Duchenne muscular dystrophy (DMD) is caused by mutations in the dystrophin gene and is characterized by skeletal and cardiac muscle disease. The progression of skeletal muscle disease from fibrosis to fatty infiltration raises the possibility of cardiac fatty replacement. 1 If fatty replacement is present, this would inform cardiac magnetic resonance imaging (CMR) findings and the approach to medical and advanced therapies.
This study is a retrospective case series of patients with DMD at Cincinnati Children's Hospital Medical Center (institutional review board approved) January 2010 to 2020 with cardiac pathology specimens available. Clinical characteristics were obtained by chart review. Consent was obtained in accordance with the institutional review board protocol. Clinical data are available from the corresponding author upon reasonable request. CMR results were included if obtained within 2 years of the tissue collection to understand the relationship of CMR findings and histology.
Cardiac tissue was obtained and stained per clinical lab protocol with hematoxylin and eosin. Ventricular assist device cores were submitted in their entirety. Autopsy tissue samples were collected at the discretion of the pathologist. For cases 5 and 11, autopsy tissue was collected in conjunction with the imaging team to obtain specimens where late gadolinium enhancement (LGE) was visible on CMR.
The median age of the 11 patients was 23.2 years, and the median time between last cardiac imaging and tissue sample was 34 days (range, 1–190 days; Figure). Four (36%) patients had a normal left ventricular ejection fraction before autopsy. Two patients (patients 3 and 11) did not have an echocardiogram within 2 years of autopsy. A CMR 179 days before autopsy on patient 3 revealed a left ventricular ejection fraction of 58%. A CMR 190 days before autopsy on patient 11 revealed a left ventricular ejection fraction of 57%.
Figure 1. Patient characteristics and representative histology samples.
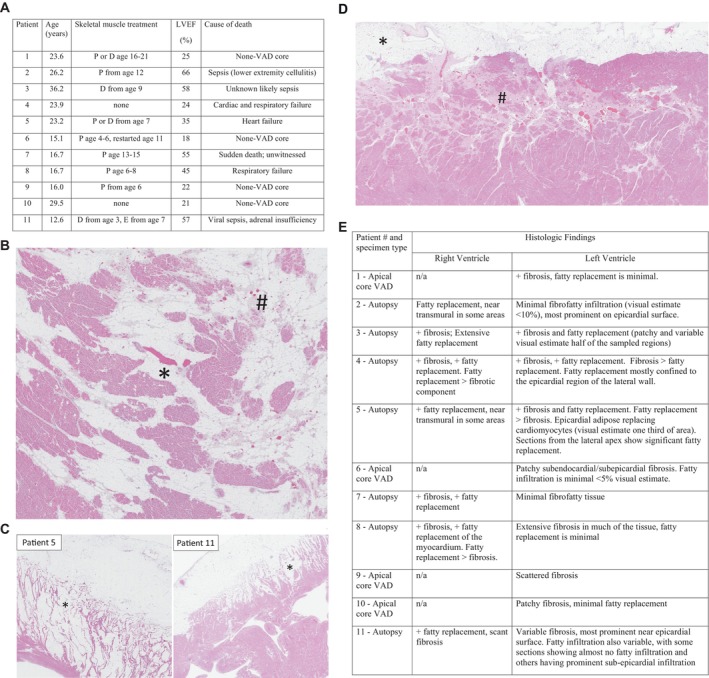
A, Patient characteristics. B, Hematoxylin and eosin stain (1.3×) depicting mixed fibrous and fatty infiltration of the left ventricle from patient 3. C, Hematoxylin and eosin stain of selected right ventricle tissue samples. Fibrofatty infiltration of the right ventricle from cases 5 (0.6×) and 11 (1×) showing fatty infiltration extending from the subepicardial surface and extending toward the endocardial surface. D, Hematoxylin and eosin stain (1×) depicting mixed fibrous (#) and fatty infiltration (*) of the left ventricle from patient 11 corresponding to areas of subepicardial late gadolinium enhancement on cardiac magnetic resonance. E, Summary of histologic findings in each ventricle. D, deflazacort; E, eteplirsen; LVEF, left ventricular ejection fraction; n/a, not available; P, prednisone/prednisolone; and VAD, ventricular assist device.
Six patients (54%) had a CMR with LGE assessment within 2 years of the histology sample (patients 5, 6, 7, 8, 10, and 11). Patient 6 had nearly transmural extension of segments of 5, 6, 9, 11, and 12. Patient 7 had a small possible area of LGE of segments 5 and 11. Patient 10 had LGE of segments 5, 6, 8, 9, 11, 12. There were scattered, nontransmural areas of LGE in segment 16 of the apex. Patient 8 had LGE visible in segments 4, 5, 6, 10, 11, and 12 with some segments demonstrating transmurality (qualitative assessment due to study quality). Patient 11 had LGE first noted at age 12.2 years. He had biventricular ejection fraction >55% on all imaging before the acute illness with sepsis.
Historically, patient 3 had a CMR 1205 days prior demonstrating LGE in segments 5, 6, 10, 11, 12, and 15. Patients 1, 2, 4, and 9 had no CMRs with contrast.
All patients/samples had left ventricular fibrosis. All patients showed evidence of fatty infiltration of the left ventricle, although to varying degrees. Selected tissue demonstrating representative sections of the left and right ventricles are demonstrated in the Figure. The 3 patients who died of infectious complications did not have histologic evidence of myocarditis.
While the majority of histology samples were from unselected areas, 2 patients (5 and 11) had cardiac tissue from specific areas. Patient 5 had LGE in segments 5, 6, 7, 10, 11, 12, 13, and 16 on his most recent CMR. Relatively low basal and mid native T1 values (949 ms and 1001 ms, respectively; institutional norms, 1018 ± 25 ms) were noted. Microscopic examination of corresponding myocardial tissue revealed diffuse fibrofatty infiltration of the left ventricle corresponding to areas of LGE.
Patient 11 had sections taken from the left ventricle corresponding to LGE. Basal and mid native T1 values were 1062 ms and 1053 ms, respectively. Tissue from the inferior right ventricle (2 cm from the apex) and additional random samples from the right and left ventricles were also obtained. The section corresponding to LGE in the mid left ventricular free wall showed fibrofatty infiltration, most prominent near the epicardial surface (Figure). The right ventricle did not show significant fibrosis, but did show extensive fatty replacement with mature adipose tissue extending deep into the myocardium (Figure). The septum showed similar changes to the left ventricle, with predominantly patchy, microscopic fibrosis.
Herein, we describe fibrofatty replacement of the left and right ventricle, including at early stages of disease and in patients with normal systolic function. Given these findings, novel strain imaging, and previous histology data, we hypothesize cardiac disease is characterized by early fibrosis and progressive fatty replacement. 2 , 3 The demonstration of fatty replacement also suggests the therapeutic window for medical interventions focused on cardiac muscle preservation may be earlier than previously thought. Future studies should focus on consistent tissue sampling to provide quantitative rather than qualitative histologic characterization of cardiac progression and to relate these findings to CMR findings.
Sources of Funding
None.
Disclosures
Dr Villa has received consultant fees from PTC Therapeutics, Capricor Therapeutics, Sarepta, Fibrogen, Pfizer, and Antisense Therapeutics. The remaining authors have no disclosures to report.
This manuscript was sent to Yen‐Hung Lin, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 4.
References
- 1. Kinali M, Arechavala‐Gomeza V, Cirak S, Glover A, Guglieri M, Feng L, Hollingsworth KG, Hunt D, Jungbluth H, Roper HP, et al. Muscle histology vs mri in duchenne muscular dystrophy. Neurology. 2011;76:346–353. doi: 10.1212/WNL.0b013e318208811f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: Epimyocardial fibrosis. Human Pathol. 1976;7:375–386. doi: 10.1016/S0046-8177(76)80053-6 [DOI] [PubMed] [Google Scholar]
- 3. Earl CC, Pyle VI, Clark SQ, Annamalai K, Torres PA, Quintero A, Damen FW, Hor KN, Markham LW, Soslow JH, et al. Localized strain characterization of cardiomyopathy in duchenne muscular dystrophy using novel 4d kinematic analysis of cine cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2023;25:14. doi: 10.1186/s12968-023-00922-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


