Abstract
Growth-differentiation factor 15 (GDF-15) is a cytokine involved in cellular stress responses and inflammation. This meta-analysis evaluates the association between circulating GDF-15 levels and functional outcomes in patients with acute ischemic stroke (AIS). A comprehensive search of Medline, Web of Science, Embase, Wanfang, and CNKI was conducted up to July 15, 2024. Observational studies with longitudinal follow-up that measured GDF-15 levels within 24 h of stroke onset and reported functional outcomes, defined as a modified Rankin Scale (mRS) score of ≥ 2, were included. Odds ratios (OR) with 95% confidence intervals (CI) were used to quantify associations. Heterogeneity was evaluated using I² statistics, and a random-effects model was used to pool the results by incorporating the influence of heterogeneity. Ten studies involving 4,231 patients were included. The pooled OR indicated that high circulating GDF-15 levels were associated with a significantly higher risk of poor functional outcomes at 3 months (OR: 2.60, 95% CI: 1.95 to 3.46, p < 0.001). Sensitivity analyses by excluding one study at a time did not significantly change the results. Subgroup analyses revealed stronger associations in studies with GDF-15 cutoff values < 1200 ng/L as compared to ≥ 1200 ng/L, and in those defining poor outcomes as mRS ≥ 3 as compared to those ≥ 2. In conclusion, elevated circulating GDF-15 levels are associated with worse functional outcomes following AIS. These findings support the potential use of GDF-15 as a prognostic biomarker in stroke patients. Further research is warranted to confirm these results and explore clinical applications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13005-024-00476-4.
Keywords: Ischemic stroke, Growth-differentiation factor 15, Functional outcome, Predictor, Meta-analysis
Introduction
Acute ischemic stroke (AIS) is a major global health concern and a leading cause of disability and death [1, 2]. It occurs when a blockage in a cerebral artery impairs blood flow to a part of the brain, leading to neuronal injury and subsequent neurological deficits [3]. According to the Global Burden of Disease Study, ischemic stroke accounts for approximately 11% of all deaths and 5% of disability-adjusted life years (DALYs) worldwide [4]. Despite advancements in acute stroke management and treatment, a substantial proportion of stroke survivors experience poor functional outcomes [5]. The modified Rankin Scale (mRS) is commonly used to assess functional outcomes post-stroke, with scores ranging from 0 (no symptoms) to 6 (death) [6]. A score of ≥ 2 indicates some degree of disability, while a score of ≥ 3 reflects significant impairment [6].
Identifying new risk factors for poor functional outcomes after AIS is critical for improving patient prognosis and tailoring treatment strategies [7]. Biomarkers, in particular, hold promise for predicting stroke severity and outcomes [8]. Growth-differentiation factor 15 (GDF-15) is a cytokine involved in cellular stress responses, inflammation, and tissue repair [9]. Elevated GDF-15 levels have been associated with adverse outcomes in various cardiovascular diseases, suggesting its potential role in stroke prognosis [9–11]. GDF-15 functions by modulating inflammatory responses and apoptosis [11, 12], which are crucial in stroke pathology. High levels of GDF-15 might reflect extensive neuronal damage and heightened inflammatory activity [13], contributing to poor functional recovery.
Recent observational studies have explored the relationship between circulating GDF-15 levels and functional outcomes following AIS [14–23]. However, a quantitative summary for the association remains lacking. In addition, the specific impact of GDF-15 levels on stroke outcomes and the influence of study characteristics, such as the methods for measuring GDF-15, cutoff values used, and patient demographics, remain inadequately explored, underscoring the need for a comprehensive meta-analysis. By aggregating data from multiple studies, this meta-analysis aims to summarize the existing evidence, clarify the robustness of the association between GDF-15 levels and poor functional outcomes, and investigate how different study characteristics might influence the observed outcomes.
Methods
The authors followed the recommendations set forth in PRISMA 2020 [24, 25] and the Cochrane Handbook for Systematic Reviews and Meta-analyses [26] during the entire meta-analysis process, including the study’s design, data acquisition, statistical evaluation, and interpretation of the findings. The protocol of the meta-analysis has been registered at PROSPERO with the identification code CRD42024581753. A PRISMA 2000 Checklist has been included in the Supplementary Material 1.
Database search
To identify studies relevant to the aim of the meta-analysis, we searched Medline, Web of Science, Embase, Wanfang and China National Knowledge Infrastructure (CNKI) utilizing comprehensive search terms involving (1) “growth differentiation factor 15” OR “macrophage inhibitory cytokine 1” OR “prostate differentiation factor” OR “GDF-15” OR “GDF 15” OR “MIC-1”; and (2) “stroke” OR “transient ischemic stroke” OR “TIA” OR “cerebral infarction” OR “cerebrovascular infarction”. The detailed search strategy for each of the included database is detailed in Supplementary Material 2. The search was limited to clinical research conducted on human subjects. We included only full-length articles published in English or Chinese in peer-reviewed journals. Additionally, we manually reviewed the references of relevant original and review articles to identify any additional pertinent studies. The literature search covered publications from the inception of the databases up to July 15, 2024.
Inclusion and exclusion criteria
The inclusion criteria for eligible studies were as follows: (1) observational studies with longitudinal follow-up published as full-length articles, including prospective and retrospective cohort studies, nested case-control studies, and post-hoc analyses of clinical trials; (2) enrollment of patients with AIS, regardless of the primary treatment method; (3) measurement of circulating GDF-15 levels at enrollment (within 24 h of admission after stroke onset) as the exposure factor; (4) reporting of the risk of poor functional outcomes during follow-up, defined as a modified Rankin Scale (mRS) score of ≥ 2 [27]; and (5) comparison of the relative risk of poor functional outcomes after IS between patients with high and low baseline GDF-15 levels. We did not apply restriction for the follow-up duration of the studies. Grey literatures such as preprints, conference abstracts, and unpublished data were not included because these literatures were generally not peer-reviewed, and inclusion of these data may affect the reliability of the meta-analysis results.
The exclusion criteria were: (1) cross-sectional or case-control studies; (2) studies involving patients with hemorrhagic stroke; (3) studies that either did not measure or report baseline circulating GDF-15 or analyzed GDF-15 only as a continuous variable; (4) studies that did not report the incidence of poor functional outcomes based on the mRS score during follow-up; and (5) studies published as conference abstracts, unpublished data, reviews, or editorials. For studies with overlapping populations, the one with the largest sample size was chosen for inclusion in the meta-analysis.
Study quality assessment and data collection
The literature search, study identification, quality evaluation, and data extraction were conducted independently by two authors (YL W and YD W). In instances of disagreement, the corresponding author was consulted to achieve consensus. The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) [28], which examines three main domains: selection of the study population, control of confounding factors, and measurement and analysis of outcomes. NOS scores range from 1 to 9, with a score of 9 representing the highest quality. Data collected from each study for subsequent analysis included study-specific information (author, year, country, and design), patient demographics (sample size, age, and sex) at admission, methods for GDF-15 measurement, criteria for determining GDF-15 cutoff values, the actual cutoff values used, follow-up period, definition of poor functional outcomes, the number of patients experiencing poor outcomes during follow-up, and the variables accounted for when analyzing the association between circulating GDF-15 levels and poor functional outcomes.
Statistical analysis
The association between circulating GDF-15 levels and poor functional outcomes after AIS was evaluated using odds ratios (OR) with corresponding 95% confidence intervals (CI), comparing patients with high versus low GDF-15 levels at admission. ORs and their standard errors were calculated from 95% CIs or p-values, followed by logarithmic transformation to stabilize variance. Study heterogeneity was assessed with the Cochrane Q test and I² statistics [29], with an I² value greater than 50% indicating significant statistical heterogeneity. The results were synthesized using a random-effects model to account for the potential impact of heterogeneity [26]. Sensitivity analyses, in which one dataset was excluded at a time, were performed to evaluate the stability of the findings. Predefined subgroup analyses were also conducted to explore how specific study characteristics might influence the results, with median values of continuous variables serving as cutoffs for subgroup definitions. Publication bias was examined using funnel plots, assessing visual symmetry [30], and confirmed with an Egger’s regression test [30]. In addition, We also performed univariate meta-regression to evaluate whether the outcome of the meta-analysis could be significantly modified by study characteristics such as publication year, sample size, mean age of the patients, proportion of men, cutoff value of GDF-15, cutoff mRS for defining a poor functional outcome, and the study quality score in NOS [26]. A p-value < 0.05 indicates significance. Statistical analyses were performed using RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corporation, College Station, TX).
Results
Study inclusion
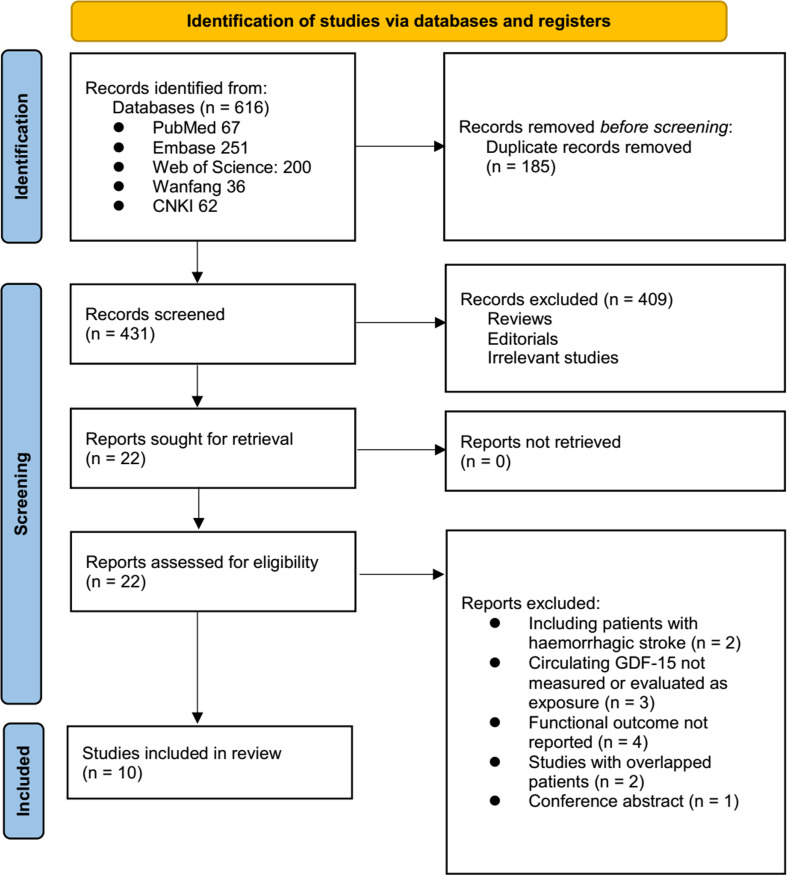
The study inclusion process is illustrated in Fig. 1. In summary, an initial search of five databases yielded 616 potentially relevant records, of which 185 were excluded due to duplication. The titles and abstracts of the remaining records were then screened, leading to the exclusion of 409 studies, primarily because they did not align with the objectives of the meta-analysis. The full texts of the remaining 22 records were reviewed by two independent authors, resulting in the exclusion of 12 studies for reasons detailed in Fig. 1. Ultimately, ten studies [14–23] were deemed appropriate for inclusion in the quantitative analyses.
Fig. 1.
PRISMA flowchart of study identification
Summary of study characteristics
Table 1 presents the overview of the characteristics of the included studies. Overall, four prospective cohort studies [14, 15, 18, 19], five retrospective cohort studies [16, 17, 21–23], and one post-hoc analysis of clinical study [20] were included in the meta-analysis. These studies were reported from 2011 to 2022 and performed in Germany, China, France, and Korea. Overall, 4231 patients with AIS were included, with mean ages varying from 58.4 to 74.6 years, and the proportion of men ranging from 39.0 to 64.1%. The circulating level of GDF-15 was measured all within 24 h after stroke onset, with enzyme-linked immunosorbent assay in seven of the included studies [17–23]. For the other three studies [14–16], other methods such as immunoradiometric assay, electrochemiluminescence immunoassay, and radioimmunoassay were used, respectively. The cutoff values for defining a high circulating GDF-15 level were derived via receiver operating characteristic (ROC) curve analysis in three studies [14, 21, 23], via median of GDF-15 in five studies [15–17, 19, 22], via the third tertile [18] and the upper limits of healthy individual [20] in the another two studies. The cutoff values for defining a high GDF-15 varying from 493 to 2088 ng/L. The patients were all followed for 3 months among the included studies. The risk of poor functional outcome at 3 months after stroke were reported in all of the included studies, which were defined as the mRS ≥ 2 in four studies [14–17] and ≥ 3 in six studies [18–23]. Accordingly, 1267 (29.9%) of the included patients had poor functional outcome 3 months after AIS. A multivariate analysis was performed in five studies when the association between circulating GDF-15 and poor functional outcome after AIS was evaluated [14, 15, 19, 20, 22], whereas a univariate analysis was performed in another five studies [16–18, 21, 23]. The NOS of the included studies were six to nine stars, suggesting overall moderate to good study quality (Table 2).
Table 1.
Characteristics of the included studies
| Study | Country | Design | Sample size | Mean age (years) | Men (%) | Timing of GDF-15 measurement | Methods of GDF-15 measurement | Methods for determining GDF-15 cutoff | Cutoff of GDF-15 | Follow-up duration (months) | Definition of poor functional outcome | No. of patients with poor functional outcome | Variables adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Worthmann [14] | Germany | PC | 57 | 74 | 47 | At admission | IRMA | ROC curve analysis | 1630 ng/l | 3 | mRS: 2–6 | 19 | Age and sex |
| Groschel [15] | Germany | PC | 264 | 70.3 | 55.3 | At admission | ECLIA | Median | 1191 ng/l | 3 | mRS: 2–6 | 113 | Age, sex, NIHSS at admission, TOAST classification, SBP, DM, CAD, HDL-C, and thrombolysis |
| Geng [16] | China | RC | 102 | 71.3 | 58.8 | At admission | RIA | Median | 1580 ng/l | 3 | mRS: 2–6 | 29 | None |
| Lu [17] | China | RC | 120 | 67.8 | 60.8 | Within 24 h of stroke onset | ELISA | Median | 1594 ng/l | 3 | mRS: 2–6 | 65 | None |
| Yin 2019 | China | Post-hoc analysis | 3066 | 62.3 | 64.1 | Within 24 h of admission | ELISA | UL of healthy individual in previous studies | 1200 ng/l | 3 | mRS: 3–6 | 762 | Age, sex, current smoking, alcohol consumption, and NIHSS at admission |
| Breniere [18] | France | PC | 173 | 74.6 | 51 | At admission | ELISA | T3 versus T1-2 | 2088 ng/l | 3 | mRS: 3–6 | 92 | None |
| Dong [19] | China | PC | 83 | 62.4 | 63.9 | At admission | ELISA | Median | 864 ng/l | 3 | mRS: 3–6 | 42 | Age, sex, comorbidities, and NIHSS at admission |
| Jeong [22] | Korea | RC | 62 | 66.2 | 39 | Within 24 h of admission | ELISA | Median | 493 ng/l | 3 | mRS: 3–6 | 20 | Age, sex, comorbidities, eGFR, and timing of recanalization |
| Cai [21] | China | RC | 148 | 58.4 | 62.2 | Within 24 h of admission | ELISA | ROC curve analysis | 826.5 ng/l | 3 | mRS: 3–6 | 61 | None |
| Li [23] | China | RC | 156 | 58.4 | 55 | Within 24 h of stroke onset | ELISA | ROC curve analysis | 802.1 ng/l | 3 | mRS: 3–6 | 64 | None |
GDF-15, growth differentiation factor 15; PC, prospective cohort; RC, retrospective cohort; IRMA, immunoradiometric assay; ECLIA, electrochemiluminescence immunoassay; ELISA, enzyme-linked immunosorbent assay; ROC, Receiver operating characteristic; UL, upper limit; T, tertile; mRS, modified Rankin Score; NIHSS, National institutes of health stroke scale; TOAST, Trial of ORG 10,172 in Acute Stroke Therapy; SBP, systolic blood pressure; CAD, coronary artery disease; DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtrating rate; RIA, radioimmunoassay
Table 2.
Study quality evaluation via the Newcastle-Ottawa Scale
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age and sex | Control for other confounding factors | Assessment of outcome | Enough long follow-up duration | Adequacy of follow-up of cohort | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Worthmann [14] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Groschel [15] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Geng [16] | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Lu [17] | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Yin [20] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Breniere [18] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Dong [19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Jeong [22] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Cai [21] | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Li [23] | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
Results of the meta-analysis and sensitivity analysis
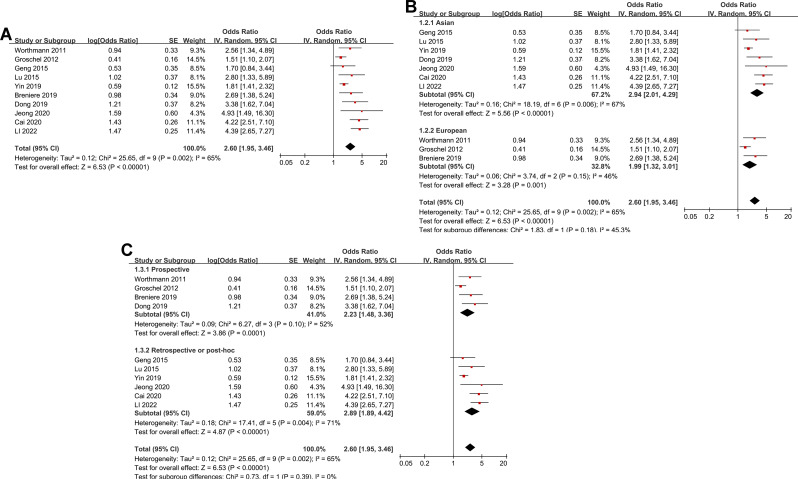
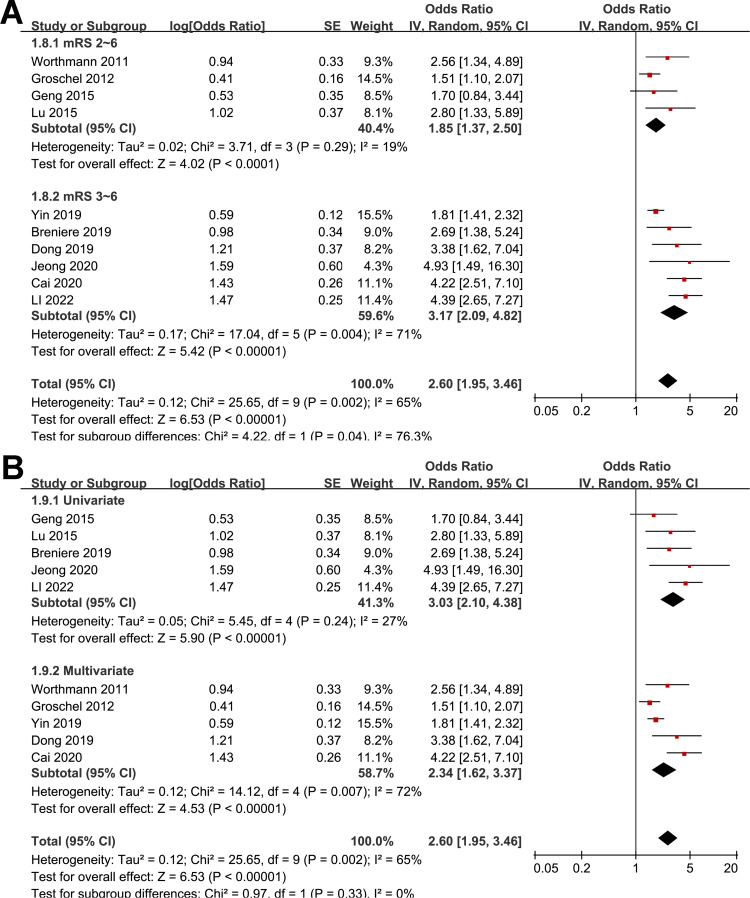
The pooled results of ten studies using a random-effects model suggested that compared to AIS patients with a low circulating GDF-15 at admission, patients with a high circulating GDF-15 were associated with an increased risk of poor functional outcome at 3 months (OR: 2.60, 95% CI: 1.95 to 3.46, p < 0.001; Fig. 2A) with moderate statistical heterogeneity (I2 = 65%). Further analysis excluding one study at a time consistently demonstrated similar results (OR: 2.38 to 2.84, all p < 0.05).
Fig. 2.
Forest plots for the meta-analysis of the association between circulating GDF-15 level at admission and functional outcome after AIS; A, overall meta-analysis; B, subgroup analysis according to study country; and C, subgroup analysis according to study design
Results of subgroup analyses and meta-regression analyses
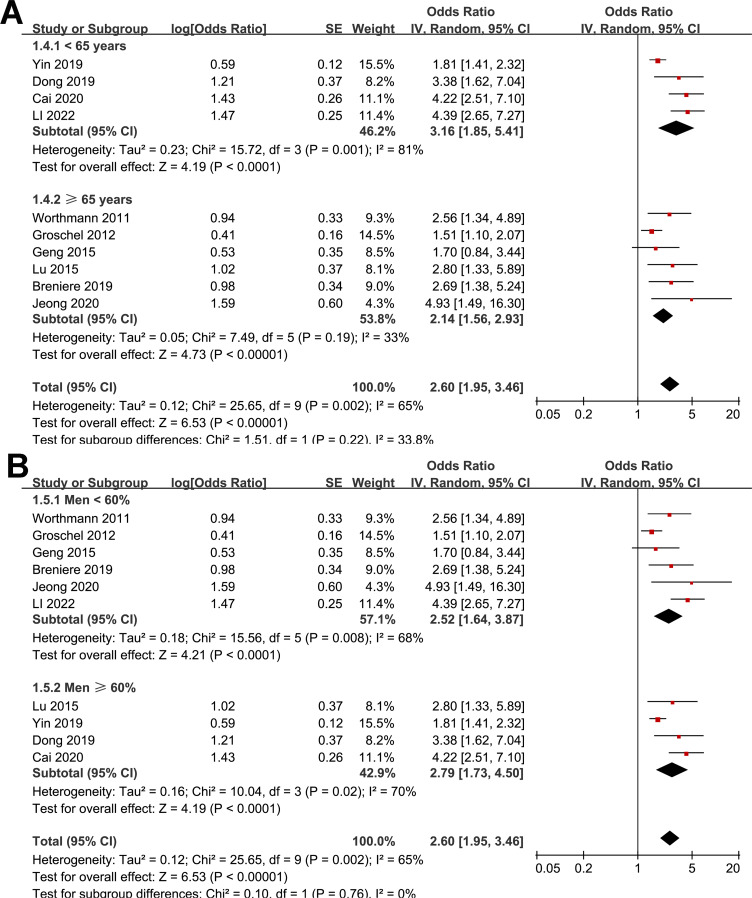
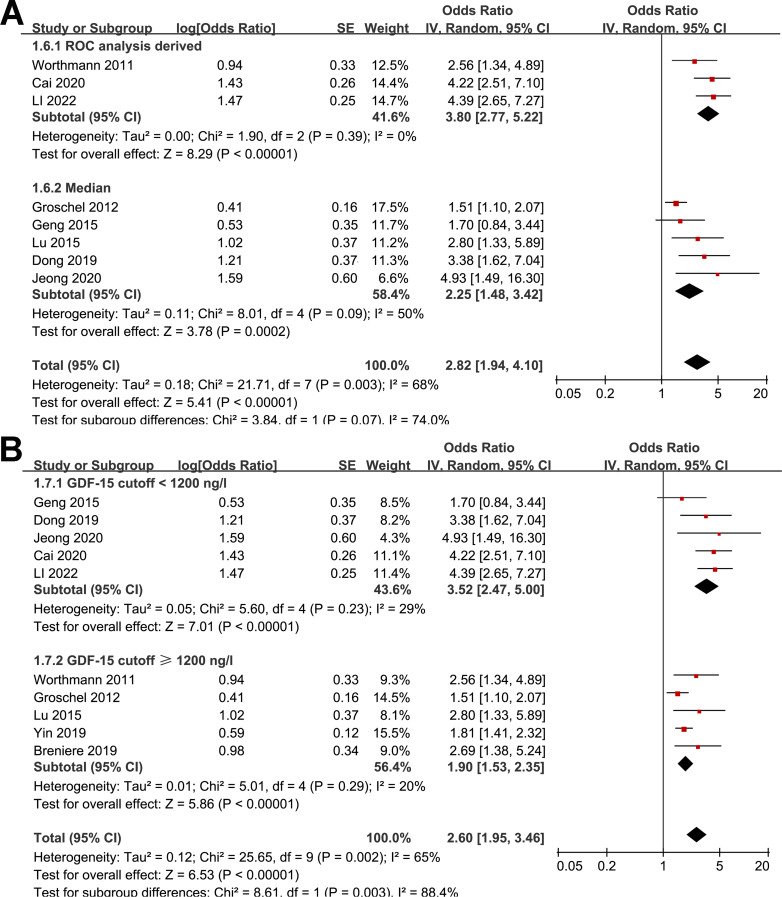
Further subgroup analyses showed similar results in studies from Asia and Europe (OR: 2.94 versus 1.99, p for subgroup difference = 0.18; Fig. 2B), in prospective and retrospective studies (OR: 2.23 versus 2.89, p for subgroup difference = 0.39; Fig. 2C), in studies with the mean age of the patients < and ≥ 65 years (OR: 3.16 versus 2.14, p for subgroup difference = 0.22; Fig. 3A), in studies with the proportion of men < and ≥ 60% (OR: 2.52 versus 2.79, p for subgroup difference = 0.76; Fig. 3B), and in studies with the cutoff of GDF-15 defined by ROC analysis and the medians (OR: 3.80 versus 2.25, p for subgroup difference = 0.07; Fig. 4A). Interestingly, the subgroup analysis suggested that the association between a high circulating GDF-15 and the risk of poor functional outcome after AIS was stronger for studies with cutoff of GDF-15 < 1200 ng/L as compared to those ≥ 1200 ng/L (OR: 3.52 versus 1.90, p for subgroup difference = 0.003; Fig. 4B), which substantially explained the source of heterogeneity. In addition, a stronger association was observed in studies with poor functional outcome define as mRS ≥ 3 compared to those as mRS ≥ 2 (OR: 3.17 versus 1.85, p for subgroup difference = 0.04; Fig. 5A). Subsequently, consistent results were obtained for studies with univariate and multivariate analysis (OR: 3.03 versus 2.34, p for subgroup difference = 0.33; Fig. 5B). Finally, the univariate meta-regression analyses did not show that study characteristics such as publication year, sample size, mean age of the patients, proportion of men, cutoff value of GDF-15, cutoff mRS for defining a poor functional outcome, or the study quality score in NOS could significantly affect the association between circulating GDF-15 at admission and the risk of poor functional outcome after AIS (p all > 0.05; Table 3). However, publication year and NOS scores have relatively higher adjusted R2 (27.4% and 28.3%, respectively), indicating they may explain more of the between-study heterogeneity compared to other factors. These findings should be interpreted cautiously, acknowledging the lack of statistical significance.
Fig. 3.
Forest plots for the subgroup analysis of the association between circulating GDF-15 level at admission and functional outcome after AIS; A, subgroup analysis according to the mean age of the patients; and B, subgroup analysis according to the proportion of men
Fig. 4.
Forest plots for the subgroup analysis of the association between circulating GDF-15 level at admission and functional outcome after AIS; A, subgroup analysis according to the methods for defining the cutoff of GDF-15; and B, subgroup analysis according to the cutoff values for defining a high circulating GDF-15 level
Fig. 5.
Forest plots for the subgroup analyses of the association between circulating GDF-15 level at admission and functional outcome after AIS; A, subgroup analysis according to the definition of poor functional outcome; and B, subgroup analysis according to the analytic models (univariate or multivariate)
Table 3.
Results of univariate meta-regression analysis
| Variables | OR for the association circulating GDF-15 and functional outcome after AIS | |||
|---|---|---|---|---|
| Coefficient | 95% CI | p values | Adjusted R2 | |
| Publication year | 0.071 | -0.011 to 0.153 | 0.11 | 27.4% |
| Sample size | -0.00015 | -0.00046 to 0.00015 | 0.28 | 2.9% |
| Mean age (years) | -0.036 | -0.090 to 0.017 | 0.16 | 19.5% |
| Men (%) | -0.012 | -0.064 to 0.040 | 0.62 | -11.3% |
| Cutoff value of GDF-15 (ng/L) | -0.00045 | -0.00123 to 0.00033 | 0.22 | 8.4% |
| Cutoff mRS of poor functional outcome | 0.45 | -0.18 to 1.09 | 0.14 | 18.7% |
| NOS | -0.19 | -0.42 to 0.04 | 0.10 | 28.3% |
GDF-15, growth differentiation factor 15; AIS, acute isckemic stroke; OR, odds ratio; CI, confidence interval; mRS, modified Rankin Score; NOS, Newcastle-Ottawa Scale
Publication bias
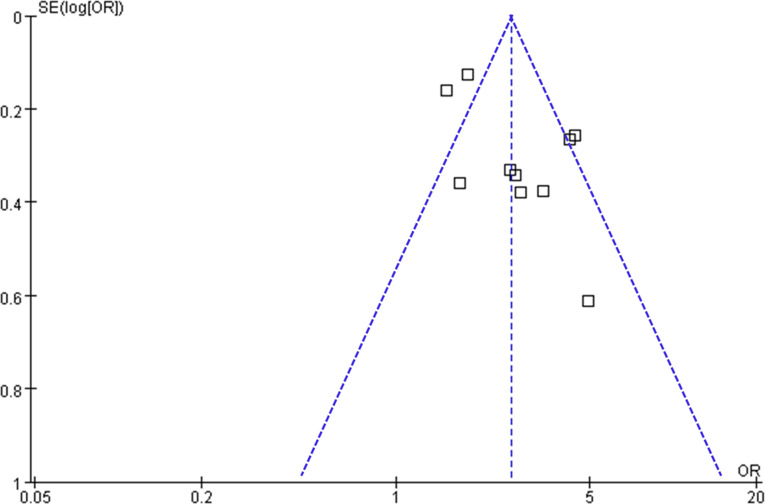
Visual inspection of the funnel plots for the meta-analysis examining the relationship between circulating GDF-15 levels and poor functional outcomes after AIS indicates symmetry, suggesting a low risk of publication bias (Fig. 6). This observation is further supported by Egger’s regression test results (p = 0.45), which also suggest a low risk of publication bias.
Fig. 6.
Funnel plots for the meta-analysis of the association between circulating GDF-15 level at admission and functional outcome after AIS
Discussion
This meta-analysis synthesizes data from ten studies to examine the relationship between circulating GDF-15 levels and functional outcomes after AIS. Our findings reveal a consistent association between elevated GDF-15 levels and an increased risk of poor functional outcomes, with a pooled OR of 2.60. This significant association underscores the potential utility of GDF-15 as a prognostic biomarker in stroke patients, helping to identify those at higher risk for adverse recovery.
GDF-15 is a member of the transforming growth factor-beta (TGF-β) superfamily and plays a critical role in cellular stress responses and inflammation [31]. Its physiological and pathological functions have been linked to various mechanisms that could explain its association with poor functional outcomes after stroke. One key mechanism involves its role in inflammation [32]. GDF-15 is upregulated in response to tissue injury and inflammation and acts as an anti-inflammatory cytokine. It modulates macrophage activation and inhibits the release of pro-inflammatory cytokines [33]. Elevated GDF-15 levels may indicate a dysregulated inflammatory response, where excessive inflammation or prolonged inflammatory signaling contributes to tissue damage and impaired recovery [34]. Additionally, GDF-15 influences apoptosis and cell survival pathways [35]. It has been shown to modulate apoptosis through the activation of the MAPK (mitogen-activated protein kinase) and PI3K/Akt (phosphoinositide 3-kinase/protein kinase B) signaling pathways [36]. These pathways are crucial in regulating cell survival and apoptosis, particularly in the context of ischemic injury [37]. Elevated GDF-15 levels might reflect enhanced cell death or apoptosis in brain tissue, contributing to poor functional outcomes. A recent study using a rat model of AIS found that GDF-15 gene expression significantly increased in the ipsilateral cortex and cerebellum, with a smaller increase observed in the contralateral cortex [38]. Additionally, GDF-15 expression was correlated with the neurological deficit score [38]. Additionally, GDF-15 is also involved in tissue repair and remodeling. After ischemic injury, the repair processes are crucial for recovery. The role of GDF-15 in modulating these processes, potentially by influencing fibroblast and endothelial cell functions, could impact the extent of tissue damage and the recovery trajectory [39, 40]. High levels of GDF-15 might signify an imbalance in repair processes, further impairing functional recovery. Studies are warranted in the future to validate these hypotheses.
Our subgroup analyses reveal important insights into the variability of the association between GDF-15 and functional outcomes after AIS. The observed stronger association in studies using cutoff values for GDF-15 < 1200 ng/L compared to ≥ 1200 ng/L suggests that lower cutoff values may better capture clinically significant elevations of GDF-15 associated with poorer outcomes. This implies that high GDF-15 levels are more predictive of adverse outcomes when measured against lower thresholds, possibly due to a more pronounced inflammatory or pathological response captured by these values. The variation in the definition of poor functional outcomes also impacts the observed associations. Studies defining poor outcomes as an mRS score ≥ 3 showed a stronger association with elevated GDF-15 levels compared to those defining poor outcomes as mRS ≥ 2. This indicates that high GDF-15 levels are more closely linked with severe disability rather than moderate disability. This finding highlights potential role of GDF-15 as a marker for severe functional impairments, providing valuable prognostic information that could guide clinical decision-making.
This meta-analysis has several strengths. We employed a comprehensive search strategy across multiple databases, ensuring a thorough identification of relevant studies. The use of a random-effects model allowed us to account for variability across studies and provided a more accurate estimate of the association between GDF-15 levels and functional outcomes. Only longitudinal observational studies were included, so as to provide a sequential relationship between high GDF-15 and increased risk of poor functional outcome after AIS. Sensitivity analyses further validated the robustness of our findings, while subgroup analyses offered insights into how study characteristics influence results. However, there are limitations to consider. The variability in measurement methods for GDF-15, cutoff values used, and definitions of poor outcomes across studies could affect the generalizability of the results. In addition, influences of concurrent medications and treatments for AIS on the results of the meta-analysis could not be determined because the influences of these factors were generally not reported among the included studies. For example, statins have been well-acknowledged to exert anti-inflammatory effect [41]. It remains to be determined if the association between a high circulating GDF-15 level and the increased risk of poor functional outcome after AIS could be significantly modified by the use of statins. Moreover, the observational nature of the included studies limits the ability to establish causality and may introduce biases. Finally, the quality of studies varied, with some having methodological limitations that could influence the findings.
From a clinical perspective, our findings suggest that elevated circulating GDF-15 levels could be a valuable biomarker for predicting poor functional outcomes in stroke patients. Identifying patients with high GDF-15 levels could help clinicians stratify risk and tailor treatment strategies accordingly. Incorporating GDF-15 into clinical assessments could improve prognosis and guide therapeutic interventions, potentially enhancing patient outcomes. Future research should focus on standardizing measurement methods for GDF-15 and defining optimal cutoff values to improve consistency across studies. Investigating the role of GDF-15 in different stroke subtypes and its interaction with other biomarkers could provide a more comprehensive understanding of its prognostic value. Additionally, prospective studies and clinical trials are needed to validate GDF-15 as a clinical tool and explore its potential therapeutic applications.
Conclusions
This meta-analysis demonstrates a significant association between elevated circulating GDF-15 levels and poor functional outcomes following AIS. The consistent findings across studies highlight the potential role of GDF-15 as a prognostic biomarker. While further research is needed to confirm these results and refine clinical applications, GDF-15 could play a crucial role in improving stroke management and patient outcomes by identifying individuals at higher risk for adverse recovery.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Yulang Wu and Fengkun Zhou designed the study. Yulang Wu and Yude Wei performed database search, literature review, study identification, study quality assessment, and data collection. Yulang Wu, Jinrong He, and Fengkun Zhou performed statistical analyses and interpreted the results. Yulang Wu drafted the manuscript. All authors revised the manuscript and approved the submission.
Funding
No funding was received for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Ethics approval was not required as this was a systematic review of published studies.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao Y, Hua X, Ren X, Ouyang M, Chen C, Li Y, et al. Increasing burden of stroke in China: a systematic review and meta-analysis of prevalence, incidence, mortality, and case fatality. Int J Stroke. 2023;18(3):259–67. 10.1177/17474930221135983. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Owolabi MO. Pragmatic solutions to reduce the global burden of stroke: a World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 2023;22(12):1160 – 206. doi: S1474-4422(23)00277-6 [pii]10.1016/S1474-4422(23)00277-6. [DOI] [PMC free article] [PubMed]
- 3.Herpich F, Rincon F. Management of Acute ischemic stroke. Crit Care Med. 2020;48(11):1654–63. 10.1097/CCM.000000000000459700003246-202011000-00013. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2133-61. doi: S0140-6736(24)00757-8 [pii]10.1016/S0140-6736(24)00757-8. [DOI] [PMC free article] [PubMed]
- 5.Harvey RL. Predictors of functional outcome following stroke. Phys Med Rehabil Clin N Am. 2015;26(4):583–98. 10.1016/j.pmr.2015.07.002. [DOI] [PubMed]
- 6.Broderick JP, Adeoye O, Elm J. Evolution of the Modified Rankin Scale and its use in future stroke trials. Stroke. 2017;48(7):2007–12. 10.1161/STROKEAHA.117.017866STROKEAHA.117.017866. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. 10.1038/s41572-019-0118-810.1038/s41572-019-0118-8. [pii]. [DOI] [PubMed]
- 8.Xu Q, Liu Y, Tian X, Xia X, Zhang Y, Zhang X, et al. Monocyte chemoattractant Protein-1, inflammatory biomarkers, and prognosis of patients with ischemic stroke or transient ischemic attack: Fndings from a Nationwide Registry Study. J Am Heart Assoc. 2024;e035820. 10.1161/JAHA.124.035820. [DOI] [PubMed]
- 9.Nyarady BB, Kiss LZ, Bagyura Z, Merkely B, Dosa E, Lang O et al. Growth and differentiation factor-15: A link between inflammaging and cardiovascular disease. Biomed Pharmacother. 2024;174:116475. doi: S0753-3322(24)00359-7 [pii]10.1016/j.biopha.2024.116475. [DOI] [PubMed]
- 10.Arkoumani M, Papadopoulou-Marketou N, Nicolaides NC, Kanaka-Gantenbein C, Tentolouris N, Papassotiriou I. The clinical impact of growth differentiation factor-15 in heart disease: a 2019 update. Crit Rev Clin Lab Sci. 2020;57(2):114–25. 10.1080/10408363.2019.1678565. [DOI] [PubMed] [Google Scholar]
- 11.Jiang WW, Zhang ZZ, He PP, Jiang LP, Chen JZ, Zhang XT, et al. Emerging roles of growth differentiation factor-15 in brain disorders (review). Exp Ther Med. 2021;22(5):1270. 10.3892/etm.2021.107051270ETM-22-5-10705. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochette L, Zeller M, Cottin Y, Vergely C. GDF15: an emerging modulator of immunity and a strategy in COVID-19 in association with iron metabolism. Trends Endocrinol Metab. 2021;32(11):875 – 89. doi: S1043-2760(21)00204-6 [pii]10.1016/j.tem.2021.08.011. [DOI] [PMC free article] [PubMed]
- 13.McGrath ER, Himali JJ, Levy D, Conner SC, DeCarli C, Pase MP, et al. Growth differentiation factor 15 and NT-proBNP as blood-based markers of Vascular Brain Injury and Dementia. J Am Heart Assoc. 2020;9(19):e014659. 10.1161/JAHA.119.014659e014659JAH35413. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worthmann H, Kempf T, Widera C, Tryc AB, Goldbecker A, Ma YT, et al. Growth differentiation factor 15 plasma levels and outcome after ischemic stroke. Cerebrovasc Dis. 2011;32(1):72–8. 10.1159/000328233000328233. [pii]. [DOI] [PubMed] [Google Scholar]
- 15.Groschel K, Schnaudigel S, Edelmann F, Niehaus CF, Weber-Kruger M, Haase B, et al. Growth-differentiation factor-15 and functional outcome after acute ischemic stroke. J Neurol. 2012;259(8):1574–9. 10.1007/s00415-011-6379-06379. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng S, Wang YL, Yin HL, Li JF, Lou JY, Zhang YZ. The relationship of plasma growth differentiation factor 15 expression level and prognosis of patients with acute cerebral infarction. Chin J Pract Nerv Dis. 2015;18(2):45–7. 10.3969/j.issn.1673-5110.2015.02.024. [Google Scholar]
- 17.Lu CJ, Wei BX. Correlation study between serum growth differentiation Factor-15 and Acute Ischemic Stroke. Chin Circ J. 2015;30(9):872–4. 10.3969/j.issn.1000-3614.2015.09.012. [Google Scholar]
- 18.Breniere C, Meloux A, Pedard M, Marie C, Thouant P, Vergely C, et al. Growth differentiation Factor-15 (GDF-15) is Associated with Mortality in ischemic stroke patients treated with Acute Revascularization Therapy. Front Neurol. 2019;10:611. 10.3389/fneur.2019.00611611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X, Nao J. Association of serum growth differentiation factor 15 level with acute ischemic stroke in a Chinese population. Int J Neurosci. 2019;129(12):1247–55. 10.1080/00207454.2019.1660327. [DOI] [PubMed] [Google Scholar]
- 20.Yin J, Zhu Z, Guo D, Wang A, Zeng N, Zheng X, et al. Increased growth differentiation factor 15 is Associated with unfavorable clinical outcomes of Acute ischemic stroke. Clin Chem. 2019;65(4):569–78. 10.1373/clinchem.2018.297879clinchem.2018.297879. [pii]. [DOI] [PubMed] [Google Scholar]
- 21.Cai WC, Liu YM, Qian CY, Chen WJ. Clinical significance of serum levels of growth differentiation factor-15, calmodulin and oxidative low density lipoprotein in patients with acute ischemic stroke. J Brain Nev Dis. 2020;28(4):208–12. [Google Scholar]
- 22.Jeong HS, Shin JW, Jeong JY, Kwon HJ, Koh HS, Kim JJ et al. Association of plasma level of growth differentiation factor-15 and clinical outcome after intraarterial thrombectomy. J Stroke Cerebrovasc Dis. 2020;29(8):104973. doi: S1052-3057(20)30391-8 [pii]10.1016/j.jstrokecerebrovasdis.2020.104973. [DOI] [PubMed]
- 23.Li XQ, Yu GL. Expression levels of serum Cav-1 and GDF-15 and their prognostic value in patients with Acute ischemic stroke. J Mod Lab Med. 2022;37(4):118–27. 10.3969/j.issn.1671-7414.2022.04.023. [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. 2021;www.training.cochrane.org/handbook
- 27.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091-6. doi: 01.STR.0000258355.23810.c6 [pii]10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed]
- 28.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010;http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liuize Abramaviciute A, Mongirdiene A, TGF-beta Isoforms. GDF-15 in the development and progression of atherosclerosis. Int J Mol Sci. 2024;25(4). 10.3390/ijms250421042104ijms25042104. [pii]ijms-25-02104 [pii]. [DOI] [PMC free article] [PubMed]
- 32.Liu DD, Mei YA. [Effects of growth differentiation factor-15 (GDF-15) on neurological systems, cardiovascular diseases, and cancer progression]. Sheng Li Xue Bao. 2017;69(1):109–21. [PubMed] [Google Scholar]
- 33.Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev. 2013;24(4):373–84. 10.1016/j.cytogfr.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Desmedt S, Desmedt V, De Vos L, Delanghe JR, Speeckaert R, Speeckaert MM. Growth differentiation factor 15: a novel biomarker with high clinical potential. Crit Rev Clin Lab Sci. 2019;56(5):333–50. 10.1080/10408363.2019.1615034. [DOI] [PubMed] [Google Scholar]
- 35.Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J Physiol Paris. 2002;96(1–2):25–30. doi: S0928-4257(01)00077-8 [pii]10.1016/s0928-4257(01)00077 – 8. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y, Liu T, Sun S, Peng Y, Huang X, Wang S, et al. Role and mechanism of growth differentiation factor 15 in chronic kidney disease. J Inflamm Res. 2024;17:2861–71. 10.2147/JIR.S451398451398. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Du T, Li B, Rong Y, Verkhratsky A, Peng L. Crosstalk between MAPK/ERK and PI3K/AKT Signal pathways during Brain Ischemia/Reperfusion. ASN Neuro. 2015;7(5). 10.1177/175909141560246317590914156024637/5/1759091415602463. [pii]10.1177_1759091415602463 [pii]. [DOI] [PMC free article] [PubMed]
- 38.Meloux A, Dogon G, Rigal E, Rochette L, Bejot Y, Vergely C. Proximal and distant expression of growth differentiation factor 15 (GDF15) correlate with neurological deficit following experimental ischemic stroke. PLoS ONE. 2024;19(7):e0307105. 10.1371/journal.pone.0307105e0307105PONE-D-24-07402. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Li M, Zhang W, Hua H, Wang N, Zhao J, et al. Growth differentiation factor 15 promotes blood vessel growth by stimulating cell cycle progression in repair of critical-sized calvarial defect. Sci Rep. 2017;7(1):9027. 10.1038/s41598-017-09210-4902710.1038/s41598-017-09210-4. [pii]9210 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patsalos A, Halasz L, Medina-Serpas MA, Berger WK, Daniel B, Tzerpos P, et al. A growth factor-expressing macrophage subpopulation orchestrates regenerative inflammation via GDF-15. J Exp Med. 2022;219(1). 10.1084/jem.20210420e20210420212883. [pii]jem.20210420 [pii]. [DOI] [PMC free article] [PubMed]
- 41.Kelly PJ, Murphy S, Coveney S, Purroy F, Lemmens R, Tsivgoulis G, et al. Anti-inflammatory approaches to ischaemic stroke prevention. J Neurol Neurosurg Psychiatry. 2018;89(2):211–8. 10.1136/jnnp-2016-314817jnnp-2016-314817. [pii]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.