Abstract
Background
This study aimed to investigate the potential utility of Epithelial-mesenchymal transition (EMT) signaling cell detection in the early diagnosis of cervical lesions.
Methods
Enrichment of cervical epithelial cells was carried out using a calibrated membrane with 8-μm diameter pores. RNA-in situ hybridization (RNA-ISH) was employed to detect and characterize EMT cells utilizing specific EMT markers.
Results
EMT cells were successfully identified in cervical samples, while none were detected in the healthy control group. Meanwhile, the number of EMT cells is not correlated with either the presence or type of HPV infection. Comparison of diagnostic tests showed the area under the curve (AUC) for HPV DNA tests, Thinprep cytologic tests (TCT), colposcopy and EMT signaling tests to be 0.758, 0.800, 0.889 and 0.992, respectively. A higher detection rate of EMT cells was observed in patients with cervical lesions aged ≥ 45 compared to those aged < 45 years (P < 0.05). In cervical cancer patients, a significantly greater number of EMT cells were found in FIGO stage II than in FIGO stage I (P < 0.05). Notably, epithelial-type EMT cells were detected at significantly higher rates in patients with high-grade squamous intraepithelial lesion (HSIL) and cervical cancer compared to those with low-grade squamous intraepithelial lesion (LSIL).
Conclusions
EMT markers demonstrate potential as effective tools for detecting cervical lesions.
Keywords: SIL, Cervical cancer, Epithelial–mesenchymal transition markers, HPV, TCT, Colposcopy
Introduction
Cervical cancer is a significant threat to women's health and is the 4th most common cancer among women [1]. Screening for cervical cancer is crucial because it allows for the prediction, prevention, and control of the disease using screening methods [2, 3].
Currently, the most commonly used methods for cervical cancer screening include HPV testing [4, 5], TCT [6, 7] and colposcopy [8]. Each screening method for cervical lesions has its advantages and limitations. HPV testing is critical for identifying human papillomavirus, which is the primary cause of cervical cancer. However, its relatively low positive predictive value for detecting cervical lesions can result in false positives, potentially leading to unnecessary treatments and increased patient anxiety. TCT is widely recognized for its ability to provide clear images of cervical cells, which aids in the detection of cellular abnormalities. Nonetheless, this method may occasionally fail to identify pathological cells due to technical limitations and the variable expertise of pathologists, which can affect the accuracy of the test. Colposcopy allows for the direct visualization of cervical lesions, facilitating detailed assessments of their severity and extent. It supports guided biopsies and histological analysis, which are crucial for determining the nature and depth of the lesions. Despite its effectiveness, colposcopy requires highly skilled practitioners and may cause discomfort or pain for patients, along with potential bleeding [9].
The adoption of a combined approach that utilizes multiple methods significantly improves the accuracy and reliability of cervical cancer screening. However, this approach can impose a financial burden on patients, especially in developing and underdeveloped countries. Consequently, there is a pressing need for more cost-effective screening strategies and technologies that align with prevention goals.
EMT is a process by which tumor cells undergo morphological changes, gaining the capacity to invade blood vessels and metastasize [10–13]. Detecting the shift from epithelial to mesenchymal cells in the bloodstream is crucial not only for confirming the presence of tumor cells but also for assessing their metastatic potential [14–17]. This detection has been effectively utilized as a molecular target for tumor monitoring [18].
Key molecular markers for identifying epithelial and mesenchymal cells in circulation include the epithelial cell adhesion molecule (EpCAM), cytokeratins (CKs), vimentin, and Twist [19]. EpCAM, a transmembrane glycoprotein, plays a role in cell adhesion within epithelial tissues and is implicated in the progression to malignancy [20]. Cytokeratins, intermediate filament proteins integral to the cytoskeleton of epithelial cells, along with EpCAM, are recognized as molecular markers for detecting epithelial tumor cells [21]. Vimentin, predominantly found in mesenchymal cells, is linked with increased tumor growth and invasiveness when overexpressed [22, 23]. Twist, a helix-loop-helix protein frequently elevated in various tumors, is associated with resistance to certain cancer therapies [24–26]. The identification of these markers can aid in confirming the presence of tumor cells and evaluating the likelihood of EMT, signaling the potential for metastasis [27, 28].
Currently, EpCAM, CKs, vimentin, and Twist are primarily targeted for monitoring circulating tumor cells (CTCs) in the bloodstream [28]. However, the utility of detecting CTCs in early-stage tumors is debated, as this method focuses on tumor cells in peripheral blood rather than at the primary tumor site. Thus, detecting EMT markers in tissues or cells shed from the primary tumor might offer greater value in the early detection of tumors.
This study aims to detect EMT markers in exfoliated cervical cells from women to investigate their potential in the early diagnosis of cervical lesions.
Materials and methods
Patients
A total of 64 patients from the Department of Gynecology of the First Affiliated Hospital of Guangzhou University of Chinese Medicine were selected for this study: 17 patients with LSIL, 30 patients with HSIL and 17 patients with cervical cancer. Prior to surgery, all patients underwent preoperative cervical biopsies for pathological confirmation and relevant tests. Postoperative pathological data were obtained. Twenty healthy women were included as controls. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine, and all the enrolled patients provided informed consent.
Cervical cell collection and enrichment
Using sterile cotton swabs, secretions were carefully removed from the cervical and external cervical areas once the cervix was fully exposed with a vaginal speculum. A cervical brush (Jianyou, Jiangsu, China) was then inserted approximately 2 cm into the cervical canal and rotated clockwise 3–5 times before being placed in a cell preservation solution. To ensure the complete release of cells from the cervical brush, the solution was vortexed for 5-10 s.
One milliliter of the cell preservation solution was mixed gently by inverting it 8-10 times in 9 mL of pre-chilled 1X PBS (Sigma, St. Louis, USA) at a temperature of 2-6 °C to wash the cervical epithelial cells. If no flocculent material was present, cell enrichment was performed. In cases where flocculent material was observed, the solution was centrifuged at 4 °C and 3000 rpm for 5 min. The supernatant was carefully removed until approximately 1 mL of liquid remained. The washing steps with 1X PBS were repeated until no visible flocculent material remained.
The cell suspension was then transferred to a filtration tube equipped with a calibrated membrane having pores 8 μm in diameter (Millipore, Billerica, USA). A vacuum pump was activated to create a pressure of at least 0.08 MPa, and the manifold vacuum plate valve was opened to commence filtration. This procedure allows the capture and enrichment of epithelial cells by filtering the solution through membrane filters calibrated for larger cell volumes. Once the liquid was removed, the pump continued to run for an additional 15 s with a vacuum no lower than −0.04 MPa to ensure firm adhesion of cells to the filter. Subsequently, the filtration pump and two-way valve were closed, and 1 mL of 4% formaldehyde solution (Sigma, St. Louis, USA) was added to the filter. The cells were then incubated for 1 h to achieve fixation.
EMT signal detection
ISH-RNA was used for the identification and characterization of cervical lesion cells based on EMT markers using branched DNA (bDNA) signal amplification technology. EMT markers were labeled with different colored probes (red probes for the epithelial markers EpCAM or CK8 and green probes for the mesenchymal markers vimentin or TWIST) to achieve tumor cell subtype classification. In addition, CD45 was labeled with white probes to prevent interference from leukocytes during detection [19].
The membrane was transferred from the filter into a 24-well plate (Corning, NY, USA) for molecular hybridization experiments. The membrane was transferred to 100 μL of x-Triton 100 working solution and incubated at room temperature for 5 min, followed by three washes with 1 mL of wash buffer I (0.1 × SSC (Sigma, St. Louis, USA)) for 2 min each to remove the x-Triton 100. Subsequently, 200 μL of pepsin working solution was added to the membrane, which was subsequently incubated at room temperature for 1 h and washed three times with 1 mL of wash buffer I for 2 min each to remove pepsin. Next, 200 μL of the probe hybridization working solution was applied to the membrane (solution I [30% horse serum (Sigma, St. Louis, USA)], 1.5% sodium dodecyl sulfate [Sigma, St. Louis, USA], 3 mM Tris–HCl [pH 8.0] [Sigma, St. Louis, USA], and 1 fmol of probe), and hybridization was conducted at 40 °C for 3 h. Subsequently, 200 μL of preamplification working solution (solution I and 0.5 fmol of preamplifier) was added, the mixture was incubated at 40 °C for 30 min, and 200 μL of amplification working solution (solution I and 1 fmol of amplifier) was added to perform signal amplification at 40 °C for 30 min. Finally, 200 μL of fluorescently labeled probe working solution (30% horse serum, 1.5% sodium dodecyl sulfate, 3 mM Tris–HCl (pH 8.0), and 0.5 fmol of fluorescently labeled probe including three types of fluorescently labeled probes conjugated with the fluorescent dye Alexa Fluor 594) was added, and the mixture was incubated at 40 °C for 20 min. After each incubation step, the membrane was washed three times with 1 mL of wash buffer (0.2 × SSC (Sigma, St. Louis, USA)) before the next experiment [19].
Finally, 20 μL of DAPI (Sigma, St. Louis, USA) was applied for counterstaining onto a dried coverslip. The membrane was inverted, allowing it to contact the target area of the coverslip, and gently pressed to prevent bubble formation.
The samples were analyzed using a fluorescence microscope (Zeiss Axio Imager. D2, Oberkochen, Germany) and an automatic recognition system (SurExam MetaSystems, Guangzhou, China). EMT cells were defined as cells without CD45 expression and expressing epithelial and/or mesenchymal markers. Cells with only red dots were defined as epithelial EMT cells, cells with only green dots were defined as mesenchymal EMT cells, and cells with both red and green dots were defined as mixed phenotypic EMT cells.
Statistical analysis
Student's t test or one-way ANOVA was used to investigate the correlation between the number of EMT cells and various clinicopathological features, as well as between the number of EMT cells and the severity of cervical lesions at different stages. Additionally, the numbers of EMT cells detected before and after cervical cancer surgery were compared. Statistical significance was set at P < 0.05. All statistical analyses were performed using the SPSS software (version 20.0; IBM, USA).
Results
Enrichment and morphological identification of exfoliated cervical cells
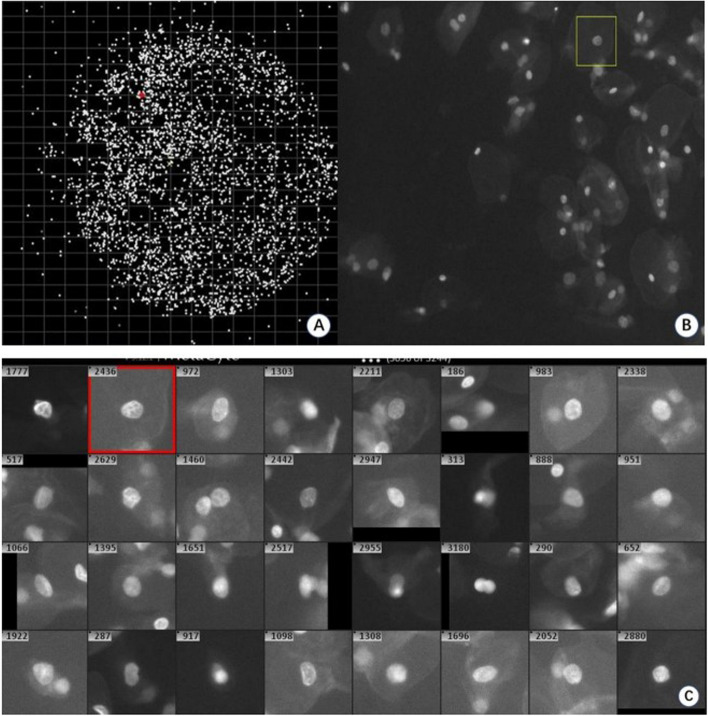
The automated scanning microscope initially performed a low-magnification (10 ×) scan of the entire filter membrane to assess the distribution and overall morphology of cells enriched on the membrane. Subsequently, each individual cell on the filter was scanned with a 20 × objective lens, allowing for detailed recording of cellular morphology. The automated detection system discriminates cells on the filter membrane by analyzing nuclear morphology, identifying potentially abnormal cells for further examination of EMT signals. Figure 1A illustrates the observed cell distribution (represented as white dots) across the entire membrane surface. Epithelial cells predominantly appeared in a single layer or in slightly overlapping configurations (Fig. 1B), maintaining an intact morphology. Additionally, the cell capture system effectively preserved the complete morphology of individual cells (Fig. 1C). In this study, the median number of cells enriched per milliliter of cell preservation fluid was 2131, with a range of 1022 to 4220. The median number of initially discriminated cells was 155, ranging from 55 to 333.
Fig. 1.
Enrichment and morphological identification of exfoliated cervix cells. The whole membrane (A) can be scanned using a fully automated fluorescence microscope(10x), which enables the assessment of the overall distribution of cells on the membrane. The cells on the membrane can be scanned at higher magnifications (20x) (B, C), allowing for preliminary determination of the possibility of abnormal cells based on nuclear morphology
EMT signal-positive cells
Utilizing RNA-in situ hybridization technology enables the differentiation of lesion cells from exfoliated cervical epithelial cells based on EMT markers (Fig. 2). EMT signal-positive cells were classified into three categories: epithelial EMT cells (indicated by red dots) (Fig. 2B), mesenchymal EMT cells (indicated by green dots) (Fig. 2C), and mixed phenotypic EMT cells (indicated by both red and green dots) (Fig. 2D). In contrast, EMT signal-negative cells exhibited only blue nuclei, identified through DAPI staining (Fig. 2A). EMT signal-positive cells were quantified when the number of EMT signal dots was ≥ 7 and CD45 signal dots were < 7, thereby meeting the established thresholds (Fig. 2).
Fig. 2.
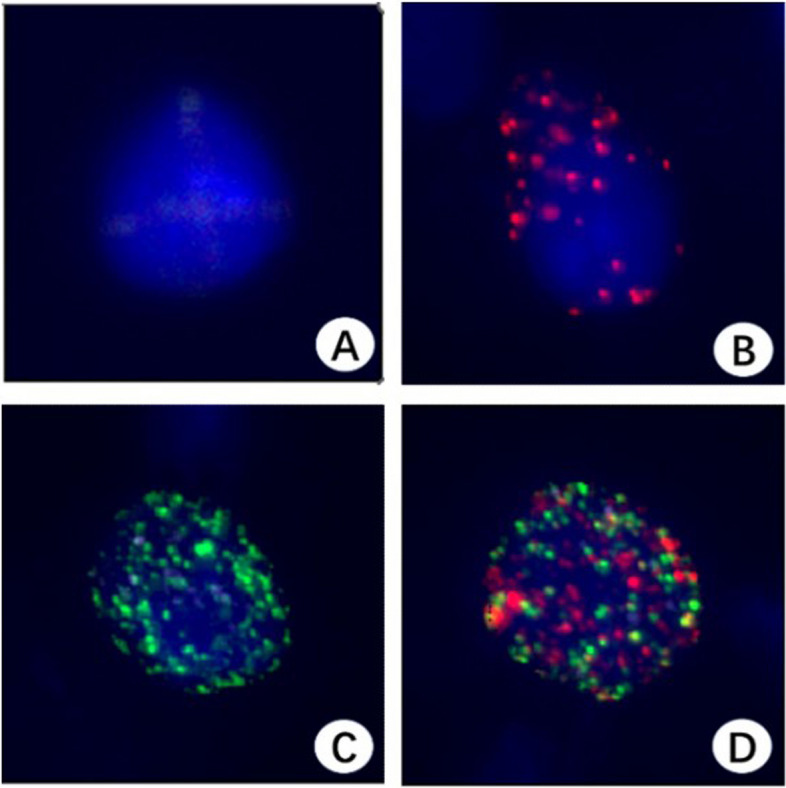
Three subtypes of EMT cells (40x). EMT signal-negative cells exhibit only blue cell nuclei (DAPI staining) (A). Epithelial EMT cells (red dots) (B). Mesenchymal EMT cells (green dots) (C). Mixed phenotypic EMT cells (red and green dots) (D)
In a study of 17 patients with LSIL, EMT cells were identified in all samples, indicating a 100% positivity rate. Every positive sample displayed both epithelial and mixed phenotypes, whereas 41.18% of samples also showed mesenchymal phenotype expression. Among 30 HSIL patients, 29 were positive for EMT cells, with a positivity rate of 96.66%. All positive samples revealed expression of epithelial and mixed phenotypes, with 56.67% exhibiting the mesenchymal phenotype. In the group of 17 cervical cancer patients, EMT cells were positively identified in all cases, reflecting a 100% positivity rate. Each positive sample had both epithelial and mixed phenotype expression, and 35.29% exhibited the mesenchymal phenotype (Table 1).
Table 1.
EMT cells positive rate detected in cervical lesion patients
| Clinical staging (TNM) | Sample No | Number Of EMT cells positive sample (%) | EMT cells counts (Mean±SD) | Range of CTCs counts | Classification of EMT cells | Enrichment cells (Mean ± SD) | Initial discrimination cells (Mean ± SD) | ||
|---|---|---|---|---|---|---|---|---|---|
| Number Of Epithelial EMT cells positive samples (%) | Number Of Mixed phenotypic EMT cells positive samples (%) | Number Of Mesenchymal EMT cells positive samples (%) | |||||||
| LSIL | 17 | 17 (100) | 27.18 ± 14.244 | 8 ~ 56 | 17(100) | 17(100) | 7(41.18) | 2443.12 ± 697.771 | 160.24 ± 65.656 |
| HSIL | 30 | 29(96.66) | 64.57 ± 52.087 | 0 ~ 173 | 29(96.66) | 29(96.66) | 17(56.67) | 2248.93 ± 684.491 | 184.9 ± 74.443 |
| Cervical cancer | 17 | 17(100) | 68.07 ± 6.86 | 7–177 | 17(100) | 17(100) | 6(35.29) | 2569.17 ± 871.934 | 155.06 ± 60.067 |
| Control | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 2354.21 ± 712.35 | 75.36 ± 34.58 |
Comparison of different examination methods for cervical lesion detection
Both enrolled patients and healthy controls underwent HPV DNA, TCT, colposcopy, and EMT signal detection. Histopathological findings served as the definitive diagnostic benchmark. The sensitivity and specificity of HPV DNA for detecting cervical lesions were 76.68% and 75.00%, respectively (AUC = 0.758). Similarly, the sensitivity and specificity of TCT for detecting cervical lesions were 75.0% and 85%(AUC = 0.8), respectively, while colposcopy demonstrated sensitivities and specificities of 82.8% and 95%(AUC = 0.889), respectively. In contrast, EMT signal detection exhibited high sensitivity (98.4%) and specificity (100%) for the detection of cervical lesions (AUC = 0.992). Notably, EMT signals were not observed in the control group. (Table 2 and Fig. 3).
Table 2.
Positive samples counts of different examination methods for cervical lesion detection
| N | HPV positive samples | TCT positive samples | Colposcopy positive samples | EMT positive samples | Histopathological | |
|---|---|---|---|---|---|---|
| LSIL | 17 | 11 | 10 | 13 | 17 | 17 |
| HSIL | 30 | 23 | 18 | 25 | 29 | 30 |
| Cervical cancer | 17 | 14 | 14 | 14 | 17 | 17 |
| Control | 20 | 5 | 3 | 1 | 0 | 0 |
| Total | 84 | 53 | 45 | 53 | 63 | 64 |
Fig. 3.
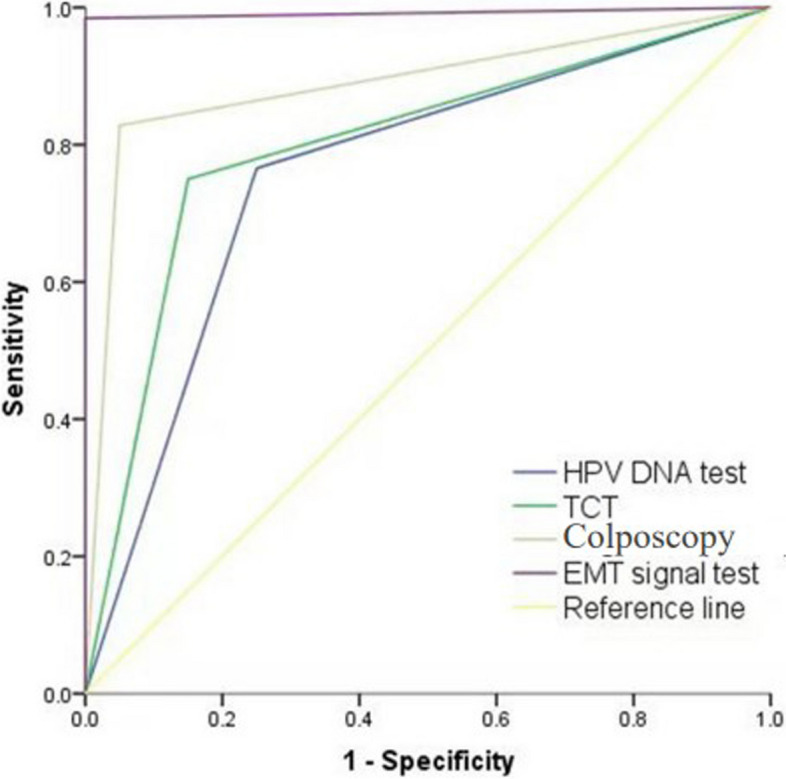
Receiver operating characteristic curve of different examination methods for cervical lesion detection. HPV DNA test AUC:0.758, TCT AUC:0.8, Colposcopy AUC:0.889, EMT signal test AUC:0.992
Correlations between specific HPV types and EMT cells
We conducted an analysis on cervical lesion patients to assess the impact of HPV infection on EMT signal detection. The findings showed that there are no statistically significant differences in the number of EMT cells detected between patients with and without HPV infection, those with single versus multiple HPV genotypes, or among patients infected with different HPV genotypes. (Table 3).
Table 3.
Correlations between specific HPV types and EMT cells
| Features | N | EMT cells counts | Epithelial EMT cells | Mixed phenotypic EMT cells | Mesenchymal EMT cells | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | t/F | p | Mean | SD | t/F | p | Mean | SD | t/F | p | Mean | SD | t/F | p | ||
| HPV positive | 48 | 46.12 | 46.369 | 0.476 | 0.636 | 7.71 | 6.047 | 0.948 | 0.346 | 37.17 | 41.176 | 0.515 | 0.608 | 1.25 | 2.159 | 0.779 | 0.439 |
| HPV negative | 16 | 40.17 | 43.684 | 6.17 | 5.68 | 31.5 | 37.466 | 0.83 | 1.15 | ||||||||
| HPV single-type infection | 40 | 49.05 | 48.938 | 1.672 | 0.111 | 8.11 | 6.285 | 1.128 | 0.265 | 39.7 | 43.549 | 1.66 | 0.117 | 1.23 | 2.078 | -0.176 | 0.862 |
| HPV multiple-type mixed infection | 8 | 30 | 24.547 | 5.5 | 4.14 | 23.25 | 21.002 | 1.38 | 2.722 | ||||||||
| HPV 16 | 12 | 41.133 | 11.874 | 1.066 | 0.389 | 4.086 | 1.18 | 2.57 | 0.056 | 37.573 | 10.846 | 0.922 | 0.463 | 2.125 | 0.613 | 0.792 | 0.539 |
| HPV 18 | 5 | 66.106 | 29.563 | 6.496 | 2.905 | 60.924 | 27.246 | 1.342 | 0.6 | ||||||||
| HPV 52 | 14 | 45.068 | 12.045 | 6.011 | 1.607 | 39.174 | 10.47 | 1.672 | 0.447 | ||||||||
| HPV 53 | 3 | 4.619 | 2.667 | 1.155 | 0.667 | 3.464 | 2 | 0 | 0 | ||||||||
| HPV 58 | 4 | 45.746 | 22.873 | 7.544 | 3.772 | 38.109 | 19.055 | 4.856 | 2.428 | ||||||||
Correlation analysis between the number of EMT cells and clinical characteristics of cervical lesion
The analysis revealed a statistically significant increase in the number of EMT cells in patients aged 45 years and older compared to those younger than 45 years with cervical lesions (P < 0.05). Interestingly, within the subset of cervical cancer patients, no significant age-related difference in EMT cell counts was found between the older (≥ 45 years) and younger (< 45 years) cohorts (P > 0.05). Furthermore, cervical cancer patients with FIGO stage II disease exhibited a significantly higher number of EMT cells compared to those with FIGO stage I (P < 0.05). The number of EMT cells did not significantly differ with respect to pelvic lymph node metastasis status or among different cancer cell types (P > 0.05). (Tables 4 and 5).
Table 4.
EMT cells counts detected in cervical lesion patients (LSIL, HSIL and cervical cancer) and correlation with clinical characteristics
| Features | N | EMT cells counts | Epithelial EMT cell | Mixed phenotypic EMT cells | Mesenchymal EMT cells | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | t | p | Mean | SD | t | p | Mean | SD | t | p | Mean | SD | t | p | ||
| Age (years) | |||||||||||||||||
| < 45 | 29 | 35 | 39.35 | 0.5 | 0.624 | 6.58 | 5.67 | −0.98 | 0.331 | 27.88 | 33.54 | −1.586 | 0.117 | 0.91 | 1.58 | −0.10 | 0.91 |
| ≥ 45 | 35 | 46.55 | 48.21 | 7.97 | 6.18 | 42.7 | 44.38 | 1.35 | 2.22 | 2 | 9 | ||||||
Table 5.
EMT cells counts detected in cervical cancer patients and correlation with clinical characteristics
| Features | N | EMT cells | Epithelial EMT cells | Mixed phenotypic EMT cells | Mesenchymal EMT cells | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | t | p | Mean | SD | t | p | Mean | SD | t | p | Mean | SD | t | p | ||
| Age (years) | |||||||||||||||||
| <45 | 6 | 35 | 39.355 | 0.5 | 0.624 | 5 | 1.414 | -0.61 | 0.551 | 28.5 | 38.847 | -0.344 | 0.737 | 1.5 | 2.811 | 0.704 | 0.492 |
| ≥45 | 11 | 46.55 | 48.219 | 6.45 | 5.663 | 35.55 | 43.098 | 1.82 | 3.601 | ||||||||
| Pelviclymphnode Metastasis | |||||||||||||||||
| With | 10 | 39 | 36.046 | 0.435 | 0.67 | 5 | 2.449 | -0.898 | 0.385 | 29.43 | 34.933 | -0.448 | 0.661 | 0.29 | 0.756 | -0.602 | 0.557 |
| Without | 7 | 49.11 | 52.413 | 7.11 | 5.798 | 39 | 47.162 | 3 | 4.093 | ||||||||
| Tumor cell type | |||||||||||||||||
| Squamous cell carcinoma | 11 | 46.08 | 49.581 | 0.508 | 0.619 | 6.08 | 5.435 | 0.191 | 0.851 | 36.67 | 45.223 | 0.556 | 0.587 | 0.83 | 2.038 | -0.333 | 0.743 |
| Adenocarcinoma | 6 | 33.8 | 31.38 | 5.6 | 1.817 | 24.4 | 28.658 | 3.8 | 4.817 | ||||||||
| FIGO | |||||||||||||||||
| I | 7 | 20.57 | 8.404 | 2.102 | 0.042 | 4.86 | 1.676 | -1.013 | 0.328 | 15.57 | 8.638 | -1.994 | 0.079 | 0.14 | 0.378 | -0.985 | 0.341 |
| II | 10 | 63.44 | 53.05 | 7.22 | 5.954 | 49.78 | 50.524 | 3.11 | 4.045 | ||||||||
Correlation analysis between the number of EMT cells and cervical lesion stage
The study demonstrated a positive correlation between the abundance of total EMT cells and the severity of cervical lesions.
The comparison of the total number of EMT cells across the three groups—cervical cancer, HSIL, and LSIL—revealed statistically significant differences. Patients diagnosed with cervical cancer exhibited significantly higher counts of epithelial or mixed phenotypic EMT cells compared to those with HSIL or LSIL. EMT cells did not show statistically significant differences among the groups. It is noteworthy that mixed phenotypic EMT cells constituted the majority of the population (80.28%), followed by epithelial EMT cells (16.35%), while mesenchymal EMT cells being relatively rare (3.36%). (Fig. 4).
Fig. 4.
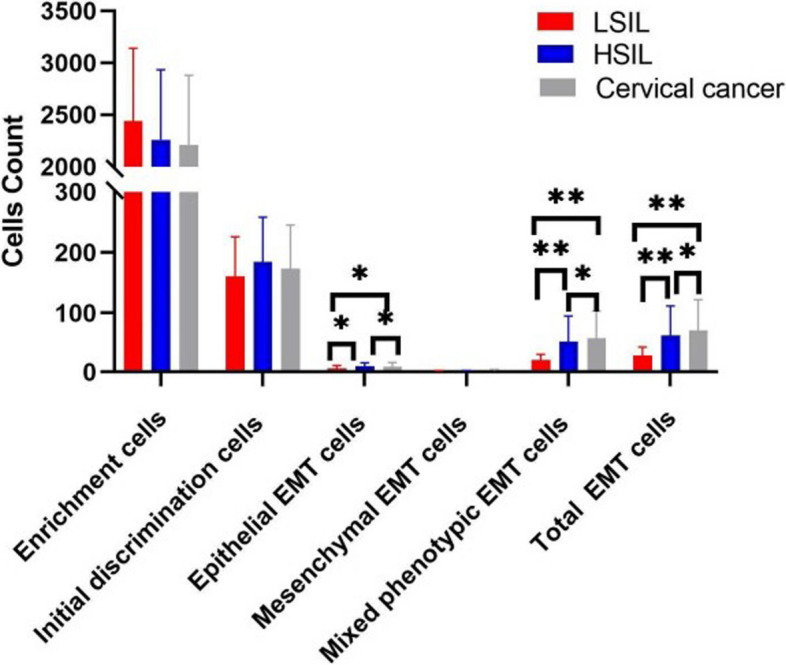
The number of EMT cells consistently positive correlates with the stages of cervical lesions. The error bars indicate standard deviations. *P<0.05, **P<0.01
Additionally, EMT cell analysis was performed on shed cervical epithelial cells from cervical cancer patients both before and after surgery. Comparisons of pre- and post-surgery samples showed no significant differences in cell enrichment or initial detection capabilities (P > 0.05). However, EMT cells were absent in post-surgery samples (Fig. 5). Intriguingly, sporadic cells exhibiting 2–7 EMT signal points were occasionally detected in post-surgery samples (data not shown).
Fig. 5.
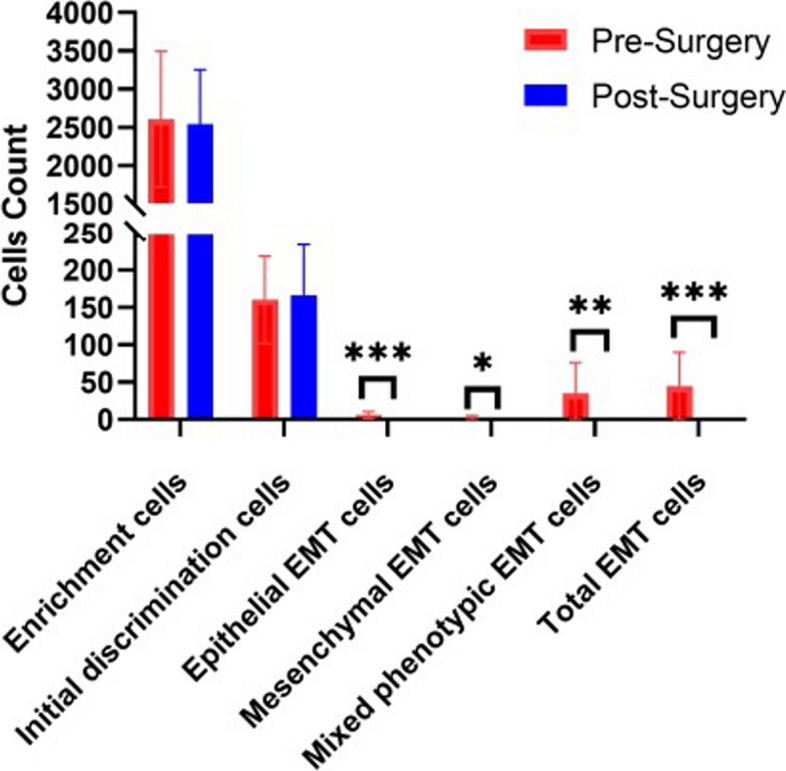
Comparison of the number of EMT cells detected pre- and post-surgery of cervical cancer. The error bars indicate standard deviations. *P<0.05, **P<0.01, ***P<0.001
Detection of EMT in cervical cancer cells
During the process of experimental optimization, exfoliated cervical cells were collected from a healthy female participant with no previous history of reproductive system diseases. The participant had recently undergone a routine health check-up, including HPV DNA test, which returned negative result. Interestingly, multiple mixed-phenotype EMT cells were detected in the sample. Based on this finding, the patient was advised to undergo a TCT, colposcopy, and additional cervical cancer-related pathological investigations. The TCT analysis identified highly abnormal epithelial cells, while the colposcopy observation indicated negative following acetic acid application. Subsequent pathological examination confirmed the presence of squamous cell carcinoma (Fig. 6).
Fig. 6.
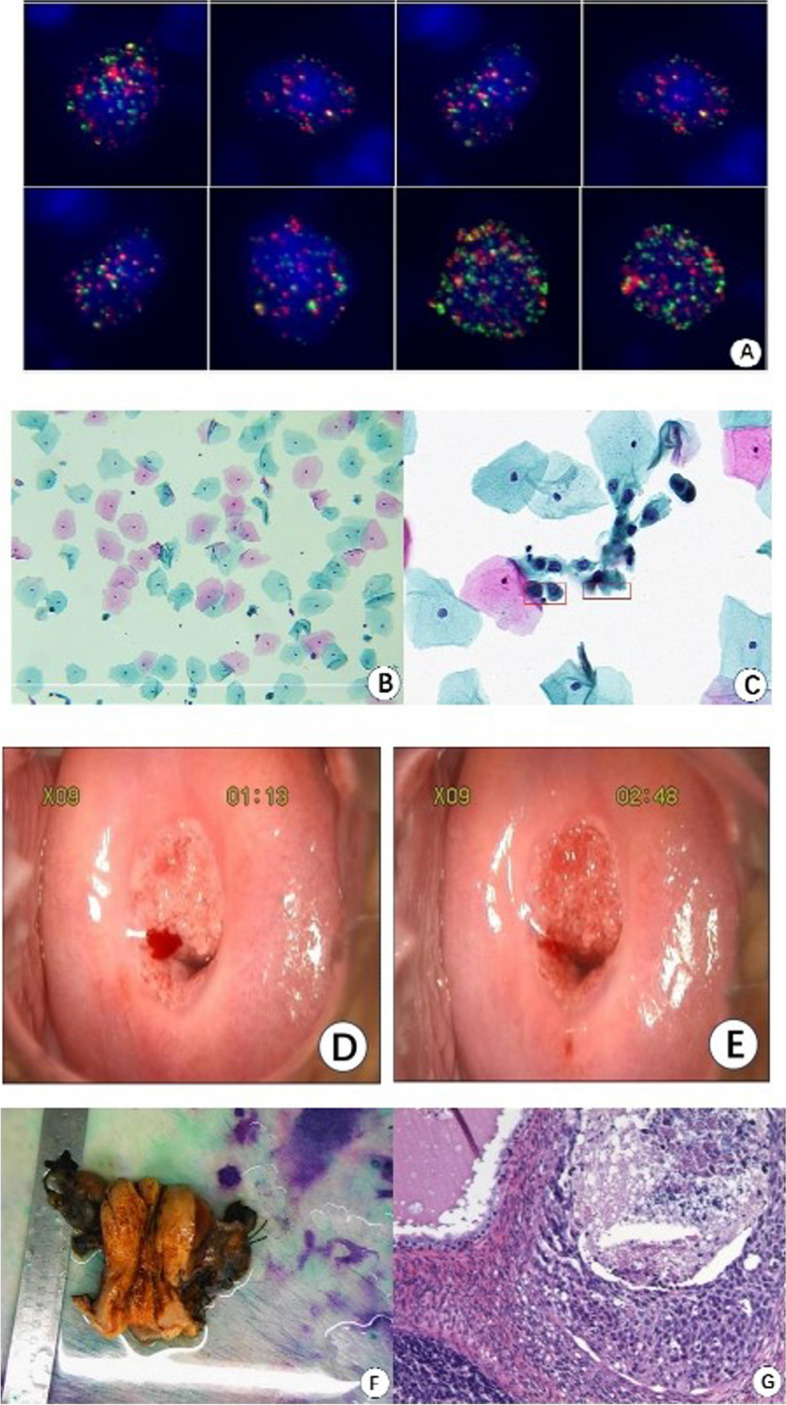
EMT cells detected and other relevant test results of a patient with cervical cancer EMT cells detected (A). TCT analysis revealed highly abnormal cells within the epithelium (B and C). Acetic acid application showed negative result (D before, E after). Pathological examination (F and G)
Discussion
The implementation of cervical cancer screening methods such as HPV DNA test, TCT, and colposcopy has significantly improved the detection and subsequent management of cervical lesions in developed nations. Recent advancements in proteomics and genomics [29, 30] are expected to further enhance these diagnostic capabilities by focusing on disease detection directly from cellular morphology and genetic profiles, rather than solely on high-risk factors [2].
Our study introduces a novel approach using EMT signaling biomarkers for detecting cervical lesion cells, an extension of methodologies applied in CTCs screening [31]. The efficiency of capturing epithelial cells with a fibrous membrane and utilizing RNA-ISH for identifying EMT markers signifies a potential shift in standard screening practices. Notably, the high sensitivity (98.4%) and specificity (100%) demonstrated by our method, substantiated by an AUC of 0.992, align well with the screening outcomes prevalent in the broader oncological context. These metrics not only reaffirm the efficacy of this method but also highlight its potential clinical application in early-stage cervical cancer detection.
Comparatively, while traditional methods focus extensively on the detection of HPV and morphological abnormalities [32, 33], our approach integrates molecular signaling, which could provide an earlier indication of malignant transformations. The absence of EMT signals in all healthy controls further emphasizes the method's discriminatory power, potentially reducing false positives prevalent in conventional screening procedures [32, 33].
However, the translation of these findings into clinical practice necessitates further exploration. It is pivotal to understand how the classification of cervical lesions through EMT signaling corresponds with traditional classifications observed in CTC screenings [34, 35]. Such investigation would not only validate the diagnostic accuracy of our method but also its reliability and applicability across different populations and settings.
The correlation between HPV infection and cervical lesions is well-recognized [36]. Our approach to identifying EMT signal detection irrespective of HPV infection status underscores the method's capability to distinguish cervical lesion cells with high specificity. This insinuates that the molecular mechanisms explored through EMT signaling are primarily associated with the pathology of the lesions themselves rather than just the presence of HPV. This distinction is crucial as it suggests that our method could be reliably utilized in broader screening scenarios where HPV status might be varied or unknown.
Analyzing the relationship between the presence of EMT cells and various clinical parameters could offer promising insights into how EMT signaling might correlate with patient demographics and cancer progression. Specifically, the increased detection of EMT signals in patients over 45 might elucidate age-related decline in immune efficacy. Such insights align with prevailing theories in immunosenescence, which postulate a degradation in immune response effectiveness with age [37–39].
Additionally, the progression of cervical cancer severity as reflected by an increase in EMT cells in later FIGO stages might point to the utility of EMT signals in not only detecting but potentially staging cervical cancer. This is significant as it could lead to earlier intervention strategies, tailored according to the stages of cervical cancer progression. However, the absence of variation in EMT cell counts with respect to lymph node metastasis suggests that while EMT signaling can indicate progression severity, it might not correlate with all forms of metastatic potential.
Drawing parallels with existing literature, our findings seem to suggest that EMT signaling might serve as an adjunct or even an alternative marker for cervical cancer staging and progression. In comparable studies where EMT has been linked with cancer progression [13], similar trends of increasing EMT signal correlation with advanced disease stages have been noted.
A similar study by Pan et al. [40] reported an elevated count of CTCs in patients with FIGO stage II cervical cancer, highlighting the marker's potential in indicating disease progression. Although there was no demonstrated correlation between CTC counts and parameters such as deep stromal invasion or age, the study confirmed the diagnostic relevance of CTCs during certain progression stages. Our findings complement this perspective by indicating a noteworthy, positive correlation between the severity of cervical neoplasia and the presence of EMT cells. These EMT cells could potentially serve comparable diagnostic and prognostic roles as CTCs, especially given their high detection rate in the early stages of cervical dysplasia and cancer in this study.
Furthermore, Pan et al. noted a high positivity rate for CTCs among early-stage cervical cancer patients, an observation that aligns with the high detection rates of EMT-positive cells in our study cohort. This underscores the potential utility of EMT cell detection as an early diagnostic tool, possibly even pacing or enhancing the detection capabilities compared to traditional methods.
The importance of such biomarkers is also evident in their potential for longitudinal monitoring. While our study captures a snapshot of EMT cell prevalence in pre and postoperative conditions, future research could benefit from a longitudinal design. Such studies would provide more dynamic insights into how EMT and CTC counts evolve with treatment and over time, and how these changes correlate with patient outcomes [12]. Although our study didn't find EMT cells in the postoperative patient samples, isolated instances of minor EMT signal points were observed. The clinical significance of these minor signals remains unclear and invites further investigation. Could these signal points potentially foreshadow the recurrence of cervical neoplasia? Exploring this could uncover more nuanced insights into the prognostic value of EMT cells.
Conclusions
In this study, we utilized acetic acid fiber membranes for the collection of cells indicative of cervical epithelial lesions, incorporating a focus on EMT-specific molecular markers. Preliminary results indicate that this method has the potential to effectively identify cervical lesions with reasonable sensitivity and specificity, including detection at early stages. It is crucial to note, however, that this technique does not offer insights into the staging of cervical lesions. While our data suggest a positive correlation between the number of EMT cells captured and lesion severity, these findings must be interpreted with caution due to the exploratory nature of the study.
Given the limitations of the study—including the small sample size and a single-center design—our conclusions regarding the effectiveness and utility of this method are provisional. We acknowledge that the sensitivity and specificity reported here may not generalize across broader, more diverse populations. Furthermore, the potential relationship between EMT cell prevalence and lesion severity requires further validation through larger-scale studies to affirm its clinical relevance and accuracy.
We recommend additional research with expanded cohorts and multicentric trials to establish the reliability and generalizability of these preliminary findings. Enhancing the scalability of this method could significantly contribute to its integration into routine cervical health screening protocols, aiding in more precise and comprehensive cervical health management.
Acknowledgements
Not applicable.
Authors’ contributions
YL, PZ, and ZZ conceived and designed the study. ZZ and CX performed the experiments and analyzed the data. SW performed gynecological examinations and collected clinical information. ZP and ZX performed the bioinformatics investigation. RZ and QX wrote the manuscript. YL and ZZ confirmed the authenticity of all the raw data. All authors have read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work is appropriately investigated and resolved.
Funding
This study was supported by Guangdong Provincial Bureau of Traditional Chinese Medicine (Grant No. 20251131) and Guangzhou University-Enterprise Joint Funding Project (Grant No. SL2024A03J01144).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and approved by the Human Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine Ethics Committee (No. k-2022-033). Written informed consent was obtained from participants or their guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhanfeng Zhang, Chuanzhen Xie and Rong Zhao contributed equally to this work.
Contributor Information
Zhanfeng Zhang, Email: zhanfengyy@yeah.net.
Peng Zhang, Email: nfyyzp@126.com.
Yuanrui Liu, Email: liuyr2303@yeah.net.
References
- 1.Vu M, Yu J, Awolude OA, Chuang L. Cervical cancer worldwide. Curr Prob Cancer. 2018;42(5):457–65. [DOI] [PubMed] [Google Scholar]
- 2.Gavinski K, DiNardo D. Cervical cancer screening. Med Clin North Am. 2023;107(2):259–69. [DOI] [PubMed] [Google Scholar]
- 3.Ngoma M, Autier P. Cancer prevention: cervical cancer. Ecancermedicalscience. 2019;13:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020;40(5):602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AA, Gheit T, Franceschi S, Tommasino M, Clifford GM. Human papillomavirus 18 genetic variation and cervical cancer risk worldwide. J Virol. 2015;89(20):10680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T, Lai Y, Yuan J. The diagnostic accuracy of TCT + HPV-DNA for cervical cancer: systematic review and meta-analysis. Ann Transl Med. 2022;10(14):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhang L, Zhao G, Che L, Zhang H, Fang J. The clinical research of Thinprep Cytology Test (TCT) combined with HPV-DNA detection in screening cervical cancer. Cell Mol Biol. 2017;63(2):92–5. [DOI] [PubMed] [Google Scholar]
- 8.Burness JV, Schroeder JM, Warren JB. Cervical colposcopy: indications and risk assessment. Am Fam Physician. 2020;102(1):39–48. [PubMed] [Google Scholar]
- 9.Tan J, Jayasinghe YL, Osinski MJ, Brotherton J, Wrede C. Recurrent post-coital bleeding: Should colposcopy still be mandatory? Aust N Z J Obstet Gynaecol. 2020;60(6):952–8. [DOI] [PubMed] [Google Scholar]
- 10.Greaves D, Calle Y. Epithelial Mesenchymal Transition (EMT) and associated invasive adhesions in solid and haematological tumours. Cells-Basel. 2022;11(4):649–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Xia L, Huang P, Wang Z, Guo Q, Huang C, Leng W, Qin S. Heterogeneity and plasticity of epithelial-mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms. Cell Proliferat. 2023;56(6):e13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfioletti G, Fedele M. Epithelial-Mesenchymal Transition (EMT). Int J Mol Sci. 2023;24(14):11386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5(2):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang SS, Mao CG, Feng YG, Jiang B, Tao SL, Tan QY, Deng B. Circulating tumor cells with epithelial-mesenchymal transition markers as potential biomarkers for the diagnosis of lung cancer. World J Clin Cases. 2021;9(12):2721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalil H, Hammam OA, Kamel A. Detection of epithelial-mesenchymal transition markers in high grade bladder cancer and special variants of urothelial carcinoma. Asian Pac J Cancer Prev. 2022;23(6):2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popiel-Kopaczyk A, Piotrowska A, Sputa-Grzegrzolka P, Smolarz B, Romanowicz H, Dziegiel P, et al. The immunohistochemical expression of epithelial-mesenchymal transition markers in precancerous lesions and cervical cancer. Int J Mol Sci. 2023;24(9):8063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solheim O, Forsund M, Trope CG, Kraggerud SM, Nesland JM, Davidson B. Epithelial-mesenchymal transition markers in malignant ovarian germ cell tumors. APMIS. 2017;125(9):781–6. [DOI] [PubMed] [Google Scholar]
- 18.Zhao XH, Wang ZR, Chen CL, Di L, Bi ZF, Li ZH, Liu YM. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J Gastroentero. 2019;25(1):138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, Yang D, Deng H, Yang N, Xu J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10(4):e123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gires O, Pan M, Schinke H, Canis M, Baeuerle PA. Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years? Cancer Metast Rev. 2020;39(3):969–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda T, Hayashi N, Iguchi T, Ito S, Eguchi H, Mimori K. Clinical and biological significance of circulating tumor cells in cancer. Mol Oncol. 2016;10(3):408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battaglia RA, Delic S, Herrmann H, Snider NT. Vimentin on the move: new developments in cell migration. F1000Res. 2018;7:1796–05. [DOI] [PMC free article] [PubMed]
- 23.Chernoivanenko IS, Minin AA, Minin AA. Role of vimentin in cell migration. Ontogenez. 2013;44(3):186–202. [DOI] [PubMed] [Google Scholar]
- 24.Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumour Biol. 2013;34(5):2497–506. [DOI] [PubMed] [Google Scholar]
- 25.Wicki A, Mandala M, Massi D, Taverna D, Tang H, Hemmings BA, Xue G. Acquired Resistance to clinical cancer therapy: a twist in physiological signaling. Physiol Rev. 2016;96(3):805–29. [DOI] [PubMed] [Google Scholar]
- 26.Kwon CH, Park HJ, Choi Y, Won YJ, Lee SJ, Park DY. TWIST mediates resistance to paclitaxel by regulating Akt and Bcl-2 expression in gastric cancer cells. Tumour Biol. 2017;39(10):1393367594. [DOI] [PubMed] [Google Scholar]
- 27.Milano A, Mazzetta F, Valente S, Ranieri D, Leone L, Botticelli A, Onesti CE, Lauro S, Raffa S, Torrisi MR, et al. Molecular detection of EMT markers in circulating tumor cells from metastatic non-small cell lung cancer patients: potential role in clinical practice. Anal Cell Pathol. 2018;2018:3506874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Zhao R, Xie Z, Pang Z, Chen S, Xu Q, Zhang Z. Significance of circulating tumor cells detection in tumor diagnosis and monitoring. BMC Cancer. 2023;23(1):1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YJ, Kim BR, Ryu JS, Lee GO, Kim HR, Choi KH, Ryu JW, Na KS, Park MC, So HS, et al. HNRNPA1, a splicing regulator, is an effective target protein for cervical cancer detection: comparison with conventional tumor markers. Int J Gynecol Cancer. 2017;27(2):326–31. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Xie X, Zhou Z, Zhang H. Expression analysis of MIST1 and EMT markers in primary tumor samples points to MIST1 as a biomarker of cervical cancer. Int J Gen Med. 2021;14:1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pipatwatcharadate C, Iyer PR, Pissuwan D. Recent update roles of magnetic nanoparticles in Circulating Tumor Cell (CTC)/Non-CTC separation. Pharmaceutics. 2023;15(10):2482–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson AA, Pantanowitz L. The evolution of cervical cancer screening. J Am Soc Cytopathol. 2024;13(1):10–5. [DOI] [PubMed] [Google Scholar]
- 33.Perkins RB, Wentzensen N, Guido RS, Schiffman M. Cervical cancer screening: a review. JAMA-J Am Med Assoc. 2023;330(6):547–58. [DOI] [PubMed] [Google Scholar]
- 34.Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, Zhu F, Zhou D, Zheng S, Chen Y, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Tar. 2021;6(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawabata N. Circulating tumor cells: from the laboratory to the cancer clinic. Cancers. 2020;12(10):3065–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterman L, Voss J. HPV, cervical cancer risks, and barriers to care for lesbian women. Nurse Pract. 2015;40(1):46–53, 53–54. [DOI] [PubMed]
- 37.Rositch AF, Koshiol J, Hudgens MG, Razzaghi H, Backes DM, Pimenta JM, Franco EL, Poole C, Smith JS. Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Int J Cancer. 2013;133(6):1271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyer BA, Zamarin D, Eskandar RN, Mayadev JM. Role of immunotherapy in the management of locally advanced and recurrent/metastatic cervical cancer. J Natl Compr Canc Netw. 2019;17(1):91–7. [DOI] [PubMed] [Google Scholar]
- 39.Song D, Li H, Li H, Dai J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol Lett. 2015;10(2):600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan L, Yan G, Chen W, Sun L, Wang J, Yang J. Distribution of circulating tumor cell phenotype in early cervical cancer. Cancer Manag Res. 2019;11:5531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.