The existence of a local brain renin–angiotensin system is a disputed topic because renin–angiotensin system peptides are often undetectable or present at low levels in brain tissue. Angiotensinogen is abundantly secreted by brain astrocytes, and angiotensin‐converting enzyme (ACE) is produced by cells of the choroid plexus, endothelium, and some astrocytes. 1 Renin is poorly expressed in the brain, and most of the protein product is projected to remain intracellularly, since an alternative promoter that causes a partial loss of renin's signal peptide drives most of brain renin transcription, rendering an interaction with secreted angiotensinogen in the brain parenchyma unlikely. 1 , 2 Recently, we have generated a transgenic mouse overexpressing the rat angiotensinogen gene specifically in brain astrocytes. Transgenic mice presented detectable levels of angiotensin II in the brain contrary to wild‐type littermates. 3 Because the pathway of angiotensin II formation in the brain remains a controversial topic, we employed this model to investigate if angiotensin II is locally produced in angiotensinogen transgenic mouse brains (deletion of endogenous angiotensinogen) and if the production of it relies on renin (deletion of renin).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We investigated if angiotensin formation in angiotensinogen transgenic mice truly occurs in the brain. We therefore generated a mouse model overexpressing angiotensinogen specifically in brain astrocytes while lacking peripheral angiotensinogen. For that, angiotensinogen transgenic mice were crossed with global angiotensinogen knockout mice, deleting the endogenous angiotensinogen gene of angiotensinogen transgenic mouse (angiotensinogen transgenic mouse/angiotensinogen knockout mouse; Figure [A]). Angiotensinogen‐knockout mice were previously generated in a mixed background strain and backcrossed to the FVB/N inbred background strains over 10 generations. 4
Figure 1. Quantification of angiotensin peptides in brain and blood as well as other renin–angiotensin system parameters.
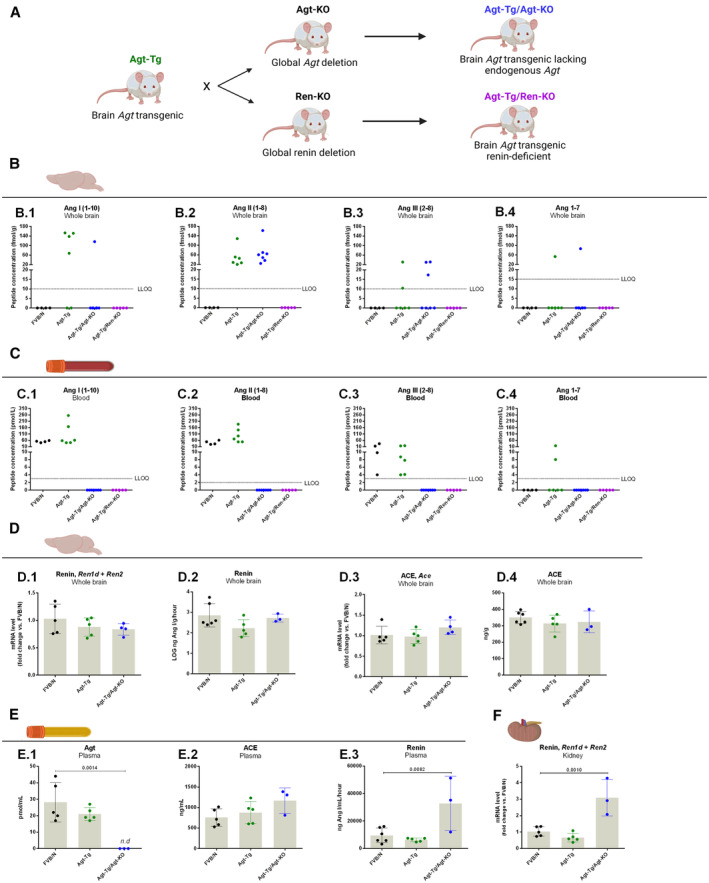
Breeding scheme for generating a mouse line overexpressing angiotensinogen specifically in the brain but lacking peripheral angiotensinogen (angiotensinogen transgenic/angiotensinogen knockout) and a mouse line overexpressing angiotensinogen but lacking renin (angiotensinogen transgenic/renin knockout) (A). Quantification of angiotensins (angiotensin I; B.1, angiotensin II; B.2, angiotensin III; B.3 and angiotensin 1–7; B.4) in whole brain of wildtype, angiotensinogen transgenic, angiotensinogen transgenic/angiotensinogen knockout and angiotensinogen transgenic/renin knockout mice (B). Quantification of angiotensins (angiotensin I; C.1, angiotensin II; C.2, angiotensin III; C.3 and angiotensin 1–7; C.4) in blood of wild‐type, angiotensinogen transgenic, angiotensinogen transgenic/angiotensinogen knockout and angiotensinogen transgenic/renin knockout mice (C). Quantification of whole brain renin mRNA levels (D.1) and concentration expressed in LOG (D.2). Quantification of whole brain ACE mRNA levels (D.3) and protein levels (D.4). Plasma angiotensinogen (E.1), plasma ACE (E.2), and plasma renin quantification (E.3), and renal renin mRNA expression (F). Data in D‐F are mean±SD each group was compared against the control (FVB/N) and analyzed by ANOVA followed by the Dunnett's post hoc test. The P values of statistically significant groups are depicted in the picture. The lower limit of quantification in brain samples was 10 fmol/g for angiotensin I, angiotensin II, and angiotensin III and 15 fmol/g for angiotensin 1–7. The lower limit of quantification for blood samples was 3 pmol/L for angiotensin I, angiotensin III, and angiotensin 1–7 and 2 pmol/L for angiotensin II. ACE indicates, angiotensin‐converting enzyme; Agt, angiotensinogen, Ang, angiotensin; LLOQ, lower limit of quantification; KO, knockout; n.d, not detected; and Ren, renin. Illustrations were created with BioRender.com.
This experiment was designed to investigate if renin is required for local brain angiotensin generation. Renin expression of angiotensinogen transgenic mice was genetically deleted by crossing this line with global renin knockout mice, producing angiotensinogen transgenic/renin‐knockout mice, a mouse line overexpressing brain angiotensinogen but lacking renin (Figure [A]). The global renin knockout mouse with targeted deletion of the Ren1c gene previously generated on the C57BL/6 genetic background was back‐crossed to the FVB/N inbred background for 8 generations. 5
Adult (15–22 weeks) male and female mice were used for the analyses. Mice were killed by isoflurane inhalation overdose, and 500 μL of blood was collected from the right ventricle into a tube containing 5 mL of ice‐cold 4 mol/L guanidine thiocyanate (for angiotensin quantification) or EDTA (other parameters). Whole brains were rapidly dissected and snap frozen in liquid nitrogen and kept at −80 °C until processing. All procedures involving animals were previously approved and performed in agreement with institutional guidelines.
Brains were pulverized under liquid nitrogen using pestle and mortar. The frozen brain powder was dissolved in 6 mol/L guanidinium chloride solution supplemented with 1% (v/v) trifluoroacetic acid (100 mg/mL). LC–MS/MS quantification of angiotensins (angiotensin I, angiotensin II, angiotensin III, and angiotensin 1–7) was performed by Attoquant Diagnostics GmbH, Austria.
Whole brains were homogenized in 0.01 mol/L phosphate buffer, pH 7.4, containing 0.15 mol/L NaCl, and the homogenates were used to measure renin and ACE. Renin in plasma and brain homogenates measured by quantifying angiotensin I generation in the presence of excess sheep angiotensinogen, both without and with the renin inhibitor 10 μmol/L, to correct for nonrenin enzymes capable of cleaving angiotensin I from angiotensinogen. ACE in plasma and brain homogenates was measured with the mouse ACE SimpleStep ELISA kit (Abcam, Cambridge, UK). Plasma angiotensinogen was quantified by measuring angiotensin I after its cleavage by excess rat renin.
mRNA of levels renin and ACE were quantified in whole brain and kidney by reverse transcription quantitative real‐time polymerase chain reaction. The cDNA was assembled in a reaction containing a SYBR Green mix and specific primers against Ren (5′‐CAAAGTCATCTTTGACACGGG‐3′ and 5′‐AGTCAGAGGACTCATAGAGGC‐3′) and Ace (5′‐TACTCCACTGGCAAGGTCTG‐3′ and 5′‐TGGCATAGCTTCGTGAGGAA‐3′). mRNA levels were calculated as 2−ΔΔCT using Gapdh (5′‐TCACCACCATGGAGAAGGC‐3′ and 5′‐GCTAAGCAGTTGGTGGTGC‐3′) as a housekeeping gene.
Among the 7 angiotensinogen transgenic/angiotensinogen knockout mice analyzed all presented detectable levels of angiotensin II in the brain (Figure [B.2]), 1 animal presented detectable levels of angiotensin I and angiotensin 1–7, and angiotensin III could be detected in 3 mice (Figure [B]). None of these peptides were detectable in blood of angiotensinogen transgenic/angiotensinogen knockout mice indicating local brain production and absence of significant spillover in the circulation (Figure [C]). Four wild‐type mice were analyzed in parallel, and these mice lacked detectable levels of any angiotensin in the brain (Figure [B]) as in our previous report. 3 However, angiotensin I, angiotensin II, and angiotensin III were detected in the blood of all 4 wild‐type mice (Figure [C]).
After confirming that angiotensin II is locally generated in the brain of transgenic mice, we deleted renin from angiotensinogen transgenic mice to answer if renin is involved in the process of angiotensin II formation in the brain. All 5 angiotensinogen transgenic/renin knockout mice lacked angiotensin peptides in both brain and blood, indicating an essential role for renin in the brain like in the periphery (Figure [B,C]). As positive controls, brain and blood of angiotensinogen transgenic littermates expressing renin were also collected, and angiotensins were quantified. In the brain, angiotensin II was detected in all samples, angiotensin I in 4 of 6, angiotensin III in 2 of 6, and angiotensin 1–7 in 1 sample (Figure [B]). In the peripheral blood, detectable levels of angiotensin I, angiotensin II, and angiotensin III were found in all samples, and angiotensin 1–7 was detectable in 2 of 6 samples (Figure [C]).
In the brain, mRNA levels of renin and ACE were not altered upon angiotensinogen transgene expression (Figure [D] and [D.3]). Brain renin concentration was not altered in the investigated lines (Figure [D.2]). Plasma renin concentration (Figure [E.3]) and renal renin expression (Figure [F]) were increased in angiotensinogen transgenic/angiotensinogen knockout mice, reflecting the lack of circulating angiotensinogen (Figure [E.1]) and angiotensin II. Importantly, the renal renin amplification exponential phase detected by reverse transcription quantitative real‐time polymerase chain reaction happened ≈10 Ct after renal renin. Finally, neither brain nor plasma ACE were altered (Figure [D.4] and [E.2]).
Our experiments, using mice with brain‐specific rat angiotensinogen overexpression, indicate that the formation of angiotensin peptides in the brain from overexpressed rat angiotensinogen depends on renin. Although locally synthesized renin in defined brain areas cannot be excluded as the source of central renin, it most likely stems from the kidney. 2 However, this was not addressed in the current study. Finally, brain angiotensin generation by renin relying on endogenous mouse angiotensinogen could not be demonstrated, in full agreement with previous studies. 2
Sources of Funding
None.
Disclosures
Drs Domenig and Poglitsch are employees of Attoquant Diagnostics. The remaining authors have no disclosures to report.
This manuscript was sent to Neel S. Singhal, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 4.
Contributor Information
André F. Rodrigues, Email: andrefelipe.rodrigues@mdc-berlin.de.
Michael Bader, Email: mbader@mdc-berlin.de.
References
- 1. Nakagawa P, Sigmund CD. How is the brain renin–angiotensin system regulated? Hypertension. 2017;70:10–18. doi: 10.1161/HYPERTENSIONAHA.117.08550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Thiel BS, Góes Martini A, te Riet L, Severs D, Uijl E, Garrelds IM, Leijten FPJ, van der Pluijm I, Essers J, Qadri F, et al. Brain renin‐angiotensin system does it exist? Hypertension. 2017;69:1136–1144. doi: 10.1161/HYPERTENSIONAHA.116.08922 [DOI] [PubMed] [Google Scholar]
- 3. Rodrigues AF, Todiras M, Qadri F, Campagnole‐Santos MJ, Alenina N, Bader M. Increased angiotensin II formation in the brain modulates cardiovascular homeostasis and erythropoiesis. Clin Sci. 2021;135:1353–1367. doi: 10.1042/CS20210072 [DOI] [PubMed] [Google Scholar]
- 4. Rodrigues AF, Todiras M, Qadri F, Alenina N, Bader M. Angiotensin deficient FVB/N mice are normotensive. Br J Pharmacol. 2023;180:1843–1861. doi: 10.1111/bph.16051 [DOI] [PubMed] [Google Scholar]
- 5. Rodrigues AF, Domenig O, Poglitsch M, Bader M, Danser AHJ. Angiotensin‐(1‐12): does it exist? A critical evaluation in humans, rats, and mice. Hypertension. 2024;81:1776–1784. doi: 10.1161/HYPERTENSIONAHA.124.22856 [DOI] [PMC free article] [PubMed] [Google Scholar]


