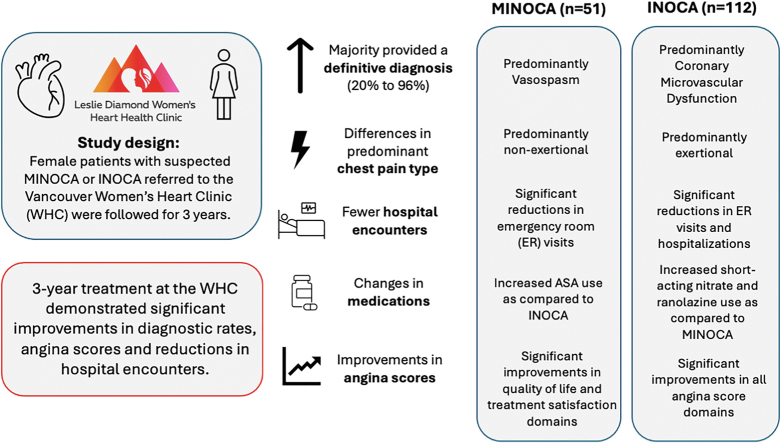
Abstract
Background
Myocardial infarction with no obstructive coronary arteries (MINOCA), and ischemia with no obstructive coronary arteries (INOCA), are female-predominant conditions; clinical trials are lacking to guide medical management for the common underlying vasomotor etiologies. Data on long-term outcomes of (M)INOCA patients following attendance at a women’s heart centre (WHC) are lacking.
Methods
Women diagnosed with MINOCA (n = 51) or INOCA (n = 112) were prospectively followed for 3 years at the Leslie Diamond WHC (LDWHC) in Vancouver. Baseline characteristics, diagnoses, chest-pain type, major adverse cardiac events, hospital encounters, medications, and Seattle Angina Questionnaire (SAQ) responses were compared between the baseline and 3-year timepoints. The χ2 test was used to compare categorical variables; the Student t test was used for continuous variables.
Results
MINOCA patients had significantly more nonexertional chest pain and more diagnoses of vasospasm than did INOCA patients, who had more exertional chest pain and more diagnoses of coronary microvascular dysfunction. Following the baseline timepoint, both groups had significant reductions in cardiovascular emergency room visits, with INOCA patients also experiencing fewer cardiovascular hospitalizations. At 3 years, the most commonly prescribed medications were calcium-channel blockers, long-acting nitrates, and beta-blockers, with MINOCA patients having more acetylsalicylic acid use, and INOCA patients having more short-acting nitrate and ranolazine prescriptions. Both groups observed significant improvements in SAQ scores, with greater improvements observed in INOCA patients. Patients with depression or who were prescribed ranolazine at 3 years had worse SAQ scores at baseline.
Conclusions
The 3-year outcomes of (M)INOCA patients indicate that the LDWHC's comprehensive care model effectively improves diagnostic clarity, reduces the number of hospital encounters, optimizes medication management, and improves self-reported patient well-being.
Graphical abstract
Résumé
Contexte
L’infarctus du myocarde sans lésion coronarienne obstructive (MINOCA, de l’anglais myocardial infarction with no obstructive coronary arteries) et l’ischémie sans lésion coronarienne obstructive (INOCA, de l’anglais ischemia with no obstructive coronary arteries) sont des affections à prédominance féminine. Or, on dispose de peu d’essais cliniques pour orienter la prise en charge médicale des causes vasomotrices sous-jacentes courantes, et les données relatives aux résultats à long terme des patientes ayant subi un MINOCA ou une INOCA et ayant été traitées dans un centre de cardiologie pour femmes sont insuffisantes.
Méthodologie
Des femmes ayant reçu un diagnostic de MINOCA (n = 51) ou d’INOCA (n = 112) ont fait l’objet d’un suivi prospectif pendant 3 ans au Leslie Diamond WHC (LDWHC) de Vancouver. On a comparé les caractéristiques initiales, le diagnostic, le type de douleur thoracique, la survenue d’une manifestation cardiovasculaire grave, les visites à l’hôpital, les médicaments et les réponses au questionnaire SAQ (Seattle Angina Questionnaire) entre le début de l’étude et le suivi après 3 ans. Le test du chi carré (χ2) a été utilisé pour comparer les variables nominales; pour les variables continues, on a utilisé le test t de Student.
Résultats
Les patientes MINOCA étaient significativement plus nombreuses à présenter des douleurs thoraciques non provoquées par l’effort et à recevoir un diagnostic de vasospasme que les patientes INOCA, chez lesquelles les douleurs thoraciques provoquées par l’effort et un diagnostic de dysfonction microcirculatoire coronaire étaient plus fréquents. Après le début de l’étude, on a observé une réduction significative du nombre de visites liées à un événement cardiovasculaire dans un service des urgences, et ce, dans les deux groupes; les patientes INOCA ayant en outre connu un plus faible nombre d’hospitalisations d’origine cardiovasculaire. Après 3 ans, les médicaments les plus fréquemment prescrits étaient les bloqueurs des canaux calciques, les dérivés nitrés à longue durée d’action et les bêta-bloquants; les patientes MINOCA ont été plus nombreuses à recourir à l’acide acétylsalicylique et les patientes INOCA, à se voir prescrire des dérivés nitrés à courte durée d’action et de la ranolazine. On a observé une amélioration significative des scores SAQ dans les deux groupes, l’amélioration étant plus marquée chez les patientes INOCA. Les patientes atteintes de dépression ou à qui on avait prescrit de la ranolazine après 3 ans affichaient de moins bons scores SAQ au départ.
Conclusions
Au bout de 3 ans, les résultats des patientes MINOCA/INOCA ont montré que le modèle de soins exhaustif offert par le LDWHC a permis d’améliorer la précision du diagnostic, de réduire le nombre de visites à l’hôpital, d’optimiser la prise en charge médicamenteuse et d’améliorer le bien-être autodéclaré des patientes.
Nonobstructive coronary syndromes, including myocardial infarction with no obstructive coronary arteries (MINOCA), and ischemia with no obstructive coronary arteries (INOCA), present unique challenges in cardiology as a result of their enigmatic nature and clinical implications. Both are characterized by less than 50% stenosis in any major coronary artery, MINOCA in the setting of myocardial infarction (MI), and INOCA with ischemia. MINOCA afflicts up to 6% of individuals experiencing an acute coronary syndrome, and a greater percentage if they are women.1,2 Similarly, two thirds of angiograms for women with suspected cardiac ischemia reveal INOCA, a prevalence twice that seen in men. Predominant underlying ischemic etiologies of these 2 syndromes (MINOCA and INOCA [(M)INOCA]) include coronary vasospasm and coronary microvascular dysfunction (CMD), conditions that are more common in women and are found to have more nontraditional risk factors compared to obstructive coronary artery disease (CAD).3
Although patients with (M)INOCA previously were considered benign and were managed conservatively, these entities have gained attention in recent years, due to their association with adverse cardiac outcomes and mortality.4,5 A 2022 systematic review and meta-analysis assessing sex-related discrepancies in MINOCA outcomes revealed a higher incidence of major adverse cardiac events (MACE) in women, compared to men, within 24 months, driven largely by a greater incidence of stroke.6 This difference holds true in INOCA patients, with a 2.55-fold higher risk of MACE in women with INOCA, compared to the risk in women with no CAD, within 1 year following angiographic investigations.7 Beyond this risk, patients with nonobstructive coronary syndromes are burdened with poor physical functioning, recurrent angina, and a reduced quality of life.8,9
At this juncture, randomized controlled trial data are lacking to provide guidance on medical treatment strategies in patients with (M)INOCA. The recent clinical practice update from the Canadian Cardiovascular Society and the Canadian Women’s Heart Health Alliance acknowledges this and the fact that, as a result, prescribing approaches are on the basis of expert opinion and a personalized strategy relating to the underlying etiology.10 This is supported by the British Heart Foundation Coronary Microvascular Angina (CorMicA) trial, which demonstrated that stratified medical therapy based on invasively diagnosed etiology significantly improved treatment satisfaction, lessened angina severity, and improved quality of life.11
Women’s heart centres (WHCs) provide specialized and comprehensive care tailored specifically to the unique needs of women.12 Recognition is growing of how specialized WHCs provide a sustainable solution to address the disparities faced in this understudied population, particularly given the ongoing confusion with regard to standardized terminology and diagnostic criteria.13 In our previous study, Parvand et al. showcased 1-year prospective data suggesting that Leslie Diamond WHC (LDWHC) enrollment resulted in a higher diagnostic yield, improved risk-factor management, and reduced angina hospitalization in women presenting with (M)INOCA.14 Furthermore, due to the heterogeneity of the (M)INOCA patient population, access to a WHC may allow for the use of tailored prescribing practices.
Overall, there remains a paucity of data evaluating the role of specialized WHCs in the longitudinal follow-up of (M)INOCA patients. The aim of the current work was to provide more detailed insights into the outcomes, diagnoses, longer-term therapeutic management, and modifiers of patient-reported outcomes for this population after 3 years of follow-up in the LDWHC.
Methods
WHC cohort
Female patients with MINOCA or INOCA who were referred to the LDWHC located in Vancouver, Canada, were identified for LDWHC registry enrollment, as approved by the University of British Columbia’s clinical research ethics board (application #H13-03322).
Patients have consented to be included in the LDWHC registry since 2015, following their baseline LDWHC consultation, with prospective data obtained thereafter for a minimum of 3 years. Patients were followed by a multidisciplinary team consisting of a cardiologist with specialized training in women’s heart health, a psychiatrist, a dietician, and 2 part-time nurse practitioners. They had access to specialized diagnostic techniques, including invasive coronary reactivity testing (CRT), adenosine stress cardiac magnetic resonance imaging (MRI), and optical coherence tomography during angiography. Patients were seen at least once annually for regular clinical follow-up, with increased frequency as needed.
Data collection
Patient data obtained from questionnaires were stored using a Research Electronic Data Capture (REDCap) database. Demographic, clinical, and female-specific variables were captured from the baseline WHC clinical consultation and/or verbal self-report to the study coordinator, and were entered into the database.
Study outcomes
Baseline characteristics
The frequencies of variables captured at baseline were compared between MINOCA and INOCA patients. These variables were as follows: age, race(s) and/or ethnicity(ies), partnered status, education level, working status, family history of premature heart disease, prior stroke or transient ischemic attack (TIA), dyslipidemia, diabetes mellitus, hypertension, obesity, smoking history, arrhythmia, depression, anxiety, thyroid dysfunction, migraine, Raynaud’s syndrome, chronic obstructive pulmonary disease, autoimmune disease, menopause status, number of children born, preeclampsia or gestational hypertension during pregnancy, and polycystic ovarian syndrome. Full definitions of each variable are described in Supplemental Appendix S1.
A key exposure variable was a history of depression, and depression was considered to be present if the patient met any of the following 3 criteria: (i) having had a recent or current clinical depression diagnosis, as indicated in the baseline consultation; (ii) having a baseline Patient Health Questionnaire (PHQ-9) score of ≥10 points15; and (iii) be currently taking (an) antidepressant(s) for depressive symptoms, as confirmed by the patient’s self-report and/or consultation notes (including referral documentation prior to patient contact with the LDWHC). This definition was used to account for self-report bias, and/or inconsistent diagnostic appraisal, which is possible among healthcare providers.16 Expansion of the criteria for defining depression to include any combination of these variables (antidepressant use, questionnaire response, and self-report data) has been shown previously to improve the validity of depression studies.17
Cardiac diagnoses
Referral letters from primary care or specialist physicians were reviewed if the patient had undiagnosed chest pain attributed to (M)INOCA. Prior to baseline, patients were appraised if either of the following was true: (i) they lacked a specialized diagnosis, and had been referred to the LDWHC for diagnostic clarity; or (ii) diagnostic evidence indicated an etiology underlying the (M)INOCA, and a request had been made for further medical opinion to aid in symptom management. The (M)INOCA etiologies were classified as being either vasomotor (CMD or coronary vasospasm) or “other,”—that is, nonvasomotor—spontaneous coronary artery dissection (SCAD), Takotsubo cardiomyopathy, myocarditis, and missed plaque rupture. Probable or confirmed CMD and coronary vasospasm were defined using the Coronary Vasomotion Disorders International Study (COVADIS) group definitions,18 and they are described further in Supplemental Appendix S1. The resulting diagnosis from cardiac investigations and/or medications prescribed during visits at the LDWHC were identified from physician consultation notes, for the baseline visit vs during 3 years of follow-up care. If patients were ascertained to have SCAD alone, they were triaged to a dedicated SCAD clinic at our centre prior to baseline LDWHC attendance and were not included. If patients had overlapping diagnoses of SCAD and an additional (M)INOCA etiology, they were included in the study. Similarly, if SCAD was identified following enrollment in the LDWHC registry, patients were included.
Clinical presentation
Baseline LDWHC physician consultation notes were reviewed for descriptors of chest pain on exertion, chest pain at rest, and/or chest pain during stress or emotional triggers. These terms were stated explicitly as such in the consultation notes, and any categorization ambiguities were clarified before categorization was made.
Cardiac testing modalities used for ischemic, structural, and overall assessment were characterized as follows: degree of stenosis from either coronary angiogram (CA) or computed tomography coronary angiography (CTCA); results from CRT with adenosine, acetylcholine, or ergonovine provocation during CA; exercise stress test results; cardiac MRI results with and without adenosine; myocardial perfusion imaging results; and echocardiography results. For the detection of microvascular dysfunction, modalities were limited to invasive CRT and stress cardiac MRI, as Doppler ultrasound and positron emission tomography scanning techniques are not available in the province of British Columbia.
Hospital encounters and MACE
Hospital encounters, defined as emergency room (ER) visits for chest pain, hospitalizations for any cardiovascular cause, and MACE, were quantified within the 3 years prior to the baseline LDWHC visit and up to 3 years post-baseline. Only electronic health record encounters from British Columbia were accessible. MACE comprised MI, stroke, heart failure, or cardiovascular death. TIAs also were separately evaluated, as they are known to be precursors to stroke within 1 year.19 For MINOCA patients, the first (index) MI was excluded from the pre–baseline hospitalization count.
Therapeutic management
Medications were quantified at baseline and at 3 years. The baseline medications reflect what the patient was taking prior to the LDWHC appointment. The 3-year medications were identified from the most-recent physician consultation notes, reflecting changes that were made in the LDWHC. For patients with medications data at both baseline and 3 years, changes in medications were analyzed on a per-individual basis.
Patient-reported outcomes
The Seattle Angina Questionnaire (SAQ) has been validated in patients with cardiac chest pain and measures 4 dimensions of self-reported symptoms, as follows: physical limitation; angina frequency; quality of life; and treatment satisfaction.20 Scores were scaled to a 100-point scale prior to analysis. Scaled scores of 0-24 represent severe angina, scores of 25-74 represent moderate angina, and scores > 75 represent mild to no angina, which corresponds to clinically relevant Canadian Cardiovascular Society angina scales.21 The SAQ was administered at baseline and at 3 years, and changes in scores were assessed using the changes that occurred on the 100-point scale. The minimal clinically important difference for the SAQ is 10 points on the 100-point scale.20
SAQ subdomain scores were stratified further and were analyzed by whether depression was present at baseline, and whether prescription of ranolazine had occurred by year 3. We hypothesized that patients with depression at baseline would have lower SAQ scores, as depression has been demonstrated to accentuate perceptions of pain.22 Further, as ranolazine is used as a second- or third-line anti-anginal agent, we hypothesized that patients prescribed this medication would have worse SAQ scores at baseline, owing to the increased challenges in managing angina in this group.
Statistical analyses
Baseline characteristics are reported as means and standard deviations for continuous variables, and as counts and percentages for categorical variables. Comparisons between MINOCA and INOCA groups were conducted using the χ2 test or the Student t test for categorical and continuous variables, respectively. Statistically significant differences in the number of ER visits, hospitalizations, and MACE between the baseline and year-3 timepoints were calculated using McNemar’s test.
Overall SAQ scores, by subdomain, were compared using the Student t tests. Two variables were investigated as modifiers of SAQ scores, at baseline and 3 years, and in the effect on the difference between baseline and 3 years, as follows: (i) depression status at baseline (yes or no) and (ii) ranolazine prescription status at 3 years (yes or no). For each analysis, the Student t test was used to compare mean questionnaire scores for each modifier variable. Baseline and year-3 scores within groups were compared using the paired Wilcoxon rank-sum test, for each subdomain, as data were non-normally distributed. To assess whether the presence of depression and ranolazine prescription impacted the change in questionnaire scores over time, differences between baseline and 3-year scores were first calculated for each patient; then, the means of each variable, stratified by the presence vs absence of each modifier variable, were compared using the Student t test. Linear regression models were plotted to display trends.
Results
Baseline characteristics
A total of 265 patients, recruited between 2015 and 2020, were considered for analysis. There were 32 excluded patients who had withdrawn or were lost to follow-up (12%), 64 who were diagnosed subsequently with a non-(M)INOCA etiology (24%), and 6 who had incomplete surveys (2%). This exclusion resulted in 163 patients (62%) who were eligible for analysis, with complete baseline and 3-year questionnaires, of whom 51 had MINOCA and 112 had INOCA. Among demographic, clinical, and female-specific variables (Table 1), no significant differences were observed between MINOCA and INOCA patients, except in relation to migraines, which were significantly more prevalent in MINOCA patients (n = 23; 45%) than they were in INOCA patients (n = 28; 25%).
Table 1.
Baseline characteristics, stratified by myocardial infarction with no obstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA) patient groups
| Variable | MINOCA patients (n = 51) | INOCA patients (n = 112) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean (SD) | 55.7 (11.1) | 58.3 (11.3) | 0.16 | |
| Race, Caucasian | 43 (84) | 95 (85) | 0.94 | |
| Partnered status, partnered | 45 (88) | 84 (75) | 0.09 | |
| Education level | High school or less | 7 (14) | 20 (18) | 0.76 |
| Some postsecondary | 29 (57) | 56 (50) | ||
| Higher than Bachelor's | 9 (18) | 25 (22) | ||
| Missing∗ | 6 (12) | 11 (10) | ||
| Working status | Employed | 31 (61) | 56 (50) | 0.36 |
| Not employed (including retired) | 15 (29) | 46 (41) | ||
| On medical leave or disability | 5 (10) | 10 (9) | ||
| Clinical | ||||
| Family history of premature heart disease | 12 (24) | 39 (35) | 0.21 | |
| Prior transient ischemic attack | 0 (0) | 2 (2) | 0.85 | |
| Dyslipidemia | 23 (45) | 60 (54) | 0.40 | |
| Diabetes | 9 (18) | 13 (12) | 0.42 | |
| Hypertension | 17 (33) | 53 (47) | 0.13 | |
| Obesity (body mass index ≥30 kg/m2) | 4 (8) | 24 (21) | 0.06 | |
| Smoking history, current or former | 9 (18) | 22 (20) | 0.93 | |
| Arrhythmia | 3 (6) | 5 (4) | 1 | |
| Depression | 19 (37) | 52 (46) | 0.36 | |
| Anxiety | 14 (27) | 26 (23) | 0.70 | |
| Thyroid dysfunction | 8 (16) | 23 (21) | 0.61 | |
| Chronic renal dysfunction | 1 (2) | 2 (2) | 1 | |
| Migraine history | 23 (45) | 28 (25) | 0.02 | |
| Raynaud's history | 7 (14) | 9 (8) | 0.40 | |
| Chronic obstructive pulmonary disease | 1 (2) | 4 (4) | 0.95 | |
| Autoimmune disease | 9 (18) | 30 (27) | 0.29 | |
| Female-specific | ||||
| Menopause status, pre/peri | 15 (29) | 30 (27) | 0.87 | |
| Number of children born | 0 or 1 | 10 (20) | 21 (19) | 0.78 |
| 2 or 3 | 31 (61) | 72 (64) | ||
| ≥ 4 | 3 (6) | 9 (8) | ||
| Missing∗ | 7 (14) | 10 (9) | ||
| Preeclampsia or hypertension in pregnancy | Yes | 10 (20) | 16 (14) | 0.39 |
| Missing∗ | 7 (14) | 10 (9) | ||
| Polycystic ovarian syndrome | Yes | 3 (6) | 12 (11) | 0.55 |
| Missing∗ | 5 (10) | 13 (12) | ||
All values are represented as n (%), unless otherwise indicated.
SD, standard deviation.
Counts of missing variables were included if > 5% were missing, and they were not included in the P-value calculations.
Cardiac diagnoses
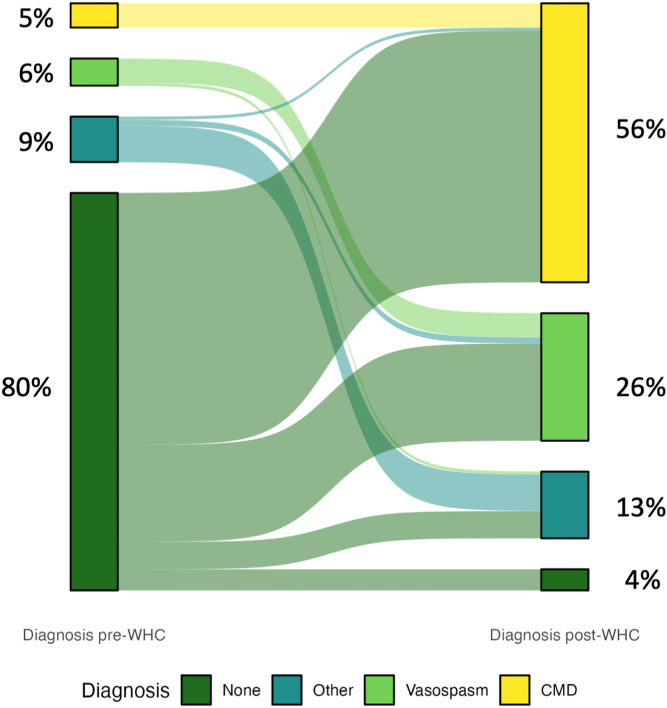
Most patients who were referred to the LDWHC with chest pain did not have a specific etiology diagnosed at entry (n = 131; 80%). A minority (n = 32; 20%) of the referrals requested medical-management consultation for chest pain with prior diagnosed CMD (n = 8; 5%), vasospasm (n = 9; 6%), or other diagnoses (n = 15; 9%).
Figure 1 demonstrates the pre- and post-LDWHC diagnostic changes that occurred, and Table 2 summarizes the specific etiologies diagnosed after 3 years of LDWHC follow-up care. Most patients had a final vasomotor diagnosis of either CMD (n = 92; 56%) or vasospasm (n = 42; 26%). The majority of INOCA patients had CMD (n = 85; 76%), followed in frequency by having vasospasm (n = 18; 16%); and the majority of MINOCA patients had vasospasm (n = 24; 47%), followed in frequency by having a nonvasomotor etiology (n = 18; 35%). Significantly more MINOCA patients had vasospasm (47%) or another nonvasomotor etiology (35%), compared to INOCA patients—16% and 4% respectively. In contrast, significantly more INOCA patients than MINOCA patients had CMD (76% vs 14%). Among the 22 patients who had "other" nonvasomotor diagnoses following LDWHC assessment, the distribution of diagnoses was as follows: 5 had Takotsubo cardiomyopathy only; 1 had Takotsubo with CMD; 2 had Takotsubo with SCAD; 5 had SCAD and CMD; 1 had SCAD and vasospasm; 1 had SCAD only; 4 had myocarditis; 1 had coronary embolism; 1 had both vasospasm and CMD; and 1 had mild plaque with aortic stenosis.
Figure 1.
Diagnoses of 163 patients prior to, and 3 years following, attendance at the Leslie Diamond Women’s Heart Centre (WHC). CMD, coronary microvascular dysfunction.
Table 2.
Comparing frequency of etiologies diagnosed following attendance at the Leslie Diamond Women's Heart Centre
| Etiology | Total (n = 163) | MINOCA patients (n = 51) | INOCA patients (n = 112) | P |
|---|---|---|---|---|
| Vasospasm | 42 (26) | 24 (47) | 18 (16) | < 0.001 |
| Definite | 8 (5) | 3 (6) | 5 (4) | — |
| Probable | 34 (21) | 21 (41) | 13 (12) | — |
| CMD | 92 (57) | 7 (14) | 85 (76) | < 0.001 |
| Definite | 14 (9) | 2 (4) | 12 (11) | — |
| Probable | 78 (48) | 5 (10) | 73 (65) | — |
| Other nonvasomotor etiology | 22 (13) | 18 (35) | 4 (4) | < 0.001 |
| Not yet diagnosed | 7 (4) | 2 (4) | 5 (4) | 1 |
All values, except P-values, are presented as n (%).
CMD, coronary microvascular dysfunction; INOCA, ischemia with no obstructive coronary arteries; MINOCA, myocardial infarction with no obstructive coronary arteries.
Clinical presentation
Significant differences occurred in the frequency of chest pain types observed between MINOCA and INOCA patients, with the majority of both groups displaying multiple types (Table 3; P < 0.001). MINOCA patients had more presentations of chest pain only at rest (20% vs 4% in INOCA patients), and INOCA patients had more chest pain only on exertion (36% vs 10% in MINOCA patients).
Table 3.
Chest pain type frequency, by myocardial infarction with no obstructive coronary arteries (MINOCA) vs ischemia with no obstructive coronary arteries (INOCA) groups
| Chest-pain component | MINOCA patients (n = 51) | INOCA patients (n = 112) | P |
|---|---|---|---|
| Combination of >1 chest-pain type | 34 (67) | 66 (59) | < 0.001 |
| Exertional only | 5 (10) | 40 (36) | |
| Nonexertional only (at rest, sleep) | 10 (20) | 5 (4) | |
| With stress and/or emotional triggers only | 2 (4) | 1 (1) |
All values, except P-value, are presented as n (%).
Within a subset of patients stratified by vasospasm or CMD etiology, significantly more patients with CMD had an exertional component to their chest pain (n = 80; 87%), compared to those with vasospasm (n =19; 45%; Table 4; P < 0.001). Conversely, significantly more patients with vasospasm had a component of nonexertional chest pain (n = 28; 67%), compared to those with CMD (n = 34; 37%). The frequency of stress- or emotional trigger–induced chest pain did not vary between groups.
Table 4.
Chest pain type frequency, by coronary microvascular dysfunction (CMD) vs vasospasm diagnosis
| Chest pain component | CMD (n = 92) | Vasospasm (n = 42) | P |
|---|---|---|---|
| Exertional | 80 (87) | 19 (45) | < 0.001 |
| Nonexertional (at rest, sleep) | 34 (37) | 28 (67) | 0.003 |
| With stress and/or emotional triggers | 24 (26) | 11 (26) | 1 |
All values, except P-values, are presented as n (%). Note that chest pain totals do not add up to 100% for each diagnosis, as some patients reported having > 1 type of chest pain.
Table 5 displays the rates of diagnostic tests for anatomic atherosclerosis and ischemia. Most patients had normal coronary arteries (MINOCA patients, 70.6%; INOCA patients, 60.7%), compared to minimal (1%-24% occlusion) or mild (25%-49% occlusion) CAD (MINOCA patients, 29.4%; INOCA patients, 33.1%). The proportion of modalities that were positive for ischemia was higher in INOCA patients, compared to that in MINOCA patients, specifically exercise stress tests (INOCA patients, 38.4% vs MINOCA patients, 5.9%; P < 0.001) and myocardial perfusion (INOCA patients, 7.1% vs MINOCA patients, 0%; P = 0.006). Wall motion abnormalities detected from echocardiography were significantly more common in MINOCA patients than they were in INOCA patients (hypokinesis, 17.6% vs 2.7%, and akinesis, 3.9% vs 0%, respectively, P < 0.001).
Table 5.
Cardiac investigations for ischemia and/or structural abnormalities in patients with undiagnosed myocardial infarction with no obstructive coronary arteries (MINOCA) vs ischemia with no obstructive coronary arteries (INOCA)
| Testing modality | MINOCA patients (n = 51) | INOCA patients (n = 112) | P | |
|---|---|---|---|---|
| Microvascular dysfunction provocation test with adenosine (CFR < 2.5 and/or IMR ≥ 25) | Positive | 2 (3.9) | 12 (10.7) | 0.20 |
| Negative | 4 (7.8) | 4 (3.6) | ||
| Not done | 45 (88.2) | 96 (85.7) | ||
| Epicardial vasospasm provocation test with ergonovine or acetylcholine (CFR < 1.5) | Positive | 3 (5.9) | 5 (4.5) | 0.59 |
| Negative | 0 | 2 (1.8) | ||
| Not done | 48 (94.1) | 105 (93.8) | ||
| Exercise stress test | Positive for ischemia | 3 (5.9) | 43 (38.4) | < 0.001 |
| Negative | 24 (47.1) | 54 (48.2) | ||
| Not done | 24 (47.1) | 15 (13.4) | ||
| Cardiac MRI | Positive for ischemia | 3 (5.9) | 6 (5.4) | 0.002 |
| Negative | 24 (47.1) | 23 (20.5) | ||
| Not done | 24 (47.1) | 83 (74.1) | ||
| Myocardial perfusion imaging | Positive for ischemia | 0 | 8 (7.1) | 0.006 |
| Negative | 6 (11.8) | 31 (27.7) | ||
| Not done | 45 (88.2) | 73 (65.2) | ||
| Highest stenosis from most recent CA and/or CTCA prior to baseline LDWHC appointment | Normal | 36 (70.6) | 68 (60.7) | 0.13 |
| Minimal (1%–24%) | 10 (19.6) | 17 (15.2) | ||
| Mild (25%–49%) | 5 (9.8) | 20 (17.9) | ||
| Not done | 0 | 7 (6.3) | ||
| Echocardiogram wall-motion findings | Normal | 29 (56.9) | 60 (53.6) | < 0.001 |
| Akinesis | 2 (3.9) | 0 | ||
| Dyskinesis | 0 | 1 (0.9) | ||
| Hypokinesis | 9 (17.6) | 3 (2.7) | ||
| Not assessed | 1 (2.0) | 0 | ||
| Not done | 10 (19.6) | 48 (42.9) | ||
All values, except P-values, are presented as n (%).
CA, coronary angiogram; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; CTCA, computed tomography coronary angiogram; IMR, index of microvascular resistance; MRI, magnetic resonance imaging; LDWHC, Leslie Diamond Women’s Heart Centre.
Hospital encounters and MACE
Significantly fewer ER visits for chest pain were observed following LDWHC attendance, compared to that observed prior to LDWHC attendance, for both MINOCA (92% vs 51%) and INOCA patients (50% vs 32%; Table 6). INOCA patients had significantly fewer hospitalizations for any cardiovascular reason post–WHC attendance (20% vs 5%). MINOCA patients did not appreciate this same reduction (4% vs 8%), although their first (index) MI hospitalization was excluded from the prebaseline hospitalization count.
Table 6.
Comparing hospital encounters at 3 years prior to baseline Leslie Diamond Women’s Heart Centre (LDWHC) appointment (appt) vs 3 years following baseline
| Group | Encounter type | Number of encounters | Prior to LDWHC baseline appt | Year 3 | P |
|---|---|---|---|---|---|
| MINOCA patients (n = 51) | ER visits | 0 | 4 (8) | 25 (49) | < 0.001 |
| 1 or 2 | 42 (82) | 22 (43) | |||
| ≥ 3 | 5 (10) | 4 (8) | |||
| Cardiovascular hospitalizations | 0 | 49 (96) | 47 (92) | 0.65 | |
| 1 | 2 (4) | 3 (6) | |||
| ≥ 2 | 0 (0) | 1 (2) | |||
| INOCA patients (n = 112) | ER visits | 0 | 56 (50) | 74 (66) | 0.03 |
| 1 or 2 | 48 (43) | 30 (27) | |||
| ≥ 3 | 8 (7) | 8 (7) | |||
| Cardiovascular hospitalizations | 0 | 90 (80) | 107 (96) | < 0.001 | |
| 1 | 20 (18) | 4 (4) | |||
| ≥ 2 | 2 (2) | 1 (1) |
Values, are represented as n (%), unless otherwise indicated. ER, emergency room; INOCA, ischemia with no obstructive coronary arteries; MINOCA, myocardial infarction with no obstructive coronary arteries.
Low rates of MACE were observed among MINOCA and INOCA patients (Table 7). All MINOCA patients’ index MI events were non–ST-segment elevation MIs. A total of 49 patients (96%) had their index MI event within 3 years prior to the baseline LDWHC visit. In the 3 years following baseline, among MINOCA patients, 1 patient had an MI and subsequent percutaneous coronary intervention, 1 (different) patient had a TIA, and no heart failure events or deaths were observed. Among INOCA patients, 2 had a TIA event post-baseline, and 1 died of an unknown cause.
Table 7.
Major adverse cardiovascular event (MACE) outcomes after 3 years of attendance at the Leslie Diamond Women’s Heart Centre (LDWHC), in myocardial infarction with no obstructive coronary arteries (MINOCA) vs ischemia with no obstructive coronary arteries (INOCA) patient groups
| Group | MACE | Pre-LDWHC | Post-LDWHC | |
|---|---|---|---|---|
| MINOCA patients (n = 51) | # of MIs | 1 | 46 (90) | 1 (2) |
| 2 | 5 (10) | 0 | ||
| TIA | 0 | 1 (2) | ||
| PCI | 0 | 1 (2) | ||
| Heart failure | 0 | 0 | ||
| Death | 0 | 0 | ||
| INOCA patients (n = 112) | MI | 0 | 0 | |
| TIA | 2 (2) | 2 (2) | ||
| PCI | 0 | 2 (2) | ||
| Heart failure | 0 | 0 | ||
| Death | — | 1 (1) | ||
All values are represented as n (%).
INOCA, ischemia with no obstructive coronary arteries; MI, myocardial infarction; MINOCA, myocardial infarction with no obstructive coronary arteries; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Therapeutic management
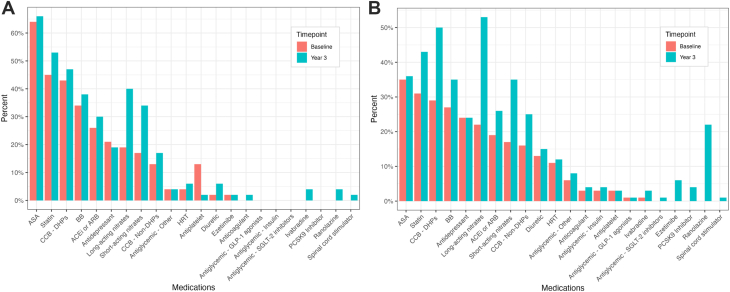
Changes in the numbers and proportions of patients on various medical therapies are summarized in Figure 2 (Supplemental Table S1). At baseline, in quantifying the 5 most frequently used medications for both MINOCA and INOCA, MINOCA patients were found to use more angiotensin-converting enzyme inhibitors and/or angiotensin II receptor antagonists than were INOCA patients, and INOCA patients had more antidepressant use. The 2 groups shared similarly high prescription levels of acetylsalicylic acid (ASA), statins, 1,4-dihydropyridine calcium-channel blockers (DHP-CCBs), and beta-blockers (BBs) at baseline. At 3 years, MINOCA patients had more ASA prescriptions than did INOCA patients, and INOCA patients had more use of short-acting nitrates. Otherwise, the 2 groups shared similarly high prescription levels for DHP-CCBs, long-acting nitrates, BBs, and statins.
Figure 2.
Proportions of therapies prescribed at the Leslie Diamond Women’s Heart Centre, at baseline and at 3 years post-baseline, in patients with (A) myocardial infarction with no obstructive coronary arteries (MINOCA) or (B) ischemia with no obstructive coronary arteries (INOCA). ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; ASA, acetylsalicylic acid; BB, beta-blocker; CCB, calcium-channel blocker; DHP, 1,4-dihydropyridine; GLP-1, glucagon-like peptide-1; HRT, hormone replacement therapy; PCSK9, proprotein convertase subtilisin/kexin type 9; SGLT-2, sodium-glucose cotransporter-2.
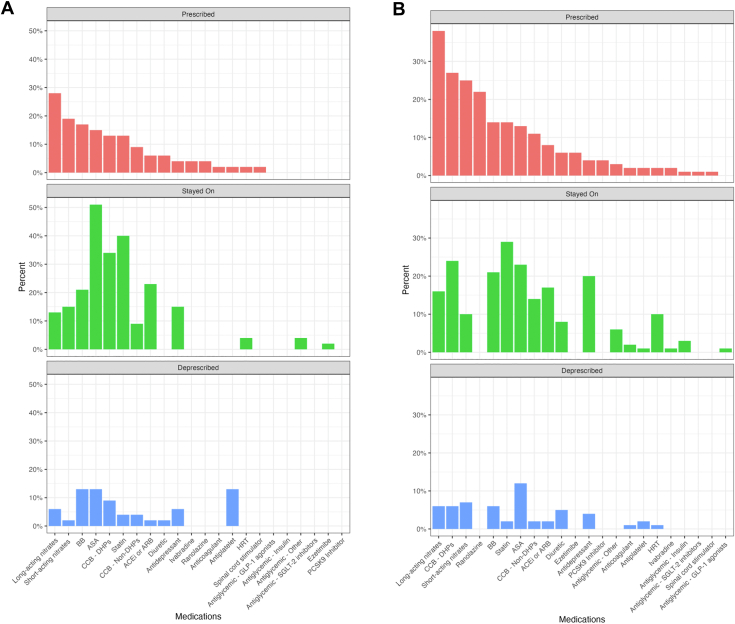
Changes in prescriptions between the baseline and 3-year points are summarized in Figure 3 (Supplemental Table S2). For MINOCA patients, the greatest increases in medications occurred for long-acting nitrates (n = 13; 28%), short-acting nitrates (n = 9; 19%), BBs (n = 8; 17%), and ASA (n = 7; 15%). Prescriptions for ASA, non-ASA antiplatelets, and BBs demonstrated the highest rates of discontinuation (n = 6 for each; 13%). For INOCA patients, the greatest increases in medication use were for long-acting nitrates (n = 41; 38%), DHP-CCBs (n = 29; 27%), short-acting nitrates (n = 27; 25%), and ranolazine (n = 24; 22%). Prescriptions for ASA (n = 13; 12%) and short-acting nitrates (n = 8; 7%) were the most frequently discontinued, followed in frequency by those for long-acting nitrates (n = 7; 6%), BBs, and DHP-CCBs (n = 6 for each; 6%). Medications that report high rates of both prescription and discontinuation are reflective of different patients being treated.
Figure 3.
Changes in medications between baseline Leslie Diamond Women’s Heart Centre visit, and visit at 3 years post-baseline, for (A) myocardial infarction with no obstructive coronary arteries (MINOCA) and (B) ischemia with no obstructive coronary arteries (INOCA) patients. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; ASA, acetylsalicylic acid; BB, beta-blocker; CCB, calcium-channel blocker; DHP, 1,4-dihydropyridine; GLP-1, glucagon-like peptide-1; HRT, hormone replacement therapy, PCSK9, proprotein convertase subtilisin/kexin type 9; SGLT-2, sodium-glucose cotransporter-2.
Patient-reported outcomes
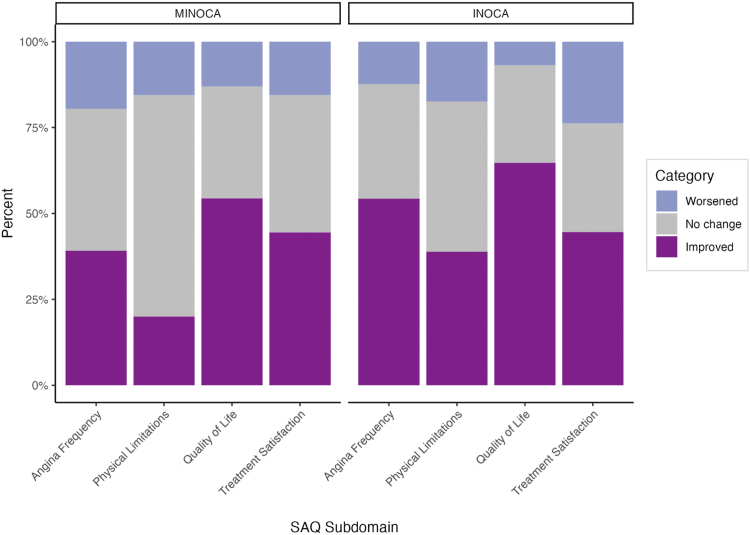
Improvements in SAQ subdomain scores were noted in both the MINOCA and INOCA patient groups between the baseline and 3-year timepoints (Table 8). Among MINOCA patients, significant improvements were observed in all subdomains, except for physical limitations. In contrast, significant improvements were observed across all domains in INOCA patients. When investigating whether minimal clinically important difference were achieved in SAQ subdomains, a higher relative proportion of INOCA patients were observed to have clinically meaningful improvements across all domains, except treatment satisfaction, compared to that for MINOCA patients (Fig. 4; Supplemental Table S3).
Table 8.
Comparing Seattle Angina Questionnaire (SAQ) subdomain scores, at baseline vs 3 years
| Group | SAQ Questionnaire subdomain | Baseline | Year 3 | P |
|---|---|---|---|---|
| MINOCA patients (n = 51) | Physical limitations | 71 (56, 86) | 71 (63, 79) | 0.47 |
| Angina frequency | 60 (37, 84) | 80 (56, 100) | 0.03 | |
| Quality of life | 50 (34, 67) | 67 (55, 80) | < 0.001 | |
| Treatment satisfaction | 76 (56, 97) | 82 (70, 94) | < 0.01 | |
| INOCA patients (n = 112) | Physical limitations | 51 (36, 66) | 60 (45, 76) | < 0.001 |
| Angina frequency | 47 (31, 64) | 67 (47, 87) | < 0.001 | |
| Quality of life | 33 (17, 50) | 47 (53, 82) | < 0.001 | |
| Treatment satisfaction | 71 (59, 83) | 82 (70, 94) | 0.02 |
All values, except P-values, are represented as median (interquartile range). INOCA, ischemia with no obstructive coronary arteries; MINOCA, myocardial infarction with no obstructive coronary arteries.
Figure 4.
Proportion of patients who had minimal clinically important differences in domain scores, between baseline and 3 years, stratified by myocardial infarction with no obstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA). SAQ, Seattle Angina Questionnaire.
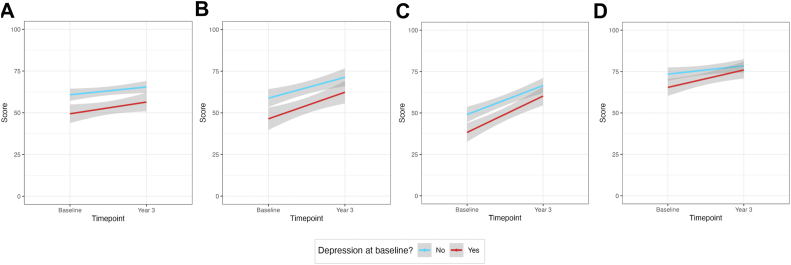
Patients who had depression at baseline had significantly worse SAQ scores, compared to patients without depression at baseline (all P < 0.05), and at 3 years, in the physical limitations (P = 0.006) and angina-frequency domains (P = 0.03; Fig. 5; Supplemental Table S4). However, SAQ scores improved at similar rates over time for the groups with and without depression. Having depression at baseline was not found to be a significant modifier for any of the SAQ subdomain scores (ΔP > 0.05).
Figure 5.
Seattle Angina Questionnaire subdomain scores at baseline Leslie Diamond Women’s Heart Centre appointment, compared to 3 years later, by depression status at baseline, for (A) physical limitations; (B) angina frequency; (C) quality of life; and (D) treatment satisfaction.
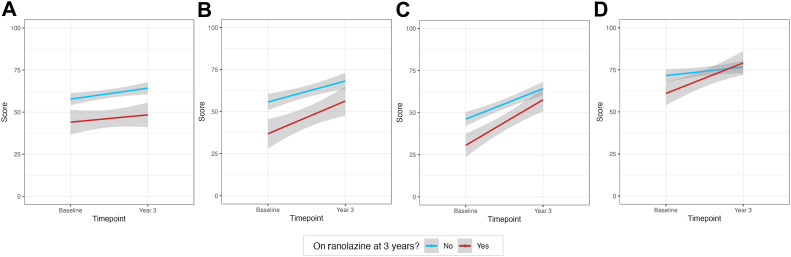
Patients prescribed ranolazine at year 3 had significantly worse SAQ scores for all subdomains at baseline, as well as for the physical limitations and angina frequency subdomains at year 3 (Fig. 6; Supplemental Table S5). SAQ scores improved at a higher rate over time in patients treated with ranolazine at year 3 for the treatment satisfaction subdomain, suggesting that ranolazine was an effect modifier (ΔP = 0.02); however, it was not associated with different rates of improvement over time in the other subdomains (ΔP > 0.05).
Figure 6.
Seattle Angina Questionnaire subdomain scores and standard deviation at baseline Leslie Diamond Women’s Heart Centre appointment, compared to 3 years later, by patients on ranolazine at year 3, compared to those not taking this drug, for (A) physical limitations; (B) angina frequency; (C) quality of life; and (D) treatment satisfaction.
Discussion
In this work, we sought to address the void of longer-term data describing (M)INOCA patients by presenting 3-year outcomes following attendance at a Canadian WHC. In summary, over 95% of both groups received a diagnosis, showing improved diagnostic accuracy following enrollment in the LDWHC. The number of hospital encounters, particularly emergency visits, significantly decreased; INOCA patients also experienced fewer cardiovascular hospitalizations. Medication adjustments were notable, with a reduction in non-ASA antiplatelets, and use of a personalized approach for BBs. Ranolazine was prescribed frequently for INOCA patients, suggesting a challenging symptom profile in this cohort that often requires second- or third-line anti-anginal agents. Mental health status at baseline was an important component in relation to self-reported angina, as patients with depression had worse baseline SAQ metrics; however, with LDWHC follow-up, they demonstrated improvements comparable to those in patients without depression.
Although 71.4% of INOCA patients and 60% of MINOCA patients had new or changed diagnoses at 1 year,14 by 3 years of follow-up, a diagnosis was achieved in 95.5% of INOCA patients and 96.1% of MINOCA patients. This improvement in diagnostic yield suggests that patients had greater access to testing and diagnostics through a longer duration of follow-up at the LDWHC, including invasive coronary reactivity testing and adenosine cardiac MRI.
Women with chest pain and nonobstructive coronary syndromes frequently utilize hospital resources.23 We demonstrated that a significant reduction in ER presentations occurred following enrollment and follow-up in the LDWHC. INOCA patients also had a significantly reduced number of cardiovascular hospitalizations, unlike MINOCA patients, who had low baseline rates excluding their index hospitalization. MINOCA patients had more diagnostic testing performed than did INOCA patients, a difference that likely can be attributed to the high-risk nature of the MI event and ability to perform more testing during their index inpatient admission.
We acknowledge the low rate of MACE endpoints, which limits our ability to detect significant differences. We suspect that this may be due to the relatively short follow-up, in contrast to that of other studies that have typically reported on a 5- to 10-year follow-up period, such as the Women’s Ischemia Syndrome Evaluation (WISE), which followed female patients with predominantly nonobstructive disease. The WISE cohort had reported MACE risks of 6.7% and 12.8% after 10 years of follow-up for women with no disease and with nonobstructive disease, respectively, with earlier timepoints not being described.5 Heart failure hospitalizations were found to have an unexpectedly high incidence in the WISE cohort (11.2%), documented as the most common MACE following a diagnosis of CMD after being followed for 4.6-8.6 years.24 Importantly, the higher proportion of hypokinesis and akinesis in our MINOCA patients suggests that they may be at a higher relative risk for subsequent heart failure than are INOCA patients, highlighting the importance of ongoing follow-up of these patients over a longer period.
After 3 years of follow-up in our LDWHC, a number of changes were observed in the use of medical therapies. Short- and long-acting nitrates were common medications prescribed for both MINOCA and INOCA patients and are well recognized for their antianginal properties.4,6 Non-ASA antiplatelets were commonly deprescribed, a finding supported by their lack of substantial benefit as seen in the available literature, particularly in INOCA patients.25 Whether to implement beta blockade in patients with MINOCA remains a nuanced decision, likely explaining why this therapy was both one of the most prescribed and deprescribed medications over 3 years of follow-up. When Lindahl et al. assessed the utility of beta blockade, a trend toward a positive effect was seen; however, the level of benefit of this approach failed to reach statistical significance.25 This potential but unclear benefit is under further review, awaiting the results of the Randomized Evaluation of Beta Blocker and ACE-Inhibitor / Angiotensin Receptor Blocker Treatment in Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA-BAT) trial.26 Calcium channel blockers were one of the most prescribed medications for patients with INOCA, but not for patients with MINOCA. This finding may be reflective of calcium channel blockers having been initiated in 42.6% of MINOCA patients prior to their LDWHC enrollment, meaning they entered the LDWHC while already receiving this appropriate therapy.
The 2023 Japanese Circulation Society, Japanese Association of Cardiovascular Intervention and Therapeutics, and Japanese College of Cardiology (JCS/CVIT/JCC) guidelines on coronary vasospasm and CMD acknowledge that approximately 20% of patients may prove refractory to these therapies and warrant alternative therapeutic considerations.27 Ranolazine is a piperazine derivative that selectively inhibits the late sodium current, with evidence suggestive of its benefit in CMD; its use can be considered as adjunctive therapy in chronic angina.28 Ranolazine was one of the most prescribed medications for INOCA patients in the LDWHC, with 22% having been initiated on the therapy compared to 4% of MINOCA patients. The reasons for this difference are likely multifactorial, owing to CMD being a more common etiology in INOCA patients and having a more challenging symptom profile necessitating additional line therapy.
Our study identified differences in angina through SAQ responses, stratified by (M)INOCA group, depression status, and prescription of ranolazine at 3 years. INOCA patients experienced significant improvements across all SAQ subdomains. MINOCA patients did not have a similar improvement in physical limitations; however, they showed significant improvement in the other subdomains of angina frequency, quality of life, and treatment satisfaction.
Patients with depression at the time of their LDWHC enrollment had significantly worse baseline SAQ scores across all metrics, including angina frequency and quality of life. This finding is reflective of the literature, wherein depression has been independently associated with angina in patients without obstructive CAD.9 Reassuringly, having depression at baseline was not a significant modifier for any subdomain over 3 years, suggesting that patients with depression improved to a degree similar to that of those without depression. Mental health status at presentation of cardiac symptoms has important implications for the interpretation of angina severity; a women’s heart health psychiatrist is an important part of our team approach in the LDWHC and may help contribute to the improvements seen in these patients. Acknowledging the bidirectional relationship between mental health and angina, attendance at a specialized clinic addressing both in unison also represents a plausible explanation for the improvements seen in these patients.
Improving mental health symptoms is an important priority for reducing not only symptom burden, but also long-term adverse cardiovascular outcomes. Appreciation of the interplay between mental health and cardiovascular health is increasing.29 From a sex-specific perspective, women are twice as likely as men to be diagnosed with depression, exemplified by the INTERHEART study of risk factors for acute myocardial infarction across 52 countries that found psychosocial factors (depression, locus of control, perceived stress, life events) increase the odds of MI in women 3.5-fold, compared to 2.6-fold in men.30
In this study, patients who were prescribed ranolazine by year 3 had significantly worse SAQ scores, for all subdomains, at the time of LDWHC enrollment. These patients subsequently improved to a similar degree across timepoints as patients not on ranolazine; for medication satisfaction, however, ranolazine was a significant modifier toward greater improvement over time. This medication, which is new to Canada, is an important adjunctive agent and should be considered early in treatment.
Limitations
WHCs are relatively novel entities, with our centre being 1 of 6 in Canada, and the only centre in British Columbia. This context, and our single-centre data collection without use of a control group, carry inherent limitations. Our patient population is predominantly Caucasian, and other ethnicities are underrepresented. Further, due to SCAD patients being referred to a dedicated SCAD clinic rather than to our WHC, this patient population is underrepresented in this dataset compared to other studies of (M)INOCA patients. Therefore, the level of appropriate generalizability of our results to other WHCs with more representation of SCAD and other ethnicities is unclear. Further, measures of gender identity were not captured. The extent to which our findings extend to male patients with nonobstructive vasomotor etiologies is unclear.
Conclusion
This study addresses a gap in longer-term data for (M)INOCA patients by presenting patients’ 3-year outcomes after specialized care in a Canadian WHC. The enhanced diagnostic clarity and the differentiation of symptom characteristics between these patient groups underscores the importance of specialized continuity of care. These findings suggest that ongoing care through a WHC, including attention to mental health and tailored medication strategies, is crucial for achieving optimal outcomes in (M)INOCA patients. Given the practical implications of having only 6 available WHCs across Canada, knowledge dissemination is imperative in bridging the gaps for patients awaiting access to specialized care. When appropriate, virtual healthcare can be beneficial. Additionally, clinical trial and observational data to evaluate the efficacy of different medication regimens on MACE outcomes are warranted across larger cohorts representing a greater diversity of gender and ethnic identities. Future directions entail the establishment of a control group in facilitating robust comparative assessments of long-term data.
Acknowledgements
The authors thank the patients attending the WHC for their participation in this research, statistician May Lee for her valuable consultation in statistical analysis, and Lily Cai, Andrew Starovoytov, Chenille Wong, and Sasha Voznyuk for data collection.
Ethics Statement
Female patients with MINOCA or INOCA who were referred to the LDWHC in Vancouver, Canada, were identified for LDWHC registry enrollment as approved by the University of British Columbia’s clinical research ethics board (application #H13-03322).
Patient Consent
The authors confirm that patient consent forms have been obtained for the WHC Registry through the University of British Columbia clinical research ethics board, application #H13-03322. Secondary consent was not required for this article, as aggregate deidentified data were used for the analyses, as is within the scope of the WHC registry protocol.
Funding Sources
This work was supported through the 2022 BC Women’s Hospital Women’s Health Research Institute Catalyst Grant, and by the Vancouver General Hospital (VGH) Foundation.
Disclosures
E.T. has received honoraria for speaking engagements, from Amicus Therapeutics. T.S. has received honoraria for speaking engagements and advisory board membership from KYE Pharmaceuticals, Sanofi, Amgen, Pfizer, HLS Therapeutics, Novartis Pharmaceuticals, Boehringer Ingelheim and Eli Lilly and Company, and Novo Nordisk. The other authors have no conflicts of interest to disclose.
Footnotes
See page 1474 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2024.08.013.
Supplementary Material
References
- 1.Maddox T.M., Stanislawski M.A., Grunwald G.K., et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan A.M., Gandhi H., Smilowitz N.R., et al. Seasonal and circadian patterns of myocardial infarction by coronary artery disease status and sex in the ACTION Registry-GWTG. Int J Cardiol. 2019;274:16–20. doi: 10.1016/j.ijcard.2018.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theberge E.T., Vikulova D.N., Pimstone S.N., et al. The importance of nontraditional and sex-specific risk factors in young women with vasomotor nonobstructive vs obstructive coronary syndromes. CJC Open. 2024;6:279–291. doi: 10.1016/j.cjco.2023.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agewall S., Beltrame J.F., Reynolds H.R., et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–153. doi: 10.1093/eurheartj/ehw149. [DOI] [PubMed] [Google Scholar]
- 5.Sharaf B., Wood T., Shaw L., et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–141. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary R., Bashline M., Novelli E.M., et al. Sex-related differences in clinical outcomes among patients with myocardial infarction with nonobstructive coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2022;369:1–4. doi: 10.1016/j.ijcard.2022.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Sedlak T.L., Lee M., Izadnegahdar M., et al. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44. doi: 10.1016/j.ahj.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Johnson B.D., Shaw L.J., Pepine C.J., et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI–sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 9.Grodzinsky A., Arnold S.V., Gosch K., et al. Angina frequency after acute myocardial infarction in patients without obstructive coronary artery disease. Eur Heart J Qual Care Clin Outcomes. 2015;1:92–99. doi: 10.1093/ehjqcco/qcv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacheco C., Coutinho T., Bastiany A., et al. Canadian Cardiovascular Society/Canadian Women's Heart Health Alliance clinical practice update on myocardial infarction with no obstructive coronary artery disease (MINOCA) Can J Cardiol. 2024;40:953–968. doi: 10.1016/j.cjca.2024.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Ford T.J., Stanley B., Good R., et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Parvand M., Ghadiri S., Théberge E., et al. Sex, gender, and women's heart health: how women's heart programs address the knowledge gap. CJC Open. 2023;6:442–453. doi: 10.1016/j.cjco.2023.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacheco C., Luu J., Mehta P.K., et al. INOCA and MINOCA: Are women's heart centres the answer to understanding and management of these increasing populations of women (and men)? Can J Cardiol. 2022;38:1611–1614. doi: 10.1016/j.cjca.2022.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parvand M., Cai L., Ghadiri S., et al. One-year prospective follow-up of women with INOCA and MINOCA at a Canadian women’s heart centre. Can J Cardiol. 2022;38:1600–1610. doi: 10.1016/j.cjca.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell R.A., Franks P., Duberstein P.R., et al. Suffering in silence: reasons for not disclosing depression in primary care. Ann Fam Med. 2011;9:439–446. doi: 10.1370/afm.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glanville K.P., Coleman J.R.I., Howard D.M., et al. Multiple measures of depression to enhance validity of major depressive disorder in the UK Biobank. BJPsych Open. 2021;7:e44. doi: 10.1192/bjo.2020.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong P., Camici P.G., Beltrame J.F., et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 19.Lioutas V.A., Ivan C.S., Himali J.J., et al. Incidence of transient ischemic attack and association with long-term risk of stroke. JAMA. 2021;325:373–381. doi: 10.1001/jama.2020.25071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spertus J.A., Winder J.A., Dewhurst T.A., et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 21.Guimarães W.V.N., Nicz P.F.G., Garcia-Garcia H.M., et al. Seattle Angina Pectoris Questionnaire and Canadian Cardiovascular Society angina categories in the assessment of total coronary atherosclerotic burden. Am J Cardiol. 2021;152:43–48. doi: 10.1016/j.amjcard.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Hermesdorf M., Berger K., Baune B.T., et al. Pain sensitivity in patients with major depression: differential effect of pain sensitivity measures, somatic cofactors, and disease characteristics. J Pain. 2016;17:606–616. doi: 10.1016/j.jpain.2016.01.474. [DOI] [PubMed] [Google Scholar]
- 23.Shaw L.J., Merz C.N.B., Pepine C.J., et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 24.Bakir M., Nelson M.D., Jones E., et al. Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the Women's Ischemia Syndrome Evaluation Study. Int J Cardiol. 2016;223:936–939. doi: 10.1016/j.ijcard.2016.07.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindahl B., Baron T., Erlinge D., et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135:1481–1489. doi: 10.1161/CIRCULATIONAHA.116.026336. [DOI] [PubMed] [Google Scholar]
- 26.Nordenskjold A.M., Agewall S., Atar D., et al. Randomized evaluation of beta blocker and ACE-inhibitor/ angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): rationale and design. Am. Heart J. 2021;231:96–104. doi: 10.1016/j.ahj.2020.10.059. [DOI] [PubMed] [Google Scholar]
- 27.Hokimoto S., Kaikita K., Yasuda S., et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. J Cardiol. 2023;82:293–341. doi: 10.1016/j.jjcc.2023.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Rayner-Hartley E., Sedlak T. Ranolazine: a contemporary review. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine G.N., Cohen B.E., Commodore-Mensah Y., et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation. 2021;143:e763–e783. doi: 10.1161/CIR.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 30.Anand SS, Islam S, Rosengren A, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.