Abstract
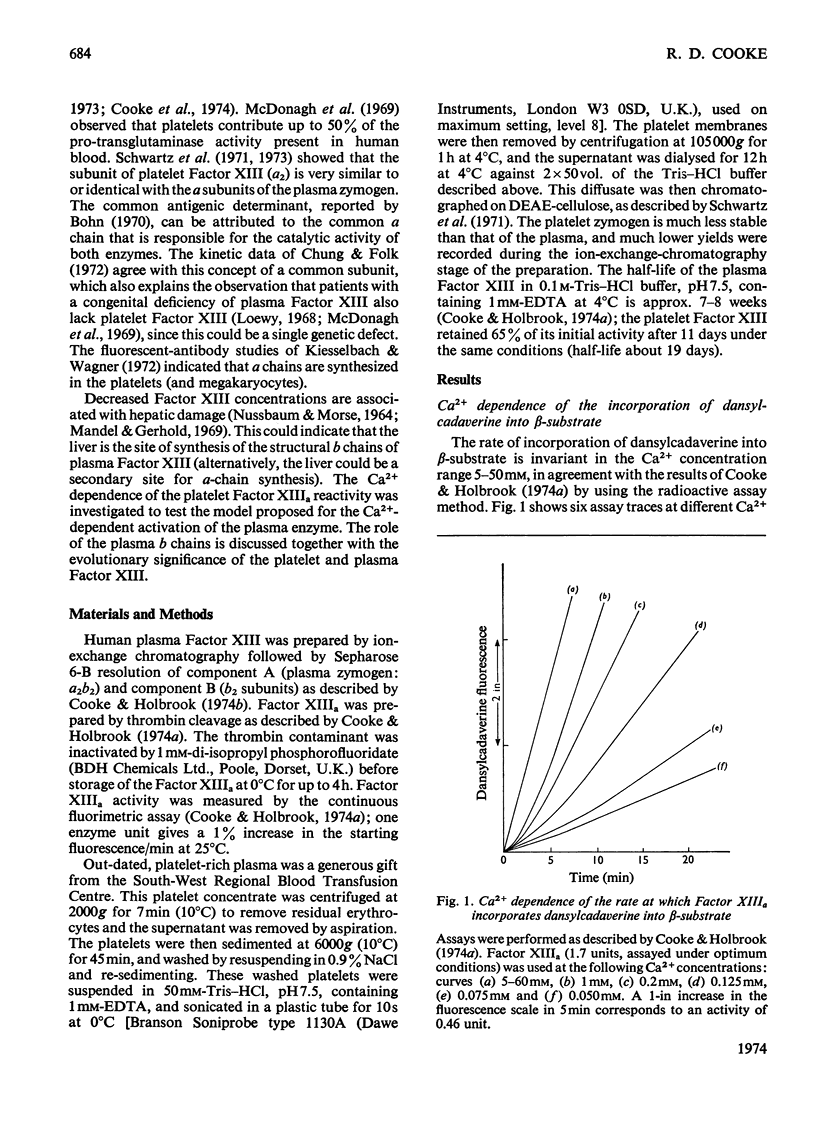
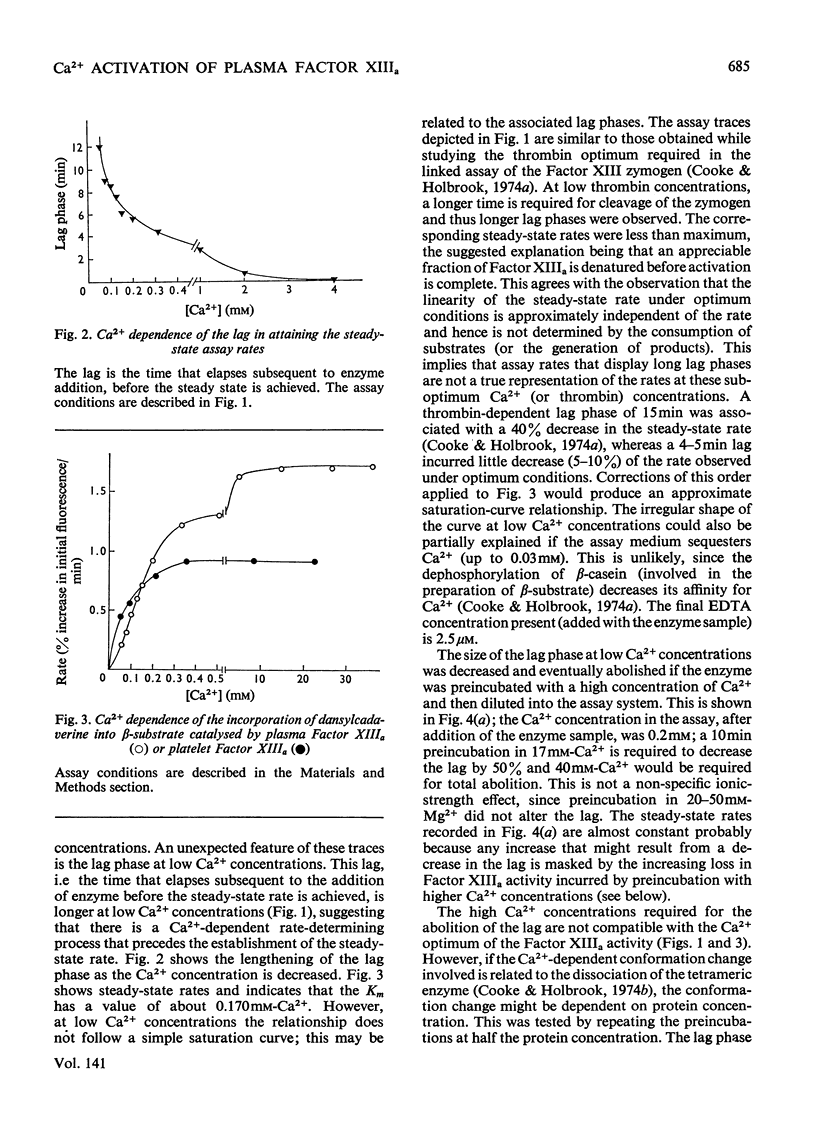
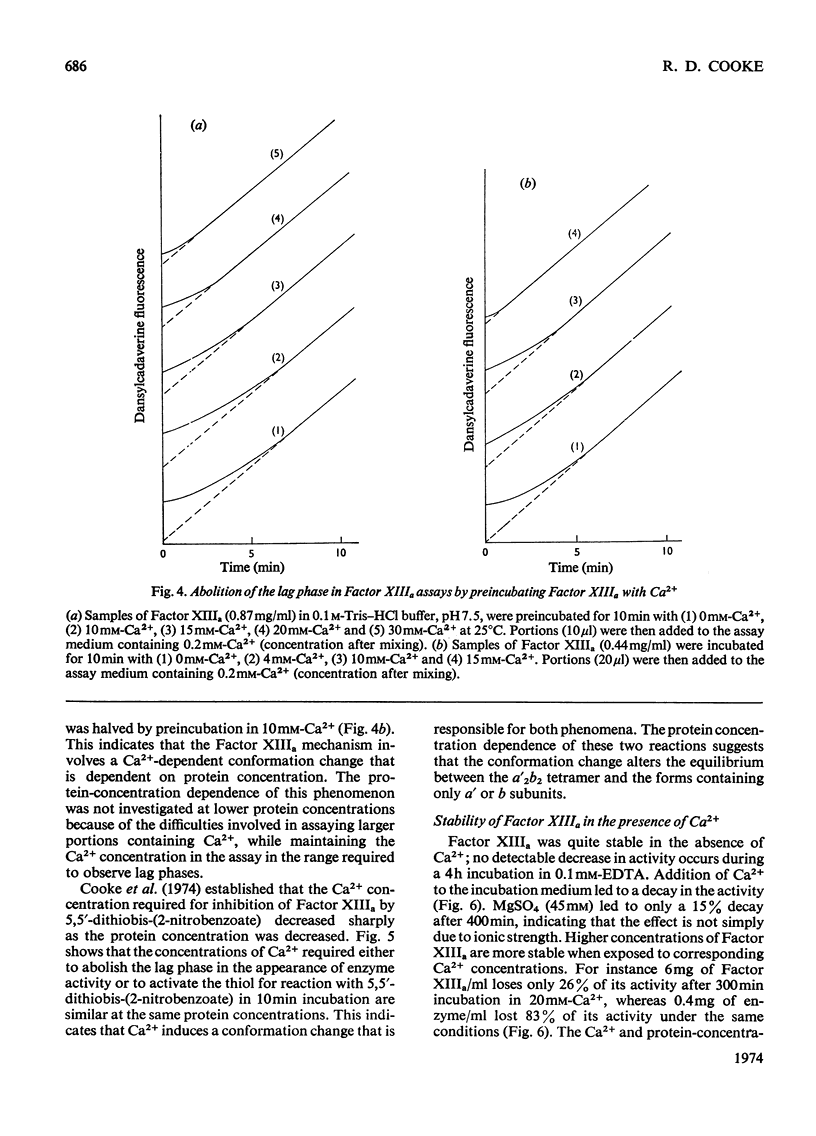
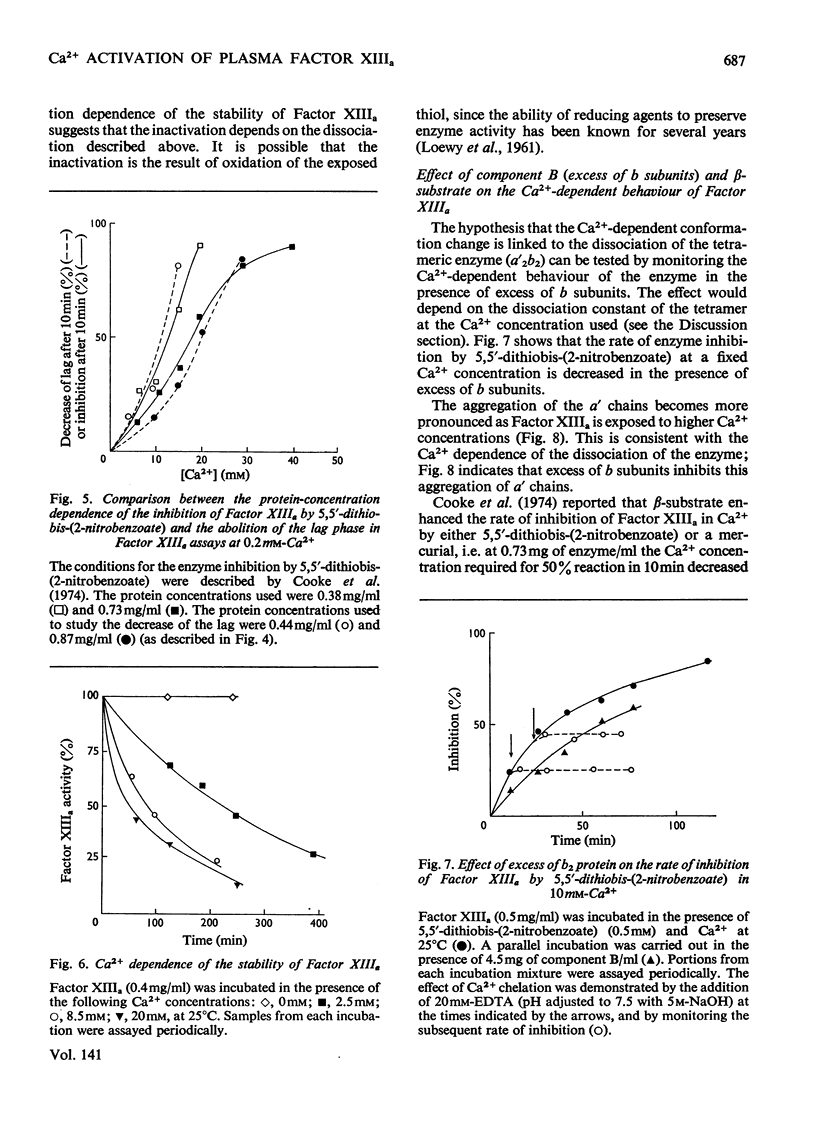
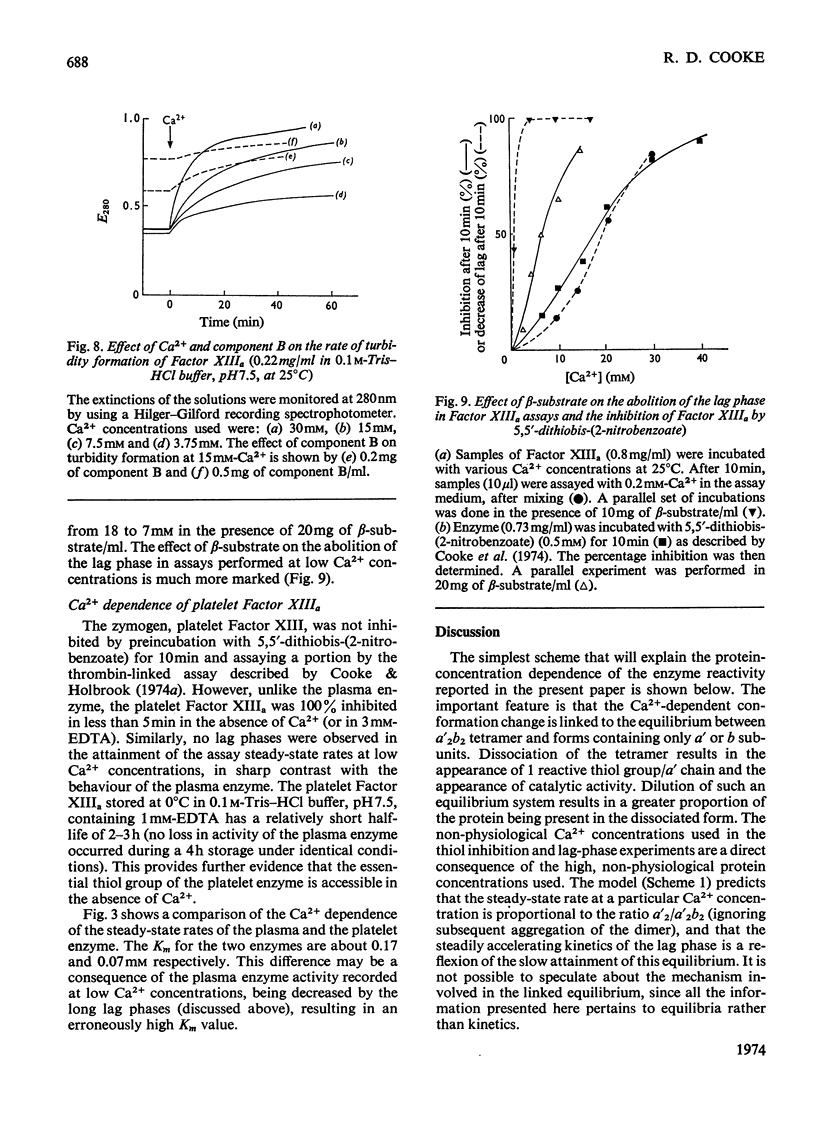
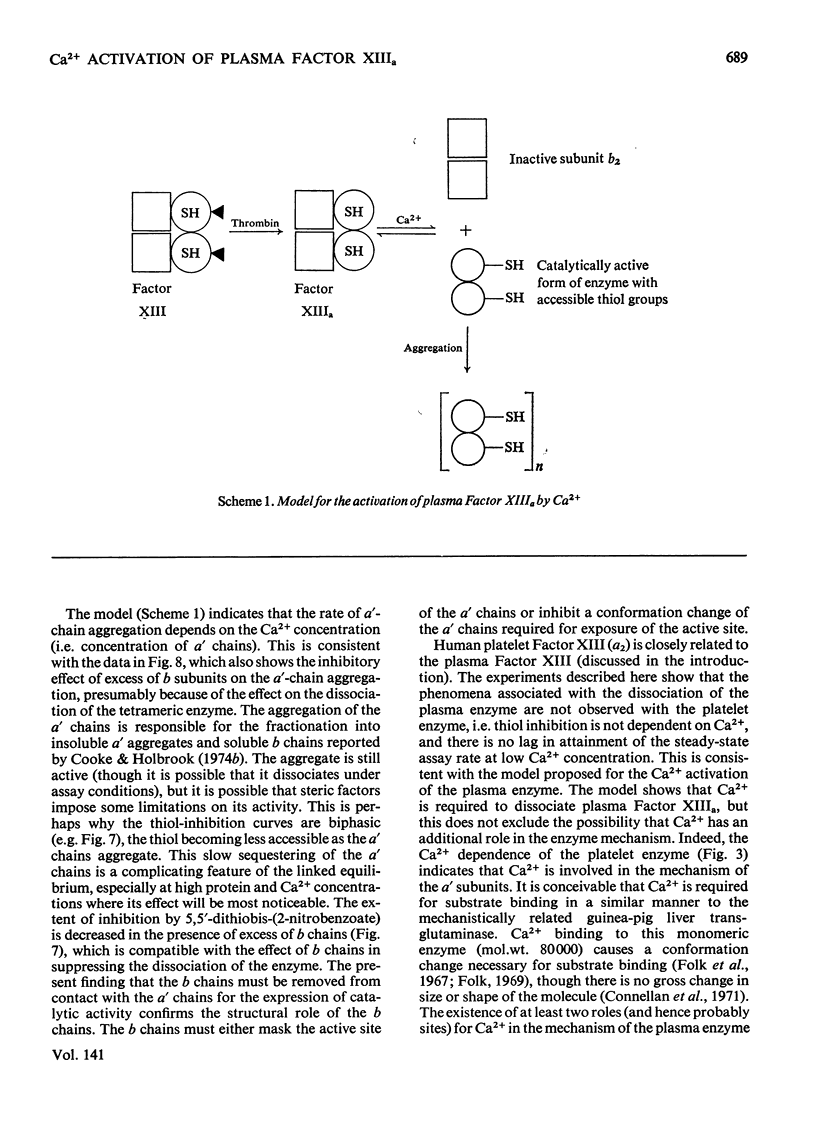
1. The Ca2+ dependence of the activity of plasma Factor XIIIa was studied by using the continuous assay based on the incorporation of dansylcadaverine into dephosphorylated acetylated β-casein (β-substrate). The Km for Ca2+ is about 0.170mm. 2. At low concentrations of Ca2+ there was a lag in attaining the steady-state rate. The size of the lag was decreased and eventually abolished if the enzyme was preincubated with a high concentration of Ca2+ before assay. The concentration of Ca2+ required to decrease the lag phase by 50% in 10min depended on the protein concentration: at 0.87mg of protein/ml it required 17mm-Ca2+ and at 0.44mg/ml it needed 10mm-Ca2+. 3. The concentrations of Ca2+ required either to abolish the lag phase in the appearance of enzyme activity or to activate the essential thiol for reaction with 5,5′-dithiobis-(2-nitrobenzoate) in 10min incubation were similar at the same protein concentration. This indicated that Ca2+ induces a conformation change that is responsible for both phenomena. A model is proposed that links this conformation change to the dissociation of the tetrameric enzyme. 4. This was supported by the observation that the addition of excess of b chains to the Factor XIIIa (a′2b2) increased the concentration of Ca2+ required to expose the reactive thiol, and inhibited the Ca2+-dependent aggregation of a′ chains. 5. Platelet Factor XIIIa (a′2) was inhibited by 5,5′-dithiobis-(2-nitrobenzoate) in the absence of Ca2+, and no lag phases were observed in attaining the steady-state rate at low Ca2+ concentrations, thus confirming the model for the activation of the plasma enzyme. 6. The Ca2+ dependence of platelet Factor XIIIa indicated that Ca2+ has an additional role in the enzyme mechanism of the plasma enzyme, perhaps being involved in substrate binding. 7. The dependence of the stability of plasma Factor XIIIa on Ca2+ and protein concentration indicates that the decay in activity is related to the tetramer dissociation. 8. β-Substrate decreased the Ca2+ concentration required for (1) abolition of the lag phase and (2) enzyme inhibition by thiol reagents. The effect on the former is greater than on the latter. 9. The role of the b chains of the plasma Factor and the evolutionary significance of the plasma and platelet Factors are considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEZKOROVAINY A., RAFELSON M. E., Jr CHARACTERIZATION OF SOME PROTEINS FROM NORMAL HUMAN PLATELETS. J Lab Clin Med. 1964 Aug;64:212–225. [PubMed] [Google Scholar]
- Bohn H., Becker W., Trobisch H. Die molekulare Struktur der fibrinstablisierenden Faktoren des Menschen. II. Vergleichennde immunologische Untersuchungen von Faktor-XIII-Mangelplasmen und Normalplasma. Blut. 1973 May;26(5):303–311. doi: 10.1007/BF01638695. [DOI] [PubMed] [Google Scholar]
- Bohn H. Comparative studies on the fibrin-stabilizing factors from human plasma, platelets and placentas. Ann N Y Acad Sci. 1972 Dec 8;202:256–272. doi: 10.1111/j.1749-6632.1972.tb16339.x. [DOI] [PubMed] [Google Scholar]
- Bohn H. Isolierung und Charakterisierung des fibrinstabilisierenden Faktors aus menschlichen Thrombozyten. Thromb Diath Haemorrh. 1970 Jun 30;23(3):455–468. [PubMed] [Google Scholar]
- Castaldi P. A., Caen J. Platelet fibrinogen. J Clin Pathol. 1965 Sep;18(5):579–585. doi: 10.1136/jcp.18.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. I., Folk J. E. Kinetic studies with transglutaminases. The human blood enzymes (activated coagulation factor 13 and the guinea pig hair follicle enzyme. J Biol Chem. 1972 May 10;247(9):2798–2807. [PubMed] [Google Scholar]
- Connellan J. M., Chung S. I., Whetzel N. K., Bradley L. M., Folk J. E. Structural properties of guinea pig liver transglutaminase. J Biol Chem. 1971 Feb 25;246(4):1093–1098. [PubMed] [Google Scholar]
- Cooke R. D., Holbrook J. J. Calcium and the assays of human plasma clotting factor XIII. Biochem J. 1974 Jul;141(1):71–78. doi: 10.1042/bj1410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Holbrook J. J. The calcium-induced dissociation of human plasma clotting factor XIII. Biochem J. 1974 Jul;141(1):79–84. doi: 10.1042/bj1410079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Pestell T. C., Holbrook J. J. Calcium and thiol reactivity of human plasma clotting factor XIII. Biochem J. 1974 Sep;141(3):675–682. doi: 10.1042/bj1410675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCHATEAU G., FLORKIN M. La coagulation du sang des arthropodes. IV. Sur le fibrinogène et sur la coaguline musculaire du homard. Bull Soc Chim Biol (Paris) 1954;36(2-3):295–305. [PubMed] [Google Scholar]
- Dvilansky A., Britten A. F., Loewy A. G. Factor XIII assay by an isotope method. I. Factor XIII (transamidase) in plasma, serum, leucocytes, erythrocytes and platelets and evaluation of screening tests of clot solubility. Br J Haematol. 1970 Apr;18(4):399–410. doi: 10.1111/j.1365-2141.1970.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Cole P. W. Identification of a functional cysteine essential for the activity of guinea pig liver transglutaminase. J Biol Chem. 1966 Jul 10;241(13):3238–3240. [PubMed] [Google Scholar]
- Folk J. E. Mechanism of action of guinea pig liver transglutaminase. VI. Order of substrate addition. J Biol Chem. 1969 Jul 10;244(13):3707–3713. [PubMed] [Google Scholar]
- Folk J. E., Mullooly J. P., Cole P. W. Mechanism of action of guinea pig liver transglutaminase. II. The role of metal in enzyme activation. J Biol Chem. 1967 Apr 25;242(8):1838–1844. [PubMed] [Google Scholar]
- Fuller G. M., Doolittle R. F. Studies of invertebrate fibrinogen. II. Transformation of lobster fibrinogen into fibrin. Biochemistry. 1971 Apr 13;10(8):1311–1315. doi: 10.1021/bi00784a006. [DOI] [PubMed] [Google Scholar]
- James H. L., Ganguly P. Studies on platelet proteins. IX. The identity of fibrinogen. Biochim Biophys Acta. 1973 Dec 6;328(2):448–455. doi: 10.1016/0005-2795(73)90279-1. [DOI] [PubMed] [Google Scholar]
- Kiesselbach T. H., Wagner R. H. Demonstration of factor XIII in human megakaryocytes by a fluorescent antibody technique. Ann N Y Acad Sci. 1972 Dec 8;202:318–328. doi: 10.1111/j.1749-6632.1972.tb16344.x. [DOI] [PubMed] [Google Scholar]
- LOEWY A. G., DAHLBERG A., DUNATHAN K., KRIEL R., WOLFINGER H. L., Jr Fibrinase. II. Some physical properties. J Biol Chem. 1961 Oct;236:2634–2643. [PubMed] [Google Scholar]
- Mandel E. E., Gerhold W. M. Plasma fibrin-stabilizing factor: acquired deficiency in various disorders. Am J Clin Pathol. 1969 Nov;52(5):547–556. doi: 10.1093/ajcp/52.5.547. [DOI] [PubMed] [Google Scholar]
- McDonagh J., McDonagh R. P., Jr, Delâge J. M., Wagner R. H. Factor XIII in human plasma and platelets. J Clin Invest. 1969 May;48(5):940–946. doi: 10.1172/JCI106053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- NUSSBAUM M., MORSE B. S. PLASMA FIBRIN STABILIZING FACTOR ACTIVITY IN VARIOUS DISEASES. Blood. 1964 May;23:669–678. [PubMed] [Google Scholar]
- Nachman R. L., Marcus A. J., Zucker-Franklin D. Immunologic studies of proteins associated with subcellular fractions of normal human platelets. J Lab Clin Med. 1967 Apr;69(4):651–658. [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. [Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine]. Science. 1968 May 24;160(3830):892–893. doi: 10.1126/science.160.3830.892. [DOI] [PubMed] [Google Scholar]
- STRAUB P. W. A study of fibrinogen production by human liver slices in vitro by an immunoprecipitin method. J Clin Invest. 1963 Jan;42:130–136. doi: 10.1172/JCI104690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S., Rapaport S. I., Chong M. M. Platelets and initiation of intrinsic clotting. Br J Haematol. 1973 May;24(5):633–642. doi: 10.1111/j.1365-2141.1973.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. The subunit structures of human plasma and platelet factor XIII (fibrin-stabilizing factor). J Biol Chem. 1971 Sep 25;246(18):5851–5854. [PubMed] [Google Scholar]
- Sheltawy M. J., Miloszewski K., Losowsky M. S. Factors affecting factor XIII assay by dansyl cadaverine incorporation. Thromb Diath Haemorrh. 1972 Dec 31;28(3):483–488. [PubMed] [Google Scholar]
- Solum N. O., Lopaciuk S. Bovine platelet proteins. 3. Some properties of platelet fibrinogen. Thromb Diath Haemorrh. 1969 Jun 15;21(3):428–440. [PubMed] [Google Scholar]
- Tyler H. M. Studies on the activation of purified human factor XIII. Biochim Biophys Acta. 1970 Nov 24;222(2):396–404. doi: 10.1016/0304-4165(70)90129-7. [DOI] [PubMed] [Google Scholar]
- Walsh P. N. The effects of collagen and kaolin on the intrinsic coagulant activity of platelets. Evidence for an alternative pathway in intrinsic coagulation not requiring factor XII. Br J Haematol. 1972 Apr;22(4):393–405. doi: 10.1111/j.1365-2141.1972.tb05687.x. [DOI] [PubMed] [Google Scholar]


