Abstract
Hormographiella aspergillata is a rare hyaline mold causing invasive fungal infection in humans, until the frequent use of antifungal prophylaxis in immunocompromised hosts. Due to the high mortality of H. aspergillata infection, early recognition and treatment are crucial. Previous case reports suggested that serum (1,3)-beta-D-Glucan (BG) is one of the diagnostic aids for H. aspergillata infection. Here we report for the first time a case of pulmonary H. aspergillata infection with a negative serum BG but positive bronchoalveolar lavage fluid (BAL) BG. This may suggest that BAL BG is a useful and additional microbiological marker for prompt identification of this fatal invasive fungal infection (IFI). But it should be interpreted together with the clinical presentation, imaging, and other laboratory results.
Keywords: Hormographiella aspergillata; (1,3)-beta-D-Glucan; Invasive fungal infection; Amphotericin B
Introduction
Invasive fungal infection (IFI) is a serious complication in patients with hematological malignancies. With the use of antifungal prophylaxis in immunocompromised hosts, there is a change in the etiology of fungal infection from Aspergillus and Candida to more resistant fungi such as Mucorales, Fusarium, Scedosporium, and Basidiomycetes [1, 2]. Pulmonary infection due to Hormographiella aspergillata is rare but associated with a high mortality rate, leading to delays in diagnosis and treatment [3]. The diagnosis of invasive fungal infection is often difficult, because of risks associated with invasive procedures such as lung biopsy or bronchoalveolar lavage in patients with coagulopathies, low sensitivity of routine fungal culture, together with the long turn-around time in confirmation of fungal identity due to lack of expertise and the need of further tests for confirmation. Previous case reports have reported that serum BG levels were elevated in patients with H. aspergillata infection, suggesting it is an important marker of H. aspergillata infection [4–7]. Here we report the first case of pulmonary H. aspergillata infection with a negative serum but a positive BAL BG, suggesting that BAL BG may be a useful and additional tool for prompt diagnosis of this fatal IFI.
Presentations
A 34-year-old Chinese female, with newly diagnosed acute lymphoblastic leukemia, was admitted to our hospital for chemotherapy . Prior to admission, she experienced a dry cough and persistent neutropenic fever for 15 days, without improvement despite broad-spectrum antibiotics coverage with biapenem, amikacin, and itraconazole in another hospital. Chemotherapy was administered according to the GRAALL-2003 regimen [8] (Intravenous vincristine, daunorubicin, pegaspargase, and cyclophosphamide; oral prednisone; intrathecal methotrexate, cytarabine, and dexamethasone) from day 6 to day 34 of hospitalization. She enjoyed good past health with no known drug allergies. She had no personal history or contact history of pulmonary tuberculosis. She kept no pets at home and had never used any over-the-counter medication or traditional Chinese medicine. She was a non-smoker and a social drinker. Her body temperature was 38.7 °C on admission, with a heart rate of 110 beats per minute, blood pressure of 112/69 mm Hg, respiratory rate of 28 breaths per minute, and oxygen saturation of 80% in room air. There were no palpable lymphadenopathies or finger clubbing. Abdominal, respiratory, and cardiovascular examinations were all unremarkable.
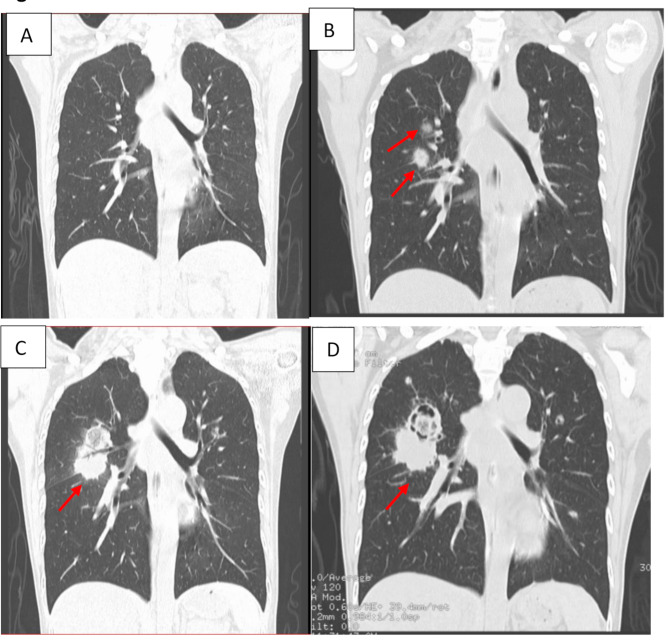
On day 9 of admission, she remained neutropenic and febrile despite broad-spectrum antibiotics (imipenem, vancomycin, trimethoprim-sulfamethoxazole, doxycycline, and levofloxacin) and itraconazole prophylaxis. Computed tomography (CT) of the thorax showed no signs of pneumonia and pulmonary lesions (Fig. 1A). In view of persistent fever, itraconazole was switched to micafungin for antifungal prophylaxis. However, fever still persisted despite adjustment of antifungal, with a repeated CT thorax (day 21) demonstrating two new pulmonary nodules in the right middle lobe with halo signs (Fig. 1B). Blood culture, sputum for acid-fast bacilli (AFB) smear, and sputum for bacterial culture were unremarkable. Sputum fungal culture was not ordered and performed. Mycobacterium tuberculosis DNA was not detected in the sputum by PCR. Serum galactomannan and BG were negative on Day 22. Bronchoscopy with biopsy was not initially performed due to refusal by the patient and her family, therefore micafungin was empirically switched to caspofungin, with the subsequent addition of voriconazole. However, reassessment CT thorax (day 33) still showed a progression of the right middle lung mass with cavitation (Fig. 1C). A bronchoscopy with lung biopsy was subsequently performed on the following day (day 34), and a mucous plug containing purulent secretions in the right posterior segmental bronchus was removed and sent for microbiological investigations. Both BAL and lung tissue bacterial cultures were negative, with Mycobacterium tuberculosis DNA not detected in the specimens. BAL galactomannan antigen was negative, but BAL BG was positive (> 600 pg/mL). Antifungal was subsequently switched to amphotericin B deoxycholate, escalating from 5 mg daily to a treatment dose of 40 mg (1 mg/kg) daily (from day 34 to day 41) to reduce the chance of acute hypersensitivity reaction. Despite the recovery of her neutrophil level and the administration of amphotericin B deoxycholate, the lung lesions continued to progress (Fig. 1D) with her clinical condition deteriorating rapidly, and the patient died from severe hemoptysis and respiratory failure on day 44.
Fig. 1.
CT thorax (sagittal view) showing the progression of the lung lesions (arrows). A: No signs of pneumonia and pulmonary lesions on day 9; B: Two new pulmonary nodules (the biggest one is 1.9 × 1.5 cm) located in the right middle lobe surrounded by a halo of ground-glass opacity on day 21; C: Progression of the right middle lung mass (the biggest one is 3.5 × 5.2 cm) with cavitation on day 33; D: Progression of the right middle lung mass(3.6 × 5.2 cm) with cavitation on day 41
Mycology and histology
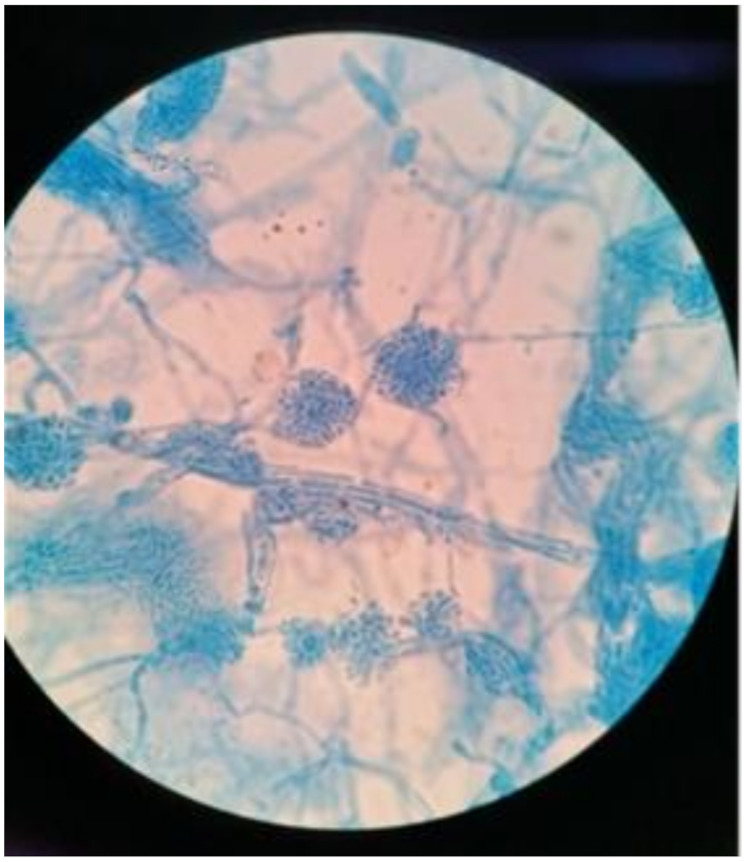
After incubating the BAL and lung tissue on Sabouraud dextrose agar at 37℃ for 48 h, white to cream-colored colonies and cottony aerial mycelium were observed. All inoculated media (Sabouraud dextrose agar, chocolate agar, and blood agar) yielded pure cultures of this fungus in the following days. Staining with lactophenol blue demonstrated septate conidiophores with clusters of smooth-walled, hyaline, and cylindrical arthroconidia (Fig. 2). Pan-fungal PCR and subsequent sequencing using the internal transcribed spacer (ITS) region of the rRNA gene of the isolates were performed. Based on the Basic Local Alignment Search Tool (BLAST) search of the sequence on the ITS region of the isolated fungus, the homology of Coprinopsis cinerea culture CBS 338.69 strain (GeneBank Accession No: MH878445.1) was 100.0% (365/ 365 bp), therefore the organism was identified as Coprinopsis cinerea (the teleomorph of Hormographiella aspergillata). Histology of the lung tissue revealed hyaline, septate, and branched hyphae with evidence of angioinvasion (Fig. 3). Antifungal susceptibility testing was performed by the E-test method on Sabouraud dextrose agar, with the minimal inhibitory concentrations (MIC) of voriconazole and amphotericin B being 32 μg/mL and 0.94 μg/mL, respectively.
Fig. 2.
Staining with lactophenol blue demonstrating septate conidiophores with clusters of smooth-walled, hyaline, and cylindrical arthroconidia (10 × 100)
Fig. 3.
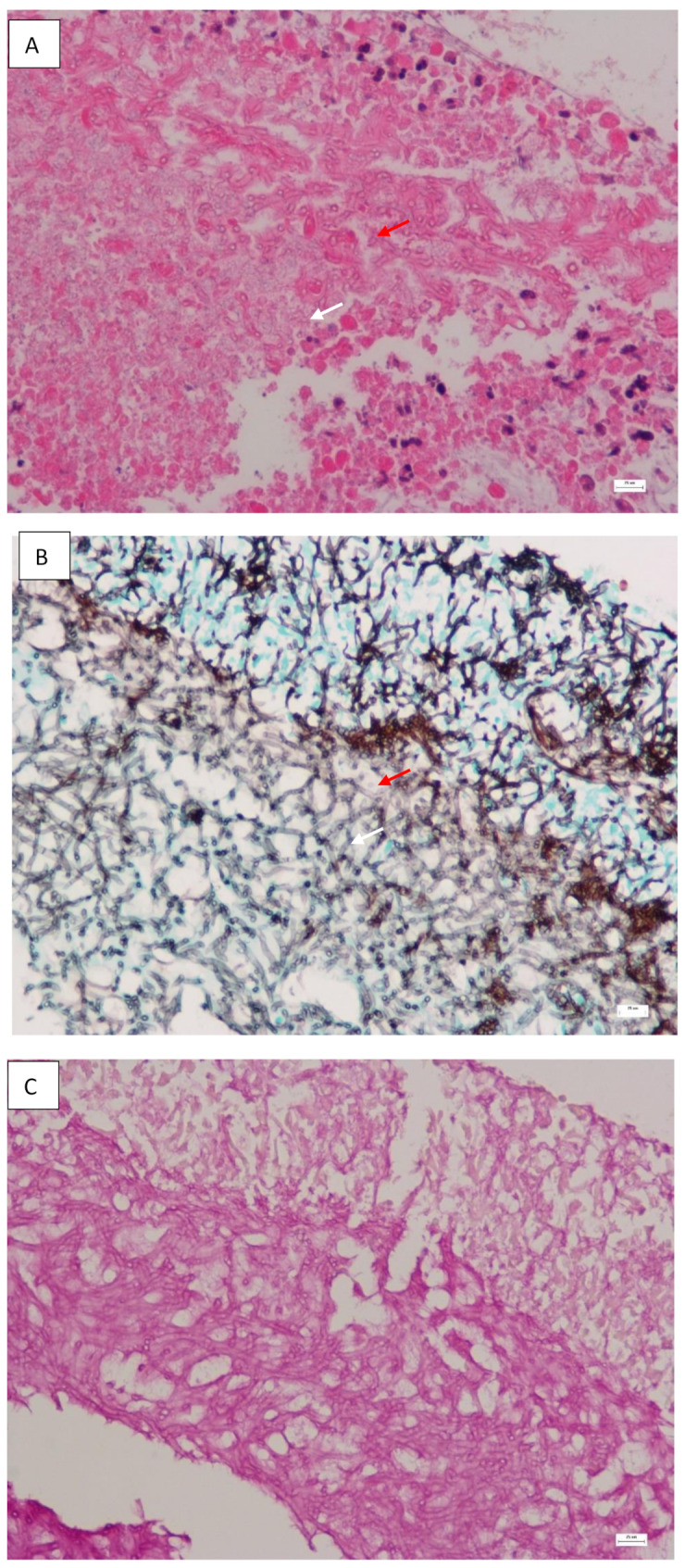
Histology of lung biopsy showing septate (red arrow) and branched hyphae with the formation of clews (white arrow); A: Hematoxylin and Eosin stain (magnification,× 400); B: Gomori-Grocott stain (magnification × 400); C: Periodic Acid-Schiff stain (magnification × 400)
Discussion
H. aspergillata belongs to the Basidiomycota division of fungi, and is the asexual form of Corprinopsis cinerea, an edible mushroom usually found in compost and other nutrient-rich substrates. H. aspergillata is a rare hyaline mold causing invasive fungal infection in humans. Twenty-seven cases were reported in the literature from 23 publications, with infection occurring mainly in patients with hematological malignancies [3–7, 9–26]. The majority of the patients were diagnosed in Europe, with other cases documented in the United States, Japan, and India. Most cases of H. aspergillata infection have been reported as pulmonary and disseminated infections, but the involvement of the skin, central nervous system, eye, and spleen was also reported in immunocompromised patients [5, 6, 12, 15]. Endocarditis and endophthalmitis have been described in immunocompetent patients [10, 20, 25, 26]. The mortality rate of H. aspergillata infection was high (78%), likely related to the delay in diagnosis and initiation of effective antifungal agents [3]. Patients who survived were managed with prompt invasive diagnostic procedures such as bronchoscopy and lung biopsy for respiratory infection, adequate surgical debridement of involved areas, prompt initiation of broad-spectrum antifungal therapy, as well as recovery of neutrophils in patients with underlying hematological malignancies.
Most cases of H. aspergillata infection were diagnosed by histology with confirmation by molecular techniques such as PCR and sequencing. Due to the high mortality of H. aspergillata infection, early recognition and treatment are crucial. We attempted to evaluate the value of galactomannan, BG, and glucuronoxylomannan (GXM) antigens from previous studies to facilitate the early diagnosis of H. aspergillata infection. GXM is the capsular antigen of Cryptococcus neoformans, that is widely used for the diagnosis of cryptococcosis. Cross-reactions with GXM have been described in the members of Basidiomycetes such as Trichosporon and Coprinopsis cinerea [27]. However, another study has demonstrated that the culture supernatants of H. aspergillata produce galactomannan and BG but not GXM [4]. This concurs with the two reported cases of H. aspergillata infection that the serum GXM antigens were negative [19, 20]. Despite the in vitro result of positive galactomannan, the diagnostic utility of galactomannan is doubtful, as all documented cases of H. aspergillata infection have negative galactomannan assay. The situation of the use of BG for diagnosis is different, with an earlier study showing that BG is an important component of the cell wall of Coprinus cinereus [28]. In five reported cases of H. aspergillata infection with serum BG checked, four (80%) of them had positive serum BG (greater than 500pg/mL) [3–7]. Serum BG was also found to be positive seven days prior to the development of radiological changes and at least one month prior to the identification of H. aspergillata in one of the case reports [7]. In our case, serum BG was negative despite positive radiological findings, but the BAL BG was found to be strongly positive (> 600 pg/mL). Although Candida spp. colonization or overgrowth at the lower respiratory tract can lead to false positive BAL BG results, yeasts have never been cultured from the respiratory specimens in our case, which further supports H. aspergillata as the cause of positive BAL BG in this case. Although BAL BG has a similar sensitivity to BAL galactomannan in the diagnosis of invasive aspergillosis and fungal infection (71%), it exhibits inferior specificity [29]. A positive BAL BG should be interpreted together with the clinical presentation, imaging, and other laboratory results for the diagnosis of IFI [30].
Currently, there is no standardized antifungal susceptibility protocol, clinical breakpoints, and treatment guidelines for H. aspergillata. Based on previous in vitro data, Jonathan T et al. summarized the antifungal susceptibility profile of 16 H. aspergillata clinical isolates reported in the literature, most cases exhibited a relatively low MIC range of voriconazole (0.015 to 1 mg/L) and amphotericin B (0.03 to 2 mg/L), except one case of amphotericin B MIC was 32 mg/L, while the MIC range of caspofungin (0.5 to 32 mg/L) and micafungin (0.25 to 16 mg/L) were relatively higher, indicating that voriconazole and amphotericin B are important antifungal agents for the treatment of H. aspergillata infection [3]. Although the method of susceptibility testing used was not standardized, the voriconazole MIC of our H. aspergillata isolate was higher than the usual reported range in the literature, which concurs with the fact that our patient did not respond to voriconazole treatment. Breakthrough infection after empirical voriconazole treatment has been reported on three occasions for H. aspergillata infection [11, 14, 17]. Based on the above factors and experience, voriconazole may not be a reliable primary regimen for H. aspergillata infection. Amphotericin B should be considered as the first-line antifungal agent.
Voriconazole or liposomal amphotericin B are preferred antifungal agents for the treatment of IFI. Amphotericin B is a polyene antifungal agent with activity in vitro against a wide variety of fungal pathogens [11, 14, 17], including most Candida spp, most hyaline and dematiaceous molds, and all dimorphic fungi. Liposomal amphotericin B has been introduced in the market to reduce the toxicities associated with amphotericin B deoxycholate. However, liposomal amphotericin B is not affordable for many patients in developing countries. According to FDA’s labeling resources for human prescription drugs, administration of amphotericin B deoxycholate should start with a test dose of 1 mg intravenously over 20 to 30 min, and healthcare workers should observe for any acute hypersensitivity reactions within 30 min. If no reactions were observed, then the remaining treatment dose can be administered. However, the practice in China is different. Amphotericin B deoxycholate is usually administered at a dosage of 1–5 mg for the first day, with a stepwise increase by 5 mg daily or alternate day based on the patient’s tolerance, until the treatment daily dosage of 0.6-1 mg/kg is reached. Further studies are required to compare the tolerance of patients with the two-dosing approach using the amphotericin B deoxycholate manufactured in China. The stepwise dosing approach delays the achievement of therapeutic concentration, leading to a delay in the management of invasive H. aspergillata infection in our patient. Therefore, we believe that in the treatment of severe and life-threatening fungal infection in neutropenic patients, the FDA recommendations of amphotericin B deoxycholate administration should be followed to ensure the timely administration of therapeutic concentrations of antifungal agents.
Conclusion
BAL BG is a useful microbiological investigation for the diagnosis of invasive fungal infection as demonstrated in our case of fatal pulmonary H. aspergillata infection, but it should be interpreted with an assessment with clinical presentation, imaging, and other laboratory results. Amphotericin B, instead of voriconazole, should be recommended as the first-line treatment for H. aspergillata infection. In the treatment of severe and life-threatening fungal infection in neutropenic patients, the FDA recommendations (test dose then treatment dose) should be followed to ensure the timely administration of therapeutic concentrations of antifungal agents.
Abbreviations
- BAL
Bronchoalveolar lavage fluid
- BG
(1,3)-beta-D-Glucan
- CT
Computed tomography
- GXM
Glucuronoxylomannan
- H. aspergillata
Hormographiella aspergillata
- IFI
Invasive fungal infection
- ITS
Internal transcribed spacer
Author contributions
HY, KH-YC and SK-PL contributed to the design of the study. JH, JL, RWP, FX, SK-PL, and RW-TL participated in the acquisition and analysis of data. HY and KH-YC drafted the manuscript, PC and JF-WC revised the manuscript. All of the authors read and approved the final draft of the manuscript.
Funding
This study was partly supported by funding from the Sanming Project of Medicine in Shenzhen, China (SZSM201911014) and the High Level-Hospital Program, Health Commission of Guangdong Province. The funding sources had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
The study was approved by the Ethics Committee of the University of Hong Kong-Shenzhen Hospital. Written informed consent was obtained from the patient’s husband.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010;50(8):1091–100. [DOI] [PubMed] [Google Scholar]
- 2.Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–703. [DOI] [PubMed] [Google Scholar]
- 3.Tschopp J, Perentes JY, Beigelman-Aubry C, et al. Invasive Hormographiella aspergillata infection in patients with acute myeloid leukemia: report of two cases successfully treated and review of the literature. Med Mycol Case Rep. 2021;32:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moniot M, Lavergne RA, Morel T, et al. Hormographiella aspergillata: an emerging basidiomycete in the clinical setting? A case report and literature review. BMC Infect Dis. 2020;20(1):945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan A, Gruenberg J, Arbefeville S, et al. Disseminated Hormographiella aspergillata infection with lung and brain involvement after allogenic hematopoietic stem-cell transplantation in a 54-year-old man. Lab Med. 2019;50(4):426–31. [DOI] [PubMed] [Google Scholar]
- 6.Nanno S, Nakane T, Okamura H, et al. Disseminated Hormographiella aspergillata infection with involvement of the lung, brain, and small intestine following allogeneic hematopoietic stem cell transplantation: case report and literature review. Transpl Infect Dis. 2016;18(4):611–6. [DOI] [PubMed] [Google Scholar]
- 7.Koncan R, Nadali G, Favuzzi V, et al. Invasive fungal infection by Hormographyella aspergillata: a tricky diagnosis triggered by (1,3)-beta-D-Glucan assay. J Microb Biochem Technol. 2016;8:4. [Google Scholar]
- 8.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911. [DOI] [PubMed] [Google Scholar]
- 9.Isabel Cristina R, Diana A, Karen A, et al. Breakthrough Hormographiella aspergillata infection in a patient with acute myeloid leukemia receiving posaconazole prophylaxis: a case report and review. Mycopathologia. 2020;185(6):1069–76. [DOI] [PubMed] [Google Scholar]
- 10.Jain N, Jinagal J, Ghosh A, et al. Ocular infection caused by Hormographiella aspergillata: a case report and review of literature. J Mycol Med. 2019;29(1):71–4. [DOI] [PubMed] [Google Scholar]
- 11.Godet C, Cateau E, Rammaert B, et al. Nebulized liposomal amphotericin B for treatment of pulmonary infection caused by hormographiella aspergillata: case report and literature review. Mycopathologia. 2017;182(7–8):709–13. [DOI] [PubMed] [Google Scholar]
- 12.Correa-Martinez C, Brentrup A, Hess K, et al. First description of a local Coprinopsis cinerea skin and soft tissue infection. New Microbes New Infect. 2017;21:102–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiblig M, Bozzoli V, Saison J, et al. Combined medico-surgical strategy for invasive sino-orbito-cerebral breakthrough fungal infection with Hormographiella aspergillata in an acute leukaemia patient. Mycoses. 2015;58(5):308–12. [DOI] [PubMed] [Google Scholar]
- 14.Corzo-Leon DE, Satlin MJ, Soave R, et al. Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-centre study with focus on emerging pathogens. Mycoses. 2015;58(6):325–36. [DOI] [PubMed] [Google Scholar]
- 15.Bojic M, Willinger B, Rath T, et al. Fatal skin and pulmonary infection caused by Hormographiella aspergillata in a leukaemic patient: case report and literature overview. Mycoses. 2013;56(6):687–9. [DOI] [PubMed] [Google Scholar]
- 16.Pang KP, Godet C, Fekkar A, et al. Breakthrough invasive mould infections in patients treated with caspofungin. J Infect. 2012;64(4):424–9. [DOI] [PubMed] [Google Scholar]
- 17.Conen A, Weisser M, Hohler D, et al. Hormographiella aspergillata: an emerging mould in acute leukaemia patients? Clin Microbiol Infect. 2011;17(2):273–7. [DOI] [PubMed] [Google Scholar]
- 18.Suarez F, Olivier G, Garcia-Hermoso D, Randriamalala E, et al. Breakthrough Hormographiella aspergillata infections arising in neutropenic patients treated empirically with caspofungin. J Clin Microbiol. 2011;49(1):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abuali MM, Posada R, Toro GD, et al. Rhizomucor variabilis var.regularior and Hormographiella aspergillata infections in a leukemic bone marrow transplant recipient with refractory neutropenia. J Clin Microbiol. 2009;47(12):4176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer EL, Kowalski TJ, Cole ML et al. Truffles revenge a pig-eating fungus. Cardiol Path. 2008;17(5):342-3. [DOI] [PubMed]
- 21.Lagrou K, Massonet C, Theunissen K, et al. Fatal pulmonary infection in a leukaemic patient caused by Hormographiella aspergillata. J Med Microbiol. 2005;54(7):685–8. [DOI] [PubMed] [Google Scholar]
- 22.Surmont I, Aelst FV, Verbanck J, et al. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med Mycol. 2002;40(2):217–9. [DOI] [PubMed] [Google Scholar]
- 23.Verweij PE, Kasteren MV, Nes J, et al. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J Clin Microbiol. 1997;35(10):2675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nenoff P, Friedrich T, Schwerke H, et al. Rare fatal simultaneous mould infection of the lung caused by Aspergillus flavus and the basidiomycete Coprinus sp. in a leukemic patient. J Med Vet Mycol. 1997;35(1):65–9. [PubMed] [Google Scholar]
- 25.Bartz-Schmidt KU, Tintelnot K, Steffen M, et al. Chronic basidiomycetous endophthalmitis after extracapsular cataract extraction and intraocular lens implantation. Graefes Arch Clin Exp Ophthalmol. 1996;234(9):591–3. [DOI] [PubMed] [Google Scholar]
- 26.Speller DE, Maclver AG. Endocarditis caused by a coprinus species a fungus of the toadstool group. J Med Microbiol. 1970;4(3):370–4. [DOI] [PubMed] [Google Scholar]
- 27.Tone K, Umeda Y, Makimura K. Cross-reactivity in cryptococcus antigen latex agglutination test in two commercial kits. Med Mycol. 2016;54(4):439–43. [DOI] [PubMed]
- 28.Sietsma JH, Wessels JG. Solubility of (1 leads to 3)-beta-D/(1leads to 6)-beta-D-glucan in fungal walls: importance of presumed linkage between glucan and chitin. J Gen Microbiol. 1981;125(1):209–12. [DOI] [PubMed] [Google Scholar]
- 29.Reischies FMJ, Prattes J, Pruller F, et al. Prognostic potential of 1,3-beta-d-glucan levels in bronchoalveolar lavage fluid samples. J Infect. 2016;72(1):29–35. [DOI] [PubMed] [Google Scholar]
- 30.Shi X, Liu Y, Gu XM, et al. Diagnostic value of (1 → 3)-β-D-glucan in bronchoalveolar lavage fluid for invasive fungal disease: a meta-analysis. Respir Med. 2016;117:48–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.