Abstract
Fruit diameter is one of important agronomy traits that has greatly impacts fruit yield and commercial value in cucumber (Cucumis sativus L.). Hence, we preliminary mapping of fruit diameter was conducted to refine its genetic locus. In this study, to genetic mapping of QTLs that control cucumber fruit diameter, a F2 population with 120 individuals was developed by the East Asian line ‘9930’ (known as narrow fruit diameter) and the European-type cucumber ‘EU224’ (known as wide fruit diameter). Then a Genotyping-by-Sequencing (GBS)-based genetic map with 5662 markers was constructed and the total length is 656.177 cM, with average marker interval of 0.116 cM. Based on this high-density genetic map, a major QTL qfd1.1 related to fruit diameter was detected with a markedly high LOD score 4.07 located approximately 300 kb interval on Chromosome 1 (located between Chr1:1654704–1958556). To confirm qfd1.1 that detected by F2 population, we performed genetic mapping of fruit diameter with an introgression line (IL) about fruit diameter. We developed two KASP markers (FD-1 and FD-2) related to the fruit diameter. Based on this, we inserted the European cucumber EU224 into the qfd1.1 range and targeted widening the fruit diameter of the 9930 cucumber variety, further indicating that qfd1.1 is a new locus regulating the fruit diameter of cucumber. Our findings will support breeders in their research on cucumber fruit diameter.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-06000-9.
Keywords: GBS, KASP, QTL mapping, Fruit diameter, Cucumber
Introduction
Cucumber (Cucumis sativus L.) is one of the most economically important vegetables globally. It is believed that cucumber originated and was domesticated in India [1]. Cucumber fruit has significant edible value and can be consumed raw or processed into pickles [2]. China is the largest producer, accounting for about 75% of the total production [3, 4]. Cucumber fruit appearance traits include length, spine density, neck length and diameter. Different cucumber types meet various market demands. For instance, spiny cucumbers are preferred by Northern Chinese consumers, whereas glossy, spineless cucumbers are favored in Europe. The fruit’s morphological characteristics directly influence economic efficiency.
Fruit diameter, in particular, significantly impacts fruit yield and commercial value. The orientation, timing, and extent of cell division and expansion significantly influence cucumber fruit shape [5–7]. Recent studies indicate that the major Quantitative Trait Locus (QTL) FS5.2 regulates both lateral and longitudinal cucumber fruit growth through auxin-mediated cell division and expansion [8, 9]. Additionally, two major QTLs (FS1.2 and FS2.1) influence cucumber fruit shape, with CsSUN identified as the candidate gene for the FS1.2 [10]. Tan et al. [11] reported that a 14 bp deletion in the CsACS2 gene, encoding a truncated, nonfunctional protein, results in an elongated cucumber fruit shape. However, the genetic and regulatory mechanisms underlying fruit diameter are poorly understood. Thus, identifying QTL of cucumber fruit diameter traits in conjunction with molecular marker-assisted breeding is essential.
In recent decades, molecular marker-assisted breeding technologies have been crucial in selecting high-yielding and high-quality crop varieties [12–14]. Enhancing fruit characteristics has consistently been key to improving cucumber fruit quality [3, 15, 16]. Understanding the genetic mechanisms underlying key cucumber phenotypes at the molecular level, and identifying and utilizing new QTLs and genes can enhance molecular breeding theory and expedite the breeding process [4, 17, 18]. Genotyping-By-Sequencing (GBS) is a cost-effective method with low DNA requirements (100ng) and technical simplicity, making it a globally recognized benchmark technology [19–21]. In cucumber, CsOr was identified as a candidate gene for orange fruit endocarp using GBS and whole-genome sequencing [22]. Similarly, GBS technology has been validated for detecting complex molecular variations among different melon varieties, making it effective for QTL mapping [23, 24]. The emergence of this technology has filled the gaps in traditional breeding methods, making it a globally applicable and practical technology.
Kompetitive Allele Specific PCR (KASP) is a high-throughput genotyping technology based on haploid SNPs. It is simple, low cost, and does not require gel electrophoresis detection methods. Currently, this technology is used for seed purity identification in agriculture, as well as for gene mapping, molecular marker-assisted breeding, and germplasm identification [25]. Shen et al. [26] used KASP technology to identify mottled skin in an F2 population of sweet melon. Zhou et al. [27] used BSA and KASP technology to finely map the hollow fruit trait in cucumber using different genetic populations. Zhang et al. [28] used a high-density genetic map to locate QTLs related to seed size in sweet melon, combining it with KASP genotyping technology and RNA-seq analysis for fine mapping. Combining molecular marker-assisted breeding technology allows elucidation of the genetic mechanisms of key cucumber phenotypes at the molecular level and exploration of important QTLs related to fruit morphology, providing a theoretical basis for developing high-yielding and high-quality cucumber varieties [29, 30].
The East Asian cucumber line 9930, characterized by the cucumber reference genome, exhibits a narrow fruit diameter [5]. In contrast, the European cucumber variety EU224 displays a wide fruit diameter [31]. We developed a segregating population (F2) by crossing the East Asian cucumber 9930 with the European cucumber EU224. Fruit diameter Introgression Lines (ILs) were developed using KASP technology. In this study, a major QTL related to cucumber fruit diameter was identified in the F2 population using GBS. Further analysis of the ILs revealed that qfd1.1 is a novel QTL regulating cucumber fruit diameter, laying the theoretical groundwork for identifying candidate genes responsible for fruit morphology traits.
Materials and methods
Plant materials
The F2 population and its two parent lines were grown at the Juxin Modern Agricultural Demonstration Park in Taigu District, Jinzhong City, Shanxi Province. The F2 population consisted of 120 cucumber plants. Female flowers were hand-pollinated, and the plants were allowed to keep only one or two fruits.
The ILs were also derived from two inbred lines, 9930 (an East Asian cucumber) and EU224 (a European cucumber). Male flowers of 9930 were used to pollinate female flowers of the F1 plants, yielding BC1 seeds. These seeds were then backcrossed with 9930 to obtain BC2 seeds. Seedlings of the BC2 progeny were screened, and plants were selected based on the following criteria: (1) Individuals exhibited heterozygous genotypes at foreground markers; (2) Individuals had the lowest possible number of introgressions in their genome. Three cycles of genotyping and selection were conducted for the progeny using KASP technology. Then, two consecutive self-crosses were performed on BC4 plants to obtain the final ILs. The ILs were developed alternating between Shanxi and Hainan provinces.
Phenotyping of fruit diameter
Fruit diameter was measured using a vernier scale (in cm) at 45 days after pollination (mature stage) for the F2 population, and at 15 days after pollination (immature stage) for the ILs. The ILs and the recurrent parent 9930 were cultivated under identical field conditions. Phenotypic data were analyzed using SPSS 22.0 software. Violin plot distributions of mature fruit diameter (MFD) in the F2 population and bar charts of immature fruit diameter (FD) in the ILs were created using GraphPad Prism 8.4.2 software.
DNA extraction, library construction, and DNA sequencing
Genomic DNA was extracted from young leaves of 120 F2 population plants and the two parental lines using the CTAB (Cetyl Trimethyl Ammonium Bromide) method for genotyping [32]. The quality of the obtained DNA samples was assessed using both agarose gel electrophoresis and NanoDrop spectrophotometry. DNA concentration was quantified using a Qubit 3.0 Fluorometer. High-quality DNA with concentrations > 50 ng/μl and a total amount > 2 μg was used for GBS analysis. For cucumber library preparation, two restriction enzymes, MseI and SacI, were used. The library was sequenced on the Illumina HiSeq platform with paired-end 150 bp (PE150) read length. Raw sequencing reads were filtered using fastp (version 0.23.0) [33]. Sequence reads with a length < 50 bp were discarded. The filtered clean reads were aligned to the reference genome sequence of the Cucumber (Chinese Long) v3 genotype using BWA software (Burrow-Wheeler Aligner) [34]. Samtools software was used to convert sequence alignment map (SAM) files to binary alignment map (BAM) format and to perform sorting [35]. SNPs and InDels were identified using the HaplotypeCaller and GenotypeGVCFs modules of GATK (v 3.7) [36]. Finally, variants were annotated using ANNOVAR (v 2016 Feb1) to predict their effects on gene function [37].
Genetic linkage map construction
Genotypes of the F2 population were inferred based on the two parental lines, and the accuracy of parental materials was verified by comparison. The genotypes of the F2 population were then imputed and corrected using HMM. Recombination rates were assessed using MSTMap. Finally, map distances between markers were computed using the Kosambi function (cM) [38, 39].
QTL analysis and candidate gene mining
Composite interval mapping was used to detect QTLs for MFD in the F2 population. QTL analysis was performed using QTL Cartographer (v 1.17j) with the Composite Interval Mapping (CIM) method. The LOD threshold was determined by 1000 permutation tests with an error rate of 0.05 [40].
Genes within the candidate regions and their functional annotation were retrieved from the Chinese Long v3 genome database (http://cucurbitgenomics.org/ftp/genome/cucumber/Chinese_long/v3/). RNA-seq data were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA381300). A heatmap of candidate genes was generated using TBtools to compare cucumber gene expression at different developmental stages (10, 20, 30, 40DAP) [41].
Development of KASP primer for MAS and genotyping
Based on the mapping results, sequences flanking the QTL qfd1.1 were used to design KASP primers. The primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd.
KASP reactions were run in a 2 μL volume containing 1 μL diluted DNA (50-100ng/μL), 1μL KASP master mix, and 0.014 μL primer mix (100 μM). The ILs were genotyped using a high-throughput genetic testing system (GeneMatrix). Fluorescence signals from each reaction were collected, and analyzed by Matrix Master.
Statistics analysis
The experiments were repeated at least three biological times. Duncan’s multiple range test (p < 0.05) was used for the significant differences analysis between the samples.
Results
Phenotyping of the fruit diameter in cucumber
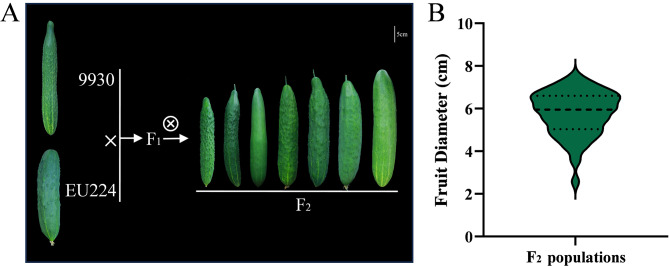
Fruit diameter directly affects yield, warranting focused study on this trait. Clear differences in fruit diameter were observed between the parents (Fig. 1A). Phenotypic differences in fruit diameter were analyzed in the F2 population. In the F2 population, fruit diameter ranged from 2.48 to 7.59 cm, with a coefficient of variation (CV) of 17.84%, indicating high diversity (Table 1). The frequency distribution of fruit diameter in the F2 population is illustrated in a violin plot (Fig. 1B). Fruit diameter exhibited a near-normal distribution in the F2 population. This result suggests that fruit diameter is a quantitative trait, and the wide segregation in the F2 population indicate its suitability for genetic mapping.
Fig. 1.
Phenotypic differences of fruit diameter in cucumber. (A) Fruit diameter assessment from two parental lines and their F2. The short fruit diameter line 9930 was crossed with the long fruit diameter line EU224 and resulting F2 hybrid plants displayed different fruit diameter. (B) Violin polt distribution of fruit diameter in F2 population
Table 1.
Variation distribution of Fruit Diameter in F2 population
| Name | Mean (cm) | SD | Range (cm) | CV |
|---|---|---|---|---|
| 9930 | 4.96 | 0.44 | 4.08–5.73 | 8.80% |
| EU224 | 7.55 | 0.19 | 7.36–7.89 | 2.51% |
| F2 population | 5.78 | 1.03 | 2.48–7.59 | 17.84% |
Construction of high-density genetic linkage map and identification of QTL associated with fruit diameter
To confirm the QTLs associated with fruit diameter in cucumber, we performed genetic mapping using F2 populations and GBS. Illumina sequencing of 120 F2 progeny generated 4.18 million paired-end (PE) reads, totaling 98.72 G clean bases, aligned to the cucumber reference genome Chinese Long v3. The average Q20 and Q30 ratios were more than 97.48% and 92.85%, respectively. The guanine-cytosine (GC) content was 41.68% (Table 2), indicating satisfactory sequencing quality. The parents generated 2.4 million and 2.2 million reads in 9930 and EU224, respectively. The mean coverage depth was 39.96 for 9930 and 13.15 for EU224. The average coverage depth for each progeny was 36.11 (Table 2). These results provided a solid foundation for SNP marker detection. As a result, 5803 variation sites were discovered across the 7 chromosomes. The distribution of SNPs was uniform (Fig. 2A), the distribution of SNPs was uniform. In total, 5182 single-nucleotide polymorphisms (SNPs) and 621 InDels were identified. Transitions, including A/G and C/T types, accounted for 63.86% of the SNPs. The remaining SNPs were transversions (A/C, A/T, C/G, G/T), accounting for 36.14%, as expected (S1 Table). After filtering out the variant loci located on the scaffold, a total of 5662 variant loci were obtained.
Table 2.
Basic statistics of the GBS data
| Name | Total reads | Q20 percentage | Q30 percentage | GC percentage | Average depth |
|---|---|---|---|---|---|
| 9930 | 2,409,801 | 97.27% | 92.47% | 42.38% | 39.96 |
| EU224 | 2,233,069 | 97.85% | 93.80% | 44.55% | 13.15 |
| F2 population | 405,461,152 | 97.48% | 92.85% | 41.68% | 36.11 |
Note: Quality evaluation of restriction-site associated DNA sequencing data in 120 hybrid seedlings derived from the cross of ‘9930’ × ‘EU224’
Fig. 2.
QTL mapping of the fruit diameter of cucumber and candidate genes related to fruit diameter in QTL intervals. (A) Distribution of SNPs and InDels on all Chromosomes. The x-axes indicates Chromosome length, the y-axes indicates the number of SNPs and InDels. (B) Distributions of bins on 7 linkage groups of the high-density genetic linkage map constructed in the F2 population. The genetic distance (cM) is denoted on the left side of each Chromosome and markers are represented by black bars. (C) LOD profiles of fruit diameter QTLs detected on the local genetic linkage map of cucumber Chromosome 1. (D) Qfd1.1 was localized to the 303.9 Kb interval of Chromosome 1 and there are 50 genes in this interval. (E) Non-synonymous SNPs and lnDels identified on exons of genes in the qfd1.1 intervals. (F) Heat map of candidate genes during the different fruit development stages in qfd1.1 intervals
5662 markers were used to construct a high-density genetic linkage map (Table 3). The SNP distribution on 7 chromosomes is illustrated, with a total genetic distance of 656.177 cM and an average marker interval of 0.116 cM, indicating high genetic map density (Fig. 2B; Table 3). Linkage group lengths ranged from 59.690 to 121.292 cM, with an average length of 93.740 cM. Markers per linkage group ranged from 634 to 1030, with Chromosome 6 having the most markers (1030 SNPs) (Table 3). The largest gap in the linkage map was 9.762 cM on Chromosome 1 (Table 3). The largest average marker interval was 0.139 cM on Chromosome 3, while Chromosome 7 had the smallest at 0.087 cM (Table 3). The unswapped markers were recombined into 1 bin, and the genetic linkage map contains 627 bins in total.
Table 3.
Information of the integrated linkage map
| Chr | Length (cM) | Number of markers |
Number of bins |
Average marker interval(cM) |
Average bin interval (cM) |
Maximum interval (cM) |
|---|---|---|---|---|---|---|
| 1 | 83.661 | 634 | 77 | 0.132 | 1.101 | 9.762 |
| 2 | 101.557 | 873 | 95 | 0.116 | 1.08 | 9.099 |
| 3 | 121.292 | 873 | 112 | 0.139 | 1.093 | 5.34 |
| 4 | 91.554 | 711 | 81 | 0.129 | 1.144 | 7.644 |
| 5 | 94.828 | 852 | 88 | 0.111 | 1.09 | 5.067 |
| 6 | 103.595 | 1,030 | 100 | 0.101 | 1.046 | 5.553 |
| 7 | 59.69 | 689 | 74 | 0.087 | 0.818 | 3.651 |
| Mean | 93.74 | 808.857 | 89.571 | 0.116 | 1.053 | 6.588 |
| Total | 656.177 | 5,662 | 627 | — | — | — |
Note: The genetic linkage maps was constructed using 5662 SNPs markers and 120 hybrid seedings crossed by ‘9930’ × ‘EU224’
Based on the phenotypic data and the genetic linkage map, we conducted QTL mapping for fruit diameter in the F2 population. We performed a whole-genome scan for QTLs using the composite interval mapping (CIM) method. One QTL was identified with details on LOD peaks, physical interval, additive effect, and dominant effect (Fig. 2C, D; Table 4). The QTL associated with fruit diameter was located at 1,654,704–1,958,556 bp on Chromosome 1 with an LOD value of 4.07 (Table 4).
Table 4.
QTL identified in the F2 population for fruit diameter
| Chr. | Pos (cM) | LOD | R² | Additive effect |
Dominant effect |
Confidence interval |
Bin interval |
Physical interval |
|
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.46 | 4.07 | 2.00% | -0.2706 | -1.1384 | 4.02–5.36 | c01b007-c01b009 | 1,654,704–1,958,556 |
Candidate genes analyses for fruit diameter
To identify candidate genes for fruit diameter in the 303 kb region on Chromosome 1, gene annotations and variation information were obtained. The functions of 50 annotated genes are listed in S2 Table. Of these genes, CsaV3_1G003030 was annotated as an “FCP1 homology domain-containing protein”, related to stem cell proliferation. In QTL qfd1.1, 550 variants were identified, including 10 non-synonymous changes in 8 genes (Fig. 2E; S3 Table). To identify candidate genes within the QTL intervals more effectively, we analyzed gene expression based on publicly available RNA-seq data from 9930 at various fruit developmental stages (Fig. 2F). The expression levels of CsaV3_1G002860, CsaV3_1G002710 and CsaV3_1G003120 were highest at 10 days post-anthesis during rapid fruit expansion, gradually decreasing at days 20, 30, and 40.
Validation of qfd1.1 associated with fruit diameter
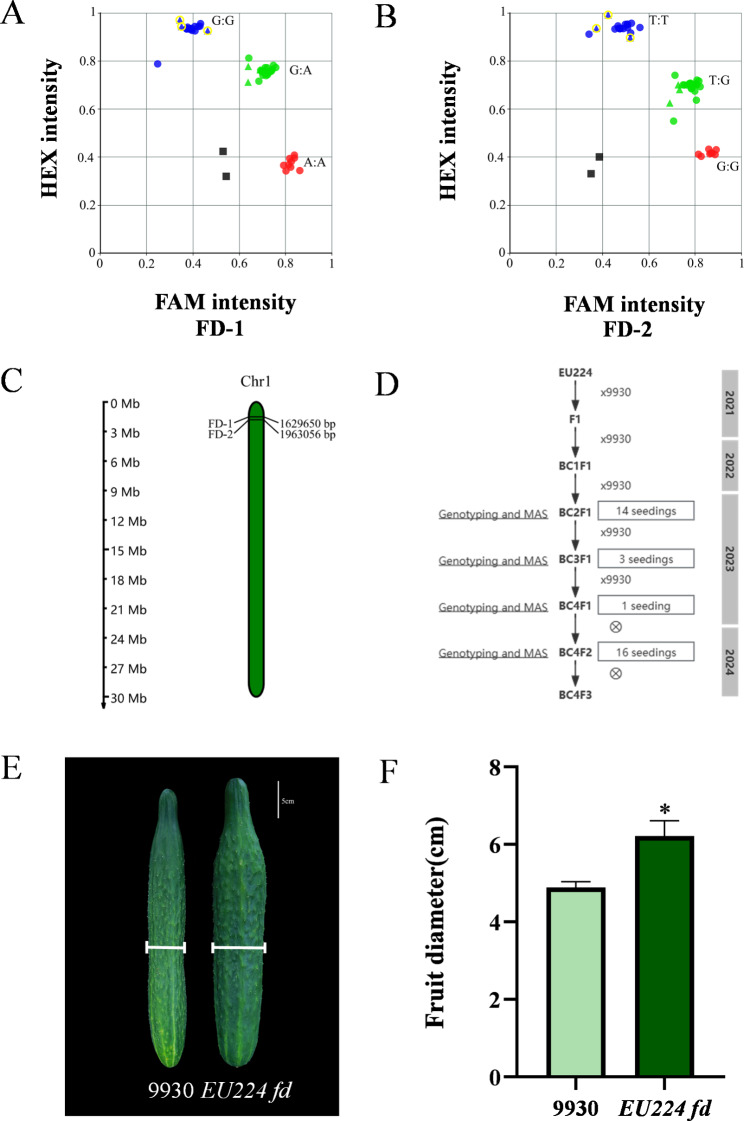
We developed KASP markers closely linked to qfd1.1. The two KASP markers FD-1 (Fig. 3A) and FD-2 (Fig. 3B), were successfully genotyped and used to construct ILs for fruit diameter. The positions of these two KASP markers on Chromosome 1 are shown in Fig. 3C. We used two KASP markers about fruit diameter and 35 background markers for molecular marker-assisted breeding and obtained an IL related to fruit diameter, which contains 16 plants (Fig. 3D; S4 Table).
Fig. 3.
Development of KASP markers and QTL validation of the fruit diameter in the Introgression lines. (A) KASP genotyping results for primers of FD-1 in the BC4F2 population. FAM intensity (A: A) corresponds to EU224 genotype, FAMHEX intensity (G: A) corresponds to F1 genotype, and HEX intensity (G: G) corresponds to 9930 genotype. (B) KASP genotyping results for primers of FD-2 in the BC4F2 population. FAM intensity (G: G) corresponds to EU224 genotype, FAMHEX intensity (T: G) corresponds to F1 genotype, and HEX intensity (T: T) corresponds to 9930 genotype. (C) Schematic representation of the location for primers of FD-1 and FD-2 on Chromosome 1. (D) Breeding scheme for the development of the Introgression lines of fruit diameter. (E) Fruit diameter, with a representation of 9930 and EU224 fd. (F) Size of fruit diameter in 9930 and EU224 fd. The asterisks (*) represent the significant differences at p < 0.05
To assess phenotypic variations, fruit diameter of 9930 and EU224 fd were measured at the immature stage. Significant differences in fruit diameter between 9930 and EU224 fd were observed (Fig. 3E). The fruit diameter range of the recipient parent 9930 was 4.72–5.05 cm, with a mean of 4.89 cm, a standard deviation of 0.16, and a coefficient of variation of 3.37%. The fruit diameter range of EU224 fd was 5.65–7.15 cm, with a mean of 6.22 cm, a standard deviation of 0.40, and a coefficient of variation of 6.42% (Table 5). EU224 fd exhibited a significant increase (27.2%) in fruit diameter compared to the recurrent parent 9930 (Fig. 3F; Table 6), indicating that QTL, qfd1.1, on Chromosome 1 controls fruit diameter.
Table 5.
Phenotypic investigation of ILs
| Name | Range (cm) | Mean (cm) | SD | CV |
|---|---|---|---|---|
| 9930 | 4.72–5.05 | 4.89 | 0.16 | 3.37% |
| EU224 fd | 5.65–7.15 | 6.22 | 0.4 | 6.42% |
Table 6.
QTL validation in the ILs for fruit diameter
| Name | Chr. | Interval (Mb) | % Difference a | p-Value |
|---|---|---|---|---|
| EU224 fd | 1 | 0-27.79 Mb | 27.2% | 0.0450 |
Discussion
QTL identifying for fruit diameter in cucumber
Fruit diameter traits are crucial factors influencing fruit yield in cucumber. Bo et al. [42] detected three QTLs (fd1.1, fd4.1, and fd6.1) based on the RIL population derived from a cross between XIS cucumber and cultivated cucumber to explain the genetic mechanism of fruit diameter. Among the three QTLs, fd4.1 is a major-effect QTL located on Chromosome 4. Previous studies identified several QTLs associated with cucumber fruit shape using F2, F3, and RIL populations derived from crosses between Gy14 and 9930. Among these, three minor-effect QTLs (qFD2.1, qFD5.1, qFD6.1) were found to control immature fruit diameter [43]. FS1.2 and FS2.1, controlling round fruit shape, were identified in a cross between WI7238 (wide fruit) and WI7239 (round fruit) inbred cucumber lines. Both genes are involved in radial growth throughout the growth process [44]. CsSUN (Cucsa.288770 or Csa1G575000) is a candidate gene associated with fruit shape for the QTL FS1.2. Cucsa.288,770 is located at 294,979–296,368 bp on scaffold02698, while Csa1G575000 is positioned at 21,748,270–21,749,865 bp on Chromosome 1 [44]. More recently, the FS5.2/CsCRC working model was identified to elucidate the regulatory mechanism of fruit diameter in cucumber. This model promotes cell division, leading to increased fruit diameter [8, 45]. Additionally, CsTRM5 is associated with cucumber fruit shape, regulating it by modulating transverse cell division, either increasing or decreasing it [46]. Variations in fruit diameter were observed among different genotypes in the F2 population. In this study, we identified a new QTL, qfd1.1, in the F2 population. This QTL is located at 1,654,704–1,958,556 bp on Chromosome 1, consistent with the phenotype of introgression line EU224 fd. Identifying qfd1.1 is crucial for improving cucumber fruit morphology.
Prediction of candidate genes for the fruit diameter of cucumber
Fifty genes were identified within the major QTL region associated with fruit diameter. We hypothesized that one of these genes may regulate immature fruit diameter in cucumber. A total of 50 genes were identified and annotated within the qfd1.1 region on Chromosome 1. Among these, CsaV3_1G003030 was annotated as “FCP1 homology domain-containing protein”. As a key transcriptional regulator, FCP1 inhibits stem cell proliferation by receiving signals from differentiating primordia [47]. Stem cells reside within specialized microenvironments known as niches, where their proliferation is regulated by signals from neighboring cells. Mounting evidence suggests that stem cells play a pivotal role in various plant growth and development processes, influencing fruit size and yield. In maize, ZmFCP1 suppresses WUS expression and stem cell proliferation via the FEA3 receptor in the SAM, thus affecting yield [48]. Previous studies reported that mutations in CLV3, which regulate stem cell production, lead to an inability to emit signals that suppress stem cell proliferation, resulting in increased fruit size in tomatoes [49]. According to previous studies [50, 51], fruit growth initiates from 0 to 3 days after anthesis (DAA), undergoes exponential growth from 3 to 12 DAA, and continues to grow at a slower rate from 12 to 30 DAA. Transcriptome analysis of cucumber fruit development stages revealed that the expression levels of CsaV3_1G002860, CsaV3_1G002710, and CsaV3_1G003120 peaked at 10 days post-anthesis and progressively decreasing thereafter. Identifying additional QTLs and genes responsible for fruit diameter is crucial for improving yield and fruit quality in cucumber. However, further experiments are needed to confirm the function of these genes in cucumber fruit diameter.
Conclusion
We constructed a high-density genetic linkage map using GBS data from the F2 population. The genetic map with 5662 markers was constructed, and one major QTL related to fruit diameter was detected, located in an approximately 303 kbp interval between 1,654,704 and 1,958,556 on Chromosome 1. Furthermore, to confirm the QTL associated with fruit diameter in cucumber, we constructed an IL associated with fruit diameter. We employed marker-assisted selection breeding to better understand the underlying causes of fruit diameter changes in cucumber. The qfd1.1 found in F2 population was validated using EU224 fd. Our findings are crucial for advancing the fine-mapping of cucumber fruit diameter and cultivating superior varieties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Hami-melon Research Center, Xinjiang Academy of Agricultural Sciences for providing the seeds of cucumber.
Author contributions
W.W and Z.X conceptualization, investigation, software and writing original draft, while L.Q, S.H and Y.N investigation and data curation. Y.W and C.S validation and visualization. B.L project administration, funding acquisition, supervision and writing review&editing.
Funding
This research was funded by Project of Xinjiang TianChi Leading Talent, the Initial Fund for Bringing in Talent at Xinjiang Academy of Agricultural Sciences (Grant no. 2022001), the Fund for Stable Support to Agricultural Sci-Tech Renovation (Grant no. xjnkywdzc-2022001-6), the Key Research and Development Program of Shanxi Province (Grant no. 202102140601013), the Natural Science Foundation of Shanxi Province (Grant no. 202303021221089), and the National Natural Science Foundation of China (Grant no. 31902029).
Data availability
All data analyzed during this study are included in this article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Experimental research on plants including the collection of plant material are comply with relevant guidelines and regulation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenjiao Wang and Bin Liu should be considered corresponding authors.
Wenjiao Wang and Zhaoying Xu contributed equally to this work.
Contributor Information
Wenjiao Wang, Email: sxndwwj@sxau.edu.cn.
Bin Liu, Email: liubincau@163.com.
References
- 1.Yundaeng C, Somta P, Tangphatsornruang S, Chankaew S, Srinives P. A single base substitution in BADH/AMADH is responsible for fragrance in cucumber (Cucumis sativus L.), and development of SNAP markers for the fragrance. Theor Appl Genet. 2015;128(9):1881–92. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Jiang L, Che G, Pan Y, Li Y, Hou Y, Zhao W, Zhong Y, Ding L, Yan S, et al. A functional allele of CsFUL1 regulates fruit length through repressing CsSUP and inhibiting Auxin Transport in Cucumber. Plant Cell. 2019;31(6):1289–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebretsadik K, Qiu X, Dong S, Miao H, Bo K. Molecular research progress and improvement approach of fruit quality traits in cucumber. Theor Appl Genet. 2021;134(11):3535–52. [DOI] [PubMed] [Google Scholar]
- 4.Feng S, Zhang J, Mu Z, Wang Y, Wen C, Wu T, Yu C, Li Z, Wang H. Recent progress on the molecular breeding of Cucumis sativus L. in China. Theor Appl Genet. 2020;133(5):1777–90. [DOI] [PubMed] [Google Scholar]
- 5.Colle M, Weng Y, Kang Y, Ophir R, Sherman A, Grumet R. Variation in cucumber (Cucumis sativus L.) fruit size and shape results from multiple components acting pre-anthesis and post-pollination. Planta. 2017;246(4):641–58. [DOI] [PubMed] [Google Scholar]
- 6.Peng Z, Li H, Liu G, Jia W, Fu D. NAC transcription factor NOR-like1 regulates tomato fruit size. Planta. 2023;258(1):9. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Lv S, Zhao T, Jiang M, Liu D, Fu S, Hu M, Huang S, Pei Y, Wang X. Modification of Threonine-825 of SlBRI1 enlarges cell size to Enhance Fruit yield by regulating the Cooperation of BR-GA Signaling in Tomato. Int J Mol Sci. 2021;22(14). [DOI] [PMC free article] [PubMed]
- 8.Pan Y, Chen B, Qiao L, Chen F, Zhao J, Cheng Z, Weng Y. Phenotypic characterization and fine mapping of a major-effect fruit shape QTL FS5.2 in Cucumber, Cucumis sativus L., with near-isogenic line-derived segregating populations. Int J Mol Sci. 2022;23(21). [DOI] [PMC free article] [PubMed]
- 9.Che G, Zhang X. Molecular basis of cucumber fruit domestication. Curr Opin Plant Biol. 2019;47:38–46. [DOI] [PubMed] [Google Scholar]
- 10.Pan Y, Wang Y, McGregor C, Liu S, Luan F, Gao M, Weng Y. Genetic architecture of fruit size and shape variation in cucurbits: a comparative perspective. Theor Appl Genet. 2020;133(1):1–21. [DOI] [PubMed] [Google Scholar]
- 11.Tan J, Tao Q, Niu H, Zhang Z, Li D, Gong Z, Weng Y, Li Z. A novel allele of monoecious (m) locus is responsible for elongated fruit shape and perfect flowers in cucumber (Cucumis sativus L). Theor Appl Genet. 2015;128(12):2483–93. [DOI] [PubMed] [Google Scholar]
- 12.Zhang RJ, Liu B, Song SS, Salah R, Song CJ, Xia SW, Hao Q, Liu YJ, Li Y, Lai YS. Lipid-related domestication accounts for the extreme cold sensitivity of semiwild and tropic Xishuangbanna Cucumber (Cucumis sativus L. var. Xishuangbannanesis). Int J Mol Sci. 2023;25(1). [DOI] [PMC free article] [PubMed]
- 13.Yuan J, Wang Z, Wang X, Zhang C, Ma F, Li M. Research advances in genetic quality of sugar content in apples. Fruit Res. 2023;3.
- 14.Gao Y, Li N, Li X, Lu Y, Feng D, Zhang X, Gu A, Ge Y, Tabusam J, Liu M, et al. Genome-wide development and utilization of simple sequence repeats in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Vegetable Res. 2022;2(1):1–7. [Google Scholar]
- 15.Shahwar D, Khan Z, Park Y. Molecular marker-assisted mapping, candidate gene identification, and breeding in Melon (Cucumis melo L.): a review. Int J Mol Sci. 2023;24(20). [DOI] [PMC free article] [PubMed]
- 16.Yu D, Gu X, Zhang S, Dong S, Miao H, Gebretsadik K, Bo K. Molecular basis of heterosis and related breeding strategies reveal its importance in vegetable breeding. Hortic Res. 2021;8. [DOI] [PMC free article] [PubMed]
- 17.Wang Y, Bo K, Gu X, Pan J, Li Y, Chen J, Wen C, Ren Z, Ren H, Chen X et al. Molecularly tagged genes and quantitative trait loci in cucumber with recommendations for QTL nomenclature. Hortic Res. 2020;7. [DOI] [PMC free article] [PubMed]
- 18.Song S, Hao Q, Su L-H, Xia S, Zhang R, Liu Y, et al. FLOWERING LOCUS T (FT) gene regulates short-day flowering in low latitude Xishuangbanna cucumber (Cucumis sativus var. Xishuangbannanesis). Vegetable Res. 2023;3:15.
- 19.Brouard JS, Boyle B, Ibeagha-Awemu EM, Bissonnette N. Low-depth genotyping-by-sequencing (GBS) in a bovine population: strategies to maximize the selection of high quality genotypes and the accuracy of imputation. BMC Genet. 2017;18(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang X, Gao M, Amanullah S, Guo Y, Liu X, Xu H, et al. Identification of QTLs linked with watermelon fruit and seed traits using GBS-based high-resolution genetic mapping. Sci Hort. 2022;303:111237.
- 21.Nyirahabimana F, Shimira F, Zahid G, Solmaz I. Recent status of genotyping by sequencing (GBS) technology in cucumber (Cucumis sativus L.): a review. Mol Biol Rep. 2022;49(6):5547–54. [DOI] [PubMed] [Google Scholar]
- 22.Kishor DS, Lee HY, Alavilli H, You CR, Kim JG, Lee SY, Kang BC, Song K. Identification of an allelic variant of the CsOr gene controlling fruit endocarp color in Cucumber (Cucumis sativus L.) using genotyping-by-sequencing (GBS) and whole-genome sequencing. Front Plant Sci. 2021;12:802864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyun DY, Sebastin R, Lee GA, Lee KJ, Kim SH, Yoo E, Lee S, Kang MJ, Lee SB, Jang I et al. Genome-wide SNP markers for genotypic and phenotypic differentiation of Melon (Cucumis melo L.) varieties using genotyping-by-sequencing. Int J Mol Sci. 2021;22(13). [DOI] [PMC free article] [PubMed]
- 24.Santo Domingo M, Areco L, Mayobre C, Valverde L, Martín-Hernández AM, Pujol M, Garcia-Mas J. Modulating climacteric intensity in melon through QTL stacking. Hortic Res. 2022;9. [DOI] [PMC free article] [PubMed]
- 25.He C, Holme J, Anthony J. SNP genotyping: the KASP assay. Methods in molecular biology. (Clifton NJ). 2014;1145:75–86. [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Xu X, Zhang Y, Niu X, Shou W. Genetic mapping and identification of the candidate genes for mottled rind in Cucumis melo L. Front Plant Sci. 2021;12:769989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou G, Chen C, Liu X, Yang K, Wang C, Lu X, Tian Y, Chen H. The formation of hollow trait in Cucumber (Cucumis sativus L.) Fruit is controlled by CsALMT2. Int J Mol Sci. 2022;23(11). [DOI] [PMC free article] [PubMed]
- 28.Zhang H, Zhang X, Li M, Yang Y, Li Z, Xu Y, Wang H, Wang D, Zhang Y, Wang H, et al. Molecular mapping for fruit-related traits, and joint identification of candidate genes and selective sweeps for seed size in melon. Genomics. 2022;114(2):110306. [DOI] [PubMed] [Google Scholar]
- 29.Bo K, Wei S, Wang W, Miao H, Dong S, Zhang S, et al. QTL mapping and genome-wide association study reveal two novel loci associated with green flesh color in cucumber. BMC plant biology. 2019;19(1):243. [DOI] [PMC free article] [PubMed]
- 30.Fu W, Huang S, Liu Z, Gao Y, Zhang M, Wang N, Qu G, Feng H. Fine mapping of Brebm6, a gene conferring the early-bolting phenotype in Chinese cabbage (Brassica rapa ssp. pekinensis). Vegetable Res. 2021;1(1):1–9. [Google Scholar]
- 31.Liu B, Weng J, Guan D, Zhang Y, Niu Q, López-Juez E, Lai Y, Garcia-Mas J, Huang D. A domestication-associated gene, CsLH, encodes a phytochrome B protein that regulates hypocotyl elongation in cucumber. Mol Hortic. 2021;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer NM. Isolation of plant DNA for PCR and genotyping using organic extraction and CTAB. Cold Spring Harbor Protoc. 2010;2010(11):pdb.prot5515. [DOI] [PubMed]
- 33.Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinf (Oxford England). 2018;34(17):i884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv: Genomics; 2013. [Google Scholar]
- 35.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinf (Oxford England). 2009;25(16):2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disratthakit A, Toyo-Oka L, Thawong P, Paiboonsiri P, Wichukjinda N, Ajawatanawong P, Thipkrua N, Suthum K, Palittapongarnpim P, Tokunaga K, et al. An optimized genomic VCF workflow for precise identification of Mycobacterium tuberculosis cluster from cross-platform whole genome sequencing data. Infect Genet evolution: J Mol Epidemiol evolutionary Genet Infect Dis. 2020;79:104152. [DOI] [PubMed]
- 37.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie W, Feng Q, Yu H, Huang X, Zhao Q, Xing Y, Yu S, Han B, Zhang Q. Parent-independent genotyping for constructing an ultrahigh-density linkage map based on population sequencing. Proc Natl Acad Sci USA. 2010;107(23):10578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Bhat PR, Close TJ, Lonardi S. Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet. 2008;4(10):e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Basten C, Zeng Z. Windows QTL cartographer version 2.5. Department of Statistics, North Carolina State University, Raleigh. 2005.
- 41.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202. [DOI] [PubMed] [Google Scholar]
- 42.Bo K, Ma Z, Chen J, Weng Y. Molecular mapping reveals structural rearrangements and quantitative trait loci underlying traits with local adaptation in semi-wild Xishuangbanna cucumber (Cucumis sativus L. var. Xishuangbannanesis Qi Et Yuan). Theor Appl Genet. 2015;128(1):25–39. [DOI] [PubMed] [Google Scholar]
- 43.Weng Y, Colle M, Wang Y, Yang L, Rubinstein M, Sherman A, Ophir R, Grumet R. QTL mapping in multiple populations and development stages reveals dynamic quantitative trait loci for fruit size in cucumbers of different market classes. Theor Appl Genet. 2015;128(9):1747–63. [DOI] [PubMed] [Google Scholar]
- 44.Pan Y, Liang X, Gao M, Liu H, Meng H, Weng Y, Cheng Z. Round fruit shape in WI7239 cucumber is controlled by two interacting quantitative trait loci with one putatively encoding a tomato SUN homolog. Theor Appl Genet. 2017;130(3):573–86. [DOI] [PubMed] [Google Scholar]
- 45.Che G, Song W, Zhang X. Gene network associates with CsCRC regulating fruit elongation in cucumber. Vegetable Res. 2023;3:1–4. [Google Scholar]
- 46.Xie Y, Liu X, Sun C, Song X, Li X, Cui H, Guo J, Liu L, Ying A, Zhang Z, et al. CsTRM5 regulates fruit shape via mediating cell division direction and cell expansion in cucumber. Hortic Res. 2023;10(3):uhad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karami O, Mueller-Roeber B, Rahimi A. The central role of stem cells in determining plant longevity variation. Plant Commun. 2023;4(5):100566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Je BI, Gruel J, Lee YK, Bommert P, Arevalo ED, Eveland AL, Wu Q, Goldshmidt A, Meeley R, Bartlett M, et al. Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat Genet. 2016;48(7):785–91. [DOI] [PubMed] [Google Scholar]
- 49.Xu C, Liberatore KL, MacAlister CA, Huang Z, Chu YH, Jiang K, Brooks C, Ogawa-Ohnishi M, Xiong G, Pauly M, et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat Genet. 2015;47(7):784–92. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Pan Y, Liu C, Ding Y, Wang X, Cheng Z, Meng H. Cucumber fruit size and shape variations explored from the aspects of morphology, histology, and endogenous hormones. Plants (Basel, Switzerland). 2020;9(6). [DOI] [PMC free article] [PubMed]
- 51.Grumet R, Lin YC, Rett-Cadman S, Malik A. Morphological and genetic diversity of Cucumber (Cucumis sativus L.) fruit development. Plants (Basel. Switzerland). 2022;12(1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this article and its supplementary information files.