Abstract
Objective
This study explored the value of stomach ultrasound reporting and data system (Su-RADS) and two-dimensional shear wave elastography (2D-SWE) in the diagnosis of benign and malignant lesions of the gastric wall, evaluating the feasibility of combining the two methods for the diagnosis of gastric wall lesions.
Methods
113 patients with gastric wall lesions were examined after oral gastric ultrasound contrast agent, and the grades of the gastric wall lesions were classified according to Su-RADS. Moreover, 2D-SWE was performed to measure the E value of the lesions. ROC curves were constructed to evaluate the diagnostic efficacy of Su-RADS, 2D-SWE and their combination for gastric wall lesions.
Results
The cutoff values for Emean and Emax were 8.01 kPa and 11.08 kPa, respectively. The sensitivity and specificity of 2D-SWE were 70.59%, 93.67% and 85.69%, 88.61%, respectively. The diagnostic sensitivity and specificity of Su-RADS were 91.18% and 82.28%, respectively. The AUC of combination of two methods was 0.951, which was greater than that of Su-RADS (0.940) or 2D-SWE alone (0.853, 0.903), and the sensitivity and specificity were 82.35% and 94.94%. The sensitivity and specificity of the combination of the two methods for the diagnosis of malignant gastric lesions were 82.35% and 94.94%, respectively. The AUC was 0.951, and the Youden index was 0.8064. The DeLong test was used to determine the AUC between the combination of two methods and 2D-SWE was P < 0.05.
Conclusion
Compared with Su-RADS or 2D-SWE alone, the combination of the two methods is more effective at diagnosing of gastric wall.And improved the specificity in the diagnosis of gastric wall lesions.
Keywords: Gastric wall lesions, Elastography, Contrast examination, Ultrasound imaging report and data system
Introduction
Gastric diseases, especially gastritis, gastric ulcers, gastric polyps and gastric cancer, are common and frequently encountered [1]. Among these malignancies, gastric cancer is one of the most common malignancies, and according to data provided in the 2020 Global Annual Report on Cancer, gastric cancer is the fourth most common cause of mortality from malignancies [2], accounting for 7.7%; it is one of the major diseases endangering people’s health and a key component in the prevention and control of cancer [3, 4]. The prognosis of gastric cancer is closely related to stage, with a cure rate of more than 90% for early-stage gastric cancer [5, 6] and a 5-year survival rate of less than 30% for patients with advanced-stage gastric cancer, even after comprehensive treatment dominated by surgery [7]. Therefore, the keys to reducing the mortality of patients with gastric cancer are early detection, early diagnosis and early treatment [8]. The diagnosis of gastric wall lesions by contrast-enhanced ultrasonography relies mainly on the thickness of the gastric wall and changes in the layer structure. The gastric wall is divided into the mucosa, submucosa, muscularis and serosa according to its histological features. Contrast-enhanced ultrasonography clearly show the structure of the gastric wall with high and low echoes. The stomach ultrasound report and data system (Su-RADS) [9] is used to grade the gastric wall and mucosal layer according to the thickness of the lesions in different parts of the stomach wall and evaluate the possibility of malignancy.
Ultrasonic elastography (UE) is a kind of ultrasonic diagnostic technique which can qualitatively describe and quantitatively measure the elasticity of biological tissues, according to the physical laws of biomechanics and elasticity, the distribution of displacement, velocity and strain will occur in the tissue, and color-coded imaging or measuring the corresponding parameters, so as to directly or indirectly judge the internal elastic modulus information. Based on physical laws such as biomechanics and elasticity, elastic modulus information has been widely used in the evaluation of liver, breast, thyroid, musculoskeletal and other tissues and lesions in adults [10, 11]. In pathological phenomena such as tumour transformation and fibrotic states, tissue changes and the elastic properties of elastography could theoretically be the basis for reliable methods for detection and quantification [12, 13]. Elastography has been proven to be valuable in the differential diagnosis of some benign and malignant lesions, but the application of 2D-SWE(2D-shear wave elastography) in the evaluation of benign and malignant lesions of the gastric wall has not been reported. The purpose of this study was to evaluate the value and feasibility of combining the gastric ultrasonography report and data system (Su-RADS) with two-dimensional shear wave elastography (2D-SWE) in the diagnosis of benign and malignant lesions of the gastric wall.
Data and methods
Study population and instrument
General data
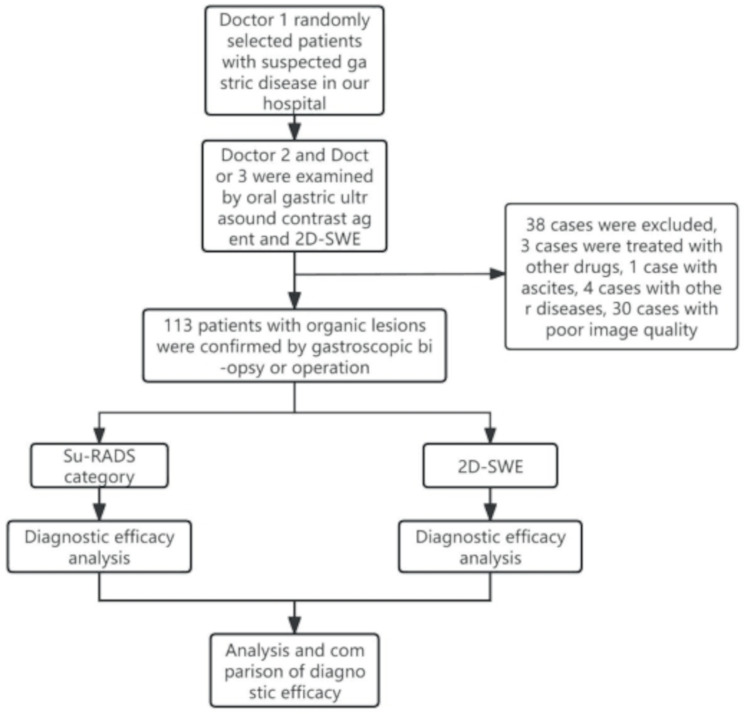
Patients with suspected gastric disease who were admitted to our hospital between May 2022 and September 2022 were randomly selected by a radiologist who was not involved in the 2023 study. A physician performed an oral gastric contrast-enhanced ultrasound without knowing the patient’s outcome or retaining images of the lesion for Su-RADS classification. The same patients were examined with 2D-SWE by another similarly qualified physician who was blinded to the outcome. The diagnostic criterion was gastroscopic biopsy or postoperative pathological diagnosis of gastric disease. Informed consent was obtained from all patients. A total of 113 patients (63 males and 50 females) were enrolled after exclusion of patients on the basis of contraindications to the examination technique and poor image quality. The inclusion criteria were as follows: ① all patients accorded with the clinical features of gastric wall lesions and were confirmed by endoscopic biopsy or pathology after operation ② all the patients did not use nonsteroidal anti-infective drugs or received anti-tumor therapy such as radiotherapy, chemotherapy and immunologic agents ③ all the patients could eat, and no upper gastrointestinal perforation and active massive bleeding and other examination contraindications. The exclusion criteria were: ① patients with severe dysfunction of heart, liver, lung and kidney, ② patients with upper gastrointestinal perforation and active massive hemorrhage, ③ patients with gastric hyperperistalsis and ascites, ④ patients with other malignant tumor except gastric tumor, ⑤ to examine the technical contraindications in this study, ⑥ patients with cognitive impairment and psychosis.These patients included 34 patients with malignant gastric wall lesions (33 patients with gastric cancer and 1 patient with gastric lymphoma) and 79 patients with benign gastric wall lesions (32 patients with gastritis, 8 patients with gastric stromal tumours, and 12 patients with gastric ulcers). The selection process is shown in Fig. 1.
Fig. 1.
Flow chart of patient enrolment
Instruments and methods
The ultrasonic detector consisted of a Mindray Resona I9s (Mindray Biomedical Electronics Co., Ltd., Shenzhen, China), an SC6-1 S convex array probe (frequency 2.0–6.0 MHz) and an L9-3 S linear array probe (9 MHz). The contrast agent used was “gastrointestinal filling ultrasound contrast agent” [F JFCM production Xu 20210012, Yanbian Junyi Medical Technology Co., Ltd. ] (500 ∼ 600 ml).
Contrast-enhanced ultrasonography and Su-RADS grading
The patients fasted for 8 h before the examination. The patient was instructed to ingest a “gastrointestinal filling ultrasound contrast agent” [FJMC production Xu 20210012, Yanbian Junyi Medical Technology Co., Ltd. ] (500 ∼ 600 ml) for ultrasound examination. During the operation, the examiner observed the gastric wall of the patient; if the findings were positive, the location and local magnification of the lesion were determined, and the characteristics of the sonogram were carefully observed. The thickness of the gastric mucosa or wall was measured. The classification was based on the criteria for determining the gastric wall mucosa or wall thickness at different sites in the gastric ultrasound reporting and data system (Su-RADS) as shown in Table 1. Category 1 is regarded as normal, categories 2–4 have the possibility of mild, moderate or severe malignancy, respectively, while Category 5 suggests a very high probability of malignancy.
Table 1.
Gastric ultrasound reporting and data system (Su-RADS)
| Categories | Assessment | TUS-OCCA report | Mucosal thicknesses(mm) | Full thickness(mm) | Necessity of additional gastroscopy | |||
|---|---|---|---|---|---|---|---|---|
| Antrum and Cardia | gastric body | fundus | ||||||
| 0 | Incomplete–need additional examination | Not satisfactory | - | - | - | - | Necessary | |
| 1 | Almost normal finding | No gastric wall thickening | ≤ 1.5 | <5 | <4 | <3 | May be unnecessary | |
| 2 | Low risk for malignancy | Mild thickening of gastric wall | 1.5-2.0 | 5–6 | 4–5 | 3–4 | Might be unnecessary | |
| 3 | Moderate risk for malignancy | Moderate thickening of gastric wall | 2.0-2.5 | 6–7 | 5–6 | 4–5 | May be necessary | |
| 4 | 4a(No mucosal ulcer was found) | High risk for malignancy | Severe thickening of gastric wall with or without ulceration | 2.5-5.0 | 7–10 | 6–9 | 5–8 | Necessary and biopsy should be performed |
| 4b (mucous ulcer) | ||||||||
| 5 | 5a(No mucosal ulcer was found) | Extremely high risk for malignancy | Extremely severe thickening of gastric wall with or without ulceration | >5 | ≥ 10 | ≥ 9 | ≥ 8 | Necessary and multiple endoscopy biopsies must be performed |
| 5b (mucous ulcer) | ||||||||
| 6 | Known biopsy-proven malignancy | Corresponded with gastric cancer | - | - | - | - | Surgical excision or other treatment | |
Note: ① for mechanical or obesity reasons, the overall gastric thickness should be judged; ② more attention should be given to whether the mucosa or gastric wall thickening is diffuse or limited
2D-SWE
The focus was placed in the image centre, the instrument was adjusted to 2D-SWE mode, and the receiver of interest (ROI) sampling frame was placed in the focus area so that the direction of the sound beam was perpendicular to the long axis of the focus. The SWE values of the lesions were measured 3 or more times, the depth was confirmed as being no more than 8.0 cm, and the mean values were obtained.
Statistical analysis
SPSS26.0 statistical software was used for the data analysis. The measurement data are expressed as the mean plus or minus the standard deviation. The normality of the data was tested by the Shapiro‒Wilk method. For comparing between two groups, two independent sample t tests were used when the data were normally distributed, the rank-sum test was used when the data were not normally distributed, and the rank-sum test was used for one-way ordered hierarchical data. The receiver operating characteristic (ROC) curve, Su-RADS grade cutoff(Category 3), sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, 2D-SWE Emean and Emax cutoff (8.01 kPa and 11.08 kPa), and negative likelihood ratio were obtained. The McNemar test was used to compare the two methods, and the Delong test was used to compare the area under the ROC curve. P < 0.05 was considered indicative of a significant difference.
Results
Su-RADS diagnostic efficacy analysis
The Su-RADS classification of 113 patients with gastric wall lesions revealed that the malignant rates in categories 1 to 5 were 0.0%, 0.0%, 12.0%, 40.0% and 92.0%, respectively. The higher the category, the greater the probability of malignancy (Table 2; Fig. 2). The area under the ROC curve was 0.929, the diagnostic sensitivity and specificity were 91.18% and 82.28%, respectively, the Youden index was 0.735, the AUC was 0.940, the positive predictive value was 68.89%, and the negative predictive value was 95.59%.The specificity of Su-RADS and 2D-SWE was 82.28 and 94.94.McNemar test showed that the specificity of Su-RADS combined with 2D-SWE was higher than that of Su-RADS alone (p < 0.05), indicating that the combination of the two methods can improve the specificity in the diagnosis of gastric wall lesions.
Table 2.
Su-RADS classification of 113 patients with gastric wall lesions
| Cases | Su-RADS Category 1 | Su-RADS Category 2 | Su-RADS Category 3 | Su-RADS Category 4 | Su-RADS Category 5 | |
|---|---|---|---|---|---|---|
| Benign | 79 | 14 | 29 | 22 | 12 | 2 |
| Malignant | 34 | 0 | 0 | 3 | 8 | 23 |
Fig. 2.
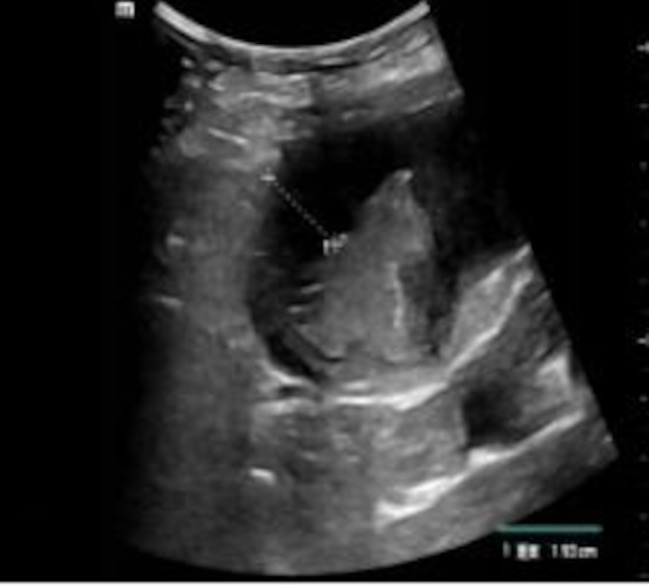
Su-RADS classification of gastric wall disease
Su-RADS category 5: malignant tumour; the thickness of the gastric wall was 19.3 mm; the pathological diagnosis via gastroscopy was gastric cancer
2D-SWE diagnostic efficacy analysis
The Emean and Emax were significantly greater for malignant lesions than for benign lesions (p < 0.05). See Fig. 3; Table 3. The mean values were 7.88 ± 3.74 kPa and 19.39 ± 7.54 kPa and 5.17 ± 1.86 kPa and 9.62 ± 3.83 kPa, respectively. The cutoff values of Emean and Emax (8.01 kPa and 11.08 kPa) could differentiate benign from malignant gastric wall lesions (AUC, sensitivity and specificity were 0.853, 70.59%, 93.67% and 0.903, 85.3%, 88.6%, respectively).
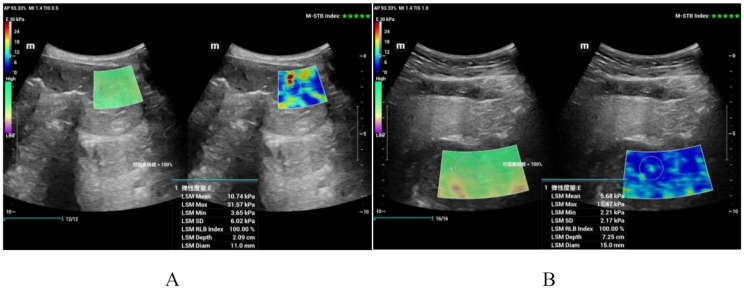
Fig. 3.
2D-SWE sonogram of gastric wall disease
Panel A shows a 2D-SWE image of gastric cancer; the Emean was 10.74 kPa, and the Emax was 31.57 kPa; panel B shows a 2D-SWE image of a gastric stromal tumour; the Emean was 5.68 kPa, and the Emax was 15.47 kPa
Table 3.
Thirteen patients with gastric wall lesions were examined by 2D-SWE
| Cases | Emean average value(kPa) | Emax average value(kPa) | Emean cut off value(kPa) | Emax cut off value(kPa) | |
|---|---|---|---|---|---|
| benign | 79 | 5.17 ± 1.86 | 9.62 ± 3.83 | 8.01 | 11.08 |
| malignant | 34 | 7.88 ± 3.74 | 19.39 ± 7.54 |
The diagnostic efficacy of Su-RADS combined with 2D-SWE
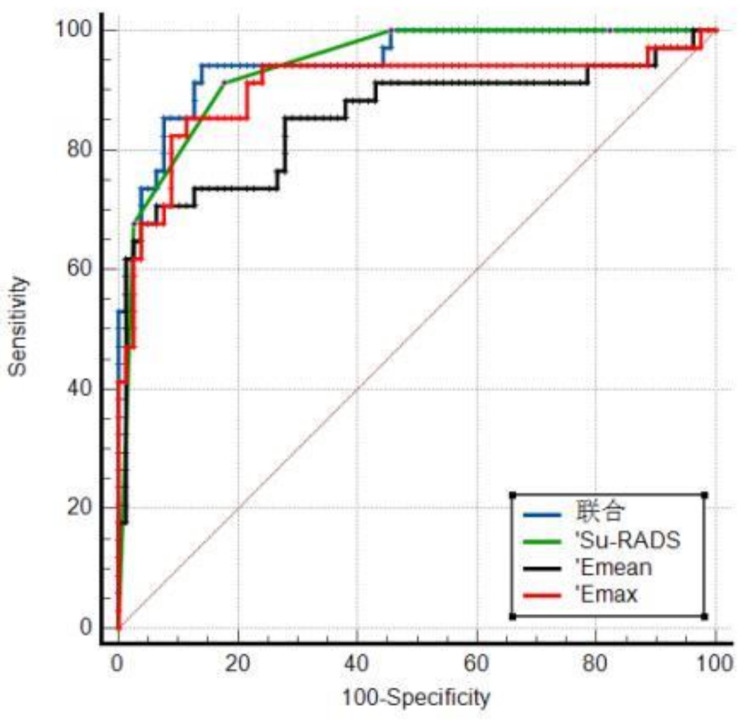
The sensitivity and specificity of Su-RADS combined with 2D-SWE were 82.35% and 94.94%, respectively; the area under the ROC curve was 0.951, and the Youden index was 0.8064. The results of the DeLong test showed that Su-RADS combined with 2D-SWE had good diagnostic efficacy. Compared with those of 2D-SWE (Emean) and 2D-SWE (Emax), the area under the curve (AUC) of Su-RADS combined with 2D-SWE was P < 0.05. Both 2D-SWE (Emean) and Su-RADS combined with 2D-SWE exhibited greater specificity than Su-RADS alone (p < 0.05) (Table 4; Fig. 4).
Table 4.
Efficacy of Su-RADS combined with 2D-SWE in the diagnosis of malignant lesions in the gastric wall
| Method | Sensitivity(%) | Specificity(%) | Positive predictive value(%) | Negative predictive value(%) | Youden index | AUC(95% CI) |
|---|---|---|---|---|---|---|
| Su-RADS | 91.18 | 82.28 | 68.89 | 95.59 | 0.7345 | 0.940(0.879, 0.976) |
| 2D-SWE (Emean) | 70.59 | 93.67* | 82.76 | 88.10 | 0.5601 | 0.853*(0.774, 0.913) |
| 2D-SWE (Emax) | 85.29 | 88.61 | 76.32 | 93.33 | 0.6807 | 0.903(0.833, 0.951) |
| Su-RADS combined with 2D-SWE | 82.35 | 94.94* | 87.50 | 92.59 | 0.8064 | 0.951#@(0.890, 0.981) |
| χ2 | 5.262 | 8.623 | 4.270 | 3.208 | ------- | ------- |
| P | 0.154 | 0.035 | 0.234 | 0.361 | ------- | ------- |
Note: *compared to Su-RADS, P < 0.05; #compared to 2D-SWE (Emean), P < 0.05; @compared to 2D-SWE (Emax), P < 0.05
Fig. 4.
The ROC curves of Su-RADS and 2D-SWE and their combination in the diagnosis of gastric cancer
Discussion
This study investigated the efficacy of Su-RADS in the diagnosis of benign and malignant lesions of the gastric wall and the benefit of 2D-SWE in the diagnosis of benign and malignant lesions of the gastric wall by transabdominal ultrasound. Our results showed that the use of a Su-RADS score greater than Category 3 as a criterion for malignancy had good diagnostic value. Using 2D-SWE, we found that the Emean and Emax were significantly greater in the malignant group than in the benign group. In addition, the diagnostic efficacy of 2D-SWE combined with Su-RADS was greater than that of Su-RADS or 2D-SWE alone, rendering the combination useful for distinguishing benign lesions from malignant lesions of the gastric wall.
Value of the Su-RADS grade in the diagnosis of gastric wall lesions
Gastric cancer and precancerous lesions of the stomach usually present as thickenings of the gastric wall and are important signs that are directly and objectively detected via routine ultrasonography [14–16]. Confined to the mucosa and submucosa is early gastric cancer, and beyond the submucosa is advanced gastric cancer. The superficial ulcer depression layer is limited to the mucosa layer, and the deep ulcer reaches the muscular layer or the serosa layer, and even penetrates the serosa. Benign gastric ulcer is characterized by local thickening of gastric wall, central mucosal collapse and depression, mucosal depression with regular shape and flat bottom The gastric ulcer type of gastric cancer is characterized by the local thickening of the gastric wall, the level of confusion, the mucosal surface appeared a huge depression, concave bottom rough.Previously, the normal value of gastric wall thickness in different parts of the stomach was not standardized. In 2018, Liu et al. [9] first proposed the establishment of the gastric ultrasound reporting and data system (Su-RADS) based on the thickness of the gastric wall and mucosa layer. A total of 2738 patients were examined via endoscopy and oral contrast-enhanced ultrasound (OCUS). The lesions were classified into 5 categories according to the thickness of the gastric wall and mucosa layer. Category 1 was considered normal, categories 2–4 were considered to indicate the possibility of mild, moderate or severe malignancy, Category 5 was considered to indicate a very high probability of malignancy, and further gastroscopy was recommended for Category 3. One of the advantages of this classification is that the thickness of the gastric wall in different parts is uniform, and the thickness of the mucosa is not affected by the thickness of the gastric wall in different parts. The diagnostic sensitivity and specificity were 95.1% and 78.6%, respectively. However, there was a lack of external validation.
In our study, patients with gastric wall lesions were classified according to Su-RADS, and the malignancy rates in patients in the 1st-5th categories were 0.0%, 0.0%, 12.0%, 40.0% and 92.0%, respectively; thus, results were similar. In this study, the diagnostic sensitivity and specificity were 91.18% and 82.28%, respectively, when > Category 3 was used as the criterion for malignancy, similar to that for > Category 2. The reasons for these differences may be that only 113 patients were included in this study and that the inclusion criteria were different according to the quality of the ultrasound diagnostic instrument. However, the classification of gastric wall lesions based on Su-RADS has good diagnostic efficacy in differentiating benign lesions from malignant gastric wall lesions. The classification not only standardized the diagnostic criteria for gastric wall lesions by contrast-enhanced ultrasonography but also provided a reference for further clinical diagnosis and treatment.
Differential diagnosis of benign and malignant lesions of the gastric wall by 2D-SWE
According to the European Federation of Ultrasound Medicine and Biology Guidelines for the Clinical Application of Ultrasound Elastography [17], ultrasound elastography methods can be divided into three broad categories according to imaging principles and modalities: one is static strain imaging techniques, the second is the quasistatic method used to induce tissue deformation/strain, and the third is the strain distribution in the region of interest (ROI), revealed by measuring the degree of change. The second is the use of acoustic radiation force pulse elastic imaging technology, which involves the use of acoustic energy mechanical excitation, in the organization of a local small range of excitation. The third method is shear wave imaging, which includes instantaneous elastic imaging, single-point shear wave elastic imaging and multidimensional shear wave imaging.
Several studies have shown that elastography has good value in the diagnosis of gastric wall lesions. Regarding the application of strain imaging to gastric wall lesions, Akbulut et al. [18] used this technique in the differential diagnosis of Helicobacter pylori gastritis and non-Helicobacter gastritis in children and reported that the AUC was 0.873, indicating that this technique had good diagnostic value. Similarly, for neoplastic disease, two case reports described strain elastography features of gastric cancer and gastrointestinal stromal tumours, respectively, with increased stiffness in both lesions [19, 20]. In addition, ARFI has been used in the diagnosis of benign and malignant lesions of the gastric wall with a sensitivity of 81.8 ∼ 87.9% and a specificity of 81.8 ∼ 92.3% [21, 22].
The value of the 2D-SWE technique in the evaluation of benign and malignant gastric wall lesions has not been previously reported. The mean values of Emean and Emax were 7.88 ± 3.74 kPa and 19.39 ± 7.54 kPa, respectively, for malignant lesions and 5.17 ± 1.86 kPa and 9.62 ± 3.83 kPa, respectively, for benign lesions. Similarly, the E value of malignant lesions was significantly greater than that of benign lesions. Using 8.01 kPa and 11.08 kPa as the cutoff values for Emean and Emax, respectively, for differentiating benign lesions from malignant lesions of the gastric wall (the area under the curve (AUC), sensitivity and specificity were 0.853, 70.59%, and 93.67%, respectively, and 0.903, 85.3%, and 88.6%, respectively) also had good diagnostic value. This is because SWE uses the “Mach cone” principle to accurately measure the velocity of shear waves and calculate the hardness of the propagation medium; without the need to compare strain imaging with surrounding reference tissues to obtain a relative ratio, elastic strain imaging techniques can be used to achieve real-time, accurate quantitative detection of tissue hardness in different regions [23]. Compared with other elastic imaging techniques, SWE does not rely on external forces to generate shear waves and can form real-time elastic imaging images and multipoint measurements of the elastic modulus. This technique has the advantages of safety, efficiency, accuracy and good repeatability [24]. Similarly, the 2D-SWE technique has been used by several scholars [25–27] and has achieved good diagnostic efficacy in the study of inflammatory bowel disease-related fibrosis. In a prospective study [28] involving 35 inflammatory bowel disease patients who underwent surgical resection within a week of receiving ultrasound elastography, it was shown that setting the e-cutoff value at 22.6 kPa made it possible to distinguish between severe and mild-moderate fibrotic inflammatory bowel disease with high accuracy (AUC, sensitivity and specificity of 0.82%, 70% and 91%, respectively). Compared with the gut, the stomach is a larger organ with less peristalsis and a thicker wall. Therefore, 2D-SWE should also be used in the diagnosis and differential diagnosis of gastric wall diseases. This study confirmed this conjecture and concluded that the Emean and Emax in malignant lesions were significantly greater than those in benign lesions and that the Emean and Emax could be used to differentiate benign lesions from malignant lesions of the gastric wall.
The diagnostic value of 2D-SWE combined with Su-RADS for detecting gastric wall lesions
The results showed that the sensitivity and specificity of the combination of Su-RADS and 2D-SWE were 82.35% and 94.94%, respectively. The area under the ROC curve was 0.951, and the Youden index was 0.8064. The sensitivity and specificity of the combination of Su-RADS and 2D-SWE for the diagnosis of malignant gastric lesions were 82.35% and 94.94%, respectively. This combination has good diagnostic efficacy, and its diagnostic efficacy is greater than that of either alone. The specificity (94.94%) was significantly greater than that of Su-RADS (82.28%) and 2D-SWE (Emean: 93.67%, Emax: 88.61%). The Su-RADS classification requires only the thickness of the gastric wall or gastric mucosa at the lesion site to be measured. This method is simple and easy to perform, and the 2D-SWE method is easier to master after training. These findings suggest that the combination of Su-RADS and 2D-SWE has good potential for the diagnosis of gastric wall lesions.
However, the study has several major limitations. First, the sample size was relatively small, and additional studies with larger sample sizes are needed to improve the accuracy of Su-RADS combined with 2D-SWE in the diagnosis of benign and malignant lesions. Second, this study focused on the diagnostic efficacy of EUS for benign and malignant lesions of the gastric wall and failed to analyse the efficacy of various gastric diseases involved.
Conclusion
This study suggested that both Su-RADS and 2D-SWE have good diagnostic efficacy for benign and malignant gastric wall lesions. Compared with Su-RADS or 2D-SWE alone, Su-RADS combined with 2D-SWE is more effective at diagnosing benign and malignant lesions of the gastric wall, especially because of its increased specificity.
Acknowledgements
The study was approved by the institutional review board at The Second Clinical Medical College of Fujian Medical University.
Abbreviations
- ARFI
Acoustic radiation force impulse
- AUC
The area under the curve
- CT
Computed tomography
- DCUS
Double contrast-enhanced ultrasound
- EFSUMB
European Federation of Societies of Ultrasound in Medicine and Biology
- IARC
International Agency for Research on Cancer
- OCUS
Oral contrast-enhanced ultrasound
- pSWE
Point shear-wave elastography
- ROC
Receiver operating characteristic
- ROI
Receiver of interest
- SE
Strain elastography
- SWE
Shear wave elastography
- SWV
Shear wave velocity
- Su-RADS
Stomach ultrasound report and data system
- TE
Transient elastography
- UE
Ultrasonic elastography
- 2D-SWE
2D-shear wave elastography
- 3D-SWE
Three dimensional shear-wave elastography
Author contributions
(I) Conception and design: J Xue, G Lyu; (II) Administrative support: S Li; (III) Provision of study materials or patients: J Xue, G Lyu; (IV) Collection and assembly of data: J Xue, G Lyu; (V) Data analysis and interpretation: J Xue, G Lyu, S Li ; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Funding
None.
Data availability
All datasets and materials used and/or analyzed during the current study are available from the corresponding authors on any reasonable request.
Declarations
Ethics approval and consent to participate
This trial was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University and carried out by the ethical standards set out in the Helsinki Declaration. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guorong Lyu and Shaohui Li contributed to the work equllly and should be regarded as co-corresponding authors.
Contributor Information
Guorong Lyu, Email: lgr_feus@sina.com.
Shaohui Li, Email: 513710286@qq.com.
References
- 1.Gong Y, Kang J, Wu R, et al. Gastroscopic results for the asymptomatic, average-risk population in Northern China: a cross-sectional study of 60,519 adults[J]. Scand J Gastroenterol. 2022;8:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: Globocan 2008[J]. Int J Cancer. 2010;127(12):2893–917. [DOI] [PubMed] [Google Scholar]
- 4.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world[J]. J Clin Oncology: Official J Am Soc Clin Oncol. 2006;24(14):2137–50. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 6.Sumiyama K. Past and current trends in endoscopic diagnosis for early stage gastric cancer in Japan[J]. Gastric Cancer: Official J Int Gastric Cancer Association Japanese Gastric Cancer Association. 2017;20(Suppl 1):20–7. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines[J]. J Natl Compr Cancer Network: JNCCN. 2013;11(5):531–46. [DOI] [PubMed] [Google Scholar]
- 8.Smyth EC, Nilsson M, Grabsch HI et al. Gastric cancer[J] Lancet 2020, 29;396(10251):635–48. [DOI] [PubMed]
- 9.Liu Z, Ren W, Guo J, et al. Preliminary opinion on assessment categories of stomach ultrasound report and data system (Su-RADS)[J]. Gastric Cancer: Official J Int Gastric Cancer Association Japanese Gastric Cancer Association. 2018;21(5):879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brener S. Transient Elastography for Assessment of Liver Fibrosis and Steatosis: An Evidence-Based Analysis[J]. Ont Health Technol Assess Ser. 2015;15(18):1–45. [PMC free article] [PubMed] [Google Scholar]
- 11.Safoiu A, Gilja OH, Sidhu PS et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018[J]. Ultraschall in Der Medizin (Stuttgart, Germany: 1980), 2019, 40(4): 425–453. [DOI] [PubMed]
- 12.Ozturk A, Grajo JR, Dhyani M, et al. Principles of ultrasound elastography[J]. Abdom Radiol (New York). 2018;43(4):773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui XW, Li KN, Yi AJ, et al. Ultrasound elastography[J]. Endoscopic Ultrasound. 2022;11(4):252–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmack SW, Genta RM, Schuler CM, et al. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients[J]. Am J Gastroenterol. 2009;104(6):1524–32. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi C, Yamamichi N, Shimamoto T, et al. Gastric polyps diagnosed by double-contrast upper gastrointestinal barium X-ray radiography mostly arise from the Helicobacter pylori-negative stomach with low risk of gastric cancer in Japan[J]. Gastric Cancer. 2017;20(2):314–21. [DOI] [PubMed] [Google Scholar]
- 16.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract[J]. Gastrointest Endosc. 2006;63(4):570–80. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich CF, Bamber J, Berzigotti A et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version)[J]. Ultraschall in Der Medizin (Stuttgart, Germany: 1980), 2017, 38(4): e16-e47. [DOI] [PubMed]
- 18.Akbulut UE, Isik IA. The usefulness of transabdominal ultrasound elastography in Helicobacter pylori gastritis in children[J]. J Ultrasonography. 2023;23(93):e61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantisani V, Rubini A, Miniagio G. CEUS and strain elastography in gastric carcinoma[J]. J Ultrasound. 2013;16(3):123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lien WC, Lee PC, Lin MT, et al. Adding trans-abdominal elastography to the diagnostic tool for an ileal gastrointestinal stromal tumor: a case report[J]. BMC Med Imaging. 2019;19(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang JH, Zheng BQ, Chen YH. etc. The diagnostic value of gastrointestinal contrast-enhanced ultrasonography combined with acoustic pulse radiography in benign and malignant gastric wall diseases [ J ]. Chinese medical equipment, 2019,16(2) : 67–70.
- 22.Wan XQ, Zhang PY, Chen J. etc. Comparative study of contrast-enhanced gastrointestinal ultrasonography and pulse radiography in the diagnosis of common benign and malignant lesions of gastric wall [ J ]. Journal of clinical ultrasound medicine, 2020,22(7) : 519–522.
- 23.Sigrist RMS, Liau J, Kaffas AE, et al. Ultrasound Elastography: Rev Techniques Clin Applications[J] Theranostics. 2017;7(5):1303–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taljanovic MS, Gimber LH, Becker GW, et al. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications[J]. Radiographics. 2017;37(3):855–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pita I, Magro F. Advanced imaging techniques for small bowel Crohn’s disease: what does the future hold?[J]. Therapeutic Adv Gastroenterol. 2018;11:1756283X18757185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pescatori LC, Mauri G, Savarino E, et al. Bowel Sonoelastography in Patients with Crohn’s Disease: A Systematic Review[J]. Ultrasound Med Biol. 2018;44(2):297–302. [DOI] [PubMed] [Google Scholar]
- 27.Lu C, Gui X, Chen W, et al. Ultrasound Shear Wave Elastography and Contrast Enhancement: Effective Biomarkers in Crohn’s Disease Strictures[J]. Inflamm Bowel Dis. 2017;23(3):421–30. [DOI] [PubMed] [Google Scholar]
- 28.Chen YJ, Mao R, Li XH, et al. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn’s Disease[J]. Inflamm Bowel Dis. 2018;24(10):2183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets and materials used and/or analyzed during the current study are available from the corresponding authors on any reasonable request.