Abstract
Background
Chest pain (CP) in patients with nonobstructive coronary artery disease is a therapeutic challenge affecting morbidity and mortality. We aimed to identify clinical factors associated with CP in this population, hypothesizing that obesity and depressive symptoms are associated with CP.
Methods and Results
In 814 patients with angiographically confirmed nonobstructive coronary artery disease, we measured sociodemographic variables, clinical risk factors, medications, and Patient Health Questionnaire 9 scores for depressive symptoms. We assessed CP frequency and prevalence by using all items from the Seattle Angina Questionnaire angina frequency domain to generate an angina frequency composite score. In the overall sample (58.8±11.7 years old, 52.6% female), 42.8% had obesity, and 71.5% had CP, with an angina frequency composite score (SD) score of 76.4 (22.1). Compared with individuals without obesity, individuals with obesity had a higher prevalence (77.6% versus 67%, P<0.001) and more frequent CP (angina frequency composite score, 74.9 [SD, 22.2] versus 78.3 [SD, 21.9], P=0.02). Obesity was independently associated with CP occurrence (odds ratio [OR], 1.7 [95% CI, 1–2.9], P=0.04). Obesity's connection with CP was only in men: men with obesity had more frequent CP (angina frequency composite score, 75.8 [SD, 20.1] versus 82.1 [SD, 19.9], P=0.002) and more prevalent CP (79.5% versus 58.2%, P<0.001) than their counterparts insofar as men with obesity had similar CP to women. Patient Health Questionnaire 9 score (OR, 1.07 [95% CI, 1.01–1.13], P=0.03) was independently associated with CP and partly mediated the association between obesity and CP.
Conclusions
Obesity and depressive symptoms were independently associated with CP in individuals with nonobstructive coronary artery disease, particularly in men, and depressive symptoms partly mediated this association.
Keywords: angina, chest pain, depression, non‐obstructive coronary artery disease, obesity
Subject Categories: Obesity, Mental Health, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- AFCS
angina frequency composite score
- CMD
coronary microvascular disease
- NOCAD
non‐obstructive coronary artery disease
- PHQ9
Patient Health Questionnaire 9
Clinical Perspective.
What Is New?
Obesity is associated with self‐reported chest pain in those with nonobstructive coronary artery disease, particularly in men, and depressive symptoms partially mediate the association between obesity and self‐reported chest pain.
What Are the Clinical Implications?
Weight loss is a potential management strategy to improve chest pain and, ultimately, quality of life in patients with nonobstructive coronary artery disease.
Treating depression could potentially reduce chest pain in patients with nonobstructive coronary artery disease.
The high prevalence and important prognostic implications of nonobstructive coronary artery disease (NOCAD) in those with ischemia or chest pain are now apparent based on mounting data over the past 3 decades. 1 , 2 , 3 , 4 , 5 , 6 , 7 Almost half of the individuals undergoing cardiac catheterization have NOCAD (usually defined as <50% stenosis), with a higher prevalence in women than men. 1 , 2 , 4 Patients with NOCAD were previously reassured without further management, but data from recent decades demonstrate higher morbidity and mortality in this group than in the general population. 3 , 4 , 5 , 6 For example, patients with NOCAD are at greater risk for myocardial infarction within 1 year of angiography than those with normal coronaries. 6
Chest pain in those with NOCAD often translates to greater usage of health care resources due to higher rates of cardiovascular and noncardiovascular hospitalizations, repeat angiograms, and use of antianginal medications compared with controls. 3 , 4 , 5 , 6 , 8 , 9 Lifetime economic costs were estimated to be >$750 000 for US women with NOCAD in 2006. 9 Furthermore, symptom burden is an important prognostic factor in patients with NOCAD, as women with NOCAD and persistent angina had greater event rates than their counterparts with NOCAD who had a resolution of their angina in 1 year. 5
Although studies have established the connection between NOCAD, angina, and worse cardiovascular disease (CVD) outcomes, they have not fully identified risk factors associated with symptoms in individuals with NOCAD. 3 , 4 , 5 , 6 , 8 , 9 Obesity is a risk factor that is becoming increasingly prevalent, estimated to affect 50% of individuals in the United States by 2030, and has been implicated as a cause of angina in population‐based studies. 10 , 11 Wilson et al. found that Framingham Heart Study participants with obesity had about a 63% increased risk of angina than Framingham Heart Study participants without obesity. 11 Furthermore, in a Swedish study, individuals with obesity who achieved weight loss via bariatric surgery reported improvement in chest pain compared with those without bariatric surgery. 12
Although studies have associated obesity with angina, these were large registry‐ or population‐based studies where CAD severity was not reported. The relationship between obesity and angina in those with established NOCAD on angiography has not been clear. Given advancements in weight loss therapies, establishing a connection between obesity and angina in patients with NOCAD could identify a therapeutic target to reduce morbidity, mortality, and health costs in this population. This study examined the relationship between obesity and responses to the Seattle Angina Questionnaire (SAQ), a validated, reproducible measure of angina, in a cohort with established NOCAD. 13 , 14 , 15 , 16 , 17 Because previous studies investigating patients with CAD and healthy individuals have suggested that individuals with depression are more prone to angina, we also examined the association of depressive symptoms with SAQ responses in these participants with NOCAD. 18 We hypothesized that obesity and depressive symptoms would be associated with a higher prevalence and frequency of self‐reported chest pain, as measured by the SAQ, in patients with NOCAD (Figure 1).
Figure 1. Hypothesized relationship between obesity, depression, and angina in patients with nonobstructive coronary artery disease.
Directed acyclic graph depicting the hypothesis that obesity and depressive symptoms are associated with angina among patients with nonobstructive coronary artery disease.
Methods
The data, analytic methods, and materials used to conduct this research and support these findings are available from the corresponding author upon reasonable request.
Study Design
Participants between the ages of 20 and 90 enrolled in the Emory Cardiovascular Biobank, a prospective registry of patients undergoing left heart catheterization for evaluation or management of CAD at 3 Emory Healthcare sites in Atlanta between 2003 and 2022, were included. 19 Nearly all registry participants included in our study underwent left heart catheterization for symptoms, abnormal stress test, suspected acute coronary syndrome, or preoperative workup as part of routine clinical care for having clinical presentations suspicious of myocardial ischemia. Patient demographic information, medical history, medication use, psychological history, and social determinants of health, including marital, employment, and education status, were gathered in precatheterization interviews and confirmed with a medical record review. At enrollment, we measured self‐reported chest pain frequency using items from the SAQ angina frequency domain. 13 A body mass index (BMI) cutoff of 30 kg/m2 was used to designate obesity status. BMI ranges between 30 and 34.9 kg/m2 were designated as obesity class 1, 35 and 39.9 kg/m2 were designated as obesity class 2, and 40 kg/m2 or more were designated as obesity class 3. Investigators blinded to the questionnaire data interpreted the angiograms, and we categorized patients having <50% epicardial coronary artery stenosis as having NOCAD. We excluded patients with obstructive (≥50% stenosis) CAD, ejection fraction <50%, history of heart failure with preserved or reduced ejection fraction, valvular heart disease, pulmonary hypertension, revascularization history, organ transplant, and dialysis from our sample. Of the total, 814 participants with NOCAD, BMI data, and SAQ data were included. The Institutional Review Board at Emory University approved the study, and patients provided informed consent.
Assessment of Chest Pain
The SAQ is a self‐administered, validated questionnaire that measures 5 components of angina: physical limitation, angina frequency, anginal stability, treatment satisfaction, and quality of life. 13 Scores from the SAQ angina frequency domain, which is composed of 2 items, have shown validity, reproducibility, and responsiveness in measuring angina and have been used as the primary measure of angina in previous studies, including those investigating individuals with NOCAD. 13 , 14 , 15 , 16 , 17 So, self‐reported chest pain on the 2 items comprising this SAQ domain served as a surrogate for angina. These 2 questions were the following: (1) how many times have you had chest pain, chest tightness, or angina in the past 4 weeks; and (2) how many times have you taken nitroglycerin (tablets or spray) for chest pain, tightness, or angina in the past 4 weeks? 13 Participant responses to each question were scored from 0 to 100 per SAQ scoring, with lower scores indicating more frequent chest pain or use of nitroglycerin and 100 indicating no chest pain or nitroglycerin use. 13 We refer to the SAQ angina frequency domain score—the average of the scores from the 2 items that comprise this domain and proxy for self‐reported chest pain frequency—as the angina frequency composite score (AFCS). The prevalence of self‐reported chest pain was measured by grouping individuals into any chest pain (AFCS <100) or no chest pain (AFCS=100) groups.
Assessment of Depressive Symptoms
We used the Patient Health Questionaire‐9 (PHQ‐9)—a validated self‐administered questionnaire comprising 9 items that screen for depression—to assess depressive symptoms. 20 , 21 , 22 Patients in the biobank registry completed this questionnaire in person upon enrollment. Each item estimates the frequency of specific depressive symptoms over the preceding 2 weeks. Scores for each item range from 0 (“not at all”) to 3 (“nearly every day”) and are aggregated to generate the total score, which ranges from 0 to 27. PHQ9 scores of ≤4 are indicative of no depression. 20 , 21 , 22 We also ascertained depression therapy by asking patients if they were receiving counseling or taking medications for depression upon enrollment.
Statistical Analysis
We present categorical variables as proportions and continuous variables as means±SD or medians [interquartile range]. We used the Shapiro–Wilk test to assess the normality of variable distributions. Mann–Whitney U, Kruskal–Wallis, and t tests were used to compare continuous variables, as appropriate. We used chi‐square tests to compare categorical variables. We performed multivariable analyses using logistic regression models, with the dichotomic occurrence of self‐reported chest pain as the outcome variable, and linear regression models, with the AFCS as the outcome variable, to identify covariates with significant associations with chest pain prevalence or frequency. Covariates in modeling included obesity or BMI, age, race, hypertension, diabetes, smoking, sex, chronic kidney disease (glomerular filtration rate <60 mL/min), low‐density lipoprotein levels, high‐density lipoprotein levels (HDL), depressive symptoms (PHQ9 score), and antianginal medications. Specific social determinants of health like marital status, education, and employment status that were available in the biobank registry were included in multivariable analyses.
Interaction analyses were performed to evaluate whether demographics, social determinants of health, traditional cardiovascular disease risk factors, or depression modified obesity's association with chest pain. In these analyses, high low‐density lipoprotein was defined as >100 mg/dL, and low HDL was defined as <40 mg/dL for men and <50 mg/dL for women. 23 All interaction effects were adjusted for the demographics, cardiovascular disease risk factors, depressive symptoms, medications, and social determinants of health. Subgroup analysis was performed on adjusted interaction effects with P values <0.1. In post hoc pairwise comparisons, P values less than the Bonferroni corrected P value of 0.008 were considered significant.
Finally, to explore how obesity is associated with self‐reported chest pain, a mediation analysis using the Monte Carlo casual‐mediation method was used. 24 This method allowed us to quantify the mediation of the association between obesity and self‐reported chest pain through obesity‐related CVD risk factors and depressive symptoms. 24 In these mediation analyses, obesity status was the independent variable, and the occurrence of any self‐reported chest pain was the outcome variable. All analyses were performed using SPSS 28.0.1.0 (Chicago, IL, USA) and R 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The mean age of the 814 included participants was 58.8 (11.7) years, 52.6% were female, and 42.7% had obesity, with a median BMI [interquartile range] of 29.3 [25.8–34.0] (Table 1). Among the 260 (31.9%) individuals for whom we have catheterization indication data, 190 received catheterization for symptoms, workup of acute coronary syndrome, or abnormal stress test, and 60 received catheterization for preoperative workup due to symptoms or findings concerning for myocardial ischemia. So, 250 of the 260 individuals (96.2%) with catheterization indication data received clinically indicated catheterization for suspected myocardial ischemia. Of the total sample, 582 (71.5%) individuals had self‐reported chest pain within the previous 4‐week period, and the mean±SD angina frequency composite score was 76.4 (22.1).
Table 1.
Baseline Characteristics
| Risk factor/medication | Total (n=814) | Without obesity (n=466) | With obesity (n=348) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y ±SD | 58.9±11.7 | 60.3±12.3 | 56.6±10.7 | <0.001 |
| Sex | 0.6 | |||
| Female | 428 (53.7%) | 238 (52.8%) | 190 (54.9%) | |
| Male | 369 (46.3%) | 213 (47.2%) | 156 (45.1%) | |
| Race and ethnicity | <0.001 | |||
| Black | 168 (20.6%) | 67 (14.4%) | 101 (29%) | |
| White | 587 (71.1%) | 355 (76.2%) | 232 (66.7%) | |
| Asian | 10 (1.2%) | 8 (1.7%) | 2 (0.6%) | |
| Hispanic | 13 (1.6%) | 8 (1.7%) | 5 (1.4%) | |
| Other | 17 (2.1%) | 13 (2.8%) | 4 (1.1%) | |
| Social determinants of health | ||||
| Marital status | 0.02 | |||
| Married | 537 (66.7%) | 323 (70.1%) | 214 (62.2%) | |
| Single | 268 (33.3%) | 138 (29.9%) | 130 (37.8%) | |
| Education | 0.006 | |||
| Below high school education | 82 (10.6%) | 35 (7.9%) | 47 (14.1%) | |
| High school education or higher | 695 (89.4%) | 409 (92.1%) | 286 (85.9%) | |
| Employment | 0.1 | |||
| Employed | 370 (46.4%) | 201 (43.9%) | 169 (49.7%) | |
| Unemployed/disabled/retired | 428 (53.6%) | 257 (56.1%) | 171 (50.3%) | |
| Cardiovascular disease risk factors | ||||
| Diabetes | 183 (24.6%) | 57 (13.6%) | 126 (38.8%) | <0.001 |
| Current smoking | 60 (7.5%) | 41 (8.9%) | 19 (5.5%) | 0.1 |
| Hypertension | 470 (61.1%) | 227 (51.8%) | 243 (73.4%) | <0.001 |
| Chronic kidney disease | 100 (12.3%) | 59 (12.7%) | 41 [11.8%) | 0.8 |
| Coronary artery disease, 30% Stenosis (%) | 145 (28.6%) | 88 (28.3%) | 57 (27.5%) | 0.7 |
| Body mass index, kg/m2 [IQR] | 29.3 [25.8–34.0] | 26.4 [24.2–28.1] | 35.0 [32.0–39.1] | <0.001 |
| Low‐density lipoprotein, mg/dL [IQR] | 102.5 [82.0–125.0] | 105.0 [85.0–128.0] | 102.0 [82.0–121.0] | 0.6 |
| High‐density lipoprotein, mg/dL [IQR] | 44.0 [36.0–56.0] | 47.0 [38.0–61.0] | 41.0 [34.0–50.0] | <0.001 |
| History of angina (chest pain) | 264 (37.4%) | 146 (36.4%) | 118 (38.8%) | 0.6 |
| Cardiac medication history | ||||
| Calcium channel blocker | 105 (12.9%) | 53 (11.4%) | 52 (14.9%) | 0.1 |
| Beta blocker | 172 (21.1%) | 99 (21.2%) | 73 (21.0%) | 1 |
| Long acting nitrates | 62 (30%) | 35 (26.9%) | 27 (35.1%) | 0.3 |
| Statin | 189 (23.2%) | 115 (24.7%) | 74 (21.3%) | 0.3 |
| Aspirin | 226 (27.8%) | 137 (29.4%) | 89 (25.6%) | 0.2 |
| Psychological risk factors | ||||
| Patient Health Questionnaire 9 score [IQR] | 3.0 [1.0, 7.0] | 3.0 [0.0, 6.0] | 4.0 [1.0, 8.0] | <0.001 |
| Depression history | 185 (22.7%) | 92 (19.7%) | 93 (26.7%) | 0.01 |
| Depression therapy | 158 (21%) | 82 (19.0%) | 76 (23.8%) | 0.07 |
P values compare individuals with and without obesity. IQR indicates interquartile range.
Individuals with obesity were younger and more likely to be Black, single, have hypertension, have diabetes, and not have a high school education than patients without obesity. Participants with obesity also had lower HDL levels and higher PHQ9 scores than their counterparts without obesity (P<0.001). Medication use was similar between the 2 groups (Table 1).
Obesity and Chest Pain Prevalence and Frequency
Chest Pain Prevalence
Individuals with obesity had a higher prevalence of self‐reported chest pain than individuals without obesity (77.6% versus 67%, P<0.001) (Figure 2A). Moreover, the prevalence of self‐reported chest pain significantly increased with increasing weight class (P=0.01) (Figure 2B). Individuals with obesity were 1.73 [1.04, 2.87] (P=0.04) times more likely to have self‐reported chest pain than individuals without obesity after adjustment for demographics, traditional CVD risk factors, social determinants of health, depressive symptoms, and antianginal medications (Table 2). Depressive symptoms were also independently associated with self‐reported chest pain prevalence (odds ratio [OR], 1.07 [95% CI, 1.01–1.13], P=0.03, Table 2). Other covariates associated with self‐reported chest pain prevalence in the fully adjusted model included younger age, smoking, and absence of diabetes (P<0.05) while being female (OR, 1.71 [95% CI, 0.96–3.05], P=0.07) had a nearly significant association with prevalence of self‐reported chest pain (Table 2).
Figure 2. Relationship between self‐reported chest pain and obesity status.
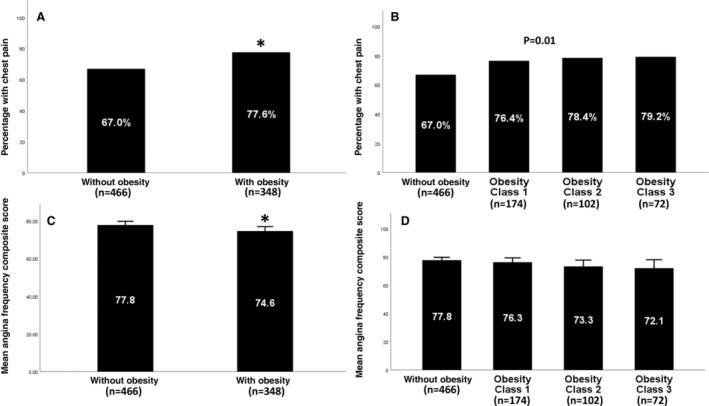
Prevalence of self‐reported chest pain by obesity status (A) and weight class (B). Mean angina frequency composite score by obesity status (C) and weight class (D). Significant P values (<0.05) are indicated with an asterisk or provided.
Table 2.
Unadjusted and Adjusted Associations Between Covariates and Self‐Reported Chest Pain Prevalence
| Covariates | Univariable odds ratio [95% CI] | Multivariable odds ratio [95% CI] |
|---|---|---|
| Obesity | 1.71 [1.24–2.35]* | 1.73 [1.04–2.87]* |
| Body mass index | 1.03 [1.01–1.06]* | 1.01 [0.98–1.05] |
| Age | 0.96 [0.95–0.97]* | 0.97 [0.95–1]* |
| Sex (male) | 1.48 [1.09–2.02]* | 1.58 [0.96–2.6] |
| Race (White) | 0.73 [0.49–1.05] | 0.73 [0.39–1.37] |
| Diabetes | 0.99 [0.69–1.44] | 0.49 [0.27–0.88]* |
| Hypertension | 0.98 [0.71–1.35] | 1.01 [0.62–1.66] |
| Smoking | 2.40 [1.15–4.96]* | 5 [1.13–22.1]* |
| Low‐density lipoprotein | 1.01 [1.00–1.01] | 1 [0.99–1.01] |
| High‐density lipoprotein | 0.99 [0.98–1.01] | 0.99 [0.97–1] |
| Chronic kidney disease | 0.92 [0.58–1.46] | 1.79 [0.84–3.83] |
| Depressive symptoms: Patient Health Questionnaire 9 score | 1.13 [1.08–1.18]* | 1.07 [1.01–1.13]* |
| Calcium channel blocker | 2.51 [1.47–4.26]* | 0.94 [0.46–1.96] |
| Beta blocker | 2.53 [1.54–4.17]* | 1.14 [0.65–2.04] |
| Education, high school education or higher | 0.46 [0.25–0.86]* | 0.61 [0.25–1.48] |
| Marital status, single | 1.11 [0.78–1.54] | 1.21 [0.71–2.08] |
| Employment status, full or partial employment | 1.57 [1.14–2.14]* | 1.22 [0.73–2.04] |
Univariable and multivariable associations between listed factors and prevalence of self‐reported chest pain. The multivariable model (n=454) included listed demographics, traditional cardiovascular risk factors, social determinants of health, depressive symptoms, and antianginal medications covariates.
Significant associations (P<0.05).
Chest Pain Frequency
Individuals with obesity had more frequent self‐reported chest pain than individuals without obesity (AFCS, 74.9 [SD, 22.2] versus 78.3 [SD, 21.9], P=0.02) (Figure 2C), with the frequency of self‐reported chest pain numerically increasing with increasing weight class (P=0.1) (Figure 2D). In the entire cohort, obesity was associated with more frequent self‐reported chest pain in univariate models (AFCS: obesity ß, −3.2 [95% CI, −6.3 to −0.1], P=0.04), but this was not significant after adjustment for demographics, CVD risk factors, social determinants of health, depressive symptoms, and antianginal medications (AFCS: obesity ß, −0.8 [95% CI, −5 to 3.3], P=0.7). In the overall sample, being female (AFCS: female ß, −4.8 [95% CI, −9 to −0.6], P=0.03), smoking (AFCS: smoking ß, −11.6 [95% CI, −18.8 to −3.3], P=0.005), and having more depressive symptoms (AFCS: PHQ9 ß, −0.8 [95% CI, −1.3 to −0.4], P<0.001) was independently associated with more frequent self‐reported chest pain.
Interaction Between Obesity, Sex, Depression, and Chest Pain
Interaction analyses were performed to investigate whether demographic features, social determinants of health, CVD risk factors, and depression (PHQ9 score >4) influenced the relationship between obesity and self‐reported chest pain. Significant interactions were observed with sex (P=0.08) and depression (P=0.08) (Figure S1).
Chest Pain Prevalence
Overall, women (75.2% versus 67.2%, P=0.02) and participants with depression (82.2% versus 65.3%, P<0.001) were more likely to have self‐reported chest pain than men and individuals without depression (Figure 3). In post hoc pairwise comparisons, obesity was associated with a significantly higher prevalence of self‐reported chest pain in men (79.5% versus 58.2%, P<0.001) and in individuals without depression (75.6% versus 58.7%, P<0.001), compared with their counterparts without obesity in these subgroups. However, there was no association between obesity and the prevalence of self‐reported chest pain in women and those with depression (Figure 3A,C).
Figure 3. Relationships between chest pain and obesity in sex and depression stratified groups.
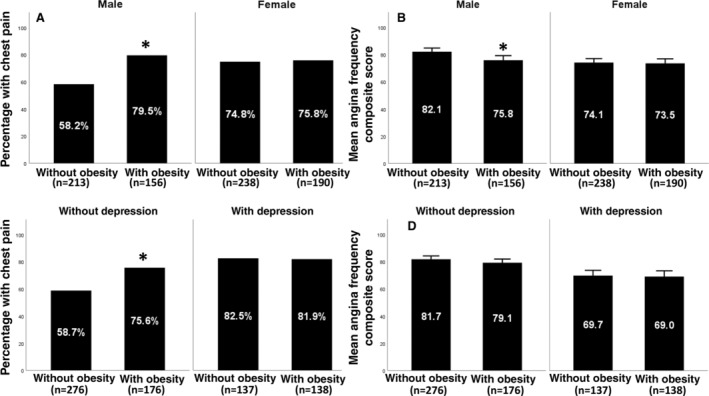
Prevalence of self‐reported chest pain (A) and mean angina frequency composite score (B) by obesity status in men vs women. Prevalence of self‐reported chest pain (C) and mean angina frequency composite score (D) by obesity status in individuals with depression (PHQ9 score >4) vs individuals without depression (PHQ9 score ≤4). Overlying asterisks indicate significant post hoc pairwise comparisons after Bonferroni correction (P<0.008). PHQ9 indicates Patient Health Questionnaire 9.
Chest Pain Frequency
As with prevalence, women (AFCS, 73.8 [SD, 23] versus 79.4 [SD, 20.6], P<0.001) and individuals with depression (AFCS, 69.4 [SD, 24.1] versus 80.7 [SD, 19.6], P<0.001) had more frequent self‐reported chest pain than men and individuals without depression (Figure 3). In post hoc analysis, the presence of obesity was significantly associated with more frequent self‐reported chest pain in men (AFCS, 75.8 [SD, 20.1] versus 82.1 [SD, 19.9], P=0.002) but had no significant association with self‐reported chest pain frequency in women and individuals with or without depression (Figure 3B,D). Notably, self‐reported chest pain frequency in women with or without obesity was similar to that of men with obesity (Figure 3B).
Mediation of the Association Between Obesity and Chest Pain Through Traditional Cardiovascular Risk Factors and Depressive Symptoms
In adjusted models examining the covariates independently associated with obesity, we found that obesity was independently associated with greater depressive symptoms (PHQ9 score), the presence of hypertension, and lower HDL levels in this cohort (P<0.05). Because obesity was independently associated with hypertension, HDL levels, and depressive symptoms, mediation analysis was performed to assess whether these covariates mediate the association between obesity and self‐reported chest pain prevalence. Monte Carlo causal‐mediation analysis revealed that only depressive symptoms had a significant mediation effect (P=0.04), accounting for 12.6% of the association between obesity and the prevalence of self‐reported chest pain. The mediation effect of obesity on self‐reported chest pain through depressive symptoms was not modified by sex.
Discussion
Main Findings
Novel findings of this study are that there is a relationship between obesity and self‐reported chest pain in patients with NOCAD, whereby individuals with obesity were more likely to have self‐reported chest pain and reported more frequent chest pain compared with individuals without obesity. The association between obesity and the prevalence of self‐reported chest pain was independent of demographics, traditional cardiovascular disease risk factors, social determinants of health, depressive symptoms, and medication use.
In this cohort of participants with NOCAD, we found obesity's association with chest pain predominantly in men and those without depression, whereby men with obesity had similar self‐reported chest pain to women. These findings may be because, regardless of obesity status, women and those with depression had a significantly higher self‐reported chest pain burden and frequency than their counterparts, making it difficult to measure a significant association between obesity and chest pain in these subgroups. Our results in this cohort with NOCAD, which suggests increased chest pain prevalence and frequency in women and those with depression, corroborate previous studies showing increased chest pain in women and those with depression in other populations. 18 , 25
Findings in our study describing an overall relationship between obesity and self‐reported chest pain are consistent with what has been previously reported in populations different from the one investigated in this study. The association between obesity and chest pain in this cohort of patients with NOCAD was similar to the association Wilson et al. reported in a cohort from the Framingham Heart Study, which found a 63% increased risk of angina among individuals with obesity versus those without obesity. 11 However, we are the first to report such an association in individuals with NOCAD diagnosed from invasive cardiac testing.
Moreover, our findings on depressive symptoms and self‐reported chest pain reflect results from previous literature on patients with NOCAD. 18 , 26 , 27 We previously showed a negative association between the SAQ angina frequency and PHQ8 scores (adjusted PHQ8 ß: −1.11, P<0.001) independent of clinical risk factors in a smaller subset of individuals without obstructive CAD. 18 We have now expanded on these findings connecting depressive symptoms to chest pain by showing that depressive symptoms partly mediate the association between obesity and self‐reported chest pain in patients with NOCAD.
Mechanistic Considerations
Previous studies have implicated coronary microvascular disease (CMD) induced oxygen‐supply demand mismatch as a driver of chest pain in patients with NOCAD. 28 , 29 By releasing certain adipokines and inflammatory cytokines, adipocytes in those with obesity inhibit endothelial‐mediated vasodilatation of coronary microvasculature, causing CMD. 30 Bajaj et al. found an inverse relationship between BMI and coronary flow reserve after 30 kg/m2, as measured by cardiac positron emission tomography. 31 Thus, obesity‐induced CMD ischemia may partly explain the association between obesity and self‐reported chest pain we observed. CMD might also explain the mediation and direct effect of depressive symptoms on chest pain in patients with NOCAD. In a study examining dizygotic twins, Vaccarino et al. found reduced coronary flow reserve in individuals with depression compared with their twin with no depression. 32 Previous literature has attributed depression‐induced alterations in vascular function to neurohormonal modulation and changes in the automatic nervous system. 33
Prior human and animal studies show that obesity increases peripheral nociceptive processing and perceived pain. 34 , 35 Rossi et al. demonstrated that mice with diet‐induced obesity had higher activation of their trigeminal nerve to capsaicin than mice fed a regular diet without obesity. 34 Using electrodes to stimulate the sural nerve in humans and electromyograms to measure the nociceptive biceps flexion reflex, Pradlier et al. showed that participants with obesity had lower nociceptive thresholds compared with participants without obesity. 35 Thus, increased nociceptive processing in patients with obesity may be an explanation for more reported chest pain in this group. In addition, reduced nociception due to peripheral nerve damage in older patients or those with diabetes may explain the lower self‐reported chest pain prevalence in these subgroups. 36 , 37
Implications
Our findings connecting obesity to self‐reported chest pain in patients with NOCAD underscore established indications of treating angina with exercise and diet, which promote weight loss. 38 Along with lifestyle modifications, the advent of surgical procedures and glucagon‐like peptide‐1 agonists have made weight loss more achievable. Karason et al. showed improved chest pain in obese patients undergoing bariatric surgery compared with those who did not receive surgical intervention. 12 We have also replicated the connection between depressive symptoms and chest pain and shown partial mediation of the association between obesity and self‐reported chest pain through depressive symptoms in patients with NOCAD, suggesting that there may be a role for antidepressants or counseling in improving the quality of life in this subgroup. 18 , 26 , 27 However, there is a paucity of evidence showing the antianginal benefits of antidepressants. One systematic review showed modest evidence of antidepressants reducing noncardiac chest pain. 39 Whether weight loss or depression interventions improve chest pain, quality of life, morbidity, mortality, and lower usage of health care resources in patients with NOCAD and chest pain remains to be studied and would be informative in managing this group of patients.
Limitations/Future Directions
There are several limitations of this cross‐sectional, registry‐based study. Although participants underwent routine clinically indicated left heart catheterization at the treating physician's discretion, detailed indication for catheterization was not collected in all patients. Given this, among participants who have catheterization indication data, almost all received catheterization for suspected myocardial ischemia, so our cohort may be more enriched for chest pain than the general population of individuals with NOCAD. Routine clinical exams evaluating angina in participants were not done in this registry. So, we used the SAQ angina frequency domain because it is a validated, reproducible measure of angina and has been used to measure angina in previous studies investigating individuals with NOCAD. 13 , 14 , 15 , 16 , 17 Consequently, we used self‐reported chest pain, as measured by the SAQ angina frequency domain, as our primary measure and surrogate of angina. However, angina is best discerned from other types of chest pain through systematic interviews with clinicians, which is challenging to do in large registries similar to the one used in this study; hence, we may not have fully captured the angina burden in our cohort.
We did not have other anthropometric measures of adiposity in our registry besides BMI. Waist circumference is a better measure of adiposity and correlates better with cardiovascular disease risk factors than BMI. 40 Future studies investigating the associations between chest pain and markers such as waist circumference, visceral fat assessment, and perivascular fat are warranted in those with NOCAD.
We did not perform comprehensive coronary vascular function testing for CMD measures or intravascular plaque imaging in this cohort. Prior work indicates that coronary endothelial dysfunction is present in at least 50% of patients with NOCAD suspected of ischemia, which could underscore our findings. 41 So, future studies investigating how CMD mediates associations found in our study would be illuminating. Although medications were included in our analysis, medication doses or granular data on depression therapy were not collected, preventing us from fully measuring their effect on self‐reported chest pain.
Conclusions
Obesity is associated with self‐reported chest pain prevalence and frequency in patients with NOCAD, wherein its association with self‐reported chest pain prevalence is independent of CVD risk factors, demographics, medications, depressive symptoms, and social determinants of health. The association between obesity and self‐reported chest pain is pronounced in men and individuals without depression. More severe depressive symptoms are independently associated with self‐reported chest pain prevalence and burden, and depressive symptoms partially mediate the association between obesity and self‐reported chest pain in patients with NOCAD.
Sources of Funding
Vatsa is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA), National Institutes of Health—National Heart, Lung, and Blood Institute grant 1R01HL157311, and 1T32HL130025. Mehta is supported by National Institutes of Health—National Heart, Lung, and Blood Institute grant 1R01HL157311. Quyyumi is supported by National Institutes of Healthgrants P01HL154996‐01A1, R33HL138657‐05, U54AG062334‐01, P30DK111024‐07S2, R61HL154116‐01, R01HL109413‐07, R01HL166004‐01.
Disclosures
None.
Supporting information
Figure S1
Acknowledgments
We acknowledge all coordinators who have assisted in gathering data for the Emory Cardiovascular Biobank.
This article was sent to Tiffany M. Powell‐Wiley, MD MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031429
For Sources of Funding and Disclosures, see page 9.
References
- 1. Davis MB, Maddox TM, Langner P, Plomondon ME, Rumsfeld JS, Duvernoy CS. Characteristics and outcomes of women veterans undergoing cardiac catheterization in the Veterans Affairs Healthcare System: insights from the VA CART Program. Circ Cardiovasc Qual Outcomes. 2015;8:S39–S47. doi: 10.1161/CIRCOUTCOMES.114.001613 [DOI] [PubMed] [Google Scholar]
- 2. Farrehi PM, Bernstein SJ, Rasak M, Dabbous SA, Stomel RJ, Eagle KA, Rubenfire M. Frequency of negative coronary arteriographic findings in patients with chest pain is related to community practice patterns. Am J Manag Care. 2002;8:643–648. [PubMed] [Google Scholar]
- 3. Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S, Prescott E. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry‐based cohort study. PLoS One. 2014;9:e93170. doi: 10.1371/journal.pone.0093170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331 [DOI] [PubMed] [Google Scholar]
- 5. Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, Rogers WJ, Mankad S, Sharaf BL, Bittner V, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH‐NHLBI‐sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040 [DOI] [PubMed] [Google Scholar]
- 6. Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. doi: 10.1001/jama.2014.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence‐based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock‐Palmer RP, Conejo T, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001030 [DOI] [PubMed] [Google Scholar]
- 9. Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health—National Heart, Lung, and Blood Institute—sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990 [DOI] [PubMed] [Google Scholar]
- 10. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. state‐level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 11. Wilson PWF, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867 [DOI] [PubMed] [Google Scholar]
- 12. Karason K, Lindroos AK, Stenlöf K, Sjöström L. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med. 2000;160:1797–1802. doi: 10.1001/archinte.160.12.1797 [DOI] [PubMed] [Google Scholar]
- 13. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9 [DOI] [PubMed] [Google Scholar]
- 14. Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle Angina Questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7:640–647. doi: 10.1161/CIRCOUTCOMES.114.000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reynolds HR, Picard MH, Spertus JA, Peteiro J, Lopez Sendon JL, Senior R, El‐Hajjar MC, Celutkiene J, Shapiro MD, Pellikka PA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO‐ISCHEMIA study. Circulation. 2021;144:1008–1023. doi: 10.1161/CIRCULATIONAHA.120.046791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimokawa H, Suda A, Takahashi J, Berry C, Camici PG, Crea F, Escaned J, Ford T, Yii E, Kaski JC, et al. Clinical characteristics and prognosis of patients with microvascular angina: an international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur Heart J. 2021;42:4592–4600. doi: 10.1093/eurheartj/ehab282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Handberg EM, Merz CNB, Cooper‐Dehoff RM, Wei J, Conlon M, Lo MC, Boden W, Frayne SM, Villines T, Spertus JA, et al. Rationale and design of the Women's Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J. 2021;237:90–103. doi: 10.1016/j.ahj.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 18. Hayek SS, Ko YA, Awad M, Del Mar SA, Ahmed H, Patel K, Yuan M, Maddox S, Gray B, Hajjari J, et al. Depression and chest pain in patients with coronary artery disease. Int J Cardiol. 2017;230:420–426. doi: 10.1016/j.ijcard.2016.12.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko YA, Hayek S, Sandesara P, Samman Tahhan A, Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB). BMJ Open. 2017;7:e018753. doi: 10.1136/bmjopen-2017-018753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire‐9. Med Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006 [DOI] [PubMed] [Google Scholar]
- 21. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spitzer RL, Kroenke K, Williams JB; Group PHQPCS . Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 23. Cleeman JI. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). International Medical Pub; 2002. [PubMed] [Google Scholar]
- 24. Tofighi D, MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hemingway H, Langenberg C, Damant J, Frost C, Pyörälä K, Barrett‐Connor E. Prevalence of angina in women versus men: a systematic review and meta‐analysis of international variations across 31 countries. Circulation. 2008;117:1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953 [DOI] [PubMed] [Google Scholar]
- 26. Wheeler A, Schrader G, Tucker G, Adams R, Tavella R, Beltrame JF. Prevalence of depression in patients with chest pain and non‐obstructive coronary artery disease. Am J Cardiol. 2013;112:656–659. doi: 10.1016/j.amjcard.2013.04.042 [DOI] [PubMed] [Google Scholar]
- 27. Mommersteeg P, Widdershoven J, Aarnoudse W, Denollet J. Personality subtypes and chest pain in patients with nonobstructive coronary artery disease from the TweeSteden Mild Stenosis study: mediating effect of anxiety and depression. Eur J Pain. 2016;20:427–437. doi: 10.1002/ejp.743 [DOI] [PubMed] [Google Scholar]
- 28. Lim PO. Angina with coronary microvascular dysfunction and its physiological assessment: a review with cases. Br J Cardiol. 2022;29:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banks K, Lo M, Khera A. Angina in women without obstructive coronary artery disease. Curr Cardiol Rev. 2010;6:71–81. doi: 10.2174/157340310790231608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bagi Z, Broskova Z, Feher A. Obesity and coronary microvascular disease—implications for adipose tissue‐mediated remote inflammatory response. Curr Vasc Pharmacol. 2014;12:453–461. doi: 10.2174/1570161112666140423221843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol. 2018;72:707–717. doi: 10.1016/j.jacc.2018.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaccarino V, Votaw J, Faber T, Veledar E, Murrah NV, Jones LR, Zhao J, Su S, Goldberg J, Raggi JP, et al. Major depression and coronary flow reserve detected by positron emission tomography. Arch Intern Med. 2009;169:1668–1676. doi: 10.1001/archinternmed.2009.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Meer RE, Maas AH. The role of mental stress in ischaemia with no obstructive coronary artery disease and coronary vasomotor disorders. Eur Cardiol. 2021;16:e37. doi: 10.15420/ecr.2021.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossi HL, Luu AK, DeVilbiss JL, Recober A. Obesity increases nociceptive activation of the trigeminal system. Eur J Pain. 2013;17:649–653. doi: 10.1002/j.1532-2149.2012.00230.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pradalier A, Willer JC, Boureau F, Dry J. Relationship between pain and obesity: an electrophysiological study. Physiol Behav. 1981;27:961–964. doi: 10.1016/0031-9384(81)90354-1 [DOI] [PubMed] [Google Scholar]
- 36. Agashe S, Petak S. Cardiac autonomic neuropathy in diabetes mellitus. Methodist Debakey Cardiovasc J. 2018;14:251–256. doi: 10.14797/mdcj-14-4-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouche P. Neuropathy of the elderly. Rev Neurol (Paris). 2020;176:733–738. doi: 10.1016/j.neurol.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 38. Ferraro R, Latina JM, Alfaddagh A, Michos ED, Blaha MJ, Jones SR, Sharma G, Trost JC, Boden WE, Weintraub WS, et al. Evaluation and management of patients with stable angina: beyond the ischemia paradigm. J Am Coll Cardiol. 2020;76:2252–2266. doi: 10.1016/j.jacc.2020.08.078 [DOI] [PubMed] [Google Scholar]
- 39. Nguyen TM, Eslick GD. Systematic review: the treatment of noncardiac chest pain with antidepressants. Aliment Pharmacol Ther. 2012;35:493–500. doi: 10.1111/j.1365-2036.2011.04978.x [DOI] [PubMed] [Google Scholar]
- 40. van Dijk SB, Takken T, Prinsen EC, Wittink H. Different anthropometric adiposity measures and their association with cardiovascular disease risk factors: a meta‐analysis. Neth Hear J. 2012;20:208–218. doi: 10.1007/s12471-011-0237-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. doi: 10.1016/j.jcin.2015.06.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


