Abstract
The chemokine CCL20, a small cytokine that belongs to the C–C chemokine family, interacts with its homologous receptor CCR6, which is expressed on wide range of cell types. According to current research, the CCL20-CCR6 has been established as acritical player in a diverse range of inflammatory, oncogenic, and autoimmune diseases. Within the respiratory system, CCL20-CCR6 demonstrates heightened expression in conditions such as allergic asthma, chronic airway inflammation, non-small cell lung cancer (NSCLC), chronic obstructive pulmonary disease (COPD), and other respiratory diseases, which is conducive to the inflammatory mediators recruitment and tumor microenvironment remodeling. Numerous studies have demonstrated that therapeutic interventions targeting CCL20 and CCR6, including antibodies and antagonists, have the potential to mitigate disease progression. Despite the promising research prospects surrounding the CCL20-CCR6 chemokine axis, the precise mechanisms underlying its action in respiratory diseases remain largely elusive. In this review, we delve into the potential roles of the CCL20-CCR6 axis within the respiratory system by synthesizing and analyzing current research findings. Our objective is to provide a comprehensive understanding of the CCL20-CCR6 axis and its implications for respiratory health and disease. And we aspire to propel research endeavors in this domain and furnish valuable insights for the development of future therapeutic strategies.
Keywords: CCL20, CCR6, Immunoreaction, Inflammation, Respiratory system
Introduction
Respiratory diseases are one of the most prevalent illnesses in daily life. As the global economic level and population grow, air pollution and environmental degradation exacerbate the incidence and impose a substantial economic burden. In the European Union, expenditures on respiratory diseases amount to approximately 6% of the annual healthcare budget [1]. Chronic Obstructive Pulmonary Disease (COPD), one of the common respiratory diseases, ranked as the fourth leading cause of death in the United States in 2018, impacting over 10% of the population [2]. Therefore, the treatment of respiratory diseases and the advancement of novel therapeutic agents remain challenging.
The role of chemokines in regulating leukocyte trafficking has been extensively documented in the scientific literature [3]. Over 20 chemokine receptors and 50 chemokine ligands have been identified and characterized until now [4]. Notably, many chemokines exhibit promiscuous binding capabilities, interacting with multiple receptors, while receptors themselves can bind to various ligands. Therefore, the highly selective interaction between CCL20 and CCR6 is a focal point of our research endeavors. It has been confirmed that bronchial and alveolar epithelial cells express CCL20 [5]. After bronchial epithelial cells were stimulated, the expression of CCL20 increased, exerting a chemotactic effect that induced the recruitment of various inflammatory factors and activated both the innate and adaptive immune systems [6].
The CCL20-CCR6 axis is expressed by a diverse array of immune cells and plays pivotal roles in numerous systems and organs throughout the human body. In the psoriasis (PS), interleukin-17A (IL-17A) stimulates the production of CCL20 by epidermal keratinocytes, recruiting CCR6+ Th17 cells to the affected skin, exacerbating the inflammatory process [7]. Furthermore, the expression of CCL20-CCR6 has been confirmed in other diseases characterized by Th1/Th17-related immune responses, such as Crohn’s disease (CD), atopic dermatitis (AD), systemic lupus erythematosus (SLE), and vitiligo [8–16]. A comprehensive understanding of the mechanisms underlying the CCL20-CCR6 axis in inflammatory responses is crucial for the identification of potential therapeutic targets. Beyond its role in inflammatory diseases, the activation of CCL20-CCR6 in the tumor microenvironment has been implicated in the proliferation, migration, and invasion of cancer cells [17]. For instance, CCL20-CCR6 promotes the development of gastric adenocarcinoma (GAC) by modulating the tumor microenvironment in gastric cancer [18]. In hepatocellular carcinoma (HCC), CCL20-CCR6 influences disease progression by regulating macrophage survival and Treg cells recruitment [19]. Collectively, these findings suggest that CCL20-CCR6 is directly involved in the pathogenesis of a wide range of diseases, sparking our interest in further exploring its functions and potential therapeutic applications.
Currently, drugs such as corticosteroids, immunosuppressants, and monoclonal antibodies available on the market may exhibit limited tolerability and efficacy [20]. Meanwhile, research on the CCL20-CCR6 axis in the respiratory system is still in nascent stages. This paper aims to summarize the advancements in the study of CCL20-CCR6 in the respiratory system, encompassing the research and developmental progress of related drug inhibitors, systematically elucidating the origin, function, and role of CCL20-CCR6 in various respiratory diseases. The feasibility of CCL20-CCR6 as a potential therapeutic target is discussed, offering a novel therapeutic perspective for the future treatment of respiratory diseases.
Method
A comprehensive literature search was conducted in the PubMed and Web of Science databases, focusing on all relevant English-language publications since January 1, 2000. The search utilized the keywords “CCL20,” “CCR6,” “respiratory diseases” and “inhibitors” to ensure a thorough capture of all pertinent studies on CCL20-CCR6 in respiratory research. Duplicate literature was meticulously eliminated to avoid redundancy. To refine the search strategy, an iterative process was employed, building upon the initial keyword search. Additionally, to trace the origins of relevant literature, earlier references were included in the review. The types of literature cited encompassed articles, reviews, letters, and other forms, ensuring a diverse and comprehensive range of sources. Two independent reviewers screened the titles, abstracts, and keywords of the shortlisted articles to ensure their relevance and quality. Articles were excluded based on the following criteria: 1. Articles with outdated results lacking a sufficient theoretical foundation. 2. Articles whose research findings aligned with the current mainstream thinking, but the knowledge points were no longer up-to-date (e.g., older views suggesting lung squamous cell carcinoma as the most prevalent type of lung cancer, whereas current understanding highlights lung adenocarcinoma as the most common). 3. Retracted articles. These exclusion criteria were applied to ensure maximum alignment with the subject matter of this study. No topics are registered for this study.
The origin of the chemokine CCL20 and its receptor CCR6
Chemokines are small cytokines that induce the directional chemotaxis of responsive cells. Based on the position of the NH2-terminal cysteine (Cys) residues in their sequences, chemokines are categorized into four main classes: C–C motif (CC), C-X-C motif (CXC), C motif (C), and CX3C chemokines [21]. The chemokine CCL20, located on human chromosome 2, is a member of the C–C chemokine family. It is also known by several other names, including liver activation-regulated chemokine (LARC), macrophage inflammatory protein-3 (MIP-3), Exodus-1, and small-inducible cytokine subfamily A member 20 (SCYA20), and identified as a chemokine through bioinformatics [22, 23]. CCL20 is primarily expressed in various epithelial cells, including keratinized cells, lung epithelial cells, and intestinal epithelial cells [24–27].
The CC chemokine receptor 6 (CCR6), alternatively known as STRL22, resides on chromosome 6q27 and functions as a homologous receptor for CCL20. It is also characterized as a 7-transmembrane domain G-protein coupled receptor, primarily expressed in leukocytes, and binds to CCL20 to mediate leukocyte migration [28]. In tissues, it is predominantly expressed in the lymph nodes, appendix, and spleen, while showing lower levels of expression in the thymus, testis, and small intestine [29].
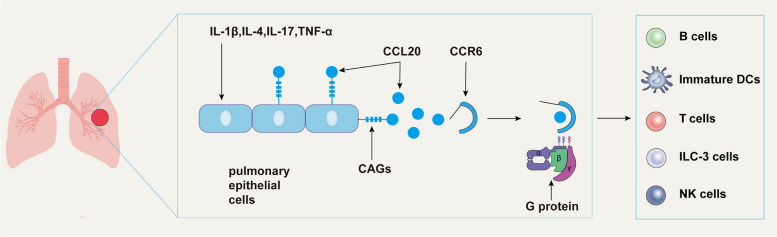
CCL20 engages in two types of interactions as a small molecule chemokine. On the one hand, the C-terminus of CCL20 binds to glycosaminoglycans (GAGs), thereby localizing CCL20 to the cell surface or extracellular matrix and creating a concentration gradient that facilitates the recruitment of its homologous receptors [30]. On the other hand, the N-terminus of CCL20 interacts with the side chain of CCR6 through hydrogen bonds and salt bridges, binding to the shallow pocket of CCR6 andactivating it [31]. This structural foundation ensures a strong binding affinity between CCL20 and CCR6 (Fig. 1). CCL20 is highly specific for CCR6 [32], which has long been recognized as its primary interacting receptor [33]. However, recent studies have identified the atypical chemokine receptor ACKR4 as an additional receptor for CCL20. β-arrestin mediates the recruitment of CCL20 to ACKR4, effectively removing excess CCL20 from the body and regulating chemokine concentration [34, 35]. Interestingly, CCL20 shares significant structural similarity with β-defensins. Certain defensins, such as human β-defensin 1 (HBD-1) and human β-defensin 2 (HBD-2), can bind to CCR6 receptors [36]. Notably, HBD-2, which boasts the highest expression level among defensins in the lung, exhibits chemotactic activity upon binding to CCR6. This interaction subsequently recruits T cells and dendritic cells (DCs) to the site of inflammation [36, 37] Additionally, mouse antimicrobial peptides β-defensins 2 and 3 also bind to CCR6, though they have a lower affinity for CCR6 compared to CCL20 [38]. This implies a potential defensin with higher CCR6 affinity could functionally replace the CCL20-CCR6 axis, warranting further investigation.
Fig. 1.
Lung epithelial cells secrete CCL20 to recruit CCR6-expressing cells. Glycosaminoglycans (GAGs) are present on the surface of lung epithelial cells. Upon stimulation, these cells secrete large amounts of CCL20. GAGs bind to CCL20 and anchor it to the cell surface. This accumulation of CCL20 creates a concentration gradient within the extracellular matrix, recruiting CCR6-expressing cells. CCR6 contains a molecular switch that remains closed in the absence of CCL20. However, upon CCL20 binding, CCR6 couples with downstream G proteins, activating signaling pathways that mediate cellular responses
The role and function of CCL20 and CCR6
CCL20 is a small-molecule peptide with diverse biological functions, including chemotaxis, and expressed by various immune cells. It is commonly associated with conditions including rheumatoid arthritis, psoriasis, and inflammatory bowel disease [39–41]. CCL20 accumulates at both sites of inflammation and immune activation, with its concentration positively correlated with inflammatory diseases severity [42]. It is stably expressed across cell types and rapidly induced upon pathogen invasion, stimulating Th17 cells to express CCR6 and recruiting CCR6-expressing cells to the inflammation site [43]. CCR6 signaling activates small GTPases and actin polymerization to regulate leukocyte migration and influence inflammation development [44]. Furthermore, recent studies have demonstrated that the homing of mature regulatory T cells (Tregs) to the thymic Treg pool occurs via a CCL20/CCR6-dependent pathway, which is crucial for maintaining immune tolerance and homeostasis [45]. This further supports the significant role of the CCL20/CCR6 axis in autoimmune inflammatory responses.
CCL20 is poorly expressed in normal tissues, but the expression of CCL20 in tumor tissues is much higher thannormal tissue, which can be used as an independent predictor of downstream cancer signaling pathways activation [46]. It has been confirmed that CCL20 plays a role in a variety of cancers, such as colorectal, pancreatic, liver, breast, ovarian, and lung cancers [47–51]. CCL20 directly targets endothelial cells through its cognate receptor, CCR6, and activates signaling pathways that can stimulate or enhance peritumoral angiogenesis in the tumor microenvironment, nourish tumor cells, and promote tumor cell metastasis and migration [52]. In addition, a recent study showed that mutations in the CCR6 gene cause abnormal activation of CCR6 and high expression in malignant B lymphocytes, leading to unbalanced cell proliferation and malignant transformation [53]. However, the exact B lymphocyte population that expresses CCR6 in a sustained T cell-dependent immune response remains to be determined.
The CCL20-CCR6 axis is intricately involved in numerous signaling pathways (Fig. 2). Specifically, it promotes osteoblast differentiation via the PI3K-AKT pathway [54]. In mouse psoriasis models, the inhibition of the JAK-STAT3 pathway has been verified to decrease CCL20 secretion [55]. Furthermore, Helicobacter pylori induces the production of CCL20 in gastric epithelial cells through NF-κB signaling dependency [56]. In lung cancer cells, CCL20 stimulates cancer cell proliferation and migration by activating the MAPK-ERK signaling pathway [57]. Additionally, Farnesyl diphosphate synthase (FDPS) induces CCL20 expression through the Wnt/β-catenin pathway, leading to the promotion of macrophage infiltration [58]. EGFR/Ras signaling plays a pivotal role in inducing CCL20 production across multiple cancer types and contributes to the establishment of the tumor microenvironment by stimulatingcytokines and chemokines secretion [52].
Fig. 2.
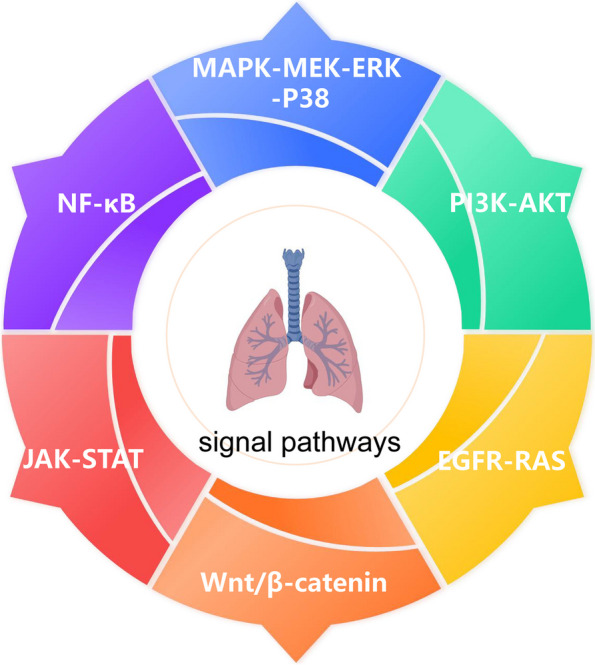
The signaling pathway involved in CCL20-CCR6 in lung disease
In summary, CCL20-CCR6 interactions have far-reaching consequences in multiple human organs health and play important roles in maintaining the integrity of the immune system in the internal environment, directing the migration of inflammatory cells in vivo, and promoting the differentiation of tumor cells.
Progress of CCL20-CCR6 in the respiratory tract
The role of CCL20-CCR6 in asthma and allergic inflammation of the airways
Asthma is a chronic inflammatory disease marked by persistent mucus hypersecretion and airway hyperresponsiveness [59]. While current treatments primarily involve steroids, some patients still experience inadequate control of their symptoms, highlighting the urgent need for new therapeutic targets [60]. The pathogenesis of asthma is complex, with the inflammatory factor IL-1β recognized as a key mediator in the disease’s inflammatory processes [61]. CCL20 is produced by airway epithelial cells [62], and its activation is closely associated with IL-1β-mediated inflammatory responses [63]. Joan Reibman et al. have demonstrated that CCL20 is regulated by pro-inflammatory cytokines such as IL-1β and TNF-α, as well as pro-allergic cytokines including IL-4 and IL-13. When the airway epithelium is exposed to inflammatory factors or environmental particles, CCL20 expression is upregulated through the ERK1/2 and p38 MAPK pathways, leading to rapid binding to the CCR6 receptor. Conversely, inhibition of the ERK1/2 and p38 MAPK pathways results in reduced CCL20 expression [5]. Additionally, Shen et al. observed in a mouse model of HDM-induced asthma that CCR6 + Treg cells were significantly increased in lung tissue and activated by CCL20. This activation led to IL-17 secretion from Th17 cells, thereby exacerbating asthma [64]. Another study investigating house dust mite (HDM)-induced CCL20 secretion in allergic asthma reported that HDM induces CCL20 secretion through the Akt-ERK1/2-C/EBPβ pathway [65]. This process recruits immature dendritic cells (DCs) to interact with airway bronchial smooth muscle cells. Conversely, anti-CCL20 treatment alleviates airway hyperresponsiveness and reduces the infiltration of inflammatory cells.
Interestingly, Zijlstra et al. have found that sputum levels of CCL20 are significantly higher in asthmatics following treatment with inhaled glucocorticoids and positively correlated with the dosage of the hormone used [66]. Alen et al. reported that CCL20 expression and airway mucus secretion were significantly increased in asthmatics in a controlled study comparing asthmatics and healthy individuals, with IL-1β induction [67]. They observed that both CCL20 expression and mucus secretion levels were greater in patients with moderate asthma compared to those with mild asthma. These findings suggest that CCL20 expression may contribute to chronic airway mucus secretion, potentially explaining why airway secretion did not improve substantially with steroid treatment in some asthma patients. Thus, the CCL20-CCR6 axis plays a critical role in asthma-related inflammation and mucus hypersecretion, and anti-CCL20 therapy could offer new approaches to asthma treatment.
Progress of CCL20-CCR6 in non-small cell lung cancer (NSCLC)
Lung cancer is a leading cause of cancer-related deaths, with approximately 90% of these deaths attributed to smoking [68]. A related study demonstrated that nicotine-derived nitrosamines (NNK) significantly increase CCL20 expression in human lung epithelial cells [69]. CCL20 expression was found to be higher in smokers compared to non-smokers, and it was elevated in non-small cell lung cancer (NSCLC) patients with a history of smoking compared to those with no smoking history [69]. Interestingly, the expression levels of CCL20 and CCR6 were higher in the adenocarcinoma (AC) group compared to the squamous cell carcinoma (SCC) group, which may account for the more aggressive nature of this histopathological subtype of lung cancer [70]. Most CCR6 mutations are nonsense mutations or frameshift insertions/deletions clustered in the C-terminal region, leading to truncated products that lack the C-terminal phosphorylation motif [71]. CCR6 mutants exhibit remarkable resistance to cell death. Upon stimulation with CCL20, CCR6 mutants W335X and R159S show enhanced transformation and proliferation compared to the wild type [53].
In lung cancer cells, the expression level of CCL20 is positively correlated with both the tissue stage and disease severity [69]. CCL20 not only promotes the proliferation and migration of lung cancer cells but serves as a significant prognostic marker in non-small cell lung cancer (NSCLC) [72]. NSCLC cells produce CCL20 through autocrine or paracrine mechanisms, which activate signaling pathways including ERK1/2-MAPK, Wnt, and PI3K, to promote epithelial-mesenchymal transition (EMT) [73]. However, the knockout of CCL20 has been observed to impair signal transduction pathways, including AKT and STAT3, ultimately inhibiting cell proliferation and migration [46]. A recent investigation has demonstrated that the use of CCR6 inhibitors in conjunction with erlotinib enhances drug sensitivity in resistant cancer cells [74]. This combination therapy exhibits a trend toward inducing cell cycle arrest and cellular growth reduction, suggesting a promising therapeutic approach. In the mouse lung cancer model, chimeric antigen receptor (CAR-T) cells expressing CCR6 significantly reduced tumor cell infiltration, lysed CCL20-secreting tumor cells, prolonged survival, and enhanced anti-lung cancer activity [75]. In conclusion, CCL20-CCR6 displays heightened expression levels in both mouse models and lung cancer patients. Blocking the expression of CCL20/CCR6 has been verified to exert specific anticancer effects.
Progress of CCL20-CCR6 in COPD
Chronic obstructive pulmonary disease (COPD) is a persistent inflammatory lung disease characterized by irreversible airflow limitation, with smoking being a major risk factor for its development [76]. Korytina et al. reported that the minor allele C of CCL20 (rs6749704) was associated with an increased risk of COPD exclusively in smokers [77]. Additionally, individuals carrying the CCR6 G allele (rs3093024) had a significantly higher smoking index. Chronic exposure to cigarette smoke promotes CCL20 expression and enhances the secretion of inflammatory cells, including macrophages, neutrophils, dendritic cells, and CD8+ T cells [27, 78–83]. These cells also secrete neutrophil elastase and matrix metalloproteinases (MMPs), which further damage lung parenchyma and contribute to COPD [84]. While previous studies have predominantly observed DC-mediated increases in CCL20 expression in asthma, Demedts et al. were the first to report that elevated CCL20 levels in COPD lead to the accumulation of dendritic cells (DCs) at sites of airway inflammation [85]. The extent of this elevation was positively correlated with COPD severity. Additionally, since CCR6 is expressed on DCs, CCR6 receptor-deficient mice exhibited significant attenuation of airway inflammation and fibrosis [86].
Airway remodeling is a key pathological feature of COPD, primarily characterized by chronic inflammation and fibrosis around the small airways [87]. During this process, Brand et al. identified CCL20-positive fibroblasts in the lamina propria of the airway epithelium [88]. They observed that CCL20 was most prominently expressed in fibroblasts that had been stimulated by the inflammatory factor IL-1β and subsequently activated through TGF-β induction by integrin αvβ8. The avβ8-mediated activation of TGF-β at the CCL20 promoter induces the formation of the SMAD4/NF-κB transcription complex, which enhances CCL20 transcription, creates a positive feedback loop, and exacerbates airway fibrosis in a murine model of COPD [89]. This provides strong evidence that CCL20 plays a crucial role in airway remodeling. Additionally, in a rat model of COPD induced by LPS, theCCL20 blocker significantly reduced dendritic cells (DCs) accumulation caused by the CCL20-CCR6 interaction and alleviated emphysema [90]. In conclusion, CCL20-CCR6 plays a certain role in the initiation and progression of COPD, albeit the research on CCL20-CCR6 in the context of COPD remains relatively scarce.
Progress of CCL20-CCR6 in pulmonary tuberculosis
Tuberculosis is caused by infection with Mycobacterium tuberculosis (Mtb), encompassing both active tuberculosis (TB) and latent tuberculosis infection (LTBI) [91]. During Mtb infection, chemokines and corresponding receptors have been extensively recognized for their role in facilitating the recruitment of inflammatory cells and the formation of granulomas at the site of infection [92]. In the Mycobacterium bovis BCG-infected mice airway model, Stolberg et al. detected that CCL20-CCR6 exhibited peak expression one week post-infection [93]. Intriguingly, their study revealed that CCR6 knockout (CCR6−/−) mice appear to play a significant antibacterial role in innate immunity, albeit not essential for adaptive immunity. Because innate immunity is considered pivotal for the establishment of early immune responses against Mycobacterium tuberculosis [94].
In tuberculosis (TB) patients, CCL20 was significantly upregulated compared to healthy controls in peripheral blood mononuclear cells (PBMCs) and monocyte-derived macrophages (MDMs) [95]. Furthermore, CCR6 expression was induced in memory T lymphocytes in a dose-dependent manner, promoting the migration of T lymphocytes to the site of infection [95]. Various types of macrophages have been implicated in TB status [96, 97]. Yang et al. employed single-cell RNA sequencing (ScRNA-seq) to identify a unique subpopulation of macrophages in bronchoalveolar lavage fluid (BALF) following Mycobacterium tuberculosis (Mtb) infection [98]. They discovered that MM macrophages in latent TB infection (LTBI) patients can regulate CD8+ T lymphocytes through the CCL20-CCR6 axis, thereby playing an anti-TB role. Additionally, several studies have demonstrated that CCR6 is expressed on the surface of CD4+ T cells in the airway during LTBI [99, 100]. Notably, CCR6−/− mice infected with Mtbare able to effectively clear the bacteria [93].
Progress of CCL20-CCR6 in pulmonary sarcoidosis
Pulmonary sarcoidosis is an interstitial lung disease characterized by a CCR6-dependent immune response involving the coactivation of CD4+ Th1 and Th17 cells [101]. Monica et al. detected elevated levels of CCL20 in bronchoalveolar lavage fluid (BALF) from patients with active pulmonary sarcoidosis [102]. They found that CCR6+ T lymphocytes recruited by CCL20 accumulated in the nodular lung microenvironment and were involved in the progression of immunoinflammatory alveolitis and granuloma formation. Ding et al. found that elevated CCL20 expression in patients with stage II nodular disease stimulated activation of the PI3K/Akt pathway, leading to a Th17/Treg imbalance and impairing the immunosuppressive effects of Treg cells, which promotes granulomatosis [103]. Recent studies have shown that serum amyloid A (SAA) upregulates CCL20 expression, which then induces migration and activation of Treg and Th17 cells by binding to the CCR6 receptor and activating the TGF-β/Smad pathway, leading to the formation of characteristic non-caseating granulomatous lesions [104]. Anti-CCL20 therapy has shown some degree of efficacy in reversing these effects.
Progress of CCL20-CCR6 in invasive pulmonary aspergillosis
Invasive pulmonary aspergillosis is commonly seen in immunocompromised individuals, with Aspergillus fumigatus being the most frequent causative agent [105]. Interestingly, studies have shown that after inducing Aspergillus fumigatus infection, CCR6 receptor-deficient mice had significantly fewer pulmonary dendritic cells (DCs) and monocytes/macrophages. These mice developed more severe infections and exhibited higher mortality rates compared to wild-type mice [106]. In contrast to previous findings, the CCL20-CCR6 axis appears to play a protective role in invasive pulmonary aspergillosis [106, 107]. Murdock et al. exposed immunocompromised mice to Aspergillus fumigatus spores and observed that it elicited Th1, Th2, and Th17-type immune responses. The CCR6 receptor, on the surface of Th17 cells, regulates their migration to the site of lungs infection by binding to the chemokine CCL20 [108]. A recent study found that when a human small airway epithelial cell line (HSAEC1-KT) was infected with Aspergillus fumigatus, it resulted in decreased levels of CCL20 in the culture supernatant [109]. This finding suggests that Aspergillus fumigatus infection depletes CCL20, thereby suppressing the host’s innate immune response and exacerbating the infection. These results underscore the importance of the CCL20-CCR6 axis as a critical host defense mechanism in Aspergillus fumigatus infection.
Progress of CCL20-CCR6 in Viral Infectious Pneumonia
Influenza viruses are common respiratory pathogens responsible for seasonal infectious pneumonia [110]. The virulence of a particular influenza strain largely depends on its ability to elicit an immune response in the host [111]. In influenza A virus (IAV)-induced pneumonia, the H1N1 strain exacerbates lung inflammation by disrupting the Th17/Treg cell balance and reducing the ability of CD8+ T cells to clear the virus, which is associated with increased expression of CCL20 and CCR6 [112]. Conversely, inhibition of CCL20-CCR6 expression affects dendritic cell (DC) recruitment, reduces the body’s ability to respond to the virus, and improves viral clearance efficiency in a model of RSV-infected pneumonia [113]. These findings suggest that targeting CCL20 and CCR6 may help attenuate pulmonary infection in viral pneumonia.
COVID-19, caused by a single-stranded RNA beta coronavirus, has been associated with severe cellular inflammatory responses and elevated chemokine levels. Studies have indicated that CCL20 is a key factor in the progression of severe COVID-19 [114–116]. High levels of CCL20 have been detected in the blood and lung tissues of critically ill patients, who are at increased risk for complications such as acute respiratory distress syndrome (ARDS) and multi-organ failure, often resulting in poor therapeutic outcomes and high mortality rates [117–119]. These findings suggest that CCL20 may have prognostic relevance in COVID-19 infection. However, the mechanisms involved are still not fully understood, and further research is needed to determine whether anti-CCL20 therapy could provide prognostic benefits for infected patients.
In this section, we will present the aforementioned information in a tabular format, as follows: (Table 1)
Table 1.
Summary table of CCL20-CCR6 in the respiratory system
| Name | frequency | Action mechanism | Effector cell | Phenotype | Reference |
|---|---|---|---|---|---|
| Asthma | ↑ | The secretion of CCL20 by airway epithelium is stimulated by MAPK and AKT pathways under the stimulation of related inflammatory factors. | DCs, Treg | Harmful | [5, 65] |
| NSCLC | ↑ | NSCLC cells promote CCL20 production and induce lung cancer cell migration through MAPK, Wnt, and PI3K pathways. | Th17, Treg, ILC3s | Harmful | [73] |
| COPD | ↑ | Activation and activation of TGF-β induced by integrin avβ8 enhances CCL20 transcription and exacerbates airway fibrosis. | DCs | Harmful | [88, 89] |
| Pulmonary tuberculosis | ↑ | Induces T lymphocytes to migrate to the site of inflammation. | CD4+ T, CD8+T | Harmful | [95] |
| Pulmonary nodular disease | ↑ | Stimulate the activation of the PI3k/Akt pathway, causing Th17/Treg imbalance; Activate the TGF-β/Smad pathway. | Treg, Th1, Th17 | Harmful | [103, 104] |
| Pulmonary aspergillosis | ↓ | By depleting CCL20, the innate immune response of the host is suppressed. | Th1, Th2, Th17 | Protective | [109] |
| Viral Infectious Pneumonia | ↑ | The imbalance of Th17/Treg cells and the decreased ability of CD8+T cells to clear the virus aggravate lung inflammation. | Treg, Th17 | Harmful | [112] |
The ↑ symbol indicates an increase in expression levels, the ↓symbol indicates a decrease in expression levels
CCL20 and CCR6 inhibitors
Inhibition of the CCL20-CCR6 axis encompasses the use of CCL20 inhibitors and CCR6 inhibitors. CCL20 and its corresponding monoclonal antibodies have demonstrated the ability to inhibit CCL20 chemotaxis and the activation of the CCL20/CCR6 pathway across a range of diseases [43]. These inhibitors have exhibited promising therapeutic effects in models of respiratory diseases, neuroinflammatory disorders, rheumatoid arthritis, breast cancer, and other conditions [65, 120, 121]. In the context of immunoinflammatory diseases, CCL20-neutralizing monoclonal antibodies effectively neutralize CCL20 chemokines, thereby preventing the recruitment of inflammation-related factors and reducing the disease’s inflammatory immune response [122]. Park et al. discovered that CCL20 regulated airway hyperresponsiveness and bronchial airway remodeling, while CCL20 inhibitors and monoclonal antibodies could ameliorate airway hyperresponsiveness, airway inflammation, and airway remodeling induced by CCL20 [65]. In further studies, anti-CCL20 therapy has been shown to alleviate smoking-induced airway inflammation and emphysema in a rat model [90]. Additionally, the application of related biologics, such as infliximab and tocilizumab, has been explored in related clinical treatments [4, 123]. Consequently, anti-CCL20 therapy has a positive impact on mitigating airway inflammation and lung tissue structural alterations.
The development of CCR6 antagonists presents a greater challenge compared to the application of various CCL20 antagonists. Sara et al. have developed a monoclonal antibody, 1C6, which targets the human CCR6 receptor (hCCR6). This antibody reduces the migration of HCCR6 to CCL20 and may potentially inhibit the recruitment of Th17 cells to inflammatory tissue [124]. However, 1C6 remains at the preclinical stage and lacks clinical validation data. ChemoCentryx has made progress in this field by developing a small molecule antagonist, CCX2553, that targets CCR6 (U.S. Patent Application No. 15/353,889) [125]. It works in a mouse model of psoriasis by preventing the accumulation of γδT17 cells in psoriatic skin. Another novel CCR6 antagonist, PF-07054894, has shown promising inhibition of T lymphocyte migration in both mice and monkeys and is currently in Phase I clinical trials [126].
Despite achieving relatively promising research outcomes in numerous studies, unfortunately, no CCR6 inhibitors have been approved for clinical use to date. However, there has been recent progress in this area with the development of the small molecule CCR6 antagonist IDOR-1117–2520, also referred to as compound 45, by Actelion-Idorsia [127]. Following a comprehensive series of experiments and model validations, IDOR-1117–2520 has demonstrated favorable activity and in vivo efficacy, suggesting its potential for future clinical application (Table 2).
Table 2.
CCL20 and CCR6-related inhibitors
| Name of Inhibitor | Mechanism of inhibitor | Type of Inhibitor | References | |
|---|---|---|---|---|
| 1 | GSK3050002 | Aggregates containing complement proteins can serve as substrates for complement activation | Humanized IgG1κ monoclonal antibody targeting CCL20 | [128] |
| 2 | Di-S-IdoA | Humanized IgG1κ monoclonal antibody targeting CCL20 | Small-molecule carbohydrates | [30] |
| 3 | NCT01984047 | Neutralization of monoclonal antibodies (mAbs) with the chemokine ligand CCL20 | A humanized anti-CCL20 monoclonal antibody (mAb) | [129] |
| 4 | CCX9664 | Targeting CCR6 reduces T lymphocyte infiltration | A small-molecule antagonist of CCR6 | [124] |
| 5 | Infliximab | TNF-induced upregulation of CCL20 is inhibited by the NF-κB pathway | Monoclonal antibody | [4, 123] |
| 6 | etanercept | Anti-TNF-α modified fusion protein | Monoclonal antibody | [4, 123] |
| 7 | Tocilizumab | Block CD4+ T lymphocyte differentiation into Th17 cells | Antibodies targeting the interleukin-6 receptor (IL-6R) production | [4, 123] |
| 8 | CO339589 | Inhibition of CCL20-mediated chemotaxis and binding in human natural killer (NK) cells and CCR6-rich peripheral blood mononuclear cells (PBMCs) | A small-molecule antagonist effective against CCR6 in both humans and mice | [4] |
| 9 | WO2017011559A1 | Competitive inhibition of CCR6 binding to CCL20 | Anti-CCL20 antibody | [130] |
| 10 | IDOR-1117–2520 | Migration of CCR6 to the bronchus and alveoli is inhibited | A highly effective small-molecule antagonist of CCR6 | [127] |
| 11 | PF-07054894 | Blocking CCR6-mediated chemotaxis and the binding of CCL20 to CCR6 | A selective small-molecule antagonist of CCR6 | [126] |
Discussion
In this paper, we demonstrate the comprehensive involvement of the CCL20-CCR6 axis in immune mechanism responses and elucidate the efficacy of anti-CCL20 and anti-CCR6 treatments in alleviating disease progression to a certain extent. This discovery is pivotal for indicating that CCL20-CCR6 may serve as a promising therapeutic target for a wide range of conditions, including inflammation, autoimmune diseases, and cancer. Our findings identify CCL20-CCR6 as a potential therapeutic target, highlighting its significant potential for transformation and development.
In the respiratory system, previous studies on the CCL20-CCR6 axis have primarily concentrated on cancer and asthma. However, our research has revealed that CCL20-CCR6 is also implicated in the pathogenesis of additional respiratory diseases, including COPD, tuberculosis, and pulmonary sarcoidosis. This review explores the role of the CCL20-CCR6 axis in the respiratory tract. In diseases such as asthma, COPD, and pneumonia, CCL20-CCR6 is related to the recruitment and activation of T lymphocytes, particularly Th17 cells. Once activated, these Th17 cells release pro-inflammatory cytokines into damaged tissues, exacerbating the inflammatory response. Furthermore, in lung cancer, the interaction between CCL20 and CCR6 can activate downstream cancer signaling pathways, induce resistance to apoptosis, and promote cancer cell proliferation and migration.
Despite the promising progress in CCL20-CCR6 research, which indicates its feasibility as an immunosuppressive target, it is crucial to acknowledge several potential limitations. For example, in studies investigating the CCL20-CCR6 axis, the absence of CCL20 knockout (CCL20−/−) mice has often led researchers to rely on CCR6 knockout (CCR6−/−) mouse models or the application of CCL20-targeting monoclonal antibodies to block CCL20 expression [123]. Currently, most CCL20-CCR6 blockers available on the market are neutralizing monoclonal antibodies, such as infliximab, tocilizumab, and etanercept [4]. Although satisfactory results are often achieved in experimental studies, the extent to which CCR6-deficient mice accurately represent CCL20 expression remains questionable. Notably, human β-defensin 2, which is structurally similar to CCL20, and its immediate homolog, murine β-defensin 4, both have the ability to bind to CCR6 and competitively inhibit CCL20 [36]. Furthermore, it is worth noting that the atypical chemokine receptor ACKR4 can also serve as a receptor for CCL20, albeit with a lower affinity compared to the CCL20-CCR6 axis [34]. This competition raises concerns about whether the interaction between CCR6 and CCL20 is fully representative in these models. Indeed, despite the presence of interfering factors, including ACKR4 and beta-defensins, it is solely the binding of CCL20 to CCR6 that initiates the classical G-protein signaling pathway. From this perspective, although small-molecule chemokine receptor antagonists have failed to demonstrate clinical efficacy in the treatment of inflammatory diseases, CCL20-CCR6 remains an attractive target for drug development research due to its specificity and potential therapeutic benefits. Consequently, while CCL20-CCR6 blockers hold promise, their application on a large scale in clinical settings remains limited, highlighting the need for further research and development to explore these new therapeutic options.
Furthermore, this paper acknowledges additional limitations. Given that CCL20-CCR6 research within the respiratory system is still an emerging field, the available literature for review is limited. Additionally, some studies date back several years, and their results may not accurately reflect the current research progress due to potential lags in publication and advancements in the field. Therefore, these findings should be interpreted with caution and considered in the context of ongoing research developments.
Conclusion
In conclusion, while the significance of the CCL20-CCR6 axis in the respiratory system is increasingly recognized, there remain substantial gaps in understanding its mechanisms of action. Further research is needed to deepen our knowledge and explore potential therapeutic applications.
Abbreviations
- CCL
C–C motif chemokine ligand
- CCR
C–C motif chemokine receptor
- CXCR
C-X-C motif chemokine receptor
- COVID-19
Coronavirus Disease 2019
- IL
Interleukin
- TNF-a
Tumour necrosis factor-a
- GAGs
Glycosaminoglycans
- HBD
Human beta-defensin
- DCs
Dendritic cells
- NSCLC
Non-small cell lung cancer
- NNK
Nicotine-derived nitrosamines
- AC
Adenocarcinoma
- SCC
Squamous cell carcinoma
- EMT
Epithelial-mesenchymal transition
- CAR-T
Chimeric antigen receptor
- COPD
Chronic obstructive pulmonary disease
- MMPs
Matrix metalloproteinases
- TGF-β
Transforming growth factor-β
- BALF
Bronchoalveolar lavage fluid
- ARDS
Acute respiratory distress syndrome
Authors’ contributions
Yong Wang and Ya-Jing Li designed the study. Ya-Jing Li wrote most of the manuscript. Wan-Li Geng, Chen-Chen Li, Jia-Hao Wu, and Fei Gao performed the study and contributed to the collection of data and analysis. The authors declare no conflicts of interest.
Funding
This research was funded by the National Natural Science Foundation of China [Grant #82070047], Anhui Province Clinical Medical Research Translation Special Program [202304295107020045], Talent Fund of the First Affiliated Hospital of Anhui Medical University [1525] and Research Fund of Anhui Medical University [2022xkj155].
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
The original version of this article has been revised: references in Table 1 have been updated.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/13/2025
A Correction to this paper has been published: 10.1186/s12950-025-00428-y
References
- 1.Boers E, et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw Open. 2023;6(12):e2346598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin S, et al. PM(2.5) and its components and respiratory disease healthcare encounters - unanticipated increased exposure-response relationships in recent years after environmental policies. Environ Pollut. 2024;360:124585. [DOI] [PubMed] [Google Scholar]
- 3.Ono SJ, et al. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003;111(6):1185–99 quiz 1200. [DOI] [PubMed] [Google Scholar]
- 4.Ranasinghe R, Eri R. Modulation of the CCR6-CCL20 axis: a potential therapeutic target in inflammation and cancer. Medicina (Kaunas). 2018;54(5):88.
- 5.Reibman J, et al. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28(6):648–54. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama WL, et al. Fine particulate matter from urban ambient and wildfire sources from California’s San Joaquin Valley initiate differential inflammatory, oxidative stress, and xenobiotic responses in human bronchial epithelial cells. Toxicol In Vitro. 2011;25(8):1895–905. [DOI] [PubMed] [Google Scholar]
- 7.Furue K, et al. The CCL20 and CCR6 axis in psoriasis. Scand J Immunol. 2020;91(3):e12846. [DOI] [PubMed] [Google Scholar]
- 8.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58(8):1152–67. [DOI] [PubMed] [Google Scholar]
- 9.Elnabawi YA, et al. CCL20 in psoriasis: a potential biomarker of disease severity, inflammation, and impaired vascular health. J Am Acad Dermatol. 2021;84(4):913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper EG, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariza ME, Williams MV. A human endogenous retrovirus K dUTPase triggers a TH1, TH17 cytokine response: does it have a role in psoriasis? J Invest Dermatol. 2011;131(12):2419–27. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura M, et al. Chemokines as novel therapeutic targets for inflammatory bowel disease. Ann N Y Acad Sci. 2009;1173:350–6. [DOI] [PubMed] [Google Scholar]
- 13.Calderón-Gómez E, et al. Commensal-Specific CD4(+) cells from patients with Crohn’s Disease Have a T-Helper 17 inflammatory profile. Gastroenterology. 2016;151(3):489–500.e3. [DOI] [PubMed] [Google Scholar]
- 14.He H, et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J Allergy Clin Immunol. 2021;147(4):1369–80. [DOI] [PubMed] [Google Scholar]
- 15.Koga T, et al. Calcium/Calmodulin-Dependent Kinase IV Facilitates the Recruitment of Interleukin-17-Producing Cells to Target Organs Through the CCR6/CCL20 Axis in Th17 Cell-Driven Inflammatory Diseases. Arthritis Rheumatol. 2016;68(8):1981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, et al. Circulating CCL20: a potential biomarker for active vitiligo together with the number of Th1/17 cells. J Dermatol Sci. 2019;93(2):92–100. [DOI] [PubMed] [Google Scholar]
- 17.Korbecki J, et al. CC Chemokines in a tumor: a review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int J Mol Sci. 2020;21(20):7619.
- 18.Liu Y, et al. PPARδ dysregulation of CCL20/CCR6 axis promotes gastric adenocarcinoma carcinogenesis by remodeling gastric tumor microenvironment. Gastric Cancer. 2023;26(6):904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Lipid droplet accumulation mediates macrophage survival and Treg recruitment via the CCL20/CCR6 axis in human hepatocellular carcinoma. Cell Mol Immunol. 2024;21(10):1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes PJ, et al. Barriers to new drug development in respiratory disease. Eur Respir J. 2015;45(5):1197–207. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9(9):949–52. [DOI] [PubMed] [Google Scholar]
- 22.Rossi DL, et al. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol. 1997;158(3):1033–6. [PubMed] [Google Scholar]
- 23.Hieshima K, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272(9):5846–53. [DOI] [PubMed] [Google Scholar]
- 24.Charbonnier A, et al. Macrophage Inflammatory protein 3α is involved in the constitutive trafficking of epidermal langerhans cells. J Exp Med. 1999;190(12):1755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka Y, et al. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999;29(2):633–42. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama T, et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13(1):95–103. [DOI] [PubMed] [Google Scholar]
- 27.Thorley AJ, et al. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol. 2005;32(4):262–7. [DOI] [PubMed] [Google Scholar]
- 28.Yoshie O, Imai T, Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. J Leukoc Biol. 1997;62(5):634–44. [DOI] [PubMed] [Google Scholar]
- 29.Varona R, et al. Molecular cloning, functional characterization and mRNA expression analysis of the murine chemokine receptor CCR6 and its specific ligand MIP-3alpha. FEBS Lett. 1998;440(1–2):188–94. [DOI] [PubMed] [Google Scholar]
- 30.Proudfoot A, et al. Glycosaminoglycan interactions with chemokines add complexity to a complex system. Pharmaceuticals. (Basel). 2017;10(3):70.
- 31.Wasilko DJ, et al. Structural basis for chemokine receptor CCR6 activation by the endogenous protein ligand CCL20. Nat Commun. 2020;11(1):3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba M, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC Chemokine LARC*. J Biol Chem. 1997;272(23):14893–8. [DOI] [PubMed] [Google Scholar]
- 33.Liao F, et al. STRL22 is a receptor for the CC chemokine MIP-3alpha. Biochem Biophys Res Commun. 1997;236(1):212–7. [DOI] [PubMed] [Google Scholar]
- 34.Matti C, et al. CCL20 is a novel ligand for the scavenging atypical chemokine receptor 4. J Leukoc Biol. 2020;107(6):1137–54. [DOI] [PubMed] [Google Scholar]
- 35.Meyrath M, et al. Systematic reassessment of chemokine-receptor pairings confirms CCL20 but not CXCL13 and extends the spectrum of ACKR4 agonists to CCL22. J Leukoc Biol. 2021;109(2):373–6. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525–8. [DOI] [PubMed] [Google Scholar]
- 37.Röhrl J, et al. Specific binding and chemotactic activity of mBD4 and its functional orthologue hBD2 to CCR6-expressing cells. J Biol Chem. 2010;285(10):7028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biragyn A, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167(11):6644–53. [DOI] [PubMed] [Google Scholar]
- 39.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martina MG, et al. Discovery of small-molecules targeting the CCL20/CCR6 axis as first-in-class inhibitors for inflammatory bowel diseases. Eur J Med Chem. 2022;243:114703. [DOI] [PubMed] [Google Scholar]
- 41.Schielke L, et al. Metabolic syndrome in psoriasis is associated with upregulation of CXCL16 on monocytes and a dysbalance in innate lymphoid cells. Front Immunol. 2022;13:916701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchida K, et al. The increased expression of CCL20 and CCR6 in rectal mucosa correlated to severe inflammation in pediatric ulcerative colitis. Gastroenterol Res Pract. 2015;2015:856532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meitei HT, Jadhav N, Lal G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun Rev. 2021;20(7):102846. [DOI] [PubMed] [Google Scholar]
- 44.Lin SL, et al. Temporal proteomics profiling of lipid rafts in CCR6-activated T cells reveals the integration of actin cytoskeleton dynamics. J Proteome Res. 2010;9(1):283–97. [DOI] [PubMed] [Google Scholar]
- 45.Peligero-Cruz C, et al. IL18 signaling promotes homing of mature Tregs into the thymus. Elife. 2020;9:e58213.
- 46.Mo M, et al. High serum CCL20 is associated with tumor progression in penile cancer. J Cancer. 2020;11(23):6812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia SN, et al. Chemokines in colon cancer progression. Semin Cancer Biol. 2022;86(Pt 3):400–7. [DOI] [PubMed] [Google Scholar]
- 48.Liu W, et al. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett. 2020;472:59–69. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R, et al. PMN-MDSCs modulated by CCL20 from cancer cells promoted breast cancer cell stemness through CXCL2-CXCR2 pathway. Signal Transduct Target Ther. 2023;8(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubie C, et al. CCL20/CCR6 expression profile in pancreatic cancer. J Transl Med. 2010;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanna DN, et al. SMAD4 suppresses colitis-associated carcinoma through inhibition of CCL20/CCR6-mediated Inflammation. Gastroenterology. 2022;163(5):1334–1350.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hippe A, et al. EGFR/Ras-induced CCL20 production modulates the tumour microenvironment. Br J Cancer. 2020;123(6):942–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korona B, et al. CCR6 activation links innate immune responses to mucosa-associated lymphoid tissue lymphoma development. Haematologica. 2022;107(6):1384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doucet M, et al. CCL20/CCR6 signaling regulates bone mass accrual in mice. J Bone Miner Res. 2016;31(7):1381–90. [DOI] [PubMed] [Google Scholar]
- 55.Lin P, et al. Centella asiatica alleviates psoriasis through JAK/STAT3-mediated inflammation: an in vitro and in vivo study. J Ethnopharmacol. 2023;317:116746. [DOI] [PubMed] [Google Scholar]
- 56.Cook KW, et al. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut. 2014;63(10):1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang XP, et al. Role of CCL20/CCR6 and the ERK signaling pathway in lung adenocarcinoma. Oncol Lett. 2017;14(6):8183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z, Chen G, Zhao H. FDPS promotes glioma growth and macrophage recruitment by regulating CCL20 via Wnt/β-catenin signalling pathway. J Cell Mol Med. 2020;24(16):9055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mims JW. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol. 2015;5(Suppl 1):S2–6. [DOI] [PubMed] [Google Scholar]
- 60.Hansbro PM, et al. Mechanisms and treatments for severe, steroid-resistant allergic airway disease and asthma. Immunol Rev. 2017;278(1):41–62. [DOI] [PubMed] [Google Scholar]
- 61.Simpson JL, et al. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014;43(4):1067–76. [DOI] [PubMed] [Google Scholar]
- 62.Starner TD, et al. CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29(5):627–33. [DOI] [PubMed] [Google Scholar]
- 63.Skovdahl HK, et al. C-C Motif Ligand 20 (CCL20) and C-C Motif Chemokine Receptor 6 (CCR6) in Human Peripheral Blood Mononuclear Cells: Dysregulated in Ulcerative Colitis and a Potential Role for CCL20 in IL-1β Release. Int J Mol Sci. 2018;19(10):3257.
- 64.Shen X, et al. Reduced CCR6(+)IL-17A(+)Treg Cells in Blood and CCR6-Dependent Accumulation of IL-17A(+)treg cells in lungs of patients with allergic asthma. Front Immunol. 2021;12:710750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park SY, et al. House dust mite-induced Akt-ERK1/2-C/EBP beta pathway triggers CCL20-mediated inflammation and epithelial-mesenchymal transition for airway remodeling. FASEB J. 2022;36(9):e22452. [DOI] [PubMed] [Google Scholar]
- 66.Zijlstra GJ, et al. Glucocorticoids induce the production of the chemoattractant CCL20 in airway epithelium. Eur Respir J. 2014;44(2):361–70. [DOI] [PubMed] [Google Scholar]
- 67.Faiz A, et al. Profiling of healthy and asthmatic airway smooth muscle cells following interleukin-1β treatment: a novel role for CCL20 in chronic mucus hypersecretion. Eur Respir J. 2018;52(2):1800310.
- 68.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang GZ, et al. Tobacco smoke induces production of chemokine CCL20 to promote lung cancer. Cancer Lett. 2015;363(1):60–70. [DOI] [PubMed] [Google Scholar]
- 70.Baran K, et al. Panel of miR-150 and linc00673, regulators of CCR6/CCL20 may serve as non-invasive diagnostic marker of non-small cell lung cancer. Sci Rep. 2023;13(1):9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moody S, et al. Novel GPR34 and CCR6 mutation and distinct genetic profiles in MALT lymphomas of different sites. Haematologica. 2018;103(8):1329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang D, et al. Serum chemokine network correlates with chemotherapy in non-small cell lung cancer. Cancer Lett. 2015;365(1):57–67. [DOI] [PubMed] [Google Scholar]
- 73.Wang B, et al. Production of CCL20 from lung cancer cells induces the cell migration and proliferation through PI3K pathway. J Cell Mol Med. 2016;20(5):920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwon EJ, Cha HJ, Lee H. Systematic omics analysis identifies CCR6 as a therapeutic target to overcome cancer resistance to EGFR inhibitors. iScience. 2024;27(4):109448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jin L, et al. Enhance anti-lung tumor efficacy of chimeric antigen receptor-T cells by ectopic expression of C-C motif chemokine receptor 6. Sci Bull (Beijing). 2021;66(8):803–12. [DOI] [PubMed] [Google Scholar]
- 76.Christenson SA, et al. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–42. [DOI] [PubMed] [Google Scholar]
- 77.Korytina GF, et al. The relationship between chemokine and chemokine receptor genes polymorphisms and chronic obstructive pulmonary disease susceptibility in tatar population from russia: a case control study. Biochem Genet. 2022;60(1):54–79. [DOI] [PubMed] [Google Scholar]
- 78.Hielpos MS, et al. CCL20 and Beta-Defensin 2 Production by Human Lung Epithelial Cells and Macrophages in Response to Brucella abortus Infection. PLoS One. 2015;10(10):e0140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Swert KO, et al. Role of the tachykinin NK1 receptor in a murine model of cigarette smoke-induced pulmonary inflammation. Respir Res. 2009;10(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Busse PJ, et al. Effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol. 2017;139(6):1808–1818.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pelletier M, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115(2):335–43. [DOI] [PubMed] [Google Scholar]
- 82.Xia L, et al. RORγt agonist enhances anti-PD-1 therapy by promoting monocyte-derived dendritic cells through CXCL10 in cancers. J Exp Clin Cancer Res. 2022;41(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song Q, et al. Transcription factor RUNX3 promotes CD8(+) T cell recruitment by CCL3 and CCL20 in lung adenocarcinoma immune microenvironment. J Cell Biochem. 2020;121(5–6):3208–20. [DOI] [PubMed] [Google Scholar]
- 84.Bracke KR, et al. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177(7):4350–9. [DOI] [PubMed] [Google Scholar]
- 85.Demedts IK, et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(10):998–1005. [DOI] [PubMed] [Google Scholar]
- 86.Hashimoto M, et al. A critical role for dendritic cells in the evolution of IL-1β-mediated murine airway disease. J Immunol. 2015;194(8):3962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–59. [DOI] [PubMed] [Google Scholar]
- 88.Brand OJ, et al. Transforming Growth Factor-β and Interleukin-1β Signaling Pathways Converge on the Chemokine CCL20 Promoter. J Biol Chem. 2015;290(23):14717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hashimoto M, et al. TGF-β-Dependent Dendritic Cell Chemokinesis in Murine Models of Airway Disease. J Immunol. 2015;195(3):1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun D, et al. Cigarette smoke-induced chronic obstructive pulmonary disease is attenuated by CCL20-blocker: a rat model. Croat Med J. 2016;57(4):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rao M, et al. Latent TB Infection (LTBI) - Mycobacterium tuberculosis pathogenesis and the dynamics of the granuloma battleground. Int J Infect Dis. 2019;80S:S58–61. [DOI] [PubMed] [Google Scholar]
- 92.Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85(2):103–11. [DOI] [PubMed] [Google Scholar]
- 93.Stolberg VR, et al. Cysteine-cysteinyl chemokine receptor 6 mediates invariant natural killer T cell airway recruitment and innate stage resistance during mycobacterial infection. J Innate Immun. 2011;3(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koeken V, et al. Trained innate immunity and resistance to Mycobacterium tuberculosis infection. Clin Microbiol Infect. 2019;25(12):1468–72. [DOI] [PubMed] [Google Scholar]
- 95.Lee JS, et al. Expression and regulation of the CC-chemokine ligand 20 during human tuberculosis. Scand J Immunol. 2008;67(1):77–85. [DOI] [PubMed] [Google Scholar]
- 96.Bo H, et al. Mycobacterium tuberculosis-macrophage interaction: Molecular updates. Front Cell Infect Microbiol. 2023;13:1062963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahmad F, et al. Macrophage: a cell with many faces and functions in tuberculosis. Front Immunol. 2022;13:747799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Q, et al. The interaction of macrophages and CD8 T cells in bronchoalveolar lavage fluid is associated with latent tuberculosis infection. Emerg Microbes Infect. 2023;12(2):2239940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shanmugasundaram U, et al. Pulmonary Mycobacterium tuberculosis control associates with CXCR3- and CCR6-expressing antigen-specific Th1 and Th17 cell recruitment. JCI Insight. 2020;5(14):e137858.
- 100.Lindestam AC, et al. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog. 2013;9(1):e1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Facco M, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66(2):144–50. [DOI] [PubMed] [Google Scholar]
- 102.Facco M, et al. Expression and role of CCR6/CCL20 chemokine axis in pulmonary sarcoidosis. J Leukoc Biol. 2007;82(4):946–55. [DOI] [PubMed] [Google Scholar]
- 103.Ding J, et al. Extensively disturbance of regulatory T cells - Th17 cells balance in stage II pulmonary sarcoidosis. Int J Med Sci. 2017;14(11):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meng K, et al. Serum amyloid A/anti-CCL20 induced the rebalance of Th17/regulatory T cells in SodA-induced sarcoidosis. Int Immunopharmacol. 2022;109:108784. [DOI] [PubMed] [Google Scholar]
- 105.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270–7. [DOI] [PubMed] [Google Scholar]
- 106.Phadke AP, et al. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med. 2007;175(11):1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gafa V, et al. In vitro infection of human dendritic cells by Aspergillus fumigatus conidia triggers the secretion of chemokines for neutrophil and Th1 lymphocyte recruitment. Microbes Infect. 2007;9(8):971–80. [DOI] [PubMed] [Google Scholar]
- 108.Thakur R, et al. Cytokines induce effector T-helper cells during invasive aspergillosis; what we have learned about T-helper cells? Front Microbiol. 2015;6:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Graf KT, et al. Depletion of Extracellular Chemokines by Aspergillus Melanin. mBio. 2023;14(3):e0019423.
- 110.Liang Y. Pathogenicity and virulence of influenza. Virulence. 2023;14(1):2223057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen X, et al. Host immune response to influenza a virus infection. Front Immunol. 2018;9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi CC, et al. Regulating the balance of Th17/Treg cells in gut-lung axis contributed to the therapeutic effect of Houttuynia cordata polysaccharides on H1N1-induced acute lung injury. Int J Biol Macromol. 2020;158:52–66. [DOI] [PubMed] [Google Scholar]
- 113.Kallal LE, et al. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur J Immunol. 2010;40(4):1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chua RL, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38(8):970–9. [DOI] [PubMed] [Google Scholar]
- 115.Hasan MZ, et al. SARS-CoV-2 infection initiates interleukin-17-enriched transcriptional response in different cells from multiple organs. Sci Rep. 2021;11(1):16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramasamy S, Subbian S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin Microbiol Rev. 2021;34(3):e00299–20.
- 117.Wilson JC, et al. Integrated miRNA/cytokine/chemokine profiling reveals severity-associated step changes and principal correlates of fatality in COVID-19. iScience. 2022;25(1):103672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hue S, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(11):1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Brabander J, et al. Persistent alveolar inflammatory response in critically ill patients with COVID-19 is associated with mortality. Thorax. 2023;78(9):912–21.
- 120.Liao LS, et al. Targeting CCL20 inhibits subarachnoid hemorrhage-related neuroinflammation in mice. Aging (Albany NY). 2020;12(14):14849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kwantwi LB, et al. Multifaceted roles of CCL20 (C-C motif chemokine ligand 20): mechanisms and communication networks in breast cancer progression. Bioengineered. 2021;12(1):6923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomson AS, et al. Structure and functional characterization of a humanized anti-CCL20 antibody following exposure to serum reveals the formation of immune complex that leads to toxicity. J Immunol. 2021;206(5):1067–76. [DOI] [PubMed] [Google Scholar]
- 123.Lee AY, et al. The relationship between CCR6 and its binding partners: does the CCR6-CCL20 axis have to be extended? Cytokine. 2015;72(1):97–101. [DOI] [PubMed] [Google Scholar]
- 124.Gómez-Melero S, et al. Amino terminal recognition by a CCR6 chemokine receptor antibody blocks CCL20 signaling and IL-17 expression via β-arrestin. BMC Biotechnol. 2021;21(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Campbell JJ, et al. IL-17-Secreting γδ T cells are completely dependent upon CCR6 for homing to inflamed skin. J Immunol. 2017;199(9):3129–36. [DOI] [PubMed] [Google Scholar]
- 126.Li W, et al. A Novel C-C Chemoattractant Cytokine (Chemokine) Receptor 6 (CCR6) Antagonist (PF-07054894) Distinguishes between Homologous Chemokine Receptors, Increases Basal Circulating CCR6(+) T Cells, and Ameliorates Interleukin-23-Induced Skin Inflammation. J Pharmacol Exp Ther. 2023;386(1):80–92. [DOI] [PubMed] [Google Scholar]
- 127.Meyer EA, et al. Discovery of the clinical candidate IDOR-1117-2520: a potent and selective antagonist of CCR6 for autoimmune diseases. J Med Chem. 2024;67(10):8077–98. [DOI] [PubMed] [Google Scholar]
- 128.Laffan SB, et al. Immune complex disease in a chronic monkey study with a humanised, therapeutic antibody against CCL20 is associated with complement-containing drug aggregates. PLoS One. 2020;15(4):e0231655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dorgham K, et al. Identification of the single immunodominant region of the native human CC chemokine receptor 6 recognized by mouse monoclonal antibodies. PLoS One. 2016;11(6):e0157740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riaz F, Huang Z, Pan F. Targeting post-translational modifications of Foxp3: a new paradigm for regulatory T cell-specific therapy. Front Immunol. 2023;14:1280741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.