., Shariati K., Wang X., Liu Q., Ma M. Hydrogels in emerging technologies for type 1 diabetes. Chem Rev. 2021;121:11458–11526. doi: 10.1021/acs.chemrev.0c01062. [DOI] [PubMed] [Google Scholar]
- 14.Chao Y., Chen Q., Liu Z. Smart injectable hydrogels for cancer immunotherapy. Adv Funct Mater. 2020;30:1902785. [Google Scholar]
- 15.Lin W., Kluzek M., Iuster N., Shimoni E., Kampf N., Goldberg R., Klein J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science. 2020;370:335–338. doi: 10.1126/science.aay8276. [DOI] [PubMed] [Google Scholar]
- 16.Lee A., Hudson A. R., Shiwarski D. J., Tashman J. W., Hinton T. J., Yerneni S., Bliley J. M., Campbell P. G., Feinberg A. W. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 17.Jin S., Choi H., Seong D., You C. L., Kang J. S., Rho S., Lee W. B., Son D., Shin M. Injectable tissue prosthesis for instantaneous closed-loop rehabilitation. Nature. 2023;623:58–65. doi: 10.1038/s41586-023-06628-x. [DOI] [PubMed] [Google Scholar]
- 18.Daly A. C., Riley L., Segura T., Burdick J. A. Hydrogel microparticles for biomedical applications. Nat Rev Mater. 2020;5:20–43. doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X., Zhou Y., Li J., Zhang C., Wang J. Opportunities and challenges of hydrogel microspheres for tendon-bone healing after anterior cruciate ligament reconstruction. J Biomed Mater Res B Appl Biomater. 2022;110:289–301. doi: 10.1002/jbm.b.34925. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki D., Horigome K., Kureha T., Matsui S., Watanabe T. Polymeric hydrogel microspheres: design, synthesis, characterization, assembly and applications. Polym J. 2017;49:695–702. [Google Scholar]
- 21.Geng Y., Dalhaimer P., Cai S., Tsai R., Tewari M., Minko T., Discher D. E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J., Chen L., Yang J., Li X., Wang J., Zhu Y., Xu X., Cui W. Injectable double positively charged hydrogel microspheres for targeting-penetration-phagocytosis. Small. 2022;18:e2202156. doi: 10.1002/smll.202202156. [DOI] [PubMed] [Google Scholar]
- 23.Han Y., Yang J., Zhao W., Wang H., Sun Y., Chen Y., Luo J., Deng L., Xu X., Cui W., Zhang H. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact Mater. 2021;6:3596–3607. doi: 10.1016/j.bioactmat.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S., Lin A., Tao Z., Fu Y., Xiao L., Ruan G., Li Y. Microsphere-containing hydrogel scaffolds for tissue engineering. Chem Asian J. 2022;17:e202200630. doi: 10.1002/asia.202200630. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z., Wang Z., Li G., Cai Z., Wu J., Wang L., Deng L., Cai M., Cui W. Injectable microfluidic hydrogel microspheres for cell and drug delivery. Adv Funct Mater. 2021;31:2103339. [Google Scholar]
- 26.Velasco D., Tumarkin E., Kumacheva E. Microfluidic encapsulation of cells in polymer microgels. Small. 2012;8:1633–1642. doi: 10.1002/smll.201102464. [DOI] [PubMed] [Google Scholar]
- 27.Mealy J. E., Chung J. J., Jeong H. H., Issadore D., Lee D., Atluri P., Burdick J. A. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv Mater. 2018;30:e1705912. doi: 10.1002/adma.201705912. [DOI] [PubMed] [Google Scholar]
- 28.Helgeson M. E., Chapin S. C., Doyle P. S. Hydrogel microparticles from lithographic processes: novel materials for fundamental and applied colloid science. Curr Opin Colloid Interface Sci. 2011;16:106–117. doi: 10.1016/j.cocis.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez C. J., Kim J. W., Ye C., Ortiz I., Rowat A. C., Marquez M., Weitz D. A microfluidic approach to encapsulate living cells in uniform alginate hydrogel microparticles. Macromol Biosci. 2012;12:946–951. doi: 10.1002/mabi.201100351. [DOI] [PubMed] [Google Scholar]
- 30.Yu L., Sun Q., Hui Y., Seth A., Petrovsky N., Zhao C. X. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. J Colloid Interface Sci. 2019;539:497–503. doi: 10.1016/j.jcis.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 31.Madrigal J. L., Sharma S. N., Campbell K. T., Stilhano R. S., Gijsbers R., Silva E. A. Microgels produced using microfluidic on-chip polymer blending for controlled released of VEGF encoding lentivectors. Acta Biomater. 2018;69:265–276. doi: 10.1016/j.actbio.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi M., Zhang H., Song T., Liu X., Gao Y., Zhou J., Li Y. Sustainable dual release of antibiotic and growth factor from ph-responsive uniform alginate composite microparticles to enhance wound healing. ACS Appl Mater Interfaces. 2019;11:22730–22744. doi: 10.1021/acsami.9b04750. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L., Chen K., Zhang H., Pang B., Choi C. H., Mao A. S., Liao H., Utech S., Mooney D. J., Wang H., Weitz D. A. Microfluidic templated multicompartment microgels for 3D encapsulation and pairing of single cells. Small. 2018;14:1702955. doi: 10.1002/smll.201702955. [DOI] [PubMed] [Google Scholar]
- 34.Mao A. S., Shin J. W., Utech S., Wang H., Uzun O., Li W., Cooper M., Hu Y., Zhang L., Weitz D. A., Mooney D. J. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat Mater. 2017;16:236–243. doi: 10.1038/nmat4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheikhi A., de Rutte J., Haghniaz R., Akouissi O., Sohrabi A., Di Carlo D., Khademhosseini A. Microfluidic-enabled bottom-up hydrogels from annealable naturally-derived protein microbeads. Biomaterials. 2019;192:560–568. doi: 10.1016/j.biomaterials.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Huang D., Wang L., Hou J., Zhang H., Li Y., Zhong S., Wang Y., Wu Y., Huang W. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J Colloid Interface Sci. 2020;574:162–173. doi: 10.1016/j.jcis.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 37.Cai Y., Wu F., Yu Y., Liu Y., Shao C., Gu H., Li M., Zhao Y. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019;84:222–230. doi: 10.1016/j.actbio.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Yang J., Han Y., Lin J., Zhu Y., Wang F., Deng L., Zhang H., Xu X., Cui W. Ball-bearing-inspired polyampholyte-modified microspheres as bio-lubricants attenuate osteoarthritis. Small. 2020;16:e2004519. doi: 10.1002/smll.202004519. [DOI] [PubMed] [Google Scholar]
- 39.Cha C., Oh J., Kim K., Qiu Y., Joh M., Shin S. R., Wang X., Camci-Unal G., Wan K. T., Liao R., Khademhosseini A. Microfluidics-assisted fabrication of gelatin-silica core-shell microgels for injectable tissue constructs. Biomacromolecules. 2014;15:283–290. doi: 10.1021/bm401533y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H., Liu H., Liu H., Su W., Chen W., Qin J. One-step generation of core–shell gelatin methacrylate (GelMA) microgels using a droplet microfluidic system. Adv Mater Technol. 2019;4:1800632. [Google Scholar]
- 41.Bian J., Cai F., Chen H., Tang Z., Xi K., Tang J., Wu L., Xu Y., Deng L., Gu Y., Cui W., Chen L. Modulation of local overactive inflammation via injectable hydrogel microspheres. Nano Lett. 2021;21:2690–2698. doi: 10.1021/acs.nanolett.0c04713. [DOI] [PubMed] [Google Scholar]
- 42.Zheng D., Chen W., Chen T., Chen X., Liang J., Chen H., Shen H., Deng L., Ruan H., Cui W. Hydrogen ion capturing hydrogel microspheres for reversing inflammaging. Adv Mater. 2024;36:e2306105. doi: 10.1002/adma.202306105. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z., Lv Z., Zhuang Y., Saiding Q., Yang W., Xiong W., Zhang Z., Chen H., Cui W., Zhang Y. Mechanical signal-tailored hydrogel microspheres recruit and train stem cells for precise differentiation. Adv Mater. 2023;35:e2300180. doi: 10.1002/adma.202300180. [DOI] [PubMed] [Google Scholar]
- 44.Yao Y., Wei G., Deng L., Cui W. Visualizable and lubricating hydrogel microspheres via nanoPOSS for cartilage regeneration. Adv Sci (Weinh) 2023;10:e2207438. doi: 10.1002/advs.202207438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muir V. G., Qazi T. H., Shan J., Groll J., Burdick J. A. Influence of microgel fabrication technique on granular hydrogel properties. ACS Biomater Sci Eng. 2021;7:4269–4281. doi: 10.1021/acsbiomaterials.0c01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J., Huang K., Chen Q., Deng C., Zhang J., Zhong Z. Tailor-making fluorescent hyaluronic acid microgels via combining microfluidics and photoclick chemistry for sustained and localized delivery of herceptin in tumors. ACS Appl Mater Interfaces. 2018;10:3929–3937. doi: 10.1021/acsami.7b15832. [DOI] [PubMed] [Google Scholar]
- 47.Griffin D. R., Weaver W. M., Scumpia P. O., Di Carlo D., Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allazetta S., Hausherr T. C., Lutolf M. P. Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules. 2013;14:1122–1131. doi: 10.1021/bm4000162. [DOI] [PubMed] [Google Scholar]
- 49.Allazetta S., Kolb L., Zerbib S., Bardy J., Lutolf M. P. Cell-instructive microgels with tailor-made physicochemical properties. Small. 2015;11:5647–5656. doi: 10.1002/smll.201501001. [DOI] [PubMed] [Google Scholar]
- 50.Foster G. A., Headen D. M., González-García C., Salmerón-Sánchez M., Shirwan H., García A. J. Protease-degradable microgels for protein delivery for vascularization. Biomaterials. 2017;113:170–175. doi: 10.1016/j.biomaterials.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Headen D. M., Aubry G., Lu H., García A. J. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv Mater. 2014;26:3003–3008. doi: 10.1002/adma.201304880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jha A. K., Malik M. S., Farach-Carson M. C., Duncan R. L., Jia X. Hierarchically structured, hyaluronic acid-based hydrogel matrices via the covalent integration of microgels into macroscopic networks. Soft Matter. 2010;6:5045–5055. doi: 10.1039/C0SM00101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia X., Yeo Y., Clifton R. J., Jiao T., Kohane D. S., Kobler J. B., Zeitels S. M., Langer R. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 54.Sonnet C., Simpson C. L., Olabisi R. M., Sullivan K., Lazard Z., Gugala Z., Peroni J. F., Weh J. M., Davis A. R., West J. L., Olmsted-Davis E. A. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J Orthop Res. 2013;31:1597–1604. doi: 10.1002/jor.22407. [DOI] [PubMed] [Google Scholar]
- 55.Ryu S., Kim H. H., Park Y. H., Lin C. C., Um I. C., Ki C. S. Dual mode gelation behavior of silk fibroin microgel embedded poly(ethylene glycol) hydrogels. J Mater Chem B. 2016;4:4574–4584. doi: 10.1039/c6tb00896h. [DOI] [PubMed] [Google Scholar]
- 56.Gansau J., Kelly L., Buckley C. T. Influence of key processing parameters and seeding density effects of microencapsulated chondrocytes fabricated using electrohydrodynamic spraying. Biofabrication. 2018;10:035011. doi: 10.1088/1758-5090/aacb95. [DOI] [PubMed] [Google Scholar]
- 57.Kim P. H., Yim H. G., Choi Y. J., Kang B. J., Kim J., Kwon S. M., Kim B. S., Hwang N. S., Cho J. Y. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J Control Release. 2014;187:1–13. doi: 10.1016/j.jconrel.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Xin S., Wyman O. M., Alge D. L. Assembly of PEG microgels into porous cell-instructive 3D scaffolds via thiol-ene click chemistry. Adv Healthc Mater. 2018;7:e1800160. doi: 10.1002/adhm.201800160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qayyum A. S., Jain E., Kolar G., Kim Y., Sell S. A., Zustiak S. P. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication. 2017;9:025019. doi: 10.1088/1758-5090/aa703c. [DOI] [PubMed] [Google Scholar]
- 60.Nichol J. W., Koshy S. T., Bae H., Hwang C. M., Yamanlar S., Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khademhosseini A., Eng G., Yeh J., Fukuda J., Blumling J., 3rd, Langer R., Burdick J. A. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res A. 2006;79:522–532. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 62.Loebel C., Broguiere N., Alini M., Zenobi-Wong M., Eglin D. Microfabrication of photo-cross-linked hyaluronan hydrogels by single- and two-photon tyramine oxidation. Biomacromolecules. 2015;16:2624–2630. doi: 10.1021/acs.biomac.5b00363. [DOI] [PubMed] [Google Scholar]
- 63.Qin X.-H., Gruber P., Markovic M., Plochberger B., Klotzsch E., Stampfl J., Ovsianikov A., Liska R. Enzymatic synthesis of hyaluronic acid vinyl esters for two-photon microfabrication of biocompatible and biodegradable hydrogel constructs. Polym Chem. 2014;5:6523–6533. [Google Scholar]
- 64.Panda P., Ali S., Lo E., Chung B. G., Hatton T. A., Khademhosseini A., Doyle P. S. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip. 2008;8:1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee S. A., Chung S. E., Park W., Lee S. H., Kwon S. Three-dimensional fabrication of heterogeneous microstructures using soft membrane deformation and optofluidic maskless lithography. Lab Chip. 2009;9:1670–1675. doi: 10.1039/b819999j. [DOI] [PubMed] [Google Scholar]
- 66.Chung S. E., Park W., Shin S., Lee S. A., Kwon S. Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nat Mater. 2008;7:581–587. doi: 10.1038/nmat2208. [DOI] [PubMed] [Google Scholar]
- 67.Dendukuri D., Pregibon D. C., Collins J., Hatton T. A., Doyle P. S. Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater. 2006;5:365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 68.Laza S. C., Polo M., Neves A. A., Cingolani R., Camposeo A., Pisignano D. Two-photon continuous flow lithography. Adv Mater. 2012;24:1304–1308. doi: 10.1002/adma.201103357. [DOI] [PubMed] [Google Scholar]
- 69.Widener A. E., Duraivel S., Angelini T. E., Phelps E. A. Injectable microporous annealed particle hydrogel based on guest-host-interlinked polyethylene glycol maleimide microgels. Adv Nanobiomed Res. 2022;2:2200030. doi: 10.1002/anbr.202200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sinclair A., O’Kelly M. B., Bai T., Hung H. C., Jain P., Jiang S. Self-healing zwitterionic microgels as a versatile platform for malleable cell constructs and injectable therapies. Adv Mater. 2018;30:e1803087. doi: 10.1002/adma.201803087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheen J. R., Mabrouk A., Garla V. V. StatPearls. StatPearls Publishing; Treasure Island (FL): 2024. Fracture healing overview. [PubMed] [Google Scholar]
- 72.Lee D. H., Kim S. J., Kim S. A., Ju G. I. Past, present, and future of cartilage restoration: from localized defect to arthritis. Knee Surg Relat Res. 2022;34:1. doi: 10.1186/s43019-022-00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu L., Bennett C. J., Lin C. H., Yan S., Yang J. Scaffold design considerations for peripheral nerve regeneration. J Neural Eng. 2024;21:041001. doi: 10.1088/1741-2552/ad628d. [DOI] [PubMed] [Google Scholar]
- 74.Lin F., Li Y., Cui W. Injectable hydrogel microspheres in cartilage repair. Biomed Technol. 2023;1:18–29. [Google Scholar]
- 75.Yu L., Martin I. J., Kasi R. M., Wei M. Enhanced intrafibrillar mineralization of collagen fibrils induced by brushlike polymers. ACS Appl Mater Interfaces. 2018;10:28440–28449. doi: 10.1021/acsami.8b10234. [DOI] [PubMed] [Google Scholar]
- 76.Pérez L. A., Hernández R., Alonso J. M., Pérez-González R., Sáez-Martínez V. Hyaluronic acid hydrogels crosslinked in physiological conditions: synthesis and biomedical applications. Biomedicines. 2021;9:1113. doi: 10.3390/biomedicines9091113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khalili M. H., Afsar A., Zhang R., Wilson S., Dossi E., Goel S., Impey S. A., Aria A. I. Thermal response of multi-layer UV crosslinked PEGDA hydrogels. Polym Degrad Stab. 2022;195:109805. [Google Scholar]
- 78.Zhou C., Cao Y., Liu C., Guo W. Microparticles by microfluidic lithography. Mater Today. 2023;67:178–202. [Google Scholar]
- 79.Gu Z., Dang T. T., Ma M., Tang B. C., Cheng H., Jiang S., Dong Y., Zhang Y., Anderson D. G. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7:6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 80.Ruan L., Su M., Qin X., Ruan Q., Lang W., Wu M., Chen Y., Lv Q. Progress in the application of sustained-release drug microspheres in tissue engineering. Mater Today Bio. 2022;16:100394. doi: 10.1016/j.mtbio.2022.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai Z., Jiang H., Lin T., Wang C., Ma J., Gao R., Jiang Y., Zhou W. Microspheres in bone regeneration: fabrication, properties and applications. Materials Today Advances. 2022;16:100315. [Google Scholar]
- 82.Hayrapetyan A., Jansen J. A., van den Beucken J. J. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng Part B Rev. 2015;21:75–87. doi: 10.1089/ten.TEB.2014.0119. [DOI] [PubMed] [Google Scholar]
- 83.Teng C., Tong Z., He Q., Zhu H., Wang L., Zhang X., Wei W. Mesenchymal stem cells-hydrogel microspheres system for bone regeneration in calvarial defects. Gels. 2022;8:275. doi: 10.3390/gels8050275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu L., Rowe D. W., Perera I. P., Zhang J., Suib S. L., Xin X., Wei M. Intrafibrillar mineralized collagen-hydroxyapatite-based scaffolds for bone regeneration. ACS Appl Mater Interfaces. 2020;12:18235–18249. doi: 10.1021/acsami.0c00275. [DOI] [PubMed] [Google Scholar]
- 85.Zhao X., Liu S., Yildirimer L., Zhao H., Ding R., Wang H., Cui W., Weitz D. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv Funct Mater. 2016;26:2809–2819. [Google Scholar]
- 86.Kanafi M. M., Ramesh A., Gupta P. K., Bhonde R. R. Dental pulp stem cells immobilized in alginate microspheres for applications in bone tissue engineering. Int Endod J. 2014;47:687–697. doi: 10.1111/iej.12205. [DOI] [PubMed] [Google Scholar]
- 87.Alipour M., Firouzi N., Aghazadeh Z., Samiei M., Montazersaheb S., Khoshfetrat A. B., Aghazadeh M. The osteogenic differentiation of human dental pulp stem cells in alginate-gelatin/Nano-hydroxyapatite microcapsules. BMC Biotechnol. 2021;21:6. doi: 10.1186/s12896-020-00666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park Y., Lin S., Bai Y., Moeinzadeh S., Kim S., Huang J., Lee U., Huang N. F., Yang Y. P. Dual delivery of BMP2 and IGF1 through injectable hydrogel promotes cranial bone defect healing. Tissue Eng Part A. 2022;28:760–769. doi: 10.1089/ten.tea.2022.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng M., Tan J., Hu C., Hou T., Peng W., Liu J., Yu B., Dai Q., Zhou J., Yang Y., Dong R., Ruan C., Dong S., Xu J. Modification of PLGA scaffold by MSC-derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF-β-induced protein. Adv Healthc Mater. 2020;9:e2000353. doi: 10.1002/adhm.202000353. [DOI] [PubMed] [Google Scholar]
- 90.Zhang C., Wang J., Xie Y., Wang L., Yang L., Yu J., Miyamoto A., Sun F. Development of FGF-2-loaded electrospun waterborne polyurethane fibrous membranes for bone regeneration. Regen Biomater. 2021;8:rbaa046. doi: 10.1093/rb/rbaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amirthalingam S., Lee S. S., Pandian M., Ramu J., Iyer S., Hwang N. S., Jayakumar R. Combinatorial effect of nano whitlockite/nano bioglass with FGF-18 in an injectable hydrogel for craniofacial bone regeneration. Biomater Sci. 2021;9:2439–2453. doi: 10.1039/d0bm01496f. [DOI] [PubMed] [Google Scholar]
- 92.Zhao Z., Li G., Ruan H., Chen K., Cai Z., Lu G., Li R., Deng L., Cai M., Cui W. Capturing magnesium ions via microfluidic hydrogel microspheres for promoting cancellous bone regeneration. ACS Nano. 2021;15:13041–13054. doi: 10.1021/acsnano.1c02147. [DOI] [PubMed] [Google Scholar]
- 93.Lu X., Shi S., Li H., Gerhard E., Lu Z., Tan X., Li W., Rahn K. M., Xie D., Xu G., Zou F., Bai X., Guo J., Yang J. Magnesium oxide-crosslinked low-swelling citrate-based mussel-inspired tissue adhesives. Biomaterials. 2020;232:119719. doi: 10.1016/j.biomaterials.2019.119719. [DOI] [PubMed] [Google Scholar]
- 94.Yu L., Tian Y., Qiao Y., Liu X. Mn-containing titanium surface with favorable osteogenic and antimicrobial functions synthesized by PIII&D. Colloids Surf B Biointerfaces. 2017;152:376–384. doi: 10.1016/j.colsurfb.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 95.Zhong Z., Wu X., Wang Y., Li M., Li Y., Liu X., Zhang X., Lan Z., Wang J., Du Y., Zhang S. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact Mater. 2022;10:195–206. doi: 10.1016/j.bioactmat.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu L., Jin G., Ouyang L., Wang D., Qiao Y., Liu X. Antibacterial activity, osteogenic and angiogenic behaviors of copper-bearing titanium synthesized by PIII&D. J Mater Chem B. 2016;4:1296–1309. doi: 10.1039/c5tb02300a. [DOI] [PubMed] [Google Scholar]
- 97.Dai M., Lin X., Hua P., Wang S., Sun X., Tang C., Zhang C., Liu L. Antibacterial sequential growth factor delivery from alginate/gelatin methacryloyl microspheres for bone regeneration. Int J Biol Macromol. 2024;275:133557. doi: 10.1016/j.ijbiomac.2024.133557. [DOI] [PubMed] [Google Scholar]
- 98.Clearfield D. S., Xin X., Yadav S., Rowe D. W., Wei M. Osteochondral differentiation of fluorescent multireporter cells on zonally-organized biomaterials. Tissue Eng Part A. 2019;25:468–486. doi: 10.1089/ten.TEA.2018.0135. [DOI] [PubMed] [Google Scholar]
- 99.Platas J., Guillén M. I., Gomar F., Castejón M. A., Esbrit P., Alcaraz M. J. Anti-senescence and anti-inflammatory effects of the C-terminal Moiety of PTHrP peptides in OA osteoblasts. J Gerontol A Biol Sci Med Sci. 2017;72:624–631. doi: 10.1093/gerona/glw100. [DOI] [PubMed] [Google Scholar]
- 100.Cibere J., Thorne A., Kopec J. A., Singer J., Canvin J., Robinson D. B., Pope J., Hong P., Grant E., Lobanok T., Ionescu M., Poole A. R., Esdaile J. M. Glucosamine sulfate and cartilage type II collagen degradation in patients with knee osteoarthritis: randomized discontinuation trial results employing biomarkers. J Rheumatol. 2005;32:896–902. [PubMed] [Google Scholar]
- 101.Korotkyi O., Huet A., Dvorshchenko K., Kobyliak N., Falalyeyeva T., Ostapchenko L. Probiotic composition and chondroitin sulfate regulate TLR-2/4-mediated NF-κB inflammatory pathway and cartilage metabolism in experimental osteoarthritis. Probiotics Antimicrob Proteins. 2021;13:1018–1032. doi: 10.1007/s12602-020-09735-7. [DOI] [PubMed] [Google Scholar]
- 102.Reyes R., Delgado A., Sánchez E., Fernández A., Hernández A., Evora C. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate-PLGA scaffold. J Tissue Eng Regen Med. 2014;8:521–533. doi: 10.1002/term.1549. [DOI] [PubMed] [Google Scholar]
- 103.Lin J., Wang L., Lin J., Liu Q. Dual delivery of TGF-β3 and ghrelin in microsphere/hydrogel systems for cartilage regeneration. Molecules. 2021;26:5732. doi: 10.3390/molecules26195732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho H., Kim J., Kim S., Jung Y. C., Wang Y., Kang B. J., Kim K. Dual delivery of stem cells and insulin-like growth factor-1 in coacervate-embedded composite hydrogels for enhanced cartilage regeneration in osteochondral defects. J Control Release. 2020;327:284–295. doi: 10.1016/j.jconrel.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 105.Yang W., Cao Y., Zhang Z., Du F., Shi Y., Li X., Zhang Q. Targeted delivery of FGF2 to subchondral bone enhanced the repair of articular cartilage defect. Acta Biomater. 2018;69:170–182. doi: 10.1016/j.actbio.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 106.Sakata R., Kokubu T., Nagura I., Toyokawa N., Inui A., Fujioka H., Kurosaka M. Localization of vascular endothelial growth factor during the early stages of osteochondral regeneration using a bioabsorbable synthetic polymer scaffold. J Orthop Res. 2012;30:252–259. doi: 10.1002/jor.21502. [DOI] [PubMed] [Google Scholar]
- 107.Vadalà G., Russo F., Musumeci M., Giacalone A., Papalia R., Denaro V. Targeting VEGF-A in cartilage repair and regeneration: state of the art and perspectives. J Biol Regul Homeost Agents. 2018;32:217–224. [PubMed] [Google Scholar]
- 108.Zhu P., Wang Z., Sun Z., Liao B., Cai Y. Recombinant platelet-derived growth factor-BB alleviates osteoarthritis in a rat model by decreasing chondrocyte apoptosis in vitro and in vivo. J Cell Mol Med. 2021;25:7472–7484. doi: 10.1111/jcmm.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Utsunomiya H., Gao X., Cheng H., Deng Z., Nakama G., Mascarenhas R., Goldman J. L., Ravuri S. K., Arner J. W., Ruzbarsky J. J., Lowe W. R., Philippon M. J., Huard J. Intra-articular injection of bevacizumab enhances bone marrow stimulation-mediated cartilage repair in a rabbit osteochondral defect model. Am J Sports Med. 2021;49:1871–1882. doi: 10.1177/03635465211005102. [DOI] [PubMed] [Google Scholar]
- 110.Tangtrongsup S., Kisiday J. D. Effects of dexamethasone concentration and timing of exposure on chondrogenesis of equine bone marrow-derived mesenchymal stem cells. Cartilage. 2016;7:92–103. doi: 10.1177/1947603515595263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y., Peng L., Li L., Huang C., Shi K., Meng X., Wang P., Wu M., Li L., Cao H., Wu K., Zeng Q., Pan H., Lu W. W., Qin L., Ruan C., Wang X. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279:121216. doi: 10.1016/j.biomaterials.2021.121216. [DOI] [PubMed] [Google Scholar]
- 112.Kulchar R. J., Denzer B. R., Chavre B. M., Takegami M., Patterson J. A review of the use of microparticles for cartilage tissue engineering. Int J Mol Sci. 2021;22:10292. doi: 10.3390/ijms221910292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shyng Y. C., Chi C. Y., Devlin H., Sloan P. Healing of tooth extraction sockets in the streptozotocin diabetic rat model: Induction of cartilage by BMP-6. Growth Factors. 2010;28:447–451. doi: 10.3109/08977194.2010.527966. [DOI] [PubMed] [Google Scholar]
- 114.García-Fernández L. Osteochondral angiogenesis and promoted vascularization: new therapeutic target. Adv Exp Med Biol. 2018;1059:315–330. doi: 10.1007/978-3-319-76735-2_14. [DOI] [PubMed] [Google Scholar]
- 115.Fransès R. E., McWilliams D. F., Mapp P. I., Walsh D. A. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthritis Cartilage. 2010;18:563–571. doi: 10.1016/j.joca.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeong S. Y., Kim D. H., Ha J., Jin H. J., Kwon S. J., Chang J. W., Choi S. J., Oh W., Yang Y. S., Kim G., Kim J. S., Yoon J. R., Cho D. H., Jeon H. B. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–2148. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]
- 117.Andrés Sastre E., Maly K., Zhu M., Witte-Bouma J., Trompet D., Böhm A. M., Brachvogel B., van Nieuwenhoven C. A., Maes C., van Osch G., Zaucke F., Farrell E. Spatiotemporal distribution of thrombospondin-4 and -5 in cartilage during endochondral bone formation and repair. Bone. 2021;150:115999. doi: 10.1016/j.bone.2021.115999. [DOI] [PubMed] [Google Scholar]
- 118.Helgeland E., Pedersen T. O., Rashad A., Johannessen A. C., Mustafa K., Rosén A. Angiostatin-functionalized collagen scaffolds suppress angiogenesis but do not induce chondrogenesis by mesenchymal stromal cells in vivo. J Oral Sci. 2020;62:371–376. doi: 10.2334/josnusd.19-0327. [DOI] [PubMed] [Google Scholar]
- 119.Dehghan-Baniani D., Mehrjou B., Wang D., Bagheri R., Solouk A., Chu P. K., Wu H. A dual functional chondro-inductive chitosan thermogel with high shear modulus and sustained drug release for cartilage tissue engineering. Int J Biol Macromol. 2022;205:638–650. doi: 10.1016/j.ijbiomac.2022.02.115. [DOI] [PubMed] [Google Scholar]
- 120.Blaney Davidson E. N., Vitters E. L., van der Kraan P. M., van den Berg W. B. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–1421. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu C., Liu J., Lu G., Xie Y., Sun Y., Wang Q., Liang J., Fan Y., Zhang X. Repair of osteochondral defects in a rabbit model with artificial cartilage particulates derived from cultured collagen-chondrocyte microspheres. J Mater Chem B. 2018;6:5164–5173. doi: 10.1039/c8tb01185k. [DOI] [PubMed] [Google Scholar]
- 122.Li F., Truong V. X., Fisch P., Levinson C., Glattauer V., Zenobi-Wong M., Thissen H., Forsythe J. S., Frith J. E. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018;77:48–62. doi: 10.1016/j.actbio.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 123.Li X., Li X., Yang J., Lin J., Zhu Y., Xu X., Cui W. Living and injectable porous hydrogel microsphere with paracrine activity for cartilage regeneration. Small. 2023;19:e2207211. doi: 10.1002/smll.202207211. [DOI] [PubMed] [Google Scholar]
- 124.Huebner E. A., Strittmatter S. M. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qian Y., Lin H., Yan Z., Shi J., Fan C. Functional nanomaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater Today. 2021;51:165–187. [Google Scholar]
- 126.Martínez de Albornoz P., Delgado P. J., Forriol F., Maffulli N. Non-surgical therapies for peripheral nerve injury. Br Med Bull. 2011;100:73–100. doi: 10.1093/bmb/ldr005. [DOI] [PubMed] [Google Scholar]
- 127.Hu Y., Wu Y., Gou Z., Tao J., Zhang J., Liu Q., Kang T., Jiang S., Huang S., He J., Chen S., Du Y., Gou M. 3D-engineering of cellularized conduits for peripheral nerve regeneration. Sci Rep. 2016;6:32184. doi: 10.1038/srep32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bellamkonda R. V. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 129.Kim Y. T., Haftel V. K., Kumar S., Bellamkonda R. V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117–3127. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Panzer K. V., Burrell J. C., Helm K. V. T., Purvis E. M., Zhang Q., Le A. D., O’Donnell J. C., Cullen D. K. Tissue engineered bands of büngner for accelerated motor and sensory axonal outgrowth. Front Bioeng Biotechnol. 2020;8:580654. doi: 10.3389/fbioe.2020.580654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee A. C., Yu V. M., Lowe J. B., 3rd, Brenner M. J., Hunter D. A., Mackinnon S. E., Sakiyama-Elbert S. E. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 132.Fine E. G., Decosterd I., Papaloïzos M., Zurn A. D., Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 133.Vögelin E., Baker J. M., Gates J., Dixit V., Constantinescu M. A., Jones N. F. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp Neurol. 2006;199:348–353. doi: 10.1016/j.expneurol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 134.Saffari T. M., Chan K., Saffari S., Zuo K. J., McGovern R. M., Reid J. M., Borschel G. H., Shin A. Y. Combined local delivery of tacrolimus and stem cells in hydrogel for enhancing peripheral nerve regeneration. Biotechnol Bioeng. 2021;118:2804–2814. doi: 10.1002/bit.27799. [DOI] [PubMed] [Google Scholar]
- 135.Zolfagharzadeh V., Ai J., Soltani H., Hassanzadeh S., Khanmohammadi M. Sustain release of loaded insulin within biomimetic hydrogel microsphere for sciatic tissue engineering in vivo. Int J Biol Macromol. 2023;225:687–700. doi: 10.1016/j.ijbiomac.2022.11.133. [DOI] [PubMed] [Google Scholar]
- 136.Kim G. B., Chen Y., Kang W., Guo J., Payne R., Li H., Wei Q., Baker J., Dong C., Zhang S., Wong P. K., Rizk E. B., Yan J., Yang J. The critical chemical and mechanical regulation of folic acid on neural engineering. Biomaterials. 2018;178:504–516. doi: 10.1016/j.biomaterials.2018.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu W., Jia S., Xu H., Gao Z., Wang Z., Lu B., Ai Y., Liu Y., Liu R., Yang T., Luo R., Hu C., Kong L., Huang D., Yan L., Yang Z., Zhu L., Hao D. Supramolecular hydrogel microspheres of platelet-derived growth factor mimetic peptide promote recovery from spinal cord injury. ACS Nano. 2023;17:3818–3837. doi: 10.1021/acsnano.2c12017. [DOI] [PubMed] [Google Scholar]
- 138.Huang F., Chen T., Chang J., Zhang C., Liao F., Wu L., Wang W., Yin Z. A conductive dual-network hydrogel composed of oxidized dextran and hyaluronic-hydrazide as BDNF delivery systems for potential spinal cord injury repair. Int J Biol Macromol. 2021;167:434–445. doi: 10.1016/j.ijbiomac.2020.11.206. [DOI] [PubMed] [Google Scholar]
- 139.Blanpain C. Stem cells: Skin regeneration and repair. Nature. 2010;464:686–687. doi: 10.1038/464686a. [DOI] [PubMed] [Google Scholar]
- 140.Chouhan D., Dey N., Bhardwaj N., Mandal B. B. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials. 2019;216:119267. doi: 10.1016/j.biomaterials.2019.119267. [DOI] [PubMed] [Google Scholar]
- 141.Clark R. A., Ghosh K., Tonnesen M. G. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018–1029. doi: 10.1038/sj.jid.5700715. [DOI] [PubMed] [Google Scholar]
- 142.Herndon D. N., Barrow R. E., Rutan R. L., Rutan T. C., Desai M. H., Abston S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann Surg. 1989;209:547–552. doi: 10.1097/00000658-198905000-00006. discussion 552-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vig K., Chaudhari A., Tripathi S., Dixit S., Sahu R., Pillai S., Dennis V. A., Singh S. R. Advances in skin regeneration using tissue engineering. Int J Mol Sci. 2017;18:789. doi: 10.3390/ijms18040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang D., Ouyang Q., Hu Z., Lu S., Quan W., Li P., Chen Y., Li S. Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int J Biol Macromol. 2021;173:591–606. doi: 10.1016/j.ijbiomac.2021.01.157. [DOI] [PubMed] [Google Scholar]
- 145.Shamloo A., Sarmadi M., Aghababaie Z., Vossoughi M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int J Pharm. 2018;537:278–289. doi: 10.1016/j.ijpharm.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 146.Liu Q., Huang Y., Lan Y., Zuo Q., Li C., Zhang Y., Guo R., Xue W. Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCS-PVA hydrogel. J Tissue Eng Regen Med. 2017;11:1562–1573. doi: 10.1002/term.2057. [DOI] [PubMed] [Google Scholar]
- 147.Kong X., Fu J., Shao K., Wang L., Lan X., Shi J. Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa. Acta Biomater. 2019;100:255–269. doi: 10.1016/j.actbio.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 148.Xiao Y., Ding T., Fang H., Lin J., Chen L., Ma D., Zhang T., Cui W., Ma J. Innovative bio-based hydrogel microspheres micro-cage for neutrophil extracellular traps scavenging in diabetic wound healing. Adv Sci (Weinh) 2024;11:e2401195. doi: 10.1002/advs.202401195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ansari S., Chen C., Xu X., Annabi N., Zadeh H. H., Wu B. M., Khademhosseini A., Shi S., Moshaverinia A. Muscle tissue engineering using gingival mesenchymal stem cells encapsulated in alginate hydrogels containing multiple growth factors. Ann Biomed Eng. 2016;44:1908–1920. doi: 10.1007/s10439-016-1594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fischer K. M., Scott T. E., Browe D. P., McGaughey T. A., Wood C., Wolyniak M. J., Freeman J. W. Hydrogels for skeletal muscle regeneration. Regen Eng Transl Med. 2021;7:353–361. [Google Scholar]
- 151.Li Y., Liu S., Zhang J., Wang Y., Lu H., Zhang Y., Song G., Niu F., Shen Y., Midgley A. C., Li W., Kong D., Zhu M. Elastic porous microspheres/extracellular matrix hydrogel injectable composites releasing dual bio-factors enable tissue regeneration. Nat Commun. 2024;15:1377. doi: 10.1038/s41467-024-45764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang R., Wang F., Lu S., Gao B., Kan Y., Yuan T., Xu Y., Yuan C., Guo D., Fu W., Yu X., Si Y. Adipose-derived stem cell/FGF19-loaded microfluidic hydrogel microspheres for synergistic restoration of critical ischemic limb. Bioact Mater. 2023;27:394–408. doi: 10.1016/j.bioactmat.2023.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiao Y., Gyawali D., Stark J. M., Akcora P., Nair P., Tran R. T., Yang J. A rheological study of biodegradable injectable PEGMC/HA composite scaffolds. Soft Matter. 2012;8:1499–1507. doi: 10.1039/C1SM05786C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Van Den Bulcke A. I., Bogdanov B., De Rooze N., Schacht E. H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 155.Lee H., Stoffey D. Polyethylene glycol diacrylate. US3769336A. 1973. [Google Scholar]
- 156.Bao B., Zeng Q., Li K., Wen J., Zhang Y., Zheng Y., Zhou R., Shi C., Chen T., Xiao C., Chen B., Wang T., Yu K., Sun Y., Lin Q., He Y., Tu S., Zhu L. Rapid fabrication of physically robust hydrogels. Nat Mater. 2023;22:1253–1260. doi: 10.1038/s41563-023-01648-4. [DOI] [PubMed] [Google Scholar]
- 157.Xu H., Yan S., Gerhard E., Xie D., Liu X., Zhang B., Shi D., Ameer G. A., Yang J. Citric acid: a nexus between cellular mechanisms and biomaterial innovations. Adv Mater. 2024;36:e2402871. doi: 10.1002/adma.202402871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang H., Huddleston S., Yang J., Ameer G. A. Enabling proregenerative medical devices via citrate-based biomaterials: transitioning from inert to regenerative biomaterials. Adv Mater. 2024;36:e2306326. doi: 10.1002/adma.202306326. [DOI] [PubMed] [Google Scholar]
- 159.Ma C., Tian X., Kim J. P., Xie D., Ao X., Shan D., Lin Q., Hudock M. R., Bai X., Yang J. Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc Natl Acad Sci U S A. 2018;115:E11741–E11750. doi: 10.1073/pnas.1813000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xie D., Guo J., Mehdizadeh M., Tran R. T., Chen R., Sun D., Qian G., Jin D., Bai X., Yang J. Development of injectable citrate-based bioadhesive bone implants. J Mater Chem B. 2015;3:387–398. doi: 10.1039/C4TB01498G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shan D., Hsieh J. T., Bai X., Yang J. Citrate-based fluorescent biomaterials. Adv Healthc Mater. 2018;7:e1800532. doi: 10.1002/adhm.201800532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen Y. R., Yan X., Yuan F. Z., Lin L., Wang S. J., Ye J., Zhang J. Y., Yang M., Wu D. C., Wang X., Yu J. K. Kartogenin-conjugated double-network hydrogel combined with stem cell transplantation and tracing for cartilage repair. Adv Sci (Weinh) 2022;9:e2105571. doi: 10.1002/advs.202105571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ren E., Chen H., Qin Z., Guan S., Jiang L., Pang X., He Y., Zhang Y., Gao X., Chu C., Zheng L., Liu G. Harnessing bifunctional ferritin with kartogenin loading for mesenchymal stem cell capture and enhancing chondrogenesis in cartilage regeneration. Adv Healthc Mater. 2022;11:e2101715. doi: 10.1002/adhm.202101715. [DOI] [PubMed] [Google Scholar]
- 164.Madrid A., Alisch R. S., Rizk E., Papale L. A., Hogan K. J., Iskandar B. J. Transgenerational epigenetic inheritance of axonal regeneration after spinal cord injury. Environ Epigenet. 2023;9:dvad002. doi: 10.1093/eep/dvad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bigham A., Rahimkhoei V., Abasian P., Delfi M., Naderi J., Ghomi M., Dabbagh Moghaddam F., Waqar T., Nuri Ertas Y., Sharifi S., Rabiee N., Ersoy S., Maleki A., Nazarzadeh Zare E., Sharifi E., Jabbari E., Makvandi P., Akbari A. Advances in tannic acid-incorporated biomaterials: infection treatment, regenerative medicine, cancer therapy, and biosensing. Chem Eng J. 2022;432:134146. [Google Scholar]
- 166.Sathishkumar G., Gopinath K., Zhang K., Kang E. T., Xu L., Yu Y. Recent progress in tannic acid-driven antibacterial/antifouling surface coating strategies. J Mater Chem B. 2022;10:2296–2315. doi: 10.1039/d1tb02073k. [DOI] [PubMed] [Google Scholar]
- 167.Zhang W., Ling C., Liu H., Zhang A., Mao L., Wang J., Chao J., Backman L. J., Yao Q., Chen J. Tannic acid-mediated dual peptide-functionalized scaffolds to direct stem cell behavior and osteochondral regeneration. Chem Eng J. 2020;396:125232. [Google Scholar]
- 168.Guo J., Tian X., Xie D., Rahn K., Gerhard E., Kuzma M. L., Zhou D., Dong C., Bai X., Lu Z., Yang J. Citrate-based tannin-bridged bone composites for lumbar fusion. Adv Funct Mater. 2020;30:2002438. [Google Scholar]
- 169.Zhang W., Fang X. X., Li Q. C., Pi W., Han N. Reduced graphene oxide-embedded nerve conduits loaded with bone marrow mesenchymal stem cell-derived extracellular vesicles promote peripheral nerve regeneration. Neural Regen Res. 2023;18:200–206. doi: 10.4103/1673-5374.343889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xia B., Gao X., Qian J., Li S., Yu B., Hao Y., Wei B., Ma T., Wu H., Yang S., Zheng Y., Gao X., Guo L., Gao J., Yang Y., Zhang Y., Wei Y., Xue B., Jin Y., Luo Z., Zhang J., Huang J. A novel superparamagnetic multifunctional nerve scaffold: a remote actuation strategy to boost in situ extracellular vesicles production for enhanced peripheral nerve repair. Adv Mater. 2024;36:e2305374. doi: 10.1002/adma.202305374. [DOI] [PubMed] [Google Scholar]
- 171.Hao R., Hu S., Zhang H., Chen X., Yu Z., Ren J., Guo H., Yang H. Mechanical stimulation on a microfluidic device to highly enhance small extracellular vesicle secretion of mesenchymal stem cells. Mater Today Bio. 2023;18:100527. doi: 10.1016/j.mtbio.2022.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zeng J., Gu C., Zhuang Y., Lin K., Xie Y., Chen X. Injectable hydrogel microspheres encapsulating extracellular vesicles derived from melatonin-stimulated NSCs promote neurogenesis and alleviate inflammation in spinal cord injury. Chem Eng J. 2023;470:144121. [Google Scholar]
- 173.Yu M., Gu G., Cong M., Du M., Wang W., Shen M., Zhang Q., Shi H., Gu X., Ding F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021;134:190–203. doi: 10.1016/j.actbio.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 174.Wang C., Wang M., Xia K., Wang J., Cheng F., Shi K., Ying L., Yu C., Xu H., Xiao S., Liang C., Li F., Lei B., Chen Q. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact Mater. 2021;6:2523–2534. doi: 10.1016/j.bioactmat.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yanatori I., Richardson D. R., Dhekne H. S., Toyokuni S., Kishi F. CD63 is regulated by iron via the IRE-IRP system and is important for ferritin secretion by extracellular vesicles. Blood. 2021;138:1490–1503. doi: 10.1182/blood.2021010995. [DOI] [PMC free article] [PubMed] [Google Scholar]