Abstract
Background
Mosaic loss of chromosome Y (mLOY) in leukocytes of men reflects genomic instability from aging, smoking, and environmental exposures. A similar mosaic loss of chromosome X (mLOX) occurs among women. However, the associations between mLOY, mLOX, and risk of incident heart diseases are unclear.
Methods and Results
We estimated associations between mLOY, mLOX, and risk of incident heart diseases requiring hospitalization, including atrial fibrillation, myocardial infarction, ischemic heart disease, cardiomyopathy, and heart failure. We analyzed 190 613 men and 224 853 women with genotyping data from the UK Biobank. Among these participants, there were 37 037 men with mLOY and 13 978 women with mLOX detected using the Mosaic Chromosomal Alterations caller. Multivariable Cox regression was used to estimate hazard ratios (HRs) and 95% CIs of each incident heart disease in relation to mLOY in men and mLOX in women. Additionally, Mendelian randomization was conducted to estimate causal associations. Among men, detectable mLOY was associated with elevated risk of atrial fibrillation (HR, 1.06 [95% CI, 1.03–1.11]). The associations were apparent in both never smokers (HR, 1.07 [95% CI, 1.01–1.14]) and ever smokers (HR, 1.05 [95% CI, 1.01–1.11]) as well as men aged >60 and ≤60 years. Mendelian randomization analyses supported causal associations between mLOY and atrial fibrillation (HRMR‐PRESSO, 1.15 [95% CI, 1.13–1.18]). Among postmenopausal women, we found a suggestive inverse association between detectable mLOX and atrial fibrillation risk (HR, 0.90 [95% CI, 0.83–0.98]). However, associations with mLOY and mLOX were not found for other heart diseases.
Conclusions
Our findings suggest that mLOY and mLOX reflect sex‐specific biological processes or exposure profiles related to incident atrial fibrillation requiring hospitalization.
Keywords: atrial fibrillation, heart disease, incident relative risk, mosaic loss of sex chromosomes, prospective cohort study
Subject Categories: Atrial Fibrillation, Risk Factors, Mechanisms
Nonstandard Abbreviations and Acronyms
- BAF
B‐allele frequency
- IVW
inverse variance weighted
- MR
Mendelian randomization
- MR‐PRESSO
Mendelian randomization pleiotropy residual sum and outlier
- MR‐RAPS
Mendelian randomization robust adjusted profile score
- mCF
mosaic cell fraction
- mLOX
mosaic loss of chromosome X
- mLOY
mosaic loss of chromosome Y
- PAR
pseudoautosomal region
Research Perspective.
What Is New?
Mosaic loss of chromosome Y (mLOY) in leukocytes of men reflects genomic instability from increasing age, smoking, and the burden of environmental exposures; a similar but less common mosaic loss of the X‐chromosome (mLOX) occurs among women.
In a population free of serious cardiovascular disease at baseline, we estimated associations between mLOY, mLOX, and risk of incident heart disease requiring hospitalization, including atrial fibrillation, myocardial infarction, ischemic heart disease, and cardiomyopathy/heart failure syndrome.
We found that detectable mLOY was associated with elevated risk of atrial fibrillation among men; conversely, among postmenopausal women, we found a suggestive inverse association between detectable mLOX and atrial fibrillation risk.
What Question Should Be Addressed Next?
Investigators should determine why mLOY and mLOX have potentially opposing associations with atrial fibrillation risk among men and women; furthermore, mechanistic studies are needed to address how mLOY and mLOX may be directly or indirectly involved in the pathogenesis of atrial fibrillation.
The Y chromosome contains genes that play crucial roles in biological processes involved in male sex determination, spermatogenesis, and sexual maturation. Furthermore, genetic variants on the Y chromosome have been linked to fundamental cellular housekeeping processes, including mitosis, cell cycle regulation, DNA damage detection and repair, and apoptosis. 1 As such, alterations to the dosage of genes located on the Y chromosome can potentially influence the pathogenesis of chronic diseases that involve these underlying biological pathways. Mosaic loss of chromosome Y (mLOY) is a common genomic alteration that manifests in a fraction of cells as men age. Depending on the study and calling methods, an estimated 7% to 57% of older men, generally aged >65 years, have detectable mLOY in circulating leukocytes. 1 , 2 , 3 , 4 , 5 , 6 , 7 Variation in mLOY has both genetic and environmental components, having associations reported with cigarette smoking, outdoor air pollution, and arsenic. 1 , 4 , 7 , 8
In population studies, mLOY has been linked to risk of various chronic diseases 9 , 10 , 11 , 12 , 13 , 14 ; however, investigations into the contribution of mLOY to the pathogenesis of cardiovascular diseases (CVDs) are still in early stages. 14 , 15 In the UK Biobank, mLOY in leukocytes was found to be cross‐sectionally associated with prevalent diabetes and heart disease. 15 Furthermore, recent analyses found that mLOY was associated with increased risk of death from both atrial fibrillation and heart failure syndrome. 16 The biological mechanisms underlying these relationships with heart diseases in humans remain unclear. However, studies have suggested links between mLOY and increased total and bioavailable testosterone, 17 metabolic biomarkers that we recently found to be associated with incident hospitalization for heart failure. 18
The X chromosome contains essential housekeeping genes responsible for basic biological function. 19 Among men, mosaic loss of chromosome X (mLOX) in leukocytes is poorly tolerated and rarely observed. 20 Among women, however, mLOX in leukocytes can change the dosage and allelic balance of genes on the maternal or paternal X chromosome. Given that 1 X chromosome is epigenetically inactivated in female diploid cells, the biological impact of mLOX is determined by which X chromosome is lost. 21 Previous analyses found that mLOX preferentially affects the inactivated X chromosome. 19 Recent meta‐analyses of large genome‐wide association studies (GWASs) conducted in multiple biobanks have identified 49 independent genetic variants associated with mLOX. 21 However, little is known about its influence on the risk of heart diseases.
Despite a growing body of literature on the relationships between mosaic loss of sex chromosomes and prevalent CVD and CVD‐related mortality, their associations with disease incidence are unclear. Investigating the impact of mosaic loss of sex chromosomes in leukocytes on risk of incident heart diseases is important for understanding the role of genomic instability and immune function in disease pathogenesis and could potentially have translational relevance toward risk prediction. To address these gaps in knowledge, we leveraged the extensive demographic, clinical, and GWAS data from the UK Biobank. In a population free of CVD at baseline, we evaluated the prospective associations between prediagnostic leukocyte mLOY among men, mLOX among women, and risk of incident atrial fibrillation, myocardial infarction, ischemic heart disease, cardiomyopathy, and heart failure syndrome requiring hospitalization.
METHODS
Study Design
The UK Biobank is a prospective cohort study and has been described in detail (http://www.ukbiobank.ac.uk/). 22 , 23 UK Biobank data are publicly available (https://ams.ukbiobank.ac.uk/ams/). Briefly, the source population included adults, aged 40 to 69 years, who lived ≤40 km of 22 study assessment centers across England, Wales, and Scotland. Nearly 9.2 million people registered in the National Health Service were mailed invitations to participate in the study, whereas 503 317 (5.5%) visited the assessment centers in 2006 to 2010 and were enrolled. 22 The volunteers completed touchscreen questionnaires, underwent physical examinations, and provided blood samples for bioassays and genomic analyses. The UK Biobank is regularly updated, and our data set from August 2022 included 502 409 subjects (project number: 28072). The data fields used in our study are shown in Table S1.
GWAS Data
GWAS data were available for 488 377 UK Biobank participants as described. 24 Briefly, genotyping was performed by the Affymetrix Research Services Laboratory (now part of Thermo Fisher Scientific) on 2 similar custom arrays. Among these subjects, 49 950 were scanned on the UK BiLEVE Axiom array, whereas the remaining 438 427 participants were scanned using the UK Biobank Axiom array. The GWAS data set combines results from both arrays, and there were 805 426 markers in the released genotype data set. Approximately 3% of eligible participants who consented to genetic analyses did not have sufficient extracted DNA to conduct the genotyping. Affymetrix used a custom genotype calling pipeline, and the genotype data were quality controlled. Furthermore, the data set was phased and nearly 96 million genotypes were imputed using computationally efficient methods combined with the Haplotype Reference Consortium and UK10K haplotype resources. Information on the quality control pipeline and population structure and relatedness has been described in detail. 24
Kinship coefficients were previously estimated for all pairs of individuals using KING software. 25 These estimates of relatedness are available in UK Biobank data field 22021. Furthermore, data field 22020 restricts to a maximal subset of unrelated (to the third degree) participants who were not sex discordant or outliers for missingness or heterozygosity. The genetic principal components (data field 22009) were generated as previously described in detail. 24
Detection of Mosaic Loss of Sex Chromosomes
Raw genotyping array intensity data were used to calculate B‐allele frequencies (BAFs) and log2 R ratios, which were then analyzed using mosaic chromosomal alterations caller v2022‐01‐12 (https://github.com/freeseek/mochawdl) to detect mosaic chromosomal alteration regions as previously described. 26 , 27 , 28 The mosaic chromosomal alteration calling was performed by Giulio Genovese (Broad Institute of Massachusetts Institute of Technology and Harvard). Mosaic chromosomal alterations caller uses Viterbi hidden Markov models to integrate log2 R ratios and BAFs, leveraging haplotype information to detect subtle imbalances between maternal and paternal allelic fractions in a cell population. SHAPEIT4 software was used for phasing to infer haplotypes. Log2 R ratio was used to determine the status of events (ie, gain, loss, or copy neutral loss of heterozygosity). We focused on subjects with a sample call rate ≥0.97 and baf_auto ≤0.03 to ensure high‐quality samples for subsequent analysis.
Postprocessing of the mosaic chromosomal alteration data to generate the mLOX and mLOY calls was performed at the National Cancer Institute. For mLOY calling, we identified male subjects exhibiting mLOY based on the established criteria outlined (https://github.com/freeseek/mocha). These criteria included a minimum event size >2 MB and a relative copy number <2.5 in the pseudoautosomal region 1 (PAR1) region. The proportion of cells with Y‐chromosome loss (“mosaic cell fraction” [mCF]) was estimated using BAF values in the mLOY region. To improve mLOY detection, mosaic fraction estimates for Y‐chromosome events were derived using BAF deviation on the pseudoautosomal 1 region. For the mLOX calling, we identified female subjects exhibiting mLOX based on the standard criteria specified (https://github.com/freeseek/mocha), including a minimum event size >100 MB and a relative copy number <2.5 on the X chromosome. The mCF with X‐chromosome loss was estimated using BAF values in the mLOX region. Recently, mCF has emerged as an important consideration in mosaic chromosomal alteration analyses, as increased proportions reflect greater white blood cell aberrations and stronger disease associations have been found at mCFs >10%. 29
Ascertainment of Heart Disease Cases
Heart disease cases were defined using inpatient hospital diagnoses coded according to International Classification of Diseases, Ninth Revision (ICD‐9), and International Classification of Diseases, Tenth Revision (ICD‐10). Atrial fibrillation was defined using ICD‐10 (I48, I48.0, I48.1, I48.2, I48.3, I48.4, or I48.9) and ICD‐9 (427.3). Myocardial infarction was defined using ICD‐10 (I21, I22, or I23) and ICD‐9 (412). Ischemic heart disease/angina pectoris was defined using ICD‐10 (I20, I24, or I25) and ICD‐9 (411.1, 413.0, 413.1, 414.0, 414.8, or 414.9). Cardiomyopathy was defined using ICD‐10 (I25.5, I42.0, I42.5, I42.9, or I43) and ICD‐9 (425.4). Heart failure syndrome was defined using ICD‐10 (I42.8, I42.1, I50, I50.1, I50.9, I11.0, I13.0, or I13.2) and ICD‐9 (428.0, 428.1).
We conducted sensitivity analysis using a smaller subgroup of participants (n~229 972, 45% of study population) in which preliminary raw primary care data were collected (https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/primary_care_data.pdf). These primary care data presumably capture more moderate cases that did not initially require hospitalization. Importantly, there is no national system for collecting or sharing primary care data, and the UK Biobank has worked with various data suppliers and other intermediaries to obtain primary care data. Participants provided written consent for linkage of primary care data to their health‐related records. Here, for example, we defined atrial fibrillation using Clinical Terms Version 3 codes that correspond to a text search for “atrial fibrillation.” In the subset with primary care data, we analyzed incident outcomes as: (1) general practitioner–diagnosed specific heart disease, (2) hospitalization for specific heart disease, and (3) combined general practitioner and hospitalization for specific heart disease.
Inclusion/Exclusion Criteria
There were 502 409 participants at baseline. We considered self‐reported sex (data field 31) acquired from the central registry at recruitment (with some data updated by the participant) as well as genetic sex (data field 22001) determined by genotyping array, then excluded 372 participants with discrepancy between genetic and self‐reported sex because of potential data quality issues. Additionally, we excluded 40 who dropped out of the study, 2257 with prevalent heart failure syndrome, and 23 199 with prevalent CVD, which left 476 541 participants (Figure 1). Among these subjects, 463 082 had genotype data for detecting mLOY or mLOX (Table 1) and were considered for further analysis.
Figure 1. Analytical flowchart.
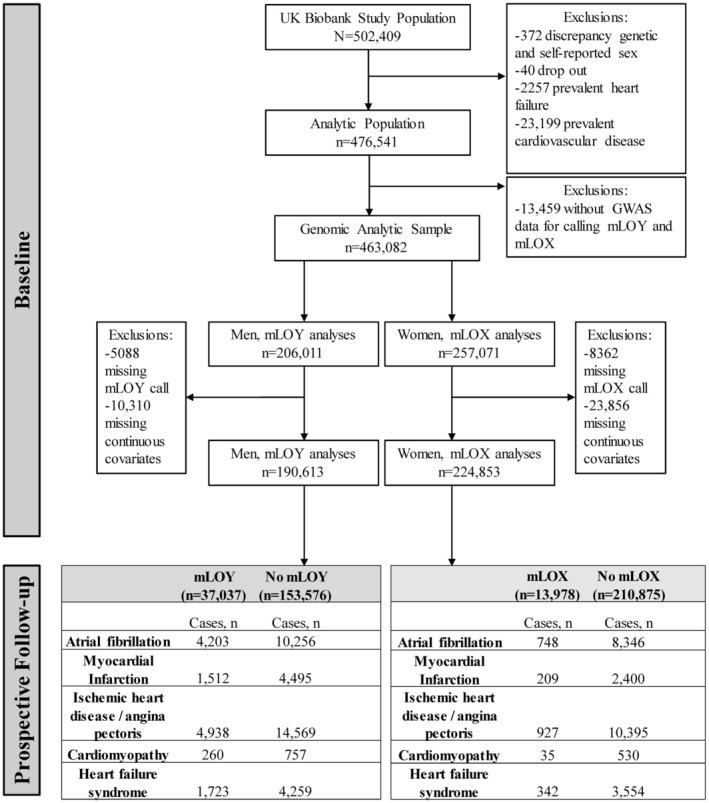
GWAS indicates genome‐wide association study; mLOX, mosaic loss of chromosome X; and mLOY, mosaic loss of chromosome Y.
Table 1.
Baseline Characteristics of UK Biobank Study Population With and Without Mosaic Loss of Sex Chromosomes (n=463 082)
| Characteristic | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detectable mLOY (n=40 043) | No mLOY (n=165 968) | Detectable mLOX (n=15 680) | No mLOX (n=241 391) | |||||||
| No. | Mean (SD) or % | No. | Mean (SD) or % | P value | No. | Mean (SD) or % | No. | Mean (SD) or % | P value | |
| Age at recruitment, y | 40 043 | 61.6 (6.0) | 165 968 | 55.1 (8.2) | <0.0001 | 15 680 | 60.6 (6.6) | 241 391 | 55.9 (8.0) | |
| Body mass index, kg/m2 | ||||||||||
| <18.5 | 90 | 0.2 | 365 | 0.2 | <0.0001 | 99 | 0.63 | 1673 | 0.7 | <0.0001 |
| ≥18.5 to <25 | 10 213 | 25.5 | 40 731 | 24.5 | 5521 | 35.21 | 93 032 | 38.5 | ||
| ≥25 to <30 | 20 139 | 50.3 | 81 156 | 48.9 | 6166 | 39.32 | 87 774 | 36.4 | ||
| ≥30 to <35 | 7254 | 18.1 | 31 954 | 19.3 | 2532 | 16.15 | 36 868 | 15.3 | ||
| ≥35 | 1659 | 4.1 | 9257 | 5.6 | 1127 | 7.19 | 18 237 | 7.6 | ||
| Missing/unknown | 688 | 1.7 | 2505 | 1.5 | 235 | 1.50 | 3807 | 1.6 | ||
| Smoking status | ||||||||||
| Never | 16 133 | 40.3 | 86 637 | 52.2 | <0.0001 | 8999 | 57.39 | 144 230 | 59.7 | <0.0001 |
| Former | 17 039 | 42.6 | 59 466 | 35.8 | 5345 | 34.09 | 74 569 | 30.9 | ||
| Current | 6674 | 16.7 | 19 018 | 11.5 | 1257 | 8.02 | 21 433 | 8.9 | ||
| Unknown | 197 | 0.5 | 847 | 0.5 | 79 | 0.50 | 1159 | 0.5 | ||
| Self‐reported race and ethnicity | ||||||||||
| European | 39 023 | 97.5 | 154 824 | 93.3 | <0.0001 | 15 369 | 98.02 | 227 217 | 94.1 | <0.0001 |
| Mixed | 96 | 0.2 | 913 | 0.6 | 51 | 0.33 | 1687 | 0.7 | ||
| South Asian | 264 | 0.7 | 3426 | 2.1 | 60 | 0.38 | 3349 | 1.4 | ||
| Black/African | 223 | 0.6 | 2949 | 1.8 | 88 | 0.56 | 4132 | 1.7 | ||
| Other groups | 239 | 0.6 | 2919 | 1.8 | 61 | 0.39 | 4039 | 1.7 | ||
| Missing/unknown | 198 | 0.5 | 937 | 0.6 | 51 | 0.33 | 967 | 0.4 | ||
| Townsend deprivation index (socioeconomic status) | 40 004 | −1.5 (3.0) | 165 747 | −1.2 (3.2) | <0.0001 | 15 666 | −1.6 (2.9) | 241 100 | −1.4 (3.0) | |
| Education (qualifications) | ||||||||||
| College or university degree | 12 180 | 30.4 | 59 183 | 35.7 | <0.0001 | 4008 | 25.6 | 77 160 | 32.0 | <0.0001 |
| Advanced levels/Advanced Subsidiary levels or equivalent | 3764 | 9.4 | 17 693 | 10.7 | 1574 | 10.0 | 29 013 | 12.0 | ||
| Ordinary levels/General Certificate of Secondary Education or equivalent | 7153 | 17.9 | 31 265 | 18.8 | 3825 | 24.4 | 56 272 | 23.3 | ||
| Certificate of Secondary Education or equivalent | 1343 | 3.4 | 10 095 | 6.1 | 672 | 4.3 | 13 243 | 5.5 | ||
| National Vocational Qualifications or Higher National Diploma or Higher National Certificate or equivalent | 4010 | 10.0 | 14 421 | 8.7 | 701 | 4.5 | 10 702 | 4.4 | ||
| Other professional qualifications | 2127 | 5.3 | 6886 | 4.1 | 1012 | 6.5 | 13 695 | 5.7 | ||
| Unknown/missing/no answer | 9466 | 23.6 | 26 425 | 15.9 | 3888 | 24.8 | 41 306 | 17.1 | ||
| Alcohol intake | ||||||||||
| Never | 827 | 2.1 | 4719 | 2.8 | <0.0001 | 846 | 5.4 | 13 701 | 5.7 | 0.02 |
| Former | 1371 | 3.4 | 5425 | 3.3 | 551 | 3.5 | 8471 | 3.5 | ||
| Current occasional | 6007 | 15.0 | 26 914 | 16.2 | 4292 | 27.4 | 67 235 | 27.9 | ||
| Current <1 drink/day | 7983 | 19.9 | 33 641 | 20.3 | 4517 | 28.8 | 70 209 | 29.1 | ||
| Current 1–3 drinks/day | 17 629 | 44.0 | 71 479 | 43.1 | 4891 | 31.2 | 72 539 | 30.1 | ||
| Current >3 drinks/day | 6150 | 15.4 | 23 251 | 14.0 | 556 | 3.5 | 8599 | 3.6 | ||
| Unknown | 76 | 0.2 | 539 | 0.3 | 27 | 0.2 | 637 | 0.3 | ||
| Self‐reported diabetes status | ||||||||||
| None | 37 368 | 93.3 | 155 269 | 93.6 | 15 036 | 95.9 | 232 269 | 96.2 | ||
| Diabetic | 2507 | 6.3 | 9837 | 5.9 | 0.0018 | 593 | 3.8 | 8265 | 3.4 | |
| Unknown | 168 | 0.4 | 862 | 0.5 | 51 | 0.3 | 857 | 0.4 | ||
| Glycated hemoglobin, mmol/mol | 38 021 | 36.8 (7.1) | 157 700 | 36.1 (7.3) | <0.0001 | 14 889 | 36.3 (5.6) | 229 328 | 35.6 (5.8) | 0.05 |
| Hypertension status | ||||||||||
| Normal | 2467 | 6.2 | 13 246 | 8.0 | <0.0001 | 2052 | 13.1 | 46 384 | 19.2 | <0.0001 |
| Elevated | 3680 | 9.2 | 17 679 | 10.7 | 1768 | 11.3 | 28 975 | 12.0 | ||
| Stage 1 or 2 | 29 507 | 73.7 | 117 793 | 71.0 | 10 082 | 64.3 | 140 702 | 58.3 | ||
| Hypertensive crisis | 1201 | 3.0 | 3398 | 2.0 | 427 | 2.7 | 4340 | 1.8 | ||
| Unknown | 3188 | 8.0 | 13 852 | 8.3 | 1351 | 8.6 | 20 990 | 8.7 | ||
| Menopausal status | N/A | N/A | ||||||||
| Premenopause | 1455 | 9.3 | 60 187 | 24.9 | <0.0001 | |||||
| Postmenopause | 11 726 | 74.8 | 143 112 | 59.3 | ||||||
| Hysterectomy | 2069 | 13.2 | 26 680 | 11.1 | ||||||
| Bilateral oophorectomy | 11 | 0.1 | 166 | 0.1 | ||||||
| Ever oral contraceptive use | Not applicable | Not applicable | ||||||||
| Yes | 3691 | 23.5 | 43 834 | 18.2 | <0.0001 | |||||
| No | 11 941 | 76.2 | 196 495 | 81.4 | ||||||
| Ever hormone replacement therapy | Not applicable | Not applicable | ||||||||
| Yes | 7801 | 49.8 | 151 542 | 62.8 | <0.0001 | |||||
| No | 7831 | 49.9 | 88 628 | 36.7 | ||||||
mLOX indicates mosaic loss of chromosome X; and mLOY, mosaic loss of chromosome Y.
Follow‐Up
For each participant, the prospective follow‐up started at the date of visit to the assessment center in 2006 to 2010 and ended at the date of first incident diagnosis (hospitalization) of heart disease, death, or administrative censoring (ie, September 20, 2021, for England and Wales and October 31, 2021, for Scotland), whichever came first. Vital status and the primary underlying cause of death for participants were provided by the National Health Service Information Centre and the National Health Service Central Register. The UK Biobank study was approved by the National Information Governance Board for Health and Social Care and the National Health Service North West Multicenter Research Ethics Committee. All participants provided electronic informed consent.
Main Analyses
Separate multivariable Cox regression models were used to estimate hazard ratios (HRs) and 95% CIs of incident hospitalization for atrial fibrillation, myocardial infarction, ischemic heart disease/angina pectoris, cardiomyopathy, and heart failure syndrome in relation to mLOY (detectable versus not detectable) among men and mLOX (detectable versus not detectable) among women. For mLOY analyses among men, we adjusted for potential confounders, including study assessment center, age at recruitment (continuous), self‐reported race and ethnicity to indirectly adjust for culture‐specific dietary patterns, environmental factors, social determinants of health (European, Black/African, mixed, South Asian, other groups [other participants who did not report themselves as European, Black/African, mixed, South Asian, or missing/unknown], or missing/unknown), 10 principal components for genetic ancestry, educational attainment (ie, qualifications), smoking status (never, former, or current, missing/unknown), body mass index (<18.5, ≥18.5 to <25, ≥25 to <30, ≥30 to <35, or ≥35 kg/m2 or missing/unknown), material deprivation (Townsend deprivation index, socioeconomic status, or continuous), alcohol intake derived by combining drinking status and amount consumed per week calculated as the sum of all alcoholic beverages consumed on average per day (never, former, current occasional, current <1 drink/day, current 1–3 drinks/day, current >3 drinks/day, unknown, or missing/unknown), self‐reported diabetes status (none, diabetic, or missing/unknown), glycated hemoglobin (mmol/mol, continuous), hypertension status based on average systolic and diastolic blood pressure at baseline (normal, elevated, stage 1 and 2 hypertension, hypertensive crisis, and unknown), total white blood cell count at enrollment, and any cancer diagnosis at enrollment. For mLOX analyses among women, we adjusted for the same covariates as men in addition to oral contraceptive use (never and ever), hormone replacement therapy use (never and ever), and menopausal status (premenopause, natural postmenopause, artificial menopause, and missing/unknown). We conducted additional analyses stratified by leukocyte mCFs, smoking status (never and ever), age groups (>60 and ≤60 years), menopausal status (premenopause and postmenopause) among women, as well as a sensitivity analysis restricted to European genetic ancestry participants determined using data field 22006 (participants who self‐identified as “White British” in data field 21000 and have similar genetic ancestry based on a principal components analysis of genotypes). Categorical variables were coded with a category for missingness. We analyzed participants with complete data on continuous independent variables. Overall and within stratified and subgroup analyses, P<0.05 was considered noteworthy, and those below a Bonferroni‐corrected α threshold of 0.01 were considered statistically significant (ie, 0.05 divided by a family of 5 tests corresponding to each heart disease outcome). We checked for violations of proportional hazards assumptions by visually inspecting the Schoenfeld residuals over the follow‐up time as well as using cross‐product terms between mLOY/mLOX and time. We did not find clear evidence of violations of proportional hazards assumptions. The P values for the cross‐product terms with time were 0.34 for mLOY and 0.78 for mLOX.
Attenuation and Sensitivity Analyses
To tightly control potential residual confounding from smoking in the main analyses, we adjusted for a 27‐category variable for smoking history and intensity. 12 , 15 Additionally, to account for nonlinear relationships with age, we included polynomial terms for age in the models. We also conducted Fine and Gray models adjusted for the same covariates to account for competing risks by all‐cause mortality and/or heart failure.
Mendelian Randomization Analyses
Among the measured mLOY, mLOX, and heart disease associations found to be statistically significant in the Cox regression analyses, we further assessed potential causal associations using several established Mendelian randomization (MR) methods. First, we used the inverse variance weighted (IVW) method, an effective approach when all included genetic variants are valid instrumental variables. 30 However, when horizonal pleiotropy is present, the estimate from IVW approach can be potentially biased with overestimation, 30 , 31 and we therefore used additional approaches as sensitivity analyses. We used the weighted median approach, which has been shown to produce valid causal estimates if the invalid instrumental variables account for <50% of the weight of the studies. 30 Next, we used MR‐Egger, which unlike other MR methods, estimates the dose‐response relationship between the genetic associations with mLOY and those with the heart disease outcome. 32 A nonzero intercept from MR‐Egger regression indicates potential horizontal pleiotropy, whereas the slope reflects the effect of mLOY on heart disease adjusted for horizontal pleiotropy. 33 Next, we used MR pleiotropy residual sum and outlier (MR‐PRESSO), an approach that effectively accounts for horizonal pleiotropy by identifying instrumental variables that are outliers. 31 By doing so, the method prevents potential horizonal pleiotropy and corrects the causal estimates. 31 Last, we used robust adjusted profile score (RAPS), which maximizes the profile likelihood of the ratio estimates while accounting for weak instrument bias, pleiotropy, and extreme outliers. 34
We used the MendelianRandomization, MR‐PRESSO, and mr.raps packages in R statistical software (version 4.2.2) to conduct MR analyses with summary GWAS data from a previously published landmark study and newly conducted analyses 1 , 35 (Data S1). A 1‐sample MR analysis was conducted using 156 previously identified independent variants (with r 2<0.05 in linkage disequilibrium (LD) clumping analyses) in the UK Biobank. 1 All 156 genetic variants were previously found to be associated with mLOY below a standard genome‐wide significance threshold of 5×10−8 in the discovery phase of the UK Biobank GWAS. 1 Notably, these genetic variants were previously validated in 757 114 men from separate European and Japanese populations. 1
RESULTS
Baseline Characteristics
The study population characteristics at baseline are shown in Table 1. Here, 19.4% of the male study participants had detectable mLOY, whereas 6.1% of the female study participants had detectable mLOX. On average, men with detectable mLOY were older (61.6 versus 55.1 years), had higher proportions of former (42.6% versus 35.8%) or current smokers (16.7% versus 11.5%), had lower proportions of non‐Europeans, and had lower proportions of college/university degree holders (30.4% versus 35.7%; Table 1). Similarly, among women, those with detectable mLOX were older (60.6 versus 55.9 years) on average, had higher proportions of former smokers (34.09% versus 30.90%), had lower proportions of non‐Europeans, and had lower proportions of college/university degree holders (25.6% versus 32.0%; Table 1).
Incident Heart Disease Cases
Among men, there were 14 459 incident cases (hospitalizations) of atrial fibrillation, 6007 cases of myocardial infarction, 19 507 cases of ischemic heart disease, 1017 cases of cardiomyopathy, and 5982 cases of heart failure syndrome during the follow‐up (Table S2). Among women, there were 9094 incident cases of atrial fibrillation, 2609 cases of myocardial infarction, 11 322 cases of ischemic heart disease, 565 cases of cardiomyopathy, and 3896 cases of heart failure syndrome during the follow‐up (Table S2). The ages at first diagnosis (hospitalization) and the follow‐up time to first diagnosis for each heart disease are shown in Table S2.
Associations of Age and Smoking With mLOY and mLOX
We conducted unadjusted analyses with age, smoking status, and sex chromosome loss at baseline to check the consistency with previous studies. Age was associated with increased prevalence odds of mLOY in men (odds ratio [OR], 1.13 per year [95% CI, 1.13–1.13]; P<0.0001) and mLOX in women (OR, 1.09 per year [95% CI, 1.09–1.10]; P<0.0001). As expected, among men, former smokers (OR, 1.54 [95% CI, 1.50–1.58]; P<0.0001) and current smokers (OR, 1.88 [95% CI, 1.82–1.95]; P<0.0001) had increased prevalence odds of mLOY compared with never smokers. Among postmenopausal women, former smoking (OR, 1.07 [95% CI, 1.03–1.11]; P=0.0014) was associated with increased prevalence odds of mLOX, but not current smoking (OR, 0.97 [95% CI, 0.90–1.04]; P=0.3865), which was similar to parallel analyses. 21
mLOY Among Men and Future Risk of Heart Disease
Among men, having detectable mLOY in leukocytes was significantly associated with elevated risk of incident atrial fibrillation requiring hospitalization (HR, 1.06 [95% CI, 1.03–1.11]; P=0.0011; Table 2), even after accounting for multiple comparisons. Furthermore, the association with atrial fibrillation strengthened nonmonotonically as the mCF increased above 20% (HR mCF=0.20‐<0.30, 1.20 [95% CI, 1.07–1.35]; HRmCF=0.30‐<0.40, 0.95 [95% CI, 1.12–1.32]; HRmCF=0.40‐<0.50, 1.38 [95% CI, 1.13–1.69]; P=0.0015; HRmCF>0.50, 1.44 [95% CI, 1.15–1.80]; P=0.0015; Figure 2). The association between detectable mLOY and atrial fibrillation was consistent across subgroups, including those of European genetic ancestry (HR, 1.08 [95% CI, 1.04–1.12]; P=0.0002), those aged >60 years (HR, 1.08 [95% CI, 1.04–1.13]; P=0.0003), and those aged ≤60 years (HR, 1.07 [95% CI, 1.02–1.11]; P=0.0021; Table S3). The atrial fibrillation associations among never smokers (HR, 1.07 [95% CI, 1.01–1.14]; P=0.0294) and ever smokers (HR, 1.05 [95% CI, 1.01–1.11]; P=0.0298) were apparent, but nonsignificant, after Bonferroni correction (Table S3). Consistent associations were not found for myocardial infarction, ischemic heart disease/angina pectoris, cardiomyopathy, and heart failure syndrome in the overall (Table 2) and stratified analyses (Table S3).
Table 2.
mLOY in Prediagnostic Leukocytes and Risk of Incident Heart Diseases in the UK Biobank
| Outcome | Detectable mLOY (total n=37 037) | No mLOY (total n=153 576) | Censored deaths, n | HR | 95% CI lower | 95% CI upper | P value |
|---|---|---|---|---|---|---|---|
| Cases, n | Cases, n | ||||||
| Atrial fibrillation | 4203 | 10 256 | 13 210 | 1.06 | 1.03 | 1.11 | 0.0011** |
| Myocardial infarction | 1512 | 4495 | 15 298 | 1.05 | 0.98 | 1.11 | 0.1448 |
| Ischemic heart disease/angina pectoris | 4938 | 14 569 | 13 387 | 1.00 | 0.96 | 1.03 | 0.8706 |
| Cardiomyopathy | 260 | 757 | 16 079 | 1.05 | 0.90 | 1.21 | 0.5579 |
| Heart failure syndrome | 1723 | 4259 | 14 320 | 1.04 | 0.98 | 1.10 | 0.2115 |
Separate multivariable Cox regression models were used to estimate HRs and 95% CIs of newly diagnosed (incident) atrial fibrillation, myocardial infarction, ischemic heart disease/angina pectoris, cardiomyopathy, and heart failure syndrome in relation to mLOY (detectable vs not detectable) among men. We adjusted for potential confounders, including study assessment center, age at recruitment (continuous), self‐reported race and ethnicity (European, Black/African, mixed, South Asian, other groups, or missing/unknown), 10 principal components for genetic ancestry, educational attainment (ie, qualifications), smoking status (never, former, current, or missing/unknown), body mass index (<18.5, ≥18.5 to <25, ≥25 to <30, ≥30 to <35, or ≥35 kg/m2 or missing/unknown), material deprivation (Townsend deprivation index, socioeconomic status, or continuous), alcohol intake (never, former, current occasional, current <1 drink/day, current 1–3 drinks/day, current >3 drinks/day, or unknown/missing), diabetes status (none, diabetic, or missing/unknown), glycated hemoglobin (mmol/mol, continuous), and hypertension status based on average systolic and diastolic blood pressure at baseline (normal, elevated, stage 1 and 2 hypertension, hypertensive crisis, and missing/unknown), total white blood cell count at enrollment, and any cancer diagnosis at enrollment.HR indicates hazard ratio; and mLOY, mosaic loss of chromosome Y.
P values below a Bonferroni‐corrected P value of 0.01 (0.05 divided by a family of 5 tests). We analyzed participants with complete mLOY and covariate data.
Figure 2. Mosaic loss of chromosome Y (mLOY) in prediagnostic leukocytes and risk of newly diagnosed atrial fibrillation, stratified by mosaic cell fractions (mCFs) with mLOY.
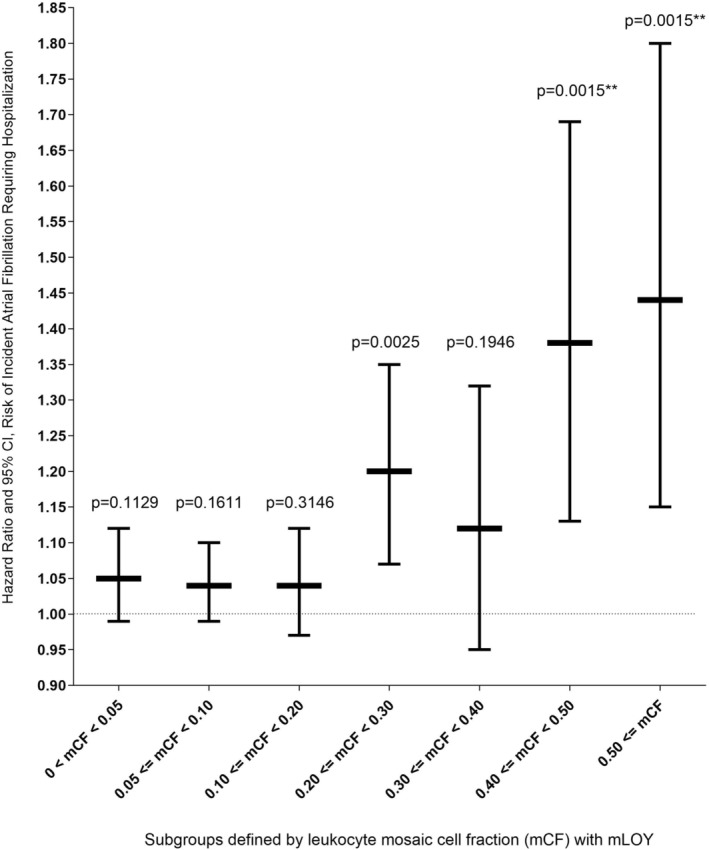
We analyzed participants with complete genotyping and covariate data. Multivariable Cox regression models were used to estimate hazard ratios and 95% CIs of newly diagnosed (incident) atrial fibrillation in relation to mLOY (detectable vs not detectable) among men. We adjusted for potential confounders, including study assessment center, age at recruitment, self‐reported race and ethnicity, 10 principal components for genetic ancestry, educational attainment (ie, qualifications), smoking status, body mass index, material deprivation, alcohol, diabetes status, glycated hemoglobin (mmol/mol), and hypertension status based on average systolic and diastolic blood pressure at baseline, total white blood cell count at enrollment, and any cancer diagnosis at enrollment. Analytic subgroup sample sizes with complete data: no detectable mLOY (comparison group): 10245 cases/153367 men; 0<mCF<0.05: 1090 cases/11531 men; 0.05≤mCF<0.10: 1649 cases/14905 men; 0.10≤mCF<0.20: 843 cases/6703 men; 0.20≤mCF<0.30: 299 cases/1972 men; 0.30≤mCF<0.40: 141 cases/939 men; 0.40≤mCF<0.50: 98 cases/533 men; and mCF<0.50: 78 cases/418 men.
Sensitivity and Attenuation Analyses
To tightly control potential residual confounding from smoking, we adjusted for a 27‐category variable for smoking history and intensity. 12 , 15 Here, the mLOY–atrial fibrillation estimates were not considerably changed (HR, 1.06 [95% CI, 1.02–1.10]; P=0.0016). Additionally, we included a quadratic term for age; however, this parameter was nonsignificant (P=0.19), and the main mLOY–atrial fibrillation association remained statistically significant (HR, 1.06 [95% CI, 1.02–1.10]; P=0.0014).
There were 3254 men who were hospitalized with both incident atrial fibrillation and heart failure during the follow‐up. Among these cases, 585 men (17.98%) had incident hospitalization for heart failure before atrial fibrillation, 1570 men (48.25%) had heart failure after atrial fibrillation, and 1099 men (33.77%) were hospitalized for both concurrently. The estimates from the Fine and Gray models for mLOY–atrial fibrillation associations accounting for competing risks by all‐cause mortality alone (Table S4), as well as heart failure and all‐cause mortality together (data not shown), were not considerably different from the cause‐specific Cox models.
When analyzing a subgroup of 87 041 participants who had primary care data and met our inclusion criteria, we found that the association between mLOY and general practitioner diagnosed incident atrial fibrillation had a similar effect size but was nonsignificant (605 cases/16749 with mLOY; 1470 cases/70292 without mLOY; HR, 1.08 [95% CI, 0.96–1.20]; P=0.19) compared with incident hospitalization for atrial fibrillation (1849 cases/16749 with mLOY; 4538 cases/70292 without mLOY; HR, 1.08 [95% CI, 1.02–1.14]; P=8.1 × 10−3). However, there was no evidence that these estimates were different (P‐difference Z score sign=1.00). When combining general practitioner and hospitalization outcome ascertainment, the association between mLOY and atrial fibrillation risk was statistically significant (1933 cases/16749 with mLOY; 4746 cases/70292 without mLOY; HR, 1.09 [95% CI, 1.03–1.16]; P=2.1 × 10−3).
MR, mLOY, and Atrial Fibrillation Risk
We conducted MR analyses to assess potential causal associations between mLOY and atrial fibrillation risk among men. Using IVW, we found evidence supporting a causal relationship between mLOY and atrial fibrillation (HRIVW, 1.15 [95% CI, 1.12–1.19]; P=1.52×10−21; Figure 3). We conducted sensitivity analyses with other MR methods and found that the weighted median (HRWM, 1.09 [95% CI, 1.05–1.14]; P=1.42×10−24; Figure 3), MR‐PRESSO (HRMR‐PRESSO, 1.15 [95% CI, 1.13–1.18]; P=5.22×10−5; Figure 3), and MR‐RAPS approaches (HRMR, 1.10 [95% CI, 1.07–1.13]; P=1.10×10−9; Figure 3) agreed with our main finding. Conversely, the MR‐Egger slope was nonsignificant (P=0.188), and the intercept was nonzero (β0=0.011, P<0.001), suggesting detection of pleiotropy.
Figure 3. Mendelian randomization (MR) analyses to assess the potential causal associations between prediagnostic leukocyte mosaic loss of chromosome Y and risk of newly diagnosed atrial fibrillation in the UK Biobank.
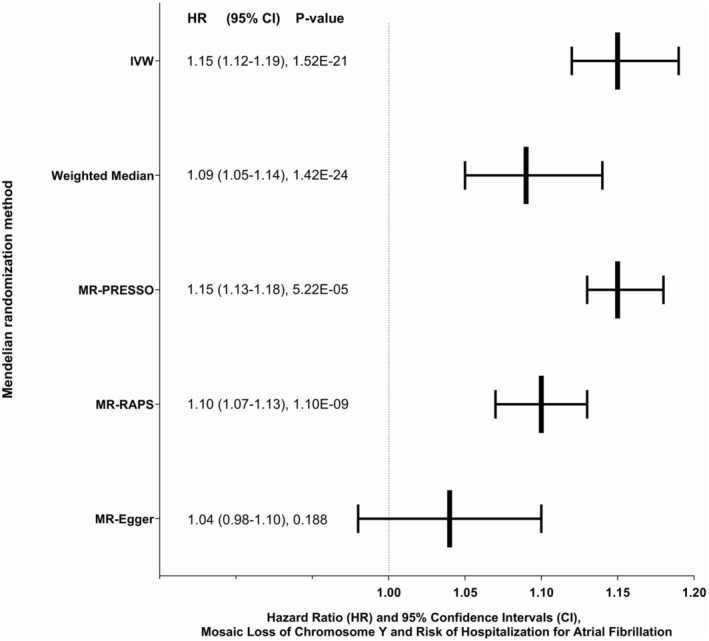
IVW indicates inverse variance weighted; MR‐PRESSO, MR pleiotropy residual sum and outlier; and MR‐RAPS, MR robust adjusted profile score.
mLOX Among Women and Future Risk of Heart Disease
Among women overall, having detectable mLOX in leukocytes was associated with decreased risk of atrial fibrillation requiring hospitalization before accounting for multiple testing; however, this association was nonsignificant after Bonferroni correction (HR, 0.91 [95% CI, 0.84–0.98]; P=0.0130; Table 3). The suggestive inverse association between mLOX and atrial fibrillation risk was observed only among postmenopausal women (HR, 0.90 [95% CI, 0.83–0.98]; P=0.0151; Table 3), who comprise most of the female participants. When further stratifying women into subgroups, the suggestive inverse association with atrial fibrillation was consistent among participants of European ancestry (HR, 0.91 [95% CI, 0.84–0.98]; P=0.0145), and those aged >60 and ≤60 years at baseline (HR, 0.90 [95% CI, 0.83–0.98]; P=0.0164; and HR, 0.89 [95% CI, 0.82–0.96]; P=0.0044, respectively) before accounting for multiple testing, but was significant only among women aged ≤60 years after Bonferroni correction (Table S5).
Table 3.
mLOX in Prediagnostic Leukocytes From Women and Risk of Incident Heart Diseases in the UK Biobank
| Heart disease | Detectable mLOX | No mLOX | Censored deaths, n | HR | 95% CI lower | 95% CI upper | P value |
|---|---|---|---|---|---|---|---|
| Cases, n | Cases, n | ||||||
| Overall women (total n of subjects) θ | 13 978 | 210 875 | |||||
| Atrial fibrillation | 748 | 8346 | 10 351 | 0.91 | 0.84 | 0.98 | 0.0130* |
| Myocardial infarction | 209 | 2400 | 11 456 | 0.97 | 0.84 | 1.12 | 0.7012 |
| Ischemic heart disease/angina pectoris | 927 | 10 395 | 10 577 | 1.00 | 0.93 | 1.07 | 0.9477 |
| Cardiomyopathy | 35 | 530 | 11 819 | 0.79 | 0.56 | 1.11 | 0.1752 |
| Heart failure syndrome | 342 | 3554 | 10 795 | 0.99 | 0.89 | 1.11 | 0.8884 |
| Postmenopause (total n of subjects) | 10 743 | 131 193 | |||||
| Atrial fibrillation | 601 | 6534 | 8064 | 0.90 | 0.83 | 0.98 | 0.0151* |
| Myocardial infarction | 170 | 1818 | 9004 | 0.98 | 0.84 | 1.15 | 0.7997 |
| Ischemic heart disease/angina pectoris | 727 | 7615 | 8326 | 1.00 | 0.93 | 1.08 | 0.9601 |
| Cardiomyopathy | 28 | 374 | 9297 | 0.84 | 0.57 | 1.23 | 0.3635 |
| Heart failure syndrome | 272 | 2750 | 8475 | 0.98 | 0.86 | 1.11 | 0.7473 |
| Premenopause (total n of subjects) | 1330 | 55 054 | |||||
| Atrial fibrillation | 18 | 468 | 851 | 1.09 | 0.68 | 1.76 | 0.7160 |
| Myocardial infarction | 7 | 216 | 876 | 0.98 | 0.45 | 2.10 | 0.9527 |
| Ischemic heart disease/angina pectoris | 40 | 918 | 839 | 1.29 | 0.93 | 1.78 | 0.1248 |
| Cardiomyopathy | 2 | 62 | 893 | 1.12 | 0.27 | 4.66 | 0.8727 |
| Heart failure syndrome | 9 | 218 | 843 | 1.05 | 0.53 | 2.11 | 0.8884 |
θ Includes women in premenopause, postmenopause, and artificial menopause.
Separate multivariable Cox regression models were used to estimate HRs and 95% CIs of newly diagnosed (incident) atrial fibrillation, myocardial infarction, ischemic heart disease/angina pectoris, cardiomyopathy, and heart failure syndrome in relation to mLOX (detectable vs not detectable) among women. We adjusted for potential confounders, including study assessment center, age at recruitment (continuous), self‐reported race and ethnicity (European, Black/African, mixed, South Asian, other groups, or missing/unknown), 10 principal components for genetic ancestry, educational attainment (ie, qualifications), smoking status (never, former, or current), body mass index (<18.5, ≥18.5 to <25, ≥25 to <30, ≥30 to <35, or ≥35 kg/m2 or missing/unknown), material deprivation (Townsend deprivation index, socioeconomic status, or continuous), alcohol intake (never, former, current occasional, current <1 drink/day, current 1–3 drinks/day, current >3 drinks/day, or missing/unknown), diabetes status (none, diabetic, or unknown/missing), glycated hemoglobin (mmol/mol, continuous), and hypertension status based on average systolic and diastolic blood pressure at baseline (normal, elevated, stage 1 and 2 hypertension, hypertensive crisis, and unknown/missing), total white blood cell count at enrollment, ever use of oral contraceptive or hormone replacement therapy, and any cancer diagnosis at enrollment. HR indicates hazard ratio; and mLOX, mosaic loss of chromosome X.
P<0.05.
There were 1835 women who were hospitalized with both incident atrial fibrillation and heart failure during the follow‐up. Among these cases, 312 women (17.00%) had incident hospitalization for heart failure before atrial fibrillation, 952 women (51.88%) had heart failure after atrial fibrillation, and 571 women (31.12%) were hospitalized with both concurrently. After accounting for the influence of all‐cause mortality alone (Table S4), as well as heart failure and all‐cause mortality together as competing risks for atrial fibrillation (data not shown), the association between mLOX and atrial fibrillation was similar to the cause‐specific Cox models.
DISCUSSION
In a large prospective cohort study from the United Kingdom, we investigated the associations between mLOY and mLOX in prediagnostic leukocytes collected at baseline and risk of incident heart disease requiring hospitalization. Among men, we found that mLOY was significantly associated with elevated risk of incident atrial fibrillation. This association was greatly strengthened among participants with increased mCF with mLOY and was largely consistent across subgroups defined by smoking, age, and race and ethnicity. Furthermore, most of the MR analyses supported causal associations between mLOY and atrial fibrillation. However, consistent associations between mLOY and other heart diseases were not detected. To our knowledge, we conducted the first study of mLOX among women and risk of newly diagnosed heart disease. Here, we found suggestive evidence for inverse associations between mLOX and atrial fibrillation risk that was nonsignificant after accounting for multiple comparisons. When stratifying by menopausal status, the suggestive association between mLOX and atrial fibrillation was only observed among postmenopausal women. Furthermore, given that mLOX is less frequent among younger women 19 and that most female UK Biobank participants were aged >50 years, we did not have sufficient sample size to make sound inferences for premenopausal women. Additionally, consistent associations were not detected between mLOX and other heart diseases among women.
Our study examines the relationships between sex chromosome mosaicisms and CVD by focusing specifically on incident heart disease hospitalization, as opposed to prevalence and mortality as in other studies. 15 , 16 , 36 We provide population‐based evidence that mLOY and mLOX could be indirectly or directly involved in the biological processes leading to atrial fibrillation occurrence. Our investigation builds on previous studies conducted in human populations. Loftfield et al reported cross‐sectional associations between prevalent heart disease and stroke and increased leukocyte mLOY at baseline. 15 Furthermore, they reported no evidence for associations between mLOY and mortality from cardiovascular disease, ischemic heart disease, and stroke. 15 Additionally, a recent study by Sano et al found evidence for associations between mLOY and death caused by diseases of the circulatory system, including hypertensive heart disease, atrial fibrillation, as well as heart failure syndrome. 16
We note that our outcome ascertainment using national hospital registries potentially enriches for more serious cases of heart disease, which can be nonfatal or fatal. As such, we conducted analyses using a subset of participants with preliminary primary care data, in which the cases had moderate, nonfatal incident heart disease. Here, we found that the associations between mLOY and general practitioner–diagnosed atrial fibrillation were similar in magnitude to the hospitalization data but were not statistically significant. This was likely attributable to limited statistical power from reduced case numbers. Important for consideration, these preliminary primary care data have not been validated or adjudicated, and we include these only for exploratory purposes and sensitivity analyses. When combining general practitioner–diagnosed and hospitalization cases in this subset, the mLOY–atrial fibrillation association was statistically significant.
Our MR analyses using IVW, weighted median, MR‐PRESSO, and MR‐RAPS strongly supported causal associations between mLOY and atrial fibrillation and had similar causal effect estimates. However, we found that the estimate from the older MR‐Egger regression was nonsignificant, and the nonzero intercept suggested some pleiotropy. MR‐Egger is different from other MR methods in that it estimates the casual effect as the slope from the weighted regression of the variant‐outcome associations on the variant‐exposure associations, with the average pleiotropic effect estimated by the intercept. MR‐Egger allows for the intercept to be nonzero, but this adds a degree of freedom that can decrease statistical efficiency compared with other methods. Furthermore, MR‐Egger is problematic because the necessary instrument strength independent of direct effect (independence between the Egger regression intercept and slope) assumption is rarely satisfied. As such, the estimates from MR‐Egger are sensitive to influential outliers and can be skewed, as well as lacking in precision when the variant‐exposure associations are similar in magnitude. Newer methods like MR‐PRESSO provide more precise and robust causal estimates by using global tests that detect horizontal pleiotropy; outlier‐corrected causal estimates that correct for the detected horizontal pleiotropy; and distortion tests for which causal estimate is significantly different. Other newer methods like MR‐RAPS down‐weights potential outlying genetic variants.
We detected associations with atrial fibrillation but not with other heart diseases. This may be because of fewer cases of myocardial infarction, cardiomyopathy, and heart failure syndrome. However, the associations for these outcomes trended in the same direction as atrial fibrillation. From a biological perspective, a plausible explanation is that mLOY or mLOX potentially reflects processes involving cardiac tissue itself, which would have greater impact on atrial fibrillation; whereas mLOY or mLOX may not reflect biological processes in vasculature, which contribute more to myocardial infarction, ischemic heart disease, and cardiomyopathy/heart failure syndrome. Mechanistic studies using mouse models provide some clues into this hypothesis. For instance, Sano et al demonstrated that mLOY promotes fibrotic phenotypes (“scarring”) in cardiac murine tissues, which potentially impairs cardiac function, such as tissue elasticity and electrical signal propagation. 16 Along those lines, Horitani et al found evidence that mLOY in mice may disrupt the ubiquitously transcribed tetratricopeptide repeat containing, Y‐linked gene, leading to epigenetic dysfunction and differentiation of profibrotic macrophages. 37 Ubiquitously transcribed tetratricopeptide repeat containing, Y‐linked is found to be expressed in cardiac tissue in humans (www.gtexportal.org).
Other studies have posited additional mechanisms of action for mLOY. Analyses of sequencing data suggests that mLOY is genetically linked to clonal hematopoiesis of indeterminate potential, 38 , 39 , 40 a mosaic expansion of hematopoietic stem cells driven by certain somatic mutations, 41 which has been recently associated with risk of CVDs and heart failure syndrome. 42 , 43 , 44 , 45 , 46 Additionally, it has been hypothesized that mLOY in leukocytes potentially reflects altered immunosurveillance and inflammatory processes, which can influence CVD pathogenesis. 3 , 47
We note that atrial fibrillation risk was positively associated with mLOY among men as expected but was potentially inversely associated with mLOX among women. It is possible that mLOY and mLOX operate in a distinctly sex‐specific manner in reflecting genomic instability from the cumulative burden of age, genetic susceptibility, and environmental exposures. Furthermore, sex chromosomes Y and X contain PARs, which contain genes homologous to those found in autosomes. These PARs may contain either protective or detrimental genes relevant to the pathogenesis of atrial fibrillation. Loss of protective genes on the PAR of chromosome Y may contribute to increased risk, whereas loss of detrimental genes on the PAR of chromosome X may confer a protective effect. However, our population study was conducted using leukocytes, and whether these are surrogates for cardiac tissue is unclear.
A recent study found that atrial fibrillation risk was elevated among 2408 patients in South Korea with Turner syndrome compared with a control group. 48 However, we found a suggestive inverse association between mLOX and atrial fibrillation risk. A possible explanation for this difference is that we examined leukocyte DNA from a relatively healthy adult population, in which a subset of people had mosaic X‐chromosome loss in a small fraction of white blood cells. This leukocyte mLOX increases with progressive age and potentially by environmental exposures. Leukocyte mLOX may be a surrogate marker for biological processes related to the pathogenesis of atrial fibrillation, but whether its directly involved is unclear. In contrast, Turner syndrome from monosomy X or partial X loss is largely a congenital disease and attributable to a random nondisjunction event during the formation of reproductive cells in an individual's parent. This kind of X‐chromosome loss would directly affect most tissues, including the heart, and a person's phenotypes would be affected at birth. These 2 biological processes are distinct and may contribute to the pathogenesis of atrial fibrillation differently.
Our study had numerous strengths. First, we used mosaic chromosomal alterations caller to call chromosomal mosaicisms from GWAS data, which is more sensitive compared with previous calling approaches. 17 Second, the prospective cohort design strengthened our inferences and is robust against disease‐effect bias of prediagnostic leukocyte mLOY and mLOX (ie, “reverse causation”). Third, we analyzed a large sample size of middle‐aged and older adults, which improved statistical power to detect modest associations.
Our study had limitations. First, we had limited power to detect modest associations among younger premenopausal women. However, we did detect suggestive but nonsignificant associations for mLOX among postmenopausal women, which warrants further exploration. Second, although we thoroughly adjusted for measured confounders, we cannot discount the possibility of unmeasured or residual confounding. However, our findings were consistent across never and ever smokers and age groups; supported by MR analyses; robust to adjustment for detailed smoking history; and consistent with previous studies. 16 Third, the study population was predominantly of European ancestry. We included available ethnic minority populations in our main analyses; however, future studies are needed in more diverse populations. Fourth, we found some evidence of horizontal pleiotropy, but only with MR‐Egger, which has been reported to be less statistically efficient and subject to skewed estimates 49 when the instrument strength independent of direct effect assumption is violated. 32 As such, the MR‐Egger results need to be taken together with those from the more robust MR‐PRESSO and MR‐RAPS methods, which did not detect pleiotropy. Fifth, UK Biobank did not have verified information on medication dose, duration, and history. Sixth, we acknowledge that some cryptic relatedness or closely related individuals may be in the study population; however, this is unlikely to influence the findings. Last, there is the possibility that healthy volunteer bias in the UK Biobank could limit generalizability; however, this does not affect the internal validity of our mechanistic study.
In conclusion, mLOY was significantly associated with elevated risk of atrial fibrillation among men. Conversely, we found suggestive evidence of an inverse association between mLOX and atrial fibrillation risk among postmenopausal women. Associations were not detected for other heart diseases. Caution is recommended when interpreting the findings, particularly for mLOX among women. Although the precise biological role of sex chromosome mosaicisms in heart disease pathogenesis remains unclear, our findings support biologic processes related to or tagged by leukocyte mLOY and mLOX are related to atrial fibrillation occurrence in human populations. If our findings are confirmed across diverse populations, they support further consideration of mLOY in atrial fibrillation risk prediction and stratification analyses among men.
Sources of Funding
This study was supported by intramural funding from the National Cancer Institute and National Heart, Lung, and Blood Institute.
Disclosures
None.
Supporting information
Data S1
Tables S1–S5
Acknowledgments
We thank Drs Qing Lan and Nathaniel Rothman for their scientific support. We extend our appreciation to Zhonghua Liu, Haoyu Zhang, and Peter Kraft for their advice on interpreting the Mendelian randomization analyses. We thank Gabriel Goodney for bioinformatics support. Additionally, we thank Lisa Finkelstein, Jillian Varonin, and the UK Biobank Access Team for helping us with international data agreements. Last, we thank Giulio Genovese and John R.B. Perry for contributing to the calling of chromosomal mosaicisms.
This manuscript was sent to Luciano A. Sposato, MD, MBA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Preprint posted on MedRxiv May 31, 2024. doi: https://doi.org/10.1101/2024.05.29.24308171.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.036984
For Sources of Funding and Disclosures, see page 14.
References
- 1. Thompson DJ, Genovese G, Halvardson J, Ulirsch JC, Wright DJ, Terao C, Davidsson OB, Day FR, Sulem P, Jiang Y, et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature. 2019;575:652–657. doi: 10.1038/s41586-019-1765-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia‐Gonzalez P, de Rojas I, Moreno‐Grau S, Montrreal L, Puerta R, Alarcon‐Martin E, Quintela I, Orellana A, Andrade V, Adami PVM, et al. Mendelian randomisation confirms the role of Y‐chromosome loss in Alzheimer's disease aetiopathogenesis in men. Int J Mol Sci. 2023;24:24. doi: 10.3390/ijms24020898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forsberg LA, Rasi C, Malmqvist N, Davies H, Pasupulati S, Pakalapati G, Sandgren J, Diaz de Stahl T, Zaghlool A, Giedraitis V, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet. 2014;46:624–628. doi: 10.1038/ng.2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong JYY, Margolis HG, Machiela M, Zhou W, Odden MC, Psaty BM, Robbins J, Jones RR, Rotter JI, Chanock SJ, et al. Outdoor air pollution and mosaic loss of chromosome Y in older men from the cardiovascular health study. Environ Int. 2018;116:239–247. doi: 10.1016/j.envint.2018.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou W, Machiela MJ, Freedman ND, Rothman N, Malats N, Dagnall C, Caporaso N, Teras LT, Gaudet MM, Gapstur SM, et al. Mosaic loss of chromosome Y is associated with common variation near TCL1A. Nat Genet. 2016;48:563–568. doi: 10.1038/ng.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forsberg LA, Halvardson J, Rychlicka‐Buniowska E, Danielsson M, Moghadam BT, Mattisson J, Rasi C, Davies H, Lind L, Giedraitis V, et al. Mosaic loss of chromosome Y in leukocytes matters. Nat Genet. 2019;51:4–7. doi: 10.1038/s41588-018-0267-9 [DOI] [PubMed] [Google Scholar]
- 7. Demanelis K, Delgado DA, Tong L, Jasmine F, Ahmed A, Islam T, Parvez F, Kibriya MG, Graziano JH, Ahsan H, et al. Somatic loss of the Y chromosome is associated with arsenic exposure among Bangladeshi men. Int J Epidemiol. 2023;52:1035–1046. doi: 10.1093/ije/dyac176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dumanski JP, Rasi C, Lonn M, Davies H, Ingelsson M, Giedraitis V, Lannfelt L, Magnusson PK, Lindgren CM, Morris AP, et al. Mutagenesis. Smoking is associated with mosaic loss of chromosome Y. Science. 2015;347:81–83. doi: 10.1126/science.1262092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown DW, Lan Q, Rothman N, Pluta J, Almstrup K, Dalgaard MD, Greene MH, Grotmol T, Loveday C, Schwartz SM, et al. Genetically inferred telomere length and testicular germ cell tumor risk. Cancer Epidemiol Biomarkers Prev. 2021;30:1275–1278. doi: 10.1158/1055-9965.EPI-20-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Machiela MJ, Dagnall CL, Pathak A, Loud JT, Chanock SJ, Greene MH, McGlynn KA, Stewart DR. Mosaic chromosome Y loss and testicular germ cell tumor risk. J Hum Genet. 2017;62:637–640. doi: 10.1038/jhg.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi T, Hachiya T, Ikehata Y, Horie S. Genetic association of mosaic loss of chromosome Y with prostate cancer in men of European and east Asian ancestries: a Mendelian randomization study. Front Aging. 2023;4:1176451. doi: 10.3389/fragi.2023.1176451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loftfield E, Zhou W, Yeager M, Chanock SJ, Freedman ND, Machiela MJ. Mosaic Y loss is moderately associated with solid tumor risk. Cancer Res. 2019;79:461–466. doi: 10.1158/0008-5472.CAN-18-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dumanski JP, Lambert JC, Rasi C, Giedraitis V, Davies H, Grenier‐Boley B, Lindgren CM, Campion D, Dufouil C; European Alzheimer's Disease Initiative I , et al. Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am J Hum Genet. 2016;98:1208–1219. doi: 10.1016/j.ajhg.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin SH, Brown DW, Rose B, Day F, Lee OW, Khan SM, Hislop J, Chanock SJ, Perry JRB, Machiela MJ. Incident disease associations with mosaic chromosomal alterations on autosomes, X and Y chromosomes: insights from a phenome‐wide association study in the UK biobank. Cell Biosci. 2021;11:143. doi: 10.1186/s13578-021-00651-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loftfield E, Zhou W, Graubard BI, Yeager M, Chanock SJ, Freedman ND, Machiela MJ. Predictors of mosaic chromosome Y loss and associations with mortality in the UK biobank. Sci Rep. 2018;8:12316. doi: 10.1038/s41598-018-30759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sano S, Horitani K, Ogawa H, Halvardson J, Chavkin NW, Wang Y, Sano M, Mattisson J, Hata A, Danielsson M, et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science. 2022;377:292–297. doi: 10.1126/science.abn3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hubbard AK, Brown DW, Zhou W, Lin SH, Genovese G, Chanock SJ, Machiela MJ. Serum biomarkers are altered in UK biobank participants with mosaic chromosomal alterations. Hum Mol Genet. 2023;32:3146–3152. doi: 10.1093/hmg/ddad133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim J, Hashemian M, Blechter B, Roger VL, Wong JYY. Pre‐diagnostic free androgen and estradiol levels influence heart failure risk in both women and men: a prospective cohort study in the UK biobank. Eur J Heart Fail. 2024;26:540–550. doi: 10.1002/ejhf.3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Machiela MJ, Zhou W, Karlins E, Sampson JN, Freedman ND, Yang Q, Hicks B, Dagnall C, Hautman C, Jacobs KB, et al. Female chromosome X mosaicism is age‐related and preferentially affects the inactivated X chromosome. Nat Commun. 2016;7:11843. doi: 10.1038/ncomms11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou W, Lin SH, Khan SM, Yeager M, Chanock SJ, Machiela MJ. Detectable chromosome X mosaicism in males is rarely tolerated in peripheral leukocytes. Sci Rep. 2021;11:1193. doi: 10.1038/s41598-020-80948-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu A, Genovese G, Zhao Y, Pirinen M, Zekavat SM, Kentistou KA, Yang Z, Yu K, Vlasschaert C, Liu X, et al. Genetic drivers and cellular selection of female mosaic X chromosome loss. Nature. 2024;631:134–141. doi: 10.1038/s41586-024-07533-7 [DOI] [PubMed] [Google Scholar]
- 22. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health‐related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O'Connell J, et al. The UK biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome‐wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loh PR, Genovese G, Handsaker RE, Finucane HK, Reshef YA, Palamara PF, Birmann BM, Talkowski ME, Bakhoum SF, McCarroll SA, et al. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature. 2018;559:350–355. doi: 10.1038/s41586-018-0321-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loh PR, Genovese G, McCarroll SA. Monogenic and polygenic inheritance become instruments for clonal selection. Nature. 2020;584:136–141. doi: 10.1038/s41586-020-2430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin SH, Khan SM, Zhou W, Brown DW, Vergara C, Wolinsky SM, Martinez‐Maza O, Margolick JB, Martinson JJ, Hussain SK, et al. Mosaic chromosomal alterations detected in men living with HIV and the relationship to non‐Hodgkin lymphoma. AIDS. 2023;37:1307–1313. doi: 10.1097/QAD.0000000000003545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zekavat SM, Lin SH, Bick AG, Liu A, Paruchuri K, Wang C, Uddin MM, Ye Y, Yu Z, Liu X, et al. Hematopoietic mosaic chromosomal alterations increase the risk for diverse types of infection. Nat Med. 2021;27:1012–1024. doi: 10.1038/s41591-021-01371-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR‐egger method. Eur J Epidemiol. 2017;32:377–389. doi: 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two‐sample summary‐data Mendelian randomization using robust adjusted profile score. The Annals of Statistics. 2020;48:1728. [Google Scholar]
- 35. Burgess SY. MendelianRandomization v0.9.0: an R package for performing Mendelian randomization analyses using summarized data. The Comprehensive R Archive Network. https://cran.r‐project.org/web/packages/MendelianRandomization/vignettes/Vignette_MR.pdf. Accessed: 10/1/2024.
- 36. Haitjema S, Kofink D, van Setten J, van der Laan SW, Schoneveld AH, Eales J, Tomaszewski M, de Jager SCA, Pasterkamp G, Asselbergs FW, et al. Loss of Y chromosome in blood is associated with major cardiovascular events during follow‐up in men after carotid endarterectomy. Circ Cardiovasc Genet. 2017;10:e001544. doi: 10.1161/CIRCGENETICS.116.001544 [DOI] [PubMed] [Google Scholar]
- 37. Horitani K, Chavkin NW, Arai Y, Wang Y, Ogawa H, Yura Y, Evans MA, Cochran JD, Thel MC, Polizio AH, et al. Disruption of the Uty epigenetic regulator locus in hematopoietic cells phenocopies the profibrotic attributes of Y chromosome loss in heart failure. Nat Cardiovasc Res. 2024;3:343–355. doi: 10.1038/s44161-024-00441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jakubek YA, Reiner AP, Honigberg MC. Risk factors for clonal hematopoiesis of indeterminate potential and mosaic chromosomal alterations. Transl Res. 2023;255:171–180. doi: 10.1016/j.trsl.2022.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walsh K, Raghavachari N, Kerr C, Bick AG, Cummings SR, Druley T, Dunbar CE, Genovese G, Goodell MA, Jaiswal S, et al. Clonal hematopoiesis analyses in clinical, epidemiologic, and genetic aging studies to unravel underlying mechanisms of age‐related dysfunction in humans. Front Aging. 2022;3:841796. doi: 10.3389/fragi.2022.841796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ljungstrom V, Mattisson J, Halvardson J, Pandzic T, Davies H, Rychlicka‐Buniowska E, Danielsson M, Lacaze P, Cavelier L, Dumanski JP, et al. Loss of Y and clonal hematopoiesis in blood‐two sides of the same coin? Leukemia. 2022;36:889–891. doi: 10.1038/s41375-021-01456-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heimlich JB, Bick AG. Somatic mutations in cardiovascular disease. Circ Res. 2022;130:149–161. doi: 10.1161/CIRCRESAHA.121.319809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu B, Roberts MB, Raffield LM, Zekavat SM, Nguyen NQH, Biggs ML, Brown MR, Griffin G, Desai P, Correa A, et al. Supplemental Association of Clonal Hematopoiesis with Incident Heart Failure. J Am Coll Cardiol. 2021;78:42–52. doi: 10.1016/j.jacc.2021.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reiner AP, Roberts MB, Honigberg MC, Kooperberg C, Desai P, Bick AG, Natarajan P, Manson JE, Whitsel EA, Eaton CB. Association of clonal hematopoiesis of indeterminate potential with incident heart failure with preserved ejection fraction. medRxiv. 2023. doi: 10.1101/2023.06.07.23291038 [DOI] [Google Scholar]
- 44. Jaiswal S, Libby P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol. 2020;17:137–144. doi: 10.1038/s41569-019-0247-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Libby P, Ebert BL. CHIP (clonal hematopoiesis of indeterminate potential): potent and newly recognized contributor to cardiovascular risk. Circulation. 2018;138:666–668. doi: 10.1161/CIRCULATIONAHA.118.034392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8 [DOI] [PubMed] [Google Scholar]
- 48. Cho JH, Choi EK, Moon IK, Jung JH, Han KD, Choi YJ, Park J, Lee E, Lee SR, Cha MJ, et al. Chromosomal abnormalities and atrial fibrillation and ischemic stroke incidence: a nationwide population‐based study. Sci Rep. 2020;10:15872. doi: 10.1038/s41598-020-72678-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qi G, Chatterjee N. Mendelian randomization analysis using mixture models for robust and efficient estimation of causal effects. Nat Commun. 2019;10:1941. doi: 10.1038/s41467-019-09432-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5


