Abstract
Background
To compare the efficacy and toxicity of docetaxel treatment regimens in metastatic castration-resistant prostate cancer (mCRPC).
Methods
We retrospectively analyzed 162 patients diagnosed with mCRPC who underwent docetaxel chemotherapy between 2009 and 2020. The patients were divided into three groups according to the dosage and interval of docetaxel (DCT) chemotherapy regimen: 30 mL/m2 weekly, 50 mL/m2 biweekly (every 2 weeks), and 75 mL/m2 triweekly (every 3 weeks).
Results
There were no significant differences in the prostate-specific antigen (PSA) response rates (P = 0.709). The median time to progression was 3.0 [interquartile range (IQR 2.0–5.3)] months, 5.0 (IQR 2.0–13.0) months, and 5.0 (IQR 3.0–12.0) months in the weekly, biweekly, and triweekly groups, respectively (P = 0.062). The median overall survival (OS) was 12.5 (IQR 6.0–14.0) months, 18.8 (IQR 5.5–23.5) months, and 22.9 (IQR 11.0–33.0) months in the weekly, biweekly, and triweekly groups, respectively (P < 0.001). There were no differences in all toxicity and Grade 3 or higher toxicity. In Cox multivariate regression analysis, the Eastern Cooperative Oncology Group performance status (ECOG-PS), response to chemotherapy, and chemotherapy cycle also affected the PFS. Age, ECOG-PS, and chemotherapy cycle affected the OS.
Conclusions
The various options for optimal chemotherapy are indicated depending on the patient’s conditions during the diagnosis of mCRPC. Treatment with DCT at 2-week or even 1-week intervals appears to be well tolerated in men diagnosed with mCRPC and represents a useful option when the conventional triweekly regimen is not tolerated due to poor patient condition.
Keywords: Biweekly, Chemotherapy, Docetaxel, Metastatic castration-resistant prostate cancer, Prostate cancer
1. Introduction
Prostate cancer is one of the most frequently diagnosed malignancies in men.1 It is the fifth leading cause of cancer-related deaths among males in the USA.1 In South Korea, prostate cancer ranks as the third most common cancer among men and is the fourth leading cause of cancer-related deaths.2 The standard treatment for localized prostate cancer is surgery and radiation therapy. However, despite standard treatments, about 10% to 20% of patients have metastatic recurrence and a few patients show local recurrence.3 In patients with progressive or metastatic disease, androgen deprivation therapy (ADT) is the standard of care. ADT alleviates symptoms in 70% to 80% of these patients but is not curative.4 Most patients are resistant to ADT after about two years and continue to progress despite the castration-level androgen deficiency.5,6 This condition known as metastatic castration-resistant prostate cancer (mCRPC) is associated with poor quality of life, severe morbidity, and poor prognosis.7 Among the several options available for the treatment of CRPC, including docetaxel, enzalutamide, abiraterone, cabazitaxel, sipuleucel-T, radium-223, and mitoxantrone. Docetaxel (DCT) is still the main treatment option in patients with mCRPC.7,8 but now there are lots of treatment options and choices according to several factors such as germline gene aberrations, toxicity, etc. Based on the results of TAX-327 and SWOG99-16 studies, DCT is the standard first-line chemotherapy regimen for CRPC patients. DCT is generally administered at a dose of 75 mg/m2 every 3 weeks (triweekly).3,9 However, the use of DCT is associated with significant drug toxicity, neutropenia, febrile neutropenia, fatigue, nausea and vomiting, diarrhea, alopecia, nail dystrophy, and sensory neuropathy.3,9 Due to these toxicities, the discontinuation rate of DCT reaches 10% to 25%, and DCT intolerance remains an important issue, which has been investigated in studies on the efficacy and safety of alternative schedules.10, 11, 12, 13, 14, 15 The PROSTY study, a randomized phase III trial, administered DCT at 50 mg/m2 every 2 weeks (biweekly) and neutropenia was reduced compared with the standard schedule.15 And Phase II trial of weekly docetaxel at 36 mg/m2 demonstrated increased time to progression and survival with minimal myelosuppression.16
However, in real-world clinical practice, we can easily encounter patients who may not be tolerated conventional triweekly or even 50 mg/m2 biweekly regimens due to poor general conditions such as higher performance status, which are not eligible to clinical trials.
Thus, we aimed to evaluate the efficacy and safety of three different docetaxel regimens to treat men with mCRPC in real-world setting, which were 30 mg/m2 every week (weekly), 50 mg/m2 every 2 weeks (biweekly), and 75 mg/m2 every 3 weeks (triweekly).
2. Materials and methods
We retrospectively analyzed the cohort diagnosed with mCRPC, 162 patients were included in the study. All patients were treated with DCT and prednisolone as first-line chemotherapy from January 2009 to December 2020. All patients had pathologically confirmed prostate adenocarcinoma based on surgery or biopsy. ADT disease progression to mCRPC was confirmed,8,16 the patients’ Eastern Cooperative Oncology Group (ECOG) status score was 0–3. We excluded patients who had previously undergone chemotherapy, other malignancies within 5 years, and underlying disease which might not fit any chemotherapy.
The standard triweekly regimen at our institution was docetaxel 75 mg/m2, the biweekly regimen was 50 mg/m2, and the weekly regimen was 30 mg/m2. Docetaxel was administered intravenously over 1 h with dexamethasone and antiemetics. Additional bisphosphonates, blood products, analgesics, and antiemetics were used, if necessary.
All patients underwent baseline laboratory tests, abdominal-pelvis-chest CT, and bone scan prior to the first dose of DCT. Prostate-specific antigen (PSA), CT, and bone scan were used as the primary method to evaluate the response and progression to DCT. Patients were admitted one week after DCT administration, and blood tests and brief medical and physical examinations were performed to evaluate the side-effects. The patient underwent a blood test every visit and computed tomography (CT) and bone scans every 3 months. Adverse events were assessed using the National Cancer Institute criteria (CTCAE) version 4.0 standard. The efficacy of DCT was analyzed by PSA response, progression-free survival (PFS), and overall survival (OS). PSA response was evaluated based on a 50% decrease compared with pretreatment. PFS is defined by PSA progression, radiographic progression, or death. Safety and tolerability of DCT regimen were analyzed according to the frequency of adverse events, especially Grade 3–4 adverse events. Intolerance was defined as the occurrence of discontinuation, dose reduction, and delay due to chemotherapy toxicity, and functional decline after chemotherapy.
The statistical procedure was expressed as a percentage of the categorical variables, and expressed as the mean, standard deviation, median, and quartile of the continuous variables. Continuous variables such as age and PSA were compared using one-way analysis of variance. Categorical variables were compared using Chi-square test, Fisher's exact test, and Pearson χ2 test. Kaplan–Meier analysis and log-rank test were used to compare the survival results of each treatment group. The Cox regression model was used to analyze the harzard ratio (HR) and 95% confidence intervals (CIs) for the factors affecting survival. All statistical analyses were performed using the SPSS software (SPSS 22.0, Chicago, IL, USA). All P values were two-sided, and P values of 0.05 were considered statistically significant.
3. Results
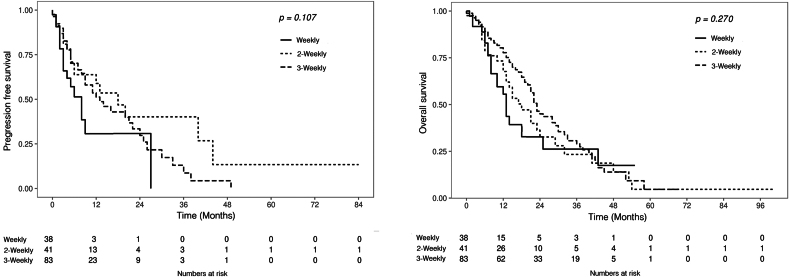
There were 38 patients in the weekly group, 41 patients in the biweekly group, and 83 patients in the triweekly group. The median age was 70.0 (IQR: 64.0–75.2) years. The mean number of DCT cycles in group 3 was 14.08 ± 2.60, 5.93 ± 4.08, and 7.89 ± 6.69 in the weekly, biweekly, and triweekly groups, respectively. Age, body mass index, and PSA at the onset of chemotherapy, sites of metastasis, and ADT duration are presented in Table 1. There was no significant difference in the PSA response rates to chemotherapy among the three groups: 36.8% (14/38) in the weekly group; 29.3% (12/41) in the biweekly group; and 36.1% (30/83) in the triweekly group, respectively (P = 0.709). The median time to progression was 3.0 (IQR 2.0-5.3) months, 5.0 (IQR 2.0-13.0) months, and 5.0 (IQR 3.0-12.0) months in the weekly, biweekly, and triweekly groups, respectively (P = 0.062). The median OS was 12.5 (IQR 6.0-14.0) months, 18.8 (IQR 5.5–23.5) months, and 22.9 (IQR 11.0–33.0) months in the weekly, biweekly, and triweekly groups, respectively (P < 0.001). The chemotherapy-related outcomes are shown in Table 2. A comparison of the survival results of PFS and OS between the three groups is presented in Fig. 1. The main reasons for discontinuing treatment were disease progression (49.5%) and chemotherapy intolerance (40.1%). There was no significant difference in the toxicity rates of the three groups, which were 55.3%, 48.9%, and 57.9%, respectively (P = 0.595). In addition, there was no significant difference in the incidence of Grade 3 or higher toxicity among the three groups. The chemotherapy-related outcome and adverse events are shown in Table 3. In Table 4, the Cox proportional hazards model was used to evaluate various factors that affect the survival of OS and PFS. Factors such as ECOG status, PSA response, and cumulative DCT cycles were significantly related to survival and progression.
Table 1.
Baseline characteristics of metastatic prostate cancer patients according to three docetaxel regimens.
| Total (N = 162) | Weekly (N = 38) | Biweekly (N = 41) | Triweekly (N = 83) | P | |
|---|---|---|---|---|---|
| Age (yr) | 69.6 ± 8.8 | 72.4 ± 7.9 | 70.2 ± 8.8 | 68.1 ± 8.9 | 0.010 |
| BMI (kg/m2) | 23.5 ± 3.8 | 22.9 ± 3.5 | 23.7 ± 3.1 | 23.6 ± 4.1 | 0.404 |
| Baseline PSA at chemotherapy (ng/mL) | 184.2 ± 659.2 | 237.1 ± 714.3 | 87.4 ± 144.0 | 207.8 ± 778.7 | 0.994 |
| ECOG-PS | <0.001 | ||||
| 0 | 80 (49.4%) | 11 (28.9%) | 17 (41.5%) | 52 (62.7%) | |
| 1 | 40 (24.7%) | 8 (21.1%) | 16 (39.0%) | 16 (19.3%) | |
| 2 | 7 (4.3%) | 4 (10.5%) | 0 (0.0%) | 3 (3.6%) | |
| 3 | 35 (21.6%) | 15 (39.5%) | 8 (19.5%) | 12 (14.5%) | |
| CCI | 0.408 | ||||
| 0 | 129 (79.6%) | 32 (84.2%) | 33 (80.5%) | 64 (77.1%) | |
| 1 | 32 (19.8%) | 6 (15.8%) | 7 (17.1%) | 19 (22.9%) | |
| ≥2 | 1 (0.6%) | 0 (0.0%) | 1 (2.4%) | 0 (0.0%) | |
| Multiple organ metastases | 0.077 | ||||
| 2 sites | 106 (65.4%) | 22 (57.9%) | 31 (75.6%) | 53 (63.9%) | |
| 3 sites | 16 (9.9%) | 8 (21.1%) | 2 (4.9%) | 6 (7.2%) | |
| 4 sites | 4 (2.5%) | 1 (2.6%) | 2 (4.9%) | 1 (1.2%) | |
| Site of metastasis | |||||
| Bone | 144 (88.9%) | 34 (89.5%) | 36 (87.8%) | 74 (89.2%) | 0.967 |
| Liver | 10 (6.2%) | 4 (10.5%) | 2 (4.9%) | 4 (4.8%) | 0.444 |
| Lung | 13 (8.0%) | 7 (18.4%) | 4 (9.8%) | 2 (2.4%) | 0.010 |
| Lymph node | 128 (79.0%) | 30 (78.9%) | 35 (85.4%) | 63 (75.9%) | 0.477 |
| Others | 13 (8.0%) | 3 (7.9%) | 5 (12.2%) | 5 (6.0%) | 0.492 |
| Initial treatment | 0.473 | ||||
| RP | 18 (11.1%) | 2 (5.3%) | 4 (9.8%) | 12 (14.5%) | |
| RT | 3 (1.9%) | 2 (5.3%) | 0 (0%) | 1 (1.2%) | |
| ADT | 141 (87.0%) | 34 (89.4%) | 37 (90.2%) | 70(84.3%) | |
| Mean ADT duration (mo) | 60.8 ± 204.2 | 25.79 ± 19.93 | 31.46 ± 32.08 | 91.31 ± 281.44 | 0.069 |
ADT, androgen deprivation therapy; BMI, body mass index; CCI, Charlson comorbidity index; ECOG-PS, eastern cooperative oncology group performance status; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy.
Table 2.
Chemotherapy-related outcomes according to the three regimens of docetaxel.
| Weekly (N = 38) | Biweekly (N = 41) | Triweekly (N = 83) | P | |
|---|---|---|---|---|
| PSA response | 14 (36.8%) | 12 (29.3%) | 30 (36.1%) | 0.709 |
| Mean total cycle | 14.1 ± 2.6 | 5.9 ± 4.1 | 7.9 ± 6.7 | <0.001 |
| Mean dose intensity (mg/m2) | 427.1 ± 92.1 | 303.2 ± 208.5 | 483.4 ± 402.7 | <0.001 |
| Dose reduction rate | 0 (0.0%) | 2 (4.9%) | 6 (7.2%) | 0.234 |
| Cause of interruption | 0.184 | |||
| Intolerance | 20 (52.6%) | 16 (39.0%) | 29 (34.9%) | |
| Disease progression | 15 (39.5%) | 18 (43.9%) | 47 (56.6%) | |
| Patients refuse | 3 (7.9%) | 7 (17.1%) | 7 (8.4%) | |
| Progression | 16 (42.1%) | 18 (43.9%) | 47 (56.6%) | 0.441 |
| Mean time to progression (months) | 4.4 ± 5.9 | 10.6 ± 15.5 | 9.2 ± 10.0 | 0.062 |
PSA, prostate-specific antigen.
Fig. 1.
Overall survival and progression-free survival in patients based on the three regimens of docetaxel.
Table 3.
Chemotherapy-related adverse events according to three regimens of docetaxel.
| Weekly (N = 38) | Biweekly (N = 41) | Triweekly (N = 83) | P | |
|---|---|---|---|---|
| Total toxicity | 21 (55.3%) | 20 (51.2%) | 36 (43.4%) | 0.453 |
| Hematologic | 3 (7.9%) | 9 (22.0%) | 15 (18.15) | 0.218 |
| Grade 1–2 | 1 (2.6%) | 3 (7.3%) | 8 (9.6%) | 0.393 |
| Grade 3–4 | 2 (5.3%) | 6 (14.6%) | 7 (8.4%) | 0.333 |
| Neurological | 1 (2.6%) | 0 (0.0%) | 1 (1.2%) | 0.571 |
| Grade 1–2 | 0 (0.0%) | 0 (0.0%) | 1 (1.2%) | 0.619 |
| Grade 3–4 | 1 (2.6%) | 0 (0.0%) | 0 (0.0%) | 0.194 |
| Respiratory | 1 (2.6%) | 0 (0.0%) | 2 (2.4%) | 0.594 |
| Grade 1–2 | 1 (2.6%) | 0 (0.0%) | 1 (1.2%) | 0.571 |
| Grade 3–4 | 0 (0.0%) | 0 (0.0%) | 1 (1.2%) | 0.619 |
| Gastrointestinal | 2 (5.3%) | 0 (0.0%) | 3 (3.6%) | 0.371 |
| Grade 1–2 | 2 (5.3%) | 0 (0.0%) | 2 (2.4%) | 0.321 |
| Grade 3–4 | 0 (0.0%) | 0 (0.0%) | 1 (1.2%) | 0.619 |
| Fatigue | 5 (13.2%) | 7 (17.1%) | 8 (9.6%) | 0.489 |
| Grade 1–2 | 5 (13.2%) | 6 (14.6%) | 8 (9.6%) | 0.684 |
| Grade 3–4 | 0 (0.0%) | 1 (2.4%) | 0 (0.0%) | 0.227 |
| Others | 9 (23.7%) | 4 (9.8%) | 19 (22.9%) | 0.176 |
| Grade 1–2 | 9 (23.7%) | 3 (7.3%) | 16 (19.3%) | 0.124 |
| Grade 3–4 | 0 (0.0%) | 1 (2.4%) | 3 (3.6%) | 0.493 |
Table 4.
Cox proportional hazards analysis of progression-free survival and overall survival.
| Variables | Progression-free survival |
Overall survival |
||
|---|---|---|---|---|
| HR (95%Cl) | P | HR (95%Cl) | P | |
| Age | 1.010 (0.984–1.036) | 0.465 | 1.036 (1.006–1.066) | 0.017 |
| ECOG status | 0.006 | 0.008 | ||
| 0 | Reference | Reference | ||
| 1 | 1.865 (1.021–3.404) | 0.042 | 0.706 (0.238–2.094) | 0.531 |
| 2 | 2.572 (1.025–6.457) | 0.044 | 1.437 (0.489–4.220) | 0.510 |
| 3 | 2.948 (1.569–5.538) | 0.001 | 3.656 (1.823–6.304) | 0.009 |
| Docetaxel regimens | 0.701 | 0.672 | ||
| Weekly | Reference | Reference | ||
| Biweekly | 1.232 (0.661–2.298) | 1.083 (0.601–1.951) | 0.791 | |
| Triweekly | 1.016 (0.579–1.782) | 0.881 (0.521–1.490) | 0.636 | |
| Chemotherapy response | 2.543 (1.305–4.953) | 0.006 | 1.881 (1.102–3.212) | 0.021 |
| Chemotherapy cycle | 0.886 (0.833–0.942) | 0.000 | 0.875 (0.828–0.925) | 0.000 |
ECOG, eastern cooperative oncology group.
4. Discussion
The TAX327 study compared triweekly regimen of DCT 75 mg/m2 with weekly DCT 30 mg/m2 and triweekly mitoxantrone 12 mg/m2 in patients with mCRPC. The weekly DCT regimen was not significantly different; however, the triweekly DCT regimen showed better survival, pain control and treatment-related quality of life than mitoxantrone.2 The SWOG99-16 also showed that triweekly DCT and estramustine treatments had a better survival benefit compared with mitoxantrone and prednisone treatments in hormone-refractory prostate cancer (HRPC) patients.8 Based on these two clinical trials, DCT treatment has been used as a first-line treatment for mCRPC patients. However, DCT chemotherapy is often associated with toxicity in elderly patients, particularly neutropenia, febrile neutropenia, fatigue, nausea and vomiting, diarrhea, alopecia, nail dystrophy, and sensory neuropathy.3,17 Studies using low-dose or adapted chemotherapy showed minimal toxicity while maintaining efficacy.11,18, 19, 20 Several studies used DCT dose and frequency-modulated methods. Of these, low-dose weekly,18, 19, 20 biweekly,11 and triweekly19 regimens showed significant efficacy and tolerability in the standard regimen. Kamiya’s study of low-dose DCT, especially in Asian patients, showed similar efficacy and better tolerability in patients treated with 60 mg/m2 compared with the standard regimen in elderly patients.21 The PROSTY study comparing biweekly docetaxel 50 mg/m2 with standard regimen showed similar efficacy and better tolerability.15 The biweekly DCT treatment delayed treatment failure and OS, and showed a similar PSA response rate. In addition, the toxicity rate of Grade 3 or higher was relatively lower than in the standard regimen. Malhotra et al compared biweekly regimen with weekly and triweekly dosages.21 In the study, the weekly intervention was associated with a low incidence of side-effects but did not show significant differences except for Grade 1/2 neuropathy. No significant difference was observed in survival outcomes among the other three groups. However, in the risk ratio of PFS and OS, the biweekly and triweekly regimens were statistically superior to the weekly regimen. This study was planned to assess the differences in DCT treatment administered weekly, or once every 2 or 3 weeks to mCRPC patients. In our study, progression and survival outcomes were similar to those reported previously. Similar to other studies, the weekly regimen showed that the low cycle dosing schedule was relatively inferior. However, our study showed a slightly lower PSA response than the previous studies, in which the PSA response rates ranged from 36% to 69%.22, 23, 24, 25 In addition, there was no significant difference in the PSA response rate according to the dose. In drug-related toxicity, previous studies reported that the low-dose was associated with a relatively lower toxicity than the standard regimen.25
Patients in the weekly and biweekly groups demonstrated inferior survival outcomes. Specifically, median overall survival and median time for progression were lower compared with those on the triweekly regimen. However, the population of our study showed a fundamental difference. The weekly group was older than the other groups, with a relatively higher number of patients with ECOG-PS 3 status and rates of lung metastasis. Although not significant, the duration of previous ADT was shorter than in other groups. At our institution, we used a weekly regimen in relatively older patients, lower performance status, and faster progression. In drug-related toxicity, previous studies reported that the low-dose regimen had a relatively low toxicity than the standard regimen. Despite this, there was no significant difference in PSA response or toxicity among the groups. In our study, tailored regimens according to patient’s conditions were a desirable option. The weekly DCT regimen was higher in dose, but the toxicity was not different and fewer cases discontinued due to intolerance.
In real-world clinical settings, a shorter interval with a lower dose of DCT may not be as effective as the conventional triweekly regimen, but it could be a viable option for CRPC patients in poor condition by reducing toxicity and treatment discontinuation.
Our study has some limitations. First, retrospective studies may have bias. In practice, there is a difference in baseline characteristics between the weekly group and the other groups. However, it might be a reflection of real clinical practice. Second, results involving a small sample size with differences between groups cannot be generalized in the absence of controls. Although these limitations cannot lead to definitive conclusions, the study did not show a significant difference in survival outcome and toxicities among the three groups.
5. Conclusions
Due to the substantial medical history of the patient during the diagnosis of CRPC, various options for optimal chemotherapy are indicated. Clinical outcomes are influenced by chemotherapy cycle, response, and patient performance status rather than by regimen. Treatment with DCT every 2 weeks or even 1 week appears to be well tolerated in men with mCRPC and is a useful option when conventional triweekly regimen is not tolerated due to poor patient condition.
Ethics approval and consent to participate
The present study does not contain clinical studies or patient data and permitted by the Institutional Review Board (IRB No. H-1908-177-1059) of Seoul National University Hospital.
Conflicts of interest
The authors declare that the research was conducted in the absence of any financial and non-financial interests that could be construed as a potential conflict of interest.
Acknowledgments
Not applicable.
Contributor Information
Hyeong Dong Yuk, Email: armenia8@snu.ac.kr.
Miso Kim, Email: misokim85@gmail.com.
Bhumsuk Keam, Email: bhumsuk@snu.ac.kr.
Ja Hyeon Ku, Email: kuuro70@snu.ac.kr.
Cheol Kwak, Email: mdrafael@snu.ac.kr.
Chang Wook Jeong, Email: drboss@snu.ac.kr.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Han S.H., Yuk H.D. Epidemiology of urologic cancer in Korea: nationwide trends in the last 2 decades. J Urol Oncol. 2023;21(1):32–44. doi: 10.22465/juo.234600080004. [DOI] [Google Scholar]
- 3.Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Damber J.E., Aus G. Prostate cancer. Lancet. 2008;371(9625):1710–1721. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberger M.A., Walsh P.C. Early androgen deprivation for prostate cancer? N Engl J Med. 1999;341(24):1837–1838. doi: 10.1056/NEJM199912093412409. [DOI] [PubMed] [Google Scholar]
- 6.Seruga B., Tannock I.F. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol. 2011;29(27):3686–3694. doi: 10.1200/JCO.2010.34.3996. [DOI] [PubMed] [Google Scholar]
- 7.Berthold D.R., Pond G.R., Roessner M., de Wit R., Eisenberger M., Tannock A.I., et al. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res. 2008;14(9):2763–2767. doi: 10.1158/1078-0432.CCR-07-0944. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y.S., Kim S.H., Tae J.H., Chang I.H., Kim T.H., Myung S.C., et al. Oral chemotherapeutic agents in metastatic hormone-sensitive prostate cancer: a network meta-analysis of randomized controlled trials. Prostate Int. 2023;11(3):159–166. doi: 10.1016/j.prnil.2023.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrylak D.P., Tangen C.M., Hussain M.H., Lara P.N., Jones J.A., Taplin M.E., et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 10.Bamias A., Bozas G., Antoniou N., Poulias I., Katsifotis H., Skolarikos A., et al. Prognostic and predictive factors in patients with androgen-independent prostate cancer treated with docetaxel and estramustine: a single institution experience. Eur Urol. 2008;53(2):323–331. doi: 10.1016/j.eururo.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 11.Hervonen P., Joensuu H., Joensuu T., Ginman C., McDermott R., Harmenberg U., et al. Biweekly docetaxel is better tolerated than conventional three-weekly dosing for advanced hormone-refractory prostate cancer. Anticancer Res. 2012;32(3):953–956. [PubMed] [Google Scholar]
- 12.Karavasilis V., Briasoulis E., Siarabi O., Pavlidis N. Biweekly administration of low-dose docetaxel in hormone-resistant prostate cancer: pilot study of an effective subtoxic therapy. Clin Prostate Cancer. 2003;2(1):46–49. doi: 10.3816/cgc.2003.n.012. [DOI] [PubMed] [Google Scholar]
- 13.Kellokumpu-Lehtinen P.L., Harmenberg U., Joensuu T., McDermott R., Hervonen P., Ginman C., et al. 2-Weekly versus 3-weekly docetaxel to treat castration-resistant prostate cancer: a randomised, phase 3 trial. Lancet Oncol. 2013;14(2):117–124. doi: 10.1016/S1470-2045(12)70537-7. [DOI] [PubMed] [Google Scholar]
- 14.Mezynski J., Pezaro C., Bianchini D., Zivi A., Sandhu S., Thompson E., et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23(11):2943–2947. doi: 10.1093/annonc/mds104. [DOI] [PubMed] [Google Scholar]
- 15.Oudard S., Fizazi K., Sengeløv L., Daugaard G., Saad F., Hansen S., et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomised phase III trial-FIRSTANA. J Clin Oncol. 2017;35(28):3189–3197. doi: 10.1200/JCO.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- 16.Culine S., Lortholary A., Voog E., Bui N.B., Kattan J., Joly F., et al. Phase II study of weekly docetaxel in patients with metastatic hormone-refractory prostate cancer. Ann Oncol. 2006;17(4):645–648. doi: 10.1093/annonc/mdj144. [DOI] [Google Scholar]
- 17.Mohler J.L., Antonarakis E.S., Armstrong A.J., D'Amico A.V., Davis B.J., Dorff T., et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 18.Oudard S., Banu E., Medioni J., Priou F., Maitre A., Madelaine I., et al. Multicentre randomised phase II trial of two sequential chemotherapy regimens in patients with docetaxel-pretreated castration-resistant prostate cancer (DCS) Eur J Cancer. 2011;47(3):209–217. doi: 10.1016/j.ejca.2010.09.018. [DOI] [Google Scholar]
- 19.Di Lorenzo G., Bracarda S., Gasparro D., Scagliarini S., Merlano M., Febbraro A., et al. The CHEIRON study: a phase II trial of low-dose docetaxel in combination with prednisone and zoledronic acid in elderly patients with castration-resistant prostate cancer. Eur Urol. 2008;53(1):106–113. doi: 10.1016/j.eururo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Sonpavde G., Pond G.R., Berry W.R., de Wit R., Armstrong A.J., Eisenberger M.A., et al. Prostate-specific antigen changes and disease progression in metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Eur Urol. 2012;62(5):921–927. doi: 10.1016/j.eururo.2012.05.015. [DOI] [Google Scholar]
- 21.Malhotra V., Perry M.C. Selective targeting by docetaxel (Taxotere): a phase I study. Semin Oncol. 1999;26(3 Suppl 9):13–18. [Google Scholar]
- 22.Matsumoto K., Ichioka K., Terai A., Yoshimura K. Low-dose docetaxel for hormone-refractory prostate cancer patients with comorbidities. Int J Clin Oncol. 2007;12(5):369–374. doi: 10.1007/s10147-007-0675-4. [DOI] [Google Scholar]
- 23.Oh W.K., Halabi S., Kelly W.K., Hayes D.F., Vogelzang N.J., Small E.J. Clinical outcomes in patients with castration-resistant prostate cancer: secondary analyses of the TAX327 study. J Clin Oncol. 2010;28(18):2822–2828. doi: 10.1200/JCO.2009.24.5127. [DOI] [Google Scholar]
- 24.Alexander M., Pook D., Hruby G., Pagano P., Aherne P., Hughes B.G., et al. Bone-targeted therapies in castration-resistant prostate cancer (CRPC) with visceral metastases: clinical outcomes in an Australian cohort. Asia Pac J Clin Oncol. 2017;13(5):327–334. doi: 10.1111/ajco.12675. [DOI] [Google Scholar]
- 25.Halabi S., Vogelzang N.J., Kornblith A.B., Ou S.S., Kantoff P.W., Dawson N.A., et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26(15):2544–2549. doi: 10.1200/JCO.2007. [DOI] [PubMed] [Google Scholar]