Abstract
Cochlear implantation (CI) is currently recognized as the most effective treatment for severe to profound sensorineural deafness and is considered one of the most successful neural prostheses. Since its inception in 1961, cochlear implantation has expanded its range of applications to encompass younger newborns, older people, and individuals with unilateral hearing loss. In addition, it has improved its surgical methods to minimize the occurrence of complications. Furthermore, notable advancements have been made in the design of electrodes, techniques for speech processing, and software for programming. Nevertheless, inflammation, fibrosis, and even ossification are observed in the cochlea of nearly all cochlear implant (CI) patients. These tissue responses might have a negative impact on the performance of the implants, residual hearing, and the results of post-operative CI rehabilitation. Animal models are significant translational tools that offer essential preclinical data for possible therapeutics. Thus, this study concentrates on the existing animal models used for cochlear implantation, highlights the advancements made in research, and offers insights into potential future research areas.
Keywords: Cochlear implantation, Animal models, Foreign body response, Fibrosis, Residual hearing
The World Health Organization (WHO) released a report on hearing in 2021, revealing that more than 1.5 billion individuals worldwide have different levels of hearing impairment, which represents 20% of the global population. Among them, approximately 30 million people have severe to profound hearing loss (World report on hearing, 2021). Individuals with this level of hearing loss generally necessitate cochlear implants due to the inadequacy of hearing aids (Carlson, 2020).
Cochlear implants have become the most effective treatment for severe to profound hearing loss after more than fifty years of development (Glennon et al., 2023).In 1961, William House and John Doyle were the first to develop a functional cochlear implant. The U.S. Food and Drug Administration (FDA) approved the commercial use of the 3M/House single-channel CI in 1984. In the same year, the first multi-channel CI system became available (Chen et al., 2019; Mudry, 2013). As of 2022, more than one million individuals globally have received cochlear implant surgery (Zeng, 2022). In 1994, China debuted the multi-channel cochlear implant technology, which was approved for the treatment of adult patients with severe to profound bilateral sensorineural hearing loss. Since 1996, this technology has been utilized to assist children with hearing loss (Implementation plan of the hearing rehabilitation program for children with disabled children in poor, Persons’ Federation, 2011). China currently has a population of more than 100,000 CI users, with 85% of them being youngsters (Li et al., 2017; Liu, 2023).
Cochlear implants are subject to increasingly stringent criteria as technology and society progress, aiming to enhance the quality of life. Despite ongoing advancements in electrode design, speech processing strategies, and programming software for cochlear implant devices, and the continuous effort to perform “soft surgery”, there are still challenges that negatively impact the outcomes of cochlear implants: Cochlear implants are commonly linked to an inflammatory/foreign body response (FBR), fibrosis, and even ossification of the cochlea. Further research and resolution are required to address the concerns of preserving residual hearing, potential limitations of implanted electrodes due to long-term use, and managing the risks of reimplantation.
This work aims to establish a basis and reference for selecting suitable animal models for cochlear implantation research. To achieve this, we conduct a comprehensive evaluation of the current animal models used in cochlear implantation research and present a summary of their characteristics and research advancements.
1. Available animal models
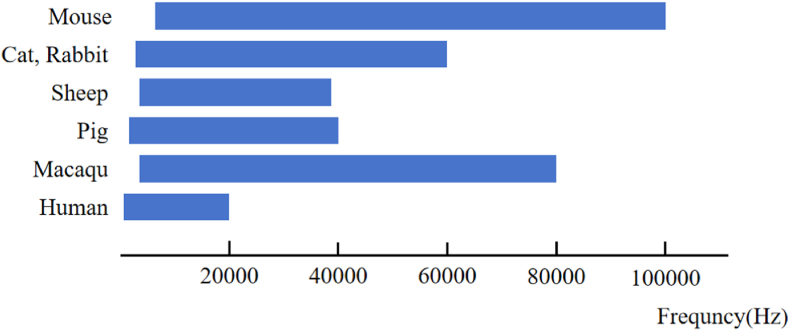
The existing animal models for cochlear implants can be categorized into two groups: rodents and non-rodents. Rodents encompass species such as mice, rats, guinea pigs, gerbils, and ferrets. On the other hand, non-rodents consist of cats, sheep, rabbits, pigs, and macaques. The auditory frequency distribution of different animal models is shown in Fig. 1. Table 1 presents a concise overview of the cochlear structural data for select species.
Fig. 1.
Auditory frequency distribution in different animals.
Table 1.
Comparison of cochlear anatomical structures across different animal modelsa.
| Species | Mouse | Rat | Guinea pig | Cat | Sheep | Rabbit | Pig | Macaque | Human |
|---|---|---|---|---|---|---|---|---|---|
| Cochlear turns | 1.75–2.2 | 2.2–2.8 | 4.0–4.5 | 3.0–3.5 | 2.5 | 2.5 | 3.5 | 2.5–2.75 | 2.75 |
| Scala tympani length (mm) | 4.6 | 7.2 | 16.2 | 23.0 | 20.7 | 15.0 | 27.5 | 27.0 | 28–36 |
Data from multiple literatures (Hatsushika S et al., 1990; J, 1999, J, 2001; Johnson et al., 2012; Martin GK, 1983; Shinomori Y, 2001; Thorne M, 1999; Trinh et al., 2022; Yi H, 2016; Yildiz et al., 2022).
1.1. Rodent models
The three most often utilized rodent models are mice, rats, and guinea pigs. The benefits of applying these three cochlear implant animal models are: a. There is a lot of research available on rodents in the field of otology, and the experimental techniques used to study hearing function, electrophysiology, and histology are well-established and widely used. b. The structure of the temporal bone is highly precise, and it conveniently houses the cochlea, semicircular canal, and vestibular system within the auditory bulla. Detailed information regarding the volume and shape of the cochlea, the size of the scala tympani and vestibuli, and the morphology of delicate structures like hair cells and spiral ganglion cells is supported by empirical evidence. c. The surgical procedure for cochlear implantation is relatively simple and straightforward. To gain entry to the auditory bubble, create an opening to reveal the round window membrane, and then insert the cochlear implant through the round window (Adunka et al., 2010; O'Leary et al., 2013; Soken et al., 2013).
However, the main constraint of the rodent model is the relatively diminutive size of the cochlea. Table 1 provides a summary of the cochlear turns in different species. In mice, the cochlear turns ranged from 1.75 to 2.2, in rats from 2.2 to 2.8, in guinea pigs from 4.0 to 4.5, and in humans from 2.5 to 3.0. The ratio between the quantities of cochlear lymphatic fluid in the three rodents and the human cochlea, based on earlier research, were 0.01:1, 0.04–0.05:1, and 0.17–0.2:1, respectively (Johnson et al., 2012; Reiss et al., 2022). Thus, the cochlea of the rodent model is incapable of accommodating a cochlear electrode array for clinical applications. In Austria, Australia, and other countries, various cochlear implant brands have developed different types of electrode arrays for experimental use in rodent models. These arrays are typically implanted at depths ranging from 1.6 mm to 3.0 mm (Claussen et al., 2019; Colesa et al., 2021; Dhanasingh and Hochmair, 2021; Irving et al., 2013), which is significantly shallower than the typical clinical implantation depths of 20 mm–25 mm or deeper (Dhanasingh and Jolly, 2017). In certain procedures in the fields of molecular biology, biochemistry, and immunogenetics, such as cochlear lymphatic fluid extraction or western blotting, it may be necessary to use many samples in order to obtain conclusive results. In order to address this issue, scientists must integrate several samples, hence augmenting the number of animals utilized in the experiment.
1.2. Non-rodent models
The most recent guidance from the FDA about animal studies specifies that the chosen animal model should effectively address the study objectives and offer the most accurate procedure that replicates the clinical scenario (General Considerations for Animal Studies Intended to Evaluate Medical Devices, U.S. Food and Drug Administration, 2023). Cochlear implant studies make use of large animal models to fulfill the requirements of translational and clinical research. In addition to their similar body size, anatomy, and physiological metabolism, which make them suitable for complete implantation of cochlear electrode arrays, they also exhibit greater similarities to humans in terms of immune response, biological characteristics, and gene expression (Lunney Jk et al., 2021; Sjostedt et al., 2020).
A comprehensive explanation has been provided regarding the nature of the inner ear, the composition of the temporal bone, and the surgical techniques used for cochlear implantation in larger species such as rabbits, sheep, cats, pigs, and macaques. Cats have served as animal models for cochlear implantation since the 1970s (P, 1980) and were the main large animal model utilized during the early stages. The cochlear turns in cats have a range of 3.0–3.5, which is comparable to the cochlear turns in pigs. In contrast, the cochlear turns in sheep, rabbits, and macaques are 2.5, which is more akin to the cochlear turns in humans (Table 1). Previous research has shown that the ratio of cochlea volume to body size is estimated to be 0.3–0.5:1 in cats and macaques, 0.5–1:1 in pigs, and 0.8–1.1:1 in sheep when compared to humans.
The surgical method used for the aforementioned big animal models is in line with the clinical cochlear implant procedure, namely using the round window approach. For illustrative purposes, the following are the procedural steps undertaken during the surgical intervention on the right side of the Bama miniature pig: Take the left lateral position and make a 5 cm incision parallel to the posterior auricular sulcus, 1 cm backward. Separate the subcutaneous tissue, parotid gland, and sternocleidomastoid muscle to expose the mastoid bone. The root of the earlobe serves as the body surface projection of the cochlea; use it as positioning to remove the mastoid bone and expose the posterior wall of the external auditory canal. Position the horizontal segment of the facial nerve, expose the tympanic chamber and the round window niche, and remove the round window niche to gain access to the round window membrane; then, implant electrodes are feasible. (Ji et al., 2022).
In recent years, our research group has found that minipigs have a similar frequency distribution and hearing threshold as humans. The cochlea of minipigs is comparable to that of humans during embryonic development, and it is fully developed at birth. Additionally, the anatomical structures of the inner ear and middle ear in minipigs are compatible with those of humans. The minipig is a highly promising large animal model for otology, whether it be for teaching otology surgical anatomy or conducting research on deafness and hearing implantation.
2. Research advances
The primary focus of cochlear implant research has predominantly been on rodent models. Studies have broadened their scope by utilizing large animal models, such as sheep, pigs, and macaques, to further improve research. Prior investigations involved the collection of temporal bone specimens from patients who underwent cochlear implantation. These specimens were subjected to CT scans and histological processing to examine the changes in the cochlear microenvironment resulting from prolonged cochlear implant stimulation. The collected data was then correlated with the patients' speech recognition outcomes during their lifetime. Nevertheless, the study's conclusions were unclear as a result of the limited sample size and the presence of uncontrollable variables. In recent times, as a result of the development of numerous animal models, the possibility and quantity of pertinent research have progressively grown. Presently, research is mostly concentrated on two primary areas.
2.1. Inner ear histopathology studies
Studies of human temporal bone specimens have confirmed that cochlear implantation can cause inflammation and foreign body reaction, which in turn leads to the development of granulomas. Subsequently, fibrosis and ossification occur as a result of this process (Benatti et al., 2013; Li Pm et al., 2007; Linthicum et al., 2017; Marsh Ma and Coker, 1992; Nadol et al., 2001; Noonan et al., 2020; Schindler RA, 1979). Nadol (Nadol et al., 2001) observed a high occurrence of the formation of a fibrous sheath around the cochlear electrode array. Linthicum (Linthicum et al., 2017) have documented similar pathological alterations in consecutive histological sections of 55 temporal bone samples. They found that the most substantial increase in fibrous tissue occurred in the basal turn of the cochlea following implantation, with minimal harm resulting from the round window method. Cochlear implants offer the benefits of excellent biocompatibility and a minimal incidence of complications. Nevertheless, the insertion of electrode arrays leads to unavoidable tissue responses. Hence, animal models are used to study the inflammation/foreign body response process and the mechanism of fibrosis and ossification. The objective is to discover novel approaches for conserving residual hearing and safeguarding the delicate structures in the cochlea.
The majority of studies investigating the histopathology of the inner ear following cochlear implantation have utilized mice as experimental models. Based on O'Leary's study (O'Leary et al., 2013), after four weeks of implantation in guinea pigs, fibrocytes expanded from the osseous spiral lamina and the lateral wall to envelop the electrodes. Furthermore, there was evidence of osteogenesis. The study conducted by Tanaka (Tanaka et al., 2014) found a significant association between hearing loss following CI and decreased vascular density as well as ossification in the area surrounding the round window niche. However, no significant correlation was observed between hearing loss and spiral ganglion neuron density, hair cell count, or fibrotic area within the cochlea. Research conducted on mice demonstrated that the occurrence of hearing loss following implantation was not correlated with the density of spiral ganglion neurons. However, the occurrence of hearing loss within two weeks after implantation was found to be associated with the severity of damage suffered by the organ of Corti (Kopelovich et al., 2015). Choong et al. used quantitative nanomechanical atomic force microscopy (QNM-AFM) to demonstrate that the basilar membrane undergoes a gradual increase in stiffness over a period of three months following implantation (Choong et al., 2020). The basal turn exhibited the greatest prominence, but the apical turn demonstrated a similar occurrence. Claussen et al. (2022) observed substantial infiltration of macrophages during the second and third weeks after implantation. They concluded that the primary cause of cochlear fibrosis was inflammation and the foreign body response, rather than electrical stimulation. Zhang et al. (2015) provided evidence at the molecular level that 14 days after implantation, there was an upregulation of cochlear inflammatory genes (TNFα, Cxcl1, IL-1b, and Tnfrsf1a/b) as well as tissue remodeling genes (MMP2, MMP9, TGF-β).
Additionally, foreign body and inflammatory responses have been documented in big animal models. In 1992, Ni et al. (Ni, et al., 1992) conducted an experiment where they inserted electrodes into the cochleae of five kittens. One month after the surgery, the cochlea showed a 100% occurrence of an inflammatory response, and the extent of hair cell loss was directly related to the intensity of the inflammatory reaction. Trinh (Trinh et al., 2022) and Kaufmann (Kaufmann et al., 2020) found that sheep cochlea can fully accommodate cochlear implant electrode arrays. Histologic analysis conducted 30 days after the implantation revealed the presence of fibrosis in the cochlea. Furthermore, cochlea electrography and auditory brainstem response (ABR) amplitude were observed to be reduced, indicating the occurrence of insertion trauma in the cochlea. Yildiz (Yildiz et al., 2022) selected minipigs as subjects for cochlear implantation. After 56 days following the surgical procedure, histological analysis of the cochlea revealed the development of a fibrous sheath and the accumulation of lymphocytes around the cochlea. Scanning electron microscopy reveals substantial damage to the cilia of inner hair cells in the basal turn of the cochlea following cochlear implantation (Liu et al., 2021).
2.2. Novel electrodes development
Glucocorticoids are commonly administered in medical settings to diminish inflammation and protect against hearing loss following cochlear implant surgery. However, there is still a lack of uniformity in the approach to drug administration, the dosage, the frequency of administration, and the range of available pharmaceuticals. Moreover, the effectiveness of these interventions is not well-supported by comprehensive evidence that includes a sufficient sample size. Medications cannot sustain a consistent level of effectiveness in the cochlea for an extended duration. As a result, throughout the past ten years or more, scientists have commenced investigations into the creation of novel electrodes utilizing animal models.
The studies conducted by Liu (Liu et al., 2015), Wilk (Wilk et al., 2016), Bas (Bas et al., 2016), Ahmadi (Ahmadi et al., 2019), and Simoni (Simoni et al., 2020) all involved the use of guinea pigs. They implanted electrodes with surface-modified dexamethasone into the cochlea. This method not only allowed for the continuous release of dexamethasone for at least one week, but also served as a protective agent for the hair cells, spiral ganglion cells, and auditory nerves. Additionally, it effectively reduced the foreign body response and the development of fibrosis. A study conducted by Ahmadi (Ahmadi et al., 2019) revealed that the impedance of electrodes changed with dexamethasone can remain stable for 120 days following implantation. Bas (Bas et al., 2016) conducted a study where they examined dexamethasone electrodes that had been modified with different concentrations of surface coating. They found that electrodes with a 10% concentration coating provided the most effective protection for residual hearing.
Liu (Liu et al., 2015) discovered that the electrodes modified with dexamethasone continued to provide protection for residual hearing for up to 12 weeks after the surgery. This resulted in a hearing threshold shift of 5.0 ± 3.4 dB SPL. On the other hand, Wilk (Wilk et al., 2016) identified that there was a positive correlation between the degree of fibrosis in the cochlea and impedance values. Regarding the innovative electrodes, surface-modified pharmaceuticals such as laminin (Bas et al., 2019), Taurodeoxycholic acid (Shah et al., 2020), brain derived neurotrophic factor (BDNF) (Rejali et al., 2007), and polymeric materials such hydrogels (Xu et al., 2018) are also included. In contrast, Matsui (Matsui et al., 2023) investigated the localization of glucocorticoid receptors (GCR) in the human inner ear using immunohistochemistry and found differences in the intensity of immunofluorescence at different locations in the cochlea. This differential expression may contribute to a deeper understanding of the mechanism of glucocorticoid in the inner ear and provide a basis for the development of more scientifically therapeutic regimens.
Eshraghi (Eshraghi et al., 2007, 2006), Vivero (Vivero et al., 2008), and Ihler (Ihler et al., 2014) researched the impact of drugs on hearing thresholds and hair cell function. They achieved this by inserting microcatheters into the cochlea through the round window and connecting them to a micro-osmotic pump. This system allowed for consistent and uninterrupted delivery of drugs to the inner ear. Eshraghi (Eshraghi et al., 2007, 2006) demonstrated that there was an initial increase in hearing thresholds at 1 kHz, 4 kHz, and 16 kHz after the surgery. However, no significant difference was observed compared to the control group at 30 days postoperatively. These results align with the findings of Vivero (Vivero et al., 2008). Ihler (Ihler et al., 2014) discovered that trauma from electrode implantation resulted in a hearing threshold shift ranging from 50.3 dB SPL to 68.0 dB SPL across all frequencies. Additionally, the administration of drugs significantly improved hearing loss following the implantation.
3. Summary and prospects
Cochlear implants are effective neuroprostheses that restore hearing in individuals with hearing loss, leading to an enhanced quality of life and alleviating the societal impact. The exact mechanisms of fibrosis ossification in the cochlea, the process by which residual hearing loss occurs, and the methods used to evaluate the new electrodes prior to clinical testing remain ambiguous. Clinical investigations frequently encounter constraints arising from various influential factors, such as age, the duration of deafness, and prior hearing disorders. Hence, it is imperative to carefully choose a suitable animal model for cochlear implantation, not only to regulate variables in scientific investigation but also to ensure preliminary validation in clinical application. There is an ample selection of animal models available for a variety of research studies. In future research, cochlear implant animal models can be effectively utilized to elucidate several aspects, including the mechanisms underlying pathological alterations following cochlear implantation, and offer novel strategies to enhance the long-term outcomes of cochlear implantation.
Declaration of generative AI and AI-assisted technologies in the writing process
While preparing this work, the authors used Grammarly to polish up the article. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the publication's content.
Fundings:
This study was supported by the following: (1) National Natural Science Foundation of China (NSFC #82000976) to Jianan Li; (2) National Key Research and Development Program of China (2022YFC2402700) to Wei Chen.
Declaration of competing Interest
The authors have no conflicts of interest to disclose.
Acknowledgments
This study was supported by the following: (1) National Key Research and Development Program of China (2021YFF0702302) to Jianan Li; (2) National Key Research and Development Program of China (2022YFC2402700) to Wei Chen.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Adunka O.F., Mlot S., Suberman T.A., Campbell A.P., Surowitz J., Buchman C.A., Fitzpatrick D.C. Intracochlear recordings of electrophysiological parameters indicating cochlear damage. Otol. Neurotol. 2010;31(8):1233–1241. doi: 10.1097/MAO.0b013e3181f1ffdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi N., Gausterer J.C., Honeder C., Motz M., Schopper H., Zhu C., Saidov N., Gabor F., Arnoldner C. Long-term effects and potential limits of intratympanic dexamethasone-loaded hydrogels combined with dexamethasone-eluting cochlear electrodes in a low-insertion trauma Guinea pig model. Hear. Res. 2019;384 doi: 10.1016/j.heares.2019.107825. [DOI] [PubMed] [Google Scholar]
- Bas E., Bohorquez J., Goncalves S., Perez E., Dinh C.T., Garnham C., Hessler R., Eshraghi A.A., Van De Water T.R. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear. Res. 2016;337:12–24. doi: 10.1016/j.heares.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Bas E., Anwar M.R., Goncalves S., Dinh C.T., Bracho O.R., Chiossone J.A., Van De Water T.R. Laminin-coated electrodes improve cochlear implant function and post-insertion neuronal survival. Neuroscience. 2019;410:97–107. doi: 10.1016/j.neuroscience.2019.04.048. [DOI] [PubMed] [Google Scholar]
- Benatti A., Castiglione A., Trevisi P., Bovo R., Rosignoli M., Manara R., Martini A. Endocochlear inflammation in cochlear implant users: case report and literature review. Int. J. Pediatr. Otorhinolaryngol. 2013;77(6):885–893. doi: 10.1016/j.ijporl.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Carlson M.L. Cochlear implantation in adults. N. Engl. J. Med. 2020;382(16):1531–1542. doi: 10.1056/NEJMra1904407. [DOI] [PubMed] [Google Scholar]
- Chen F., Ni W., Li W., Li H. Cochlear implantation and rehabilitation. Adv. Exp. Med. Biol. 2019;1130:129–144. doi: 10.1007/978-981-13-6123-4_8. [DOI] [PubMed] [Google Scholar]
- Choong J.K., Hampson A.J., Brody K.M., Lo J., Bester C.W., Gummer A.W., Reynolds N.P., O'Leary S.J. Nanomechanical mapping reveals localized stiffening of the basilar membrane after cochlear implantation. Hear. Res. 2020;385 doi: 10.1016/j.heares.2019.107846. [DOI] [PubMed] [Google Scholar]
- Claussen A.D., Vielman Quevedo R., Mostaert B., Kirk J.R., Dueck W.F., Hansen M.R. A mouse model of cochlear implantation with chronic electric stimulation. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0215407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen A.D., Quevedo R.V., Kirk J.R., Higgins T., Mostaert B., Rahman M.T., Oleson J., Hernandez R., Hirose K., Hansen M.R. Chronic cochlear implantation with and without electric stimulation in a mouse model induces robust cochlear influx of CX3CR1(+/GFP) macrophages. Hear. Res. 2022;426 doi: 10.1016/j.heares.2022.108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colesa D.J., Devare J., Swiderski D.L., Beyer L.A., Raphael Y., Pfingst B.E. Development of a chronically-implanted mouse model for studies of cochlear health and implant function. Hear. Res. 2021;404 doi: 10.1016/j.heares.2021.108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasingh A., Hochmair I. Drug delivery in cochlear implantation. Acta Otolaryngol. 2021;141(Suppl. 1):135–156. doi: 10.1080/00016489.2021.1888505. [DOI] [PubMed] [Google Scholar]
- Dhanasingh A., Jolly C. An overview of cochlear implant electrode array designs. Hear. Res. 2017;356:93–103. doi: 10.1016/j.heares.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Eshraghi A.A., H J., Mou C.H., et al. D-JNKI-1 treatment prevents the progression of hearing loss in a model of cochlear implantation trauma. Otol. Neurotol. 2006;27(4):504–511. doi: 10.1097/01.mao.0000217354.88710.13. [DOI] [PubMed] [Google Scholar]
- Eshraghi A.A., A E., He J., Graves R., Balkany T.J., Van De Water TR. Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma-induced hearing loss. Otol. Neurotol. 2007;28(6):842–849. doi: 10.1097/mao.0b013e31805778fc. [DOI] [PubMed] [Google Scholar]
- Glennon E., Valtcheva S., Zhu A., Wadghiri Y.Z., Svirsky M.A., Froemke R.C. Locus coeruleus activity improves cochlear implant performance. Nature. 2023;613(7943):317–323. doi: 10.1038/s41586-022-05554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsushika S S.R., Tong Y.C., Clark G.M., Funasaka S. Dimensions of the scala tympani in the human and cat with reference to cochlear implants. Ann. Otol. Rhinol. Laryngol. 1990;99(11):871–876. doi: 10.1177/000348949009901104. [DOI] [PubMed] [Google Scholar]
- Ihler F., Pelz S., Coors M., Matthias C., Canis M. Application of a TNF-alpha-inhibitor into the scala tympany after cochlear electrode insertion trauma in Guinea pigs: preliminary audiologic results. Int. J. Audiol. 2014;53(11):810–816. doi: 10.3109/14992027.2014.938369. [DOI] [PubMed] [Google Scholar]
- Irving S., Trotter M.I., Fallon J.B., Millard R.E., Shepherd R.K., Wise A.K. Cochlear implantation for chronic electrical stimulation in the mouse. Hear. Res. 2013;306:37–45. doi: 10.1016/j.heares.2013.09.005. [DOI] [PubMed] [Google Scholar]
- J W. Dimensions of the human vestibular and tympanic scalae. Hear. Res. 1999;135(1–2):39–46. doi: 10.1016/s0378-5955(99)00088-x. [DOI] [PubMed] [Google Scholar]
- J W. Dimensions of the vestibular and tympanic scalae of the cochlea in selected mammals. Hear. Res. 2001;161(1–2):1–9. doi: 10.1016/s0378-5955(01)00314-8. [DOI] [PubMed] [Google Scholar]
- Ji X., Luo Y., Guo W., Ji F., Yuan S., Xu L., Chen W. The miniature pig: a large animal model for cochlear implant research. J. Vis. Exp. 2022;185 doi: 10.3791/64174. [DOI] [PubMed] [Google Scholar]
- Johnson L.A., Della Santina C.C., Wang X. Temporal bone characterization and cochlear implant feasibility in the common marmoset (Callithrix jacchus) Hear. Res. 2012;290(1–2):37–44. doi: 10.1016/j.heares.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann C.R., Tejani V.D., Fredericks D.C., Henslee A.M., Sun D.Q., Abbas P.J., Hansen M.R. Pilot evaluation of sheep as in vivo model for cochlear implantation. Otol. Neurotol. 2020;41(5):596–604. doi: 10.1097/MAO.0000000000002587. [DOI] [PubMed] [Google Scholar]
- Kopelovich J.C., Robinson B.K., Soken H., Verhoeven K.J., Kirk J.R., Goodman S.S., Hansen M.R. Acoustic hearing after murine cochlear implantation: effects of trauma and implant type. Ann. Otol. Rhinol. Laryngol. 2015;124(12):931–939. doi: 10.1177/0003489415592162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.N., Chen S., Zhai L., Han D.Y., Eshraghi A.A., Feng Y., Yang S.M., Liu X.Z. The advances in hearing rehabilitation and cochlear implants in China. Ear Hear. 2017;38(6):647–652. doi: 10.1097/AUD.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Pm S.M., Eddington D.K., Nadol J.B., Jr. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann. Otol. Rhinol. Laryngol. 2007;116(10):731–738. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- Linthicum F.H., Jr., Doherty J.K., Lopez I.A., Ishiyama A. Cochlear implant histopathology. World J Otorhinolaryngol Head Neck Surg. 2017;3(4):211–213. doi: 10.1016/j.wjorl.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Point to consider in cochlear implantation for patients with microtia and atresia. Chinese Scientifific Journal of Hearing and Speech Rehabilitation. 2023;21(2):121–124. doi: 10.3969/j.issn.1672-4933.2023.02.003. [DOI] [Google Scholar]
- Liu Y., Jolly C., Braun S., Janssen T., Scherer E., Steinhoff J., Ebenhoch H., Lohner A., Stark T., Kiefer J. Effects of a dexamethasone-releasing implant on cochleae: a functional, morphological and pharmacokinetic study. Hear. Res. 2015;327:89–101. doi: 10.1016/j.heares.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Liu Q., Guo W., Yang S., Ji X., Lin C., Chen W. Electrophysiological and histomorphological changes of cochlea in miniature pigs after abrasion of round window niches. Acta Otolaryngol. 2021;141(6):557–566. doi: 10.1080/00016489.2021.1899281. [DOI] [PubMed] [Google Scholar]
- Lunney Jk V.G.A., Walker K.E., Hailstock T., Franklin J., Dai C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021;13(621) doi: 10.1126/scitranslmed.abd5758. [DOI] [PubMed] [Google Scholar]
- Marsh Ma J.H., Coker N.J. Histopathology of the temporal bone following multichannel cochlear implantation. Arch. Otolaryngol. Head Neck Surg. 1992;118(11):1257–1265. doi: 10.1001/archotol.1992.01880110125022. [DOI] [PubMed] [Google Scholar]
- Martin Gk S.D., Dobie R.A., Lonsbury-Martin B.L. Endolymphatic hydrops in the rabbit: auditory brainstem responses and cochlear morphology. Hear. Res. 1983;12(1):65–87. doi: 10.1016/0378-5955(83)90119-3. [DOI] [PubMed] [Google Scholar]
- Matsui H., Lopez I.A., Ishiyama G., Ishiyama A. Immunohistochemical localization of glucocorticoid receptors in the human cochlea. Brain Res. 2023;1806 doi: 10.1016/j.brainres.2023.148301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudry A M.M. The early history of the cochlear implant: a retrospective. JAMA Otolaryngol Head Neck Surg. 2013;139(5):446–453. doi: 10.1001/jamaoto.2013.293. [DOI] [PubMed] [Google Scholar]
- Nadol J.B., Jr S.J., Burgess B.J., et al. Histopathology of cochlear implants in humans. Ann. Otol. Rhinol. Laryngol. 2001;110(9):883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Ni D S.R., Seldon H.L., Xu S.A., Clark G.M., Millard R.E. Cochlear pathology following chronic electrical stimulation of the auditory nerve. I: normal hearing kittens. Hear. Res. 1992;62(1):63–81. doi: 10.1016/0378-5955(92)90203-y. [DOI] [PubMed] [Google Scholar]
- Noonan K.Y., Lopez I.A., Ishiyama G., Ishiyama A. Immune response of macrophage population to cochlear implantation: cochlea immune cells. Otol. Neurotol. 2020;41(9):1288–1295. doi: 10.1097/MAO.0000000000002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary S.J., Monksfield P., Kel G., Connolly T., Souter M.A., Chang A., Marovic P., O'Leary J.S., Richardson R., Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear. Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- P F. Auditory prostheses--a challenge for neurophysiology. Audiology. 1980;19(2):176–187. doi: 10.3109/00206098009072659. [DOI] [PubMed] [Google Scholar]
- Persons’ Federation China Disabled. Implementation plan of the hearing rehabilitation program for children with disabled children in poor. China Disabled Persons’ Federation. 2011 https://www.cdpf.org.cn/ Retrieved June 26 from. [Google Scholar]
- Reiss L.A.J., Kirk J., Claussen A.D., Fallon J.B. Animal models of hearing loss after cochlear implantation and electrical stimulation. Hear. Res. 2022;426 doi: 10.1016/j.heares.2022.108624. [DOI] [PubMed] [Google Scholar]
- Rejali D., Lee V.A., Abrashkin K.A., Humayun N., Swiderski D.L., Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear. Res. 2007;228(1–2):180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler Ra B.B. Traumatic intracochlear electrode implantation. Laryngoscope. 1979;89(5 pt 1):752–758. doi: 10.1288/00005537-197905000-00012. [DOI] [PubMed] [Google Scholar]
- Shah V., Mittal R., Shahal D., Sinha P., Bulut E., Mittal J., Eshraghi A.A. Evaluating the efficacy of taurodeoxycholic acid in providing otoprotection using an in vitro model of electrode insertion trauma. Front. Mol. Neurosci. 2020;13:113. doi: 10.3389/fnmol.2020.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomori Y S.D., Jones D.D., Kimura R.S. Volumetric and dimensional analysis of the Guinea pig inner ear. Ann. Otol. Rhinol. Laryngol. 2001;110(1):91–98. doi: 10.1177/000348940111000117. [DOI] [PubMed] [Google Scholar]
- Simoni E., Gentilin E., Candito M., Borile G., Romanato F., Chicca M., Nordio S., Aspidistria M., Martini A., Cazzador D., Astolfi L. Immune response after cochlear implantation. Front. Neurol. 2020;11:341. doi: 10.3389/fneur.2020.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostedt E., Zhong W., Fagerberg L., Karlsson M., Mitsios N., Adori C., Oksvold P., Edfors F., Limiszewska A., Hikmet F., Huang J., Du Y., Lin L., Dong Z., Yang L., Liu X., Jiang H., Xu X., Wang J., Yang H., Bolund L., Mardinoglu A., Zhang C., von Feilitzen K., Lindskog C., Ponten F., Luo Y., Hokfelt T., Uhlen M., Mulder J. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science. 2020;367(6482) doi: 10.1126/science.aay5947. [DOI] [PubMed] [Google Scholar]
- Soken H., Robinson B.K., Goodman S.S., Abbas P.J., Hansen M.R., Kopelovich J.C. Mouse cochleostomy: a minimally invasive dorsal approach for modeling cochlear implantation. Laryngoscope. 2013;123(12):E109–E115. doi: 10.1002/lary.24174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C., Nguyen-Huynh A., Loera K., Stark G., Reiss L. Factors associated with hearing loss in a normal-hearing Guinea pig model of Hybrid cochlear implants. Hear. Res. 2014;316:82–93. doi: 10.1016/j.heares.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne M S.A., DeMott J.E., Henson M.M., Henson O.W., Jr., Gewalt S.L. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999;109(10):1661–1668. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- Trinh T.T., Cohen C., Boullaud L., Cottier J.P., Bakhos D. Sheep as a large animal model for cochlear implantation. Braz J Otorhinolaryngol. 2022;88(Suppl. 1):S24–S32. doi: 10.1016/j.bjorl.2021.02.014. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . U.S. Food and Drug Administration; 2023. General Considerations for Animal Studies Intended to Evaluate Medical Devices.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-considerations-animal-studies-intended-evaluate-medical-devices Retrieved July 1st from. [Google Scholar]
- Vivero R.J., Joseph D.E., Angeli S., He J., Chen S., Eshraghi A.A., Balkany T.J., Van de Water T.R. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope. 2008;118(11):2028–2035. doi: 10.1097/MLG.0b013e31818173ec. [DOI] [PubMed] [Google Scholar]
- Wilk M., Hessler R., Mugridge K., Jolly C., Fehr M., Lenarz T., Scheper V. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can Be reduced using a dexamethasone eluting electrode. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World report on hearing . 2021. Geneva: World Health Organization. [Google Scholar]
- Xu M., Ma D., Chen D., Cai J., He Q., Shu F., Tang J., Zhang H. Preparation, characterization and application research of a sustained dexamethasone releasing electrode coating for cochlear implantation. Mater. Sci. Eng., C. 2018;90:16–26. doi: 10.1016/j.msec.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Yi H., G W., Chen W., Chen L., Ye J., Yang S. Miniature pigs: a large animal model of cochlear implantation. Am J Transl Res. 2016;8(12):5494–5502. [PMC free article] [PubMed] [Google Scholar]
- Yildiz E., Gerlitz M., Gadenstaetter A.J., Landegger L.D., Nieratschker M., Schum D., Schmied M., Haase A., Kanz F., Kramer A.M., Glueckert R., Staecker H., Honeder C., Arnoldner C. Single-incision cochlear implantation and hearing evaluation in piglets and minipigs. Hear. Res. 2022;426 doi: 10.1016/j.heares.2022.108644. [DOI] [PubMed] [Google Scholar]
- Zeng F.-G. Celebrating the one millionth cochlear implant. JASA Express Letters. 2022;2(7) doi: 10.1121/10.0012825. [DOI] [PubMed] [Google Scholar]
- Zhang H., Stark G., Reiss L. Changes in gene expression and hearing thresholds after cochlear implantation. Otol. Neurotol. 2015;36(7):1157–1165. doi: 10.1097/MAO.0000000000000787. [DOI] [PMC free article] [PubMed] [Google Scholar]