Highlights
-
•
Prenatal alcohol exposure (PAE) is associated with alterations in brain development.
-
•
New normative MRI databases aid in evaluating brain structure while adjusting for sex and age.
-
•
We found evidence for atypical development in important subcortical brain regions in FASD.
-
•
PAE is particularly associated with atypical development in hippocampus and caudate.
-
•
Findings have implications for understanding cognitive deficits that are common in FASD.
Keywords: FASD, Structural MRI, Subcortical gray matter, Neurodevelopment
Abstract
Aim
To quantify regional subcortical brain volume anomalies in youth with fetal alcohol spectrum disorder (FASD), assess the relative sensitivity and specificity of abnormal volumes in FASD vs. a comparison group, and examine associations with cognitive function.
Method
Participants: 47 children with FASD and 39 typically-developing comparison participants, ages 8–17 years, who completed physical evaluations, cognitive and behavioral testing, and an MRI brain scan. A large normative MRI dataset that controlled for sex, age, and intracranial volume was used to quantify the developmental status of 7 bilateral subcortical regional volumes. Z-scores were calculated based on volumetric differences from the normative sample. T-tests compared subcortical volumes across groups. Percentages of atypical volumes are reported as are sensitivity and specificity in discriminating groups. Lastly, Pearson correlations examined the relationships between subcortical volumes and neurocognitive performance.
Results
Participants with FASD demonstrated lower mean volumes across a majority of subcortical regions relative to the comparison group with prominent group differences in the bilateral hippocampi and bilateral caudate. More individuals with FASD (89%) had one or more abnormally small volume compared to 72% of the comparison group. The bilateral hippocampi, bilateral putamen, and right pallidum were most sensitive in discriminating those with FASD from the comparison group. Exploratory analyses revealed associations between subcortical volumes and cognitive functioning that differed across groups.
Conclusion
In this sample, youth with FASD had a greater number of atypically small subcortical volumes than individuals without FASD. Findings suggest MRI may have utility in identifying individuals with structural brain anomalies resulting from PAE.
1. Introduction
Fetal alcohol spectrum disorders (FASD) are common neurodevelopmental conditions resulting from prenatal alcohol exposure (PAE) and are associated with lifelong neurocognitive and neurobehavioral impairment (Mattson et al., 2019). FASDs affect approximately 1.1 % to 5 % of school-aged children in the United States and up to 6 % in some European countries, making them among the most common neurodevelopmental disorders (Lange et al., 2017, May et al., 2018). Individuals with PAE/FASD demonstrate brain anomalies including microcephaly (small head and brain size) (Jones et al., 1973, Treit et al., 2016), abnormalities in cortical thickness (Gimbel et al., 2023, Treit et al., 2014) and smaller white matter and gray matter volumes at the whole-brain and regional levels (Lebel et al., 2011). Subcortical brain structures are thought to be particularly vulnerable to the effects of PAE (Lebel et al., 2011, Moore et al., 2014, Nardelli et al., 2011). For example, an MRI study employing a multivariate approach to separate children with FASD from a typically-developing comparison group found that right and left caudate volume and left globus pallidus volume contributed heavily to the classification model (volumes were lower in the FASD group) (Little and Beaulieu 2020). Subcortical shape abnormalities and small volumes have been reported in youth with PAE for deep gray matter structures in some but not all studies (for comprehensive review see (Lebel et al., 2011, Moore et al., 2014)). Although the frequencies of various brain structural anomalies following PAE are not precisely known, one study reported high rates of volumetric anomalies (two or more standard deviations below the mean of a comparison group) in individuals with fetal alcohol syndrome (FAS)/partial FAS (78 %), static encephalopathy/alcohol exposed (58 %), and neurodevelopmental disorder/alcohol exposed (43 %) (Astley et al. 2009). Another study employing clinical neuroradiologic reviews of MRI scans found a three-fold increase in abnormalities for those with PAE (Treit et al. 2020). Another study employing retrospective examinations of brains obtained at autopsy that were known to have been exposed prenatally to alcohol found microcephaly in 20.8 %, corpus callosum defects in 4.0 %, and atypical cortical smoothness in 0.7 % of the sample (Jarmasz et al. 2017).
Across the lifespan, findings have been mixed with regard to whether subcortical volumetric abnormalities are observed both with and without correction for total brain volume (TBV) or total intracranial volume (ICV). Youth with PAE/FASD show smaller volumes in regions such as the hippocampus, putamen, pallidum, caudate, and thalamus (Archibald et al., 2001, Astley et al., 2009, Boateng et al., 2023, Gautam et al., 2015, Nardelli et al., 2011, Riikonen et al., 2005, Roussotte et al., 2012, Willoughby et al., 2008). Some studies reported atypically small hippocampal volume even after adjusting for ICV (Nardelli et al., 2011, Willoughby et al., 2008, Boateng et al., 2023) whereas others did not find smaller hippocampi when accounting for ICV (Riikonen et al., 2005, Astley et al., 2009, Coles et al., 2011, Joseph et al., 2014, Archibald et al., 2001, Roussotte et al., 2012, Biffen et al., 2018). Another study found smaller right and left hippocampi in children with FASD relative to a comparison group, but only the right side remained significantly smaller after correcting for ICV (Little and Beaulieu 2020). One study of children with PAE reported atypically small caudate, hippocampus, and putamen volumes compared to non-exposed children even after controlling for ICV (Nardelli et al., 2011). Adults with FASD also show smaller absolute hippocampal volumes than unexposed comparisons (Coles et al., 2011) and both smaller absolute and relative volumes of the caudate, putamen, and pallidum (Bischoff-Grethe et al., 2024). Some studies have also identified a relationship between structural anomalies in subcortical regions and facial dysmorphology in youth with PAE (Suttie et al., 2018, Roussotte et al., 2012, Astley et al., 2009). For example, Astley et al (2009) found that lower caudate volume was associated with a higher degree of facial dysmorphology. Importantly, a number of studies in FASD have demonstrated that structural brain anomalies (e.g., cortical surface area, white matter microstructure) can occur even in the absence of facial dysmorphology (Wozniak et al., 2009, Wozniak et al., 2006, Rajaprakash et al., 2014).
Importantly, a large body of accumulating research indicates that deep subcortical brain structures play an integral role in both lower- and higher-order cognitive functions (Janacsek et al., 2022), suggesting implications for understanding neurocognitive and neurobehavioral impairment in youth with PAE. In fact, a number of studies have shown associations between atypical subcortical volumes and associated cognitive deficits in those with PAE, including in the caudate (Astley et al., 2009, Fryer et al., 2012, Suttie et al., 2018) and the hippocampus (Dodge et al., 2020, Dudek et al., 2014, Gross et al., 2018). For example, smaller caudate volume (accounting for ICV) was associated with worse performance on a measure of verbal learning in youth with FASD (Fryer et al. 2012). Similarly, small right and left hippocampal volumes correlated with lower performance on a measure of spatial working memory (virtual water maze) in children (Dodge et al. 2020). Among children with FASD, smaller right and left hippocampal volume were associated with worse long-term verbal recall, and right hippocampal volume was correlated with delayed recall on a visual memory task (Willoughby et al. 2008). Lastly, another study in adults with FASD found that right hippocampal volume was associated with both verbal and non-verbal memory (Coles et al. 2011). Based on the prior literature, we hypothesized that atypically small norm-adjusted subcortical volumes would also be associated with cognitive deficits in our sample of children with FASD. In addition to measures of memory and working memory, we tested for associations between subcortical volumes and IQ based on prior studies showing relationships between amygdala, thalamus, basal ganglia volumes and IQ in children with very low birth weight (VLBW)/preterm birth—another condition with implications for neurodevelopment (Rimol et al., 2023, Bjuland et al., 2014). Similarly, a study of individuals with extreme prematurity found associations between smaller hippocampal volume and lower IQ (Fernández de Gamarra-Oca et al. 2023). We also hypothesized that deficits in attention and executive functioning would be associated with small subcortical volumes based on a study showing altered developmental trajectories in caudate, putamen, pallidum, thalamus, and hippocampus in children with attention-deficit hyperactivity disorder (ADHD) (Wang et al. 2021) and another study showing a correlation between executive function (spatial working memory) and putamen volume in typically developing children (Pangelinan et al. 2011).
Recently published normative modeling tools based on large numbers of individuals provide a novel approach to quantifying variation in health-related variables (e.g., brain volumes) at the individual level (Bethlehem et al., 2022, Ge et al., 2024, Rutherford et al., 2023). These normative modeling tools have led to novel methods for quantifying intra- and inter-individual brain atypicality for whole-brain and regional volumes using “brain growth charts.” This approach offers benefits over using small, potentially unrepresentative comparison groups and case-control methodologies that assume within-group homogeneity (Rutherford et al., 2022). In a recent study of an overlapping sample of youth with FASD and typically-developing comparison participants age 8 to 17 years (Gimbel et al., 2024), we computed (per)centiles for total cortical gray matter, total cortical white matter, and total subcortical gray matter and found that each was significantly smaller in youth with FASD relative to comparison participants. In addition, brain volume centiles accounted for a greater proportion of variance in IQ than occipito-frontal head circumference (HC), which is typically used as a proxy of brain anomalies in FASD diagnosis (Hoyme et al. 2016). Brain volume centiles also demonstrated increased relative sensitivity to discriminating youth with FASD from the comparison group, demonstrating that direct measurement of the brain using MRI could potentially be used to improve FASD diagnosis. In addition, FASD participants with at least one whole-brain volume anomaly (≤10th centile, which mirrors the Institute of Medicine criteria for HC and growth anomalies (Hoyme et al., 2016) had significantly lower performance on measures of IQ and executive function than FASD participants with “typical” brain volumes (>10th centile), demonstrating the clinical relevance of MRI-derived volumetric measures.
In the current study, we extend this approach by examining regional subcortical volume anomalies in youth with FASD. Using a sample of youth with FASD and typically-developing comparisons ages 8–17 years, we calculated z-scores adjusted for age, sex, and total intracranial volume (ICV) for volumes of 7 bilateral subcortical regions including the amygdala, caudate, hippocampus, pallidum, putamen, thalamus, and accumbens area. We hypothesized: 1) regional subcortical volume z-scores would be lower for youth with FASD compared to participants in the comparison group, 2) youth with FASD would demonstrate between-group regional variability in subcortical volume anomalies, and 3) larger subcortical volume z-scores would be associated with better neurocognitive and neurobehavioral functioning.
2. Method
2.1. Participants
Participants were children and adolescents, ages 8–17, who were part of a Collaborative Initiative on FASD (CIFASD) study (see www.cifasd.org) (Mattson et al., 2010). Data from this study are part of CIFASD Phase 4 (2017–2022). We used data collected at the University of Minnesota during the first visit of the larger longitudinal study. Participants and their parent or guardian completed assent and consent processes, respectively, and received monetary compensation. All study procedures were approved by the University of Minnesota Institutional Review Board.
Exclusion criteria (both FASD and comparison groups) included neurological and developmental disorders (e.g., seizure disorders and autism). Primary visual impairment and hearing impairment were exclusionary as these conditions would have interfered significantly with neuropsychological testing. Participants were also excluded for drug/alcohol use, severe psychiatric disabilities preventing participation (e.g., psychotic illness), very low birth weight (<1500 g), and MRI contraindications (e.g., braces, non-MR-safe medical devices, claustrophobia). Attention deficit/hyperactivity disorder was not exclusionary for the FASD group. Prenatal polysubstance exposure (including substances other than alcohol) was not exclusionary for the FASD group as this is common. Prenatal alcohol and other drug exposure (excluding tobacco and caffeine) were exclusionary for participants in the comparison group.
2.2. FASD diagnosis
Prior to enrollment, phone screening and record review were conducted to determine PAE history. To be eligible for the FASD group, documented evidence of heavy PAE was required (i.e., ≥ 13 drinks per week or ≥ 4 drinks in succession at least once per week during pregnancy). However, individuals who did not meet this criterion were included if they met diagnostic criteria for fetal alcohol syndrome (FAS) or partial fetal alcohol syndrome (PFAS) based on dysmorphology and growth characteristics because these are relatively specific indicators of PAE. All participants completed a physical assessment conducted by one of two trained investigators (Kenneth Lyons Jones or JRW) to obtain ratings of the upper lip and philtrum, measurements of palpebral fissure length (PFL), height, weight, and HC. Diagnostic classification was completed in accordance with The Modified Institute of Medicine (IOM) criteria for FASD (Hoyme et al., 2016). For HC measurements, normative data were taken from (Nellhaus, 1968). Growth deficiency was classified by ≤ 10th percentile in height or weight based on CDC Growth Charts (Grummer-Strawn et al., 2010). In accordance with the IOM diagnostic criteria, neurobehavioral impairment was defined by global intellectual impairment (IQ ≤ 78, equivalent to ≥ 1.5 standard deviations [SD] below the mean) or standard scores ≥ 1.5 SD below the mean on ≥ 2 neuropsychological and parent-rated assessments in different domains (e.g. memory, executive function, etc.). All participants in the FASD group met IOM criteria for neurobehavioral impairment.
2.3. Evaluations
Participants completed evaluations at the University of Minnesota and were administered the following assessments: Wechsler Intelligence Scale for Children, 5th Edition (WISC-V) (Wechsler et al., 2014) or Wechsler Adult Intelligence Scale 4th Edition (WAIS-IV) (Wechsler, 2008) for participants age 17 years (measuring general intelligence [FSIQ, hereafter IQ]) (M = 100, SD = 15); NIH Toolbox (Weintraub et al., 2013) Picture Sequence Memory subtest T-score (PSMT; measuring visual memory) (M = 50, SD = 10); and the Delis-Kaplan Executive Functioning System (Delis et al. 2001) Trail-Making Test and Color-Word Interference Test (D-KEFS; measuring inhibitory control, attention, sequencing, and cognitive flexibility) (M = 10, SD = 3). In order to minimize the number of comparisons, the following variables were examined in the current study: IQ, working memory (WISC-V/WAIS-IV Digit Span), visual memory (NIH Toolbox PSMT), and executive functioning (D-KEFS Trail-Making Test Number-Letter Switching and D-KEFS Color-Word Interference Test Inhibition-Switching). All standard scores were converted to z-scores (M = 0, SD = 1) for ease of comparison.
2.4. MRI acquisition and processing
MRI data were acquired on a Siemens 3T Prisma scanner (Siemens, Erlangen, Germany) equipped with a standard 32-channel head coil at the University of Minnesota’s Center for Magnetic Resonance Research. Custom pulse sequences with automatic real-time motion detection and k-space line rejection and replacement were used to acquire T1-weighted and T2-weighted scans chosen to match those used in the Lifespan Human Connectome Project Development sub-project (HCP-D) (Harms et al., 2018). Processing of structural images was completed using the PreFreeSurfer and FreeSurfer (version 6.0) stages of the HCP Minimal Preprocessing Pipeline (v4.0.1) (Glasser et al., 2013). Notably, the training data used in creating the CentileBrain model were primarily collected from the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) Consortium (Ge et al., 2024). The ENIGMA group shares tools and guidelines for visual quality inspection of FreeSurfer outputs. Data were visually inspected by a trained operator (DJR) and research staff (KAT) to ensure accuracy. The ENIGMA quality control protocols were also used (Thompson et al., 2020). Three participants (comparison group) demonstrated significantly aberrant FreeSurfer processing (such as failed boundary identification) and they were excluded from analyses. Five additional participants (three comparison, two FASD) were excluded on the basis of motion during the visual inspection of the images or for being an extreme outlier in regional volume metrics. To assess for group differences in within-scanner motion in the remaining participants, we used the Euler value (Dale et al., 1999) averaged across hemispheres. Measurements were obtained for regional subcortical gray matter volumes of the bilateral amygdala, caudate, hippocampus, pallidum, putamen, thalamus, and accumbens area (Fig. 1a). These 7 regions were chosen based on prior literature showing selective impacts from PAE (Archibald et al., 2001, Bookstein et al., 2001, Gautam et al., 2015, Krueger et al., 2020, Mattson et al., 1996, Mattson et al., 1994, Nardelli et al., 2011, Roussotte et al., 2012).
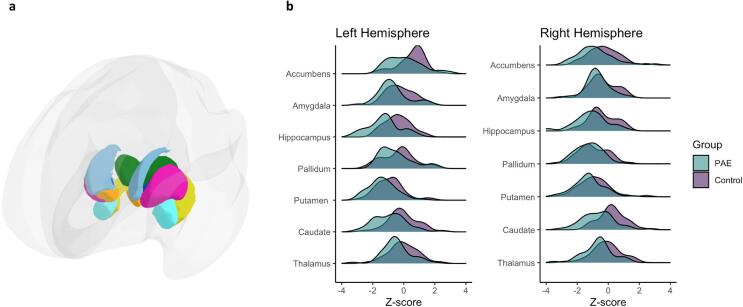
Fig. 1.
Panel a: Three dimensional illustration of parcellation used to generate regional subcortical volumes for the bilateral amygdala (teal), caudate (light blue), hippocampus (yellow), pallidum (dark blue), putamen (pink), thalamus (green), and accumbens area (orange); Panel b: Z-score distributions and group comparisons of the normed volumes for each of the subcortical regions by hemisphere. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Derivation of norm-adjusted volumetric scores
Individual regional volumetric z-scores were generated using CentileBrain (Ge et al., 2024), an open source online software tool that leverages an aggregated neuroimaging dataset with a large number of individuals (37,407 comparison brains). Z-scores (M = 0, SD = 1) were calculated for each of the 7 bilateral subcortical regions (Fig. 1a). These scores represent the degree of each participant’s regional volumetric (dis)similarity to typically-developing individuals of the same age and sex, adjusted for total intracranial volume (ICV). As a result of extensive modeling of possible covariates completed during development of the tool, CentileBrain uses ICV, which includes white matter, gray matter, cerebrospinal fluid, vasculature, blood, and other tissue (Ge et al. 2024). We examined both right and left subcortical regions separately because of well-known asymmetries in brain structure that are present in childhood and that are associated with brain function and dysfunction (Toga and Thompson, 2003, Kong et al., 2022). For example, subcortical volume asymmetries were atypically pronounced in youth with attention-deficit hyperactivity disorder (ADHD) compared to a typically-developing group (Douglas et al. 2018). Similarly, pediatric obsessive–compulsive disorder was associated with abnormal subcortical asymmetries (Kong et al. 2022). Most relevant to the current study, children with PAE have previously been shown to lack the right-left asymmetry in caudate volume seen in typically-developing children (Suttie et al. 2018). A specific Laterality Working Group within the ENIGMA consortium that developed the CentileBrain tool we employed here conducted six studies of gray matter asymmetries, concluded that asymmetries are critical in understanding brain function and pathology, and built their tool accordingly to provide normative data for both right and left volumes independently (Kong et al. 2022).
2.6. Statistical analysis
Statistical analyses were completed with R version 4.1.1 (R Core Team, n.d.). As a first step in determining the appropriateness of examining both right and left subcortical volumes, mean differences in regional volume z-scores for each left and right hemisphere region were examined across the entire sample with paired samples t-test. Participant characteristics and group differences in subcortical volume z-scores were examined using chi-square tests and independent samples t-tests. Independent samples t-tests were used to examine group differences in within-scanner motion (Euler values). In supplemental analyses, independent samples t-tests and chi-square tests were used to examine demographic differences between included participants and those excluded on the basis of visual inspection for excess within-scanner motion/poor image quality. Regional volume anomalies were characterized as “small” (z-scores ≤ -1.3, equivalent to the 10th percentile) and “large” (z-scores ≥ 1.3, equivalent to the 90th percentile). These thresholds for volumetric anomalies were selected in order to mirror IOM (Hoyme et al., 2016) diagnostic criteria for FASD with regard to head circumference and growth anomalies (e.g., height and weight) in which a threshold of ≤ 10th percentile is used. To test for potential confounding effects of prenatal substance exposures in addition to alcohol (cannabis, opioids, cocaine, and/or methamphetamine were reported), we used independent samples t-tests to compare subcortical volumes for two subgroups of the PAE group (those with other substance exposure [n = 12] vs. those without other exposure [n = 35]). For the primary analyses, we compared the relative frequencies of anomalous subcortical volumes across groups using sensitivity and specificity metrics strictly for the convenience of conveying results. It is important to note that, here, we only use these metrics for relative comparisons and are not suggesting that these measures would be used as a screening or diagnostic tool (for which the terms sensitivity and specificity are most often used). Using the total number of small regions, sensitivity (true positive rate), specificity (true negative rate), positive predictive value (probability of condition being present with a positive test), negative predictive value (probability of condition not being present with a negative test), and accuracy (overall probability of correct classification) were examined with regard to discriminating FASD and comparison participants. These metrics were also calculated for the total number of large regions in an identical manner. Sensitivity and specificity (for relative comparisons) were also calculated for small volumes across each subcortical region. In exploratory analyses, Pearson correlations (uncorrected for multiple comparisons given the exploratory nature of these analyses) were used to examine the relationship between the 7 subcortical region z-scores and neurocognitive function standard scores (Wechsler IQ, Wechsler Digit Span, NIH Toolbox PSMT, D-KEFS Trail-Making Test Number-Letter Switching, D-KEFS Color-Word Interference Test Inhibition-Switching). Given previous work identifying relationships between facial dysmorphology and subcortical anomalies (Roussotte et al., 2012, Suttie et al., 2018), within the FASD group, independent samples t-tests were used to compare subcortical volume z-scores for participants with and without a dysmorphic face (i.e., at least two of the following: palpebral fissure length ≤ 10 %ile, thin vermillion border, smooth philtrum [4 or 5 on lipometer scale]). Pearson correlations (uncorrected for multiple comparisons given the exploratory nature of these analyses) were used to examine the relationship between subcortical volume z-scores and height and weight percentile. Lastly, within the FASD group, a difference by sex in the total number of extreme volumes (i.e., regions classified as “small” or “large”) was examined with an independent sample t-test.
3. Results
3.1. Subject characteristics
A total of 100 participants were enrolled. Six comparison participants were excluded for not completing an MRI. Eight participants (6 comparison, 2 FASD) were excluded due to excess head motion (based on visual inspection), poor image quality (also based on visual inspection), or being an extreme outlier in regional volume metrics. Therefore, a total of 86 participants were included in the analyses (FASD = 47, comparison = 39). Participant demographic information is summarized in Table 1. Within the FASD group, there were 35 participants with alcohol-related neurodevelopmental disorder (ARND), 11 with partial fetal alcohol syndrome (PFAS), and 1 with fetal alcohol syndrome (FAS). FASD and comparison groups did not differ significantly regarding age, sex, ethnicity, handedness, or growth deficiency. Significantly more FASD participants had microcephaly and dysmorphic facial features than the comparison group. As expected, the FASD group had lower IQ scores compared to the comparison group. Groups differed significantly in terms of racial identity with more FASD participants than participants in the comparison group identifying as American Indian/Alaska Native, Black or African American, and Multiracial. A greater proportion of participants in the comparison group identified as White compared to the FASD group. There was no significant group difference in within-scanner motion as estimated with Euler values, t (56) = 1.39, p = 0.171. In supplemental analyses, there were no significant differences between included participants and those excluded due to excess within-scanner motion or poor MRI image quality with regard to age, t (8) = 0.29, p = 0.779, sex, χ2 (1) = 2.38, p = 0.123, IQ, t (8) = 0.79, p = 0.453, race (White vs. non-White), 2 (1) = 1.25, p = 0.264, ethnicity, 2 (1) = 0.90, p = 0.344, or handedness, 2 (2) = 0.244, p = 0.885. Tests for the potential confounding effects of other substance exposure (comparing subcortical volumes for participants in the PAE group with other substance exposure vs. participants in the PAE group without) revealed no significant differences (Cohen’s d’s ranged from 0.002 to 0.58; all p’s > 0.05). Because it was not a confounding variable, other substance exposure was not included in any subsequent analyses. Across the entire sample, volume z-scores were significantly smaller in the left compared to the right hemisphere for the caudate, t (85) = -6.32, p = 0.000, accumbens, t (85) = 8.30, p = 0.000, and at the trend-level for the hippocampus, t (85) = -1.89, p = 0.063. Volume z-scores were significantly smaller in the right hemisphere compared to the left for the thalamus, t (85) = 2.59, p = 0.011, and the pallidum, t (85) = 9.20, p = 0.000. These findings provide justification for examining left and right hemisphere volume z-scores separately (rather than averaging the two) in diagnostic group comparisons (FASD vs comparison).
Table 1.
Demographic characteristics of participants.
| FASD (n = 47) | Comparison (n = 39) | Statistical Test | |
|---|---|---|---|
| Age [M(SD)] | 12.30 (2.36) | 12.82 (2.50) | t(79) = 0.98, p = 0.330 |
| Intelligence Quotient [M(SD)] | 93.11 (15.03) | 115.39 (12.61) | t(83) = 7.43, p < 0.001 |
| Sex [n(%Female)] | 25 (53 %) | 21 (54 %) | χ2 = 0.00, p = 0.952 |
| Ethnicity [n(%Hispanic)] | 2 (4 %) | 2 (5 %) | χ2 = 0.04, p = 0.848 |
| Race* | |||
| [n(%American Indian/Alaska Native)] | 4 (9 %) | 0 | χ2 = 6.77, p = 0.009 |
| [n(%Asian)] | 2 (4 %) | 1 (3 %) | χ2 = 1.28, p = 0.258 |
| 1[n(%Black or AfricanAmerican)] | 12 (26 %) | 0 | χ2 = 17.20, p < 0.001 |
| [n(%Native Hawaiian/Other Pacific Islander)] | 1 | 0 | χ2 = 1.84, p = 0.175 |
| [n(%White)] | 21 (45 %) | 38 (97 %) | χ2 = 25.54, p < 0.001 |
| [n(%Multiracial)] | 7 (15 %) | 0 | χ2 = 11.04, p < 0.001 |
| Handedness [n(%Right)]† | 34 (72 %) | 33 (85 %) | χ2 = 1.04, p = 0.594 |
| Physical characteristics | |||
| aGrowth Deficiency | 5 (11 %) | 3 (8 %) | χ2 = 0.22, p = 0.640 |
| bMicrocephaly | 5 (11 %) | 0 | χ2 = 3.92, p = 0.048 |
| cDysmorphic Face | 12 (26 %) | 2 (5 %) | χ2 = 5.49, p = 0.019 |
FASD: Fetal Alcohol Spectrum Disorder group.
Chi square tests reflect comparisons of each racial group to the proportion of participants who identified as White (e.g., proportion of participants who identified as Multiracial to proportion of those who identified as White).
Handedness information was not available for 5 participants (4 PAE, 1 comparison).
Height or weight ≤ 10 %ile.
Head circumference ≤ 10 %ile.
At least two of the following: Palpebral fissure length ≤ 10 %ile, thin vermillion border, smooth philtrum (4 or 5 on lipometer scale). The two comparison participants who had “dysmorphic faces” had scores of 4 on the philtrum and 4 on the vermillion border; neither had any other facial features nor abnormal growth parameters.
3.2. Group differences in subcortical volumes
Participants in the FASD group demonstrated lower mean z-scores than the comparison group across 6 of the 7 subcortical regions (Fig. 1b and Table 2) including the bilateral thalami, bilateral caudate, left putamen, bilateral hippocampi, left amygdala, and right accumbens area. No significant group difference was observed for the right putamen, bilateral pallidum, right amygdala, and left accumbens area. Effect sizes were largest for the bilateral caudate and bilateral hippocampi. A total of 89 % of participants in the FASD group demonstrated one or more small subcortical volume compared to 72 % of the comparison group. A total of 9 % of participants in the FASD group and 15 % of the comparison group showed one or more large volume. Participants in the FASD group demonstrated a greater mean number of small subcortical volumes (M = 4.53, SD = 3.13) compared to the comparison group (M = 2.46, SD = 2.30; t [83] = −3.53, p < 0.001, d = −0.74). There was no significant difference in the mean number of large volumes between participants in the FASD group (M = 0.19; SD = 0.74) and the comparison group (M = 0.15, SD = 0.37; t [70] = −0.31, p = 0.760, d = −0.06).
Table 2.
Diagnostic group differences in regional subcortical volume z-scores.
| Mean (SD) | FASD | Comparison | Statistic (t) | Significance (p-value) | Effect Size (Cohen’s d) |
|---|---|---|---|---|---|
| Left thalamus | −0.46 (0.92) | −0.02 (0.91) | 2.22 | 0.029 | 0.48 |
| Right thalamus | −0.65 (0.95) | −0.17 (0.87) | 2.42 | 0.017 | 0.52 |
| Left caudate | −0.87 (1.09) | −0.01 (0.93) | 3.97 | < 0.001 | 0.85 |
| Right caudate | −0.62 (1.09) | 0.26 (0.84) | 4.24 | < 0.001 | 0.90 |
| Left putamen | −1.36 (0.89) | −0.92 (0.91) | 2.28 | 0.025 | 0.50 |
| Right putamen | −1.25 (0.96) | −0.96 (1.05) | 1.31 | 0.193 | 0.29 |
| Left pallidum | −0.49 (1.08) | −0.39 (1.05) | 0.45 | 0.656 | 0.10 |
| Right pallidum | −1.25 (0.80) | −0.99 (0.98) | 1.33 | 0.188 | 0.29 |
| Left hippocampus | −1.31 (1.10) | −0.48 (0.80) | 4.05 | < 0.001 | 0.85 |
| Right hippocampus | −1.09 (1.09) | −0.47 (0.88) | 2.96 | 0.004 | 0.63 |
| Left amygdala | −0.69 (0.99) | −0.25 (0.98) | 2.07 | 0.042 | 0.45 |
| Right amygdala | −0.67 (0.76) | −0.46 (0.86) | 1.17 | 0.246 | 0.26 |
| Left accumbens | 0.07 (1.14) | 0.48 (0.84) | 1.94 | 0.056 | 0.41 |
| Right accumbens | −0.83 (1.17) | −0.27 (1.0) | 2.38 | 0.019 | 0.51 |
NOTE: The z-scores in the table are standardized scores, with a mean of 0 and a standard deviation of 1, representing the amount of deviation from the normative mean for each subcortical volume. All normative means have been adjusted for age, sex, and intracranial volume (ICV). All p-values are unadjusted.
3.3. Relative sensitivity and specificity analyses
Small volumes (z-score ≤ −1.3) of the bilateral putamen, bilateral hippocampi, right pallidum, and right accumbens area had the highest relative sensitivity, each correctly identifying 40 to 50 percent of FASD participants (Table 3). Smaller volumes in the bilateral caudate, right thalamus, and left amygdala, while relatively less sensitive, were relatively specific to the FASD group, each identifying approximately 20 to 30 percent of FASD participants. Relative specificity for smaller volumes across a majority of regions was generally high reflecting that fewer comparison group participants demonstrate small volumes.
Table 3.
Relative sensitivity and specificity of small regional subcortical volumes.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Left thalamus | 17 % | 90 % | 67 % | 47 % | 50 % |
| Right thalamus | 23 % | 92 % | 79 % | 50 % | 55 % |
| Left caudate | 36 % | 92 % | 85 % | 55 % | 62 % |
| Right caudate | 28 % | 100 % | 100 % | 53 % | 60 % |
| Left putamen | 53 % | 62 % | 63 % | 52 % | 57 % |
| Right putamen | 47 % | 67 % | 63 % | 51 % | 56 % |
| Left pallidum | 26 % | 77 % | 57 % | 46 % | 49 % |
| Right pallidum | 45 % | 54 % | 54 % | 45 % | 49 % |
| Left hippocampus | 49 % | 82 % | 77 % | 57 % | 64 % |
| Right hippocampus | 43 % | 87 % | 80 % | 56 % | 63 % |
| Left amygdala | 23 % | 90 % | 73 % | 49 % | 53 % |
| Right amygdala | 13 % | 85 % | 50 % | 45 % | 45 % |
| Left accumbens | 11 % | 92 % | 63 % | 46 % | 48 % |
| Right accumbens | 40 % | 85 % | 76 % | 54 % | 60 % |
Note: PPV Positive predictive value; NPV Negative predictive value; small volumes reflect z-scores ≤ -1.3.
3.4. Association of subcortical volumes to neurocognitive function
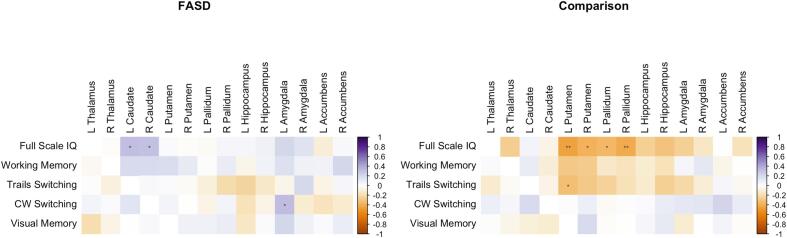
Uncorrected exploratory correlations conducted separately for FASD and comparison groups revealed significant positive correlations between bilateral caudate volumes and both IQ and cognitive flexibility (D-KEFS Color-Word Interference Test Switching) for FASD participants (Fig. 2). For Comparison participants, significant negative correlations were observed between IQ and volumes of the bilateral putamen and pallidum and between left putamen volume and a measure of visual-motor speed and cognitive flexibility (D-KEFS Trail-Making Test Number-Letter Switching).
Fig. 2.
Exploratory (uncorrected) correlations between regional subcortical volume z-scores and neurocognitive and neurobehavioral function. Blue-shaded cells indicate positive correlations; red-shaded cells indicate negative correlations. IQ = Intelligence Quotient; CW = D-KEFS Color-Word Interference Test. *p < 0.05, **p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Association of subcortical volumes to facial features, growth, and sex within FASD
There were no significant differences between participants with and without dysmorphic faces in regional subcortical volume z-scores. There were no significant correlations between subcortical volumes and height or weight percentile. Lastly, there was no significant difference in the total number of extreme volumes by sex.
4. Discussion
We demonstrate the value of normative MRI “brain growth charts” in identifying and quantifying regional subcortical brain anomalies in youth with FASD at the individual level. Building on previous findings of reduced whole-brain volumes for cortical white matter and cortical and subcortical gray matter in FASD (Gimbel et al., 2024), we demonstrate smaller regional subcortical volumes in youth with FASD after applying normative corrections accounting for age, sex, and ICV. In addition, we show that abnormal subcortical volumes in several specific regions, including the hippocampus and putamen, are common in FASD and could potentially help identify neurodevelopmental anomalies that would otherwise go unidentified. Finally, we find that subcortical volumes are associated with cognitive skills important for supporting lifelong independent functioning and well-being in youth with FASD.
4.1. Group differences in subcortical volumes
As expected, participants in the FASD group demonstrated lower mean subcortical volume z-scores (adjusted for age, sex, and ICV) across a majority of regions compared to typically-developing comparison participants, consistent with evidence from some human and animal studies suggesting vulnerability of subcortical structures to the teratogenic insult of PAE (Lebel et al., 2011, Moore et al., 2014, Petrelli et al., 2018). Specifically, participants in the FASD group demonstrated lower mean volume z-scores than the comparison group in 6 out of 7 subcortical regions of interest including the bilateral thalami, caudate, and hippocampi and lateralized smaller volumes in the left amygdala, left putamen, and right accumbens area. In total, 89 percent of individuals in the FASD group demonstrated one or more small subcortical volume compared to 72 percent of the comparison group.
These findings are consistent with prior work in which uncorrected neuroimaging data features were used (e.g., values for regional volumes without adjusting for age and sex using large normative datasets). Specifically, some studies of children and adolescents with FASD/PAE have documented smaller subcortical volumes compared to unexposed comparisons even after controlling for whole brain volumes (i.e., accounting for TBV or ICV) in regions such as the caudate (Astley et al., 2009, Nardelli et al., 2011, Boateng et al., 2023, Archibald et al., 2001), putamen (Roussotte et al., 2012, Nardelli et al., 2011), pallidum (Roussotte et al., 2012, Boateng et al., 2023, Nardelli et al., 2011), thalamus (Boateng et al., 2023, Nardelli et al., 2011), and hippocampus (Boateng et al., 2023, Willoughby et al., 2008, Nardelli et al., 2011). However, it is important to note that several other child and adolescent studies have found either no such group differences across subcortical regions (Subramoney et al. 2022) or only smaller subcortical volumes without controlling for TBV or ICV (Riikonen et al., 2005, Biffen et al., 2018). We observed the largest mean group differences in z-scores for volumes of the bilateral hippocampi and bilateral caudate—regions in which smaller volumes have been repeatedly demonstrated in individuals with FASD (Lebel et al., 2011, Mattson et al., 2019, Moore et al., 2014). Together, results of diagnostic group comparisons in regional subcortical volumes derived from a novel normative modeling approach are consistent with previous work using uncorrected brain volume data, highlighting widespread structural brain anomalies in youth with FASD. The specific regions in which the largest differences were observed here, the caudate nuclei and the hippocampi (both part of the mesolimbic dopamine system and hypothalamic–pituitary–adrenal [HPA] axis), serve important information processing and behavior/emotion regulation functions that are highly relevant to FASD (Mattson et al., 1996, Krueger et al., 2020, Saint-Cyr, 2003). For example, the basal ganglia, including the caudate nuclei, play an important role in modulating attentional control and learning among other functions (Saint-Cyr, 2003). In one study, caudate volume (after accounting for ICV) was significantly associated with elements of cognitive control and memory (perseverations, false positive errors, and total memory performance) in a sample of children and adolescents with heavy PAE (Fryer et al., 2012). In another study, adolescents with depressive disorders were found to have smaller volumes in the hippocampus, amygdala, and putamen (Whittle et al., 2014). The hippocampi are critically important for memory functioning (Hannula and Duff, 2018, Li et al., 2019), and previous studies have shown associations between atypical hippocampal structure and cognitive functioning in FASD (Roediger et al., 2021, Willoughby et al., 2008). Thus, the findings are consistent with the literature and suggest possible relationships with neurobehavioral challenges that are commonly seen in FASD.
Unexpectedly, our participants (including those in the comparison group) demonstrated smaller than expected norm-corrected subcortical volumes (mean z-scores for those in the comparison group were negative as opposed to zero) in several regions including the left and right putamen and the right pallidum. These lower than expected z-scores could be due to differences in image acquisition and processing. The CentileBrain model is based on structural imaging data from 87 datasets. These datasets are heterogeneous and include a variety of MR pulse sequences, magnetic field strengths, vendors, resolutions, and preprocessing steps. Our acquisition and preprocessing decisions were aligned with the Human Connectome Project (Glasser et a., 2013, Harms et al., 2018) rather than aiming to “match” the normative data. Our preprocessing workflow included some steps (i.e., intensity normalization and gradient distortion correction using the proprietary Siemens gradient table), which may not have been performed in some of the datasets that informed the CentileBrain model. Additionally, while the HCP Minimal Preprocessing Pipelines are based around FreeSurfer version 6.0, many of the datasets used for CentileBrain were processed using either older or more recent versions of FreeSurfer (≤ 5.3 or ≥ 7.1) (Ge et al., 2024). Small differences across FreeSurfer versions, as well as the optional arguments used during processing, may have a significant impact on morphometric analyses and may explain the smaller than expected volumes we observed across both groups of participants in some subcortical structures (Filip et al., 2022). In direct comparisons of FreeSurfer versions, prominent differences in regional subcortical volumes have been reported between versions 5.3 and 6.0, while versions 6.0 and 7.1 are reported to be relatively consistent (Bigler et al., 2018, Haddad et al., 2022). Thus, we expect that the systematic differences observed between our data and the normative data were largely caused by the inclusion of many older (versions ≤ 5.3) FreeSurfer outputs in the CentileBrain training set. Despite the systematic difference between our data and the normative data, we were still able to detect that the FASD group had smaller subcortical volumes than the comparison group.
In order to further parse individual variation in subcortical volume differences, we examined associations of norm-corrected subcortical volume z-scores with demographic variables within the FASD group. We observed no significant differences in the number of abnormal subcortical volumes by sex or facial dysmorphology, and there were no significant associations between subcortical volume z-scores and growth abnormalities in height and weight. Interestingly, previous work has documented an association between the degree of regional subcortical anomalies and dysmorphic facial features such as ratings of the upper lip, philtrum, and measurements of the eye opening (palpebral fissure length) (Roussotte et al., 2012). Future research should examine relationships between clinical characteristics and structural brain differences at the level of the individual.
4.2. Relative proportions of subcortical anomalies in FASD vs. A comparison group
Normative modeling of neuroimaging data may offer several benefits over traditional approaches using uncorrected data features by offering a method for characterizing within-group and within-individual variations in brain structure, which is obscured in traditional case-control studies that (perhaps erroneously) assume within-group homogeneity (Rutherford et al., 2022). Normative modeling may also function to reduce sources of noise or variance in the data (e.g., due to age, sex, and within-scanner motion) (Rutherford et al., 2023) offering psychometric advantages over uncorrected data features. We leverage this novel normative modeling approach to demonstrate the relative proportions of atypically small regional subcortical volumes and the potential utility of MRI evidence in characterizing underlying neurodevelopmental anomalies. Small volumes (z-scores ≤ -1.3) in the bilateral putamen, bilateral hippocampi, right pallidum, and right accumbens occurred in 40 to 50 percent of FASD participants. Small volumes in the bilateral caudate, right thalamus, or left amygdala were relatively specific to the FASD group. Similarly, a study using a multivariate classification approach to analyzing MRI-derived regional brain volumes in two independent samples of youth with FASD (ages 5–18 years and 6–19 years, respectively) found that among the most heavily weighted brain regions in the model were the left globus pallidus and left and right caudate (Little and Beaulieu, 2020). Our findings are consistent with our previous work (Gimbel et al., 2024) showing that smaller whole-brain volumes are common in youth with FASD and suggest that smaller regional subcortical volumes could play a role in characterizing neurodevelopmental effects of PAE. These findings highlight the importance of examining the variability of structural brain anomalies in youth with FASD at the individual level. Further research with larger samples is needed and may offer valuable insights into risk and protective factors that are associated with individual variation in structural brain anomalies (e.g., timing and amount of PAE, maternal nutrition, genetic variation).
4.3. Associations of subcortical volumes to cognitive functioning
In exploratory analyses, we show that regional subcortical volumes adjusted for ICV, age, and sex are associated with aspects of neurocognitive functioning with diverging patterns across diagnostic groups. For youth with FASD, smaller bilateral caudate volume z-scores were associated with lower IQ and lower executive functioning (i.e., a measure of cognitive flexibility). These findings are consistent with previous work identifying the caudate as an important structure in neurocognitive functions (e.g., intellectual performance, executive functioning skills such as planning and working memory; learning/memory; and reward processing) (Janacsek et al., 2022, Pangelinan et al., 2011), as well as findings of a relationship between reduced caudate volumes and deficits in cognitive control and verbal learning and recall in youth with FASD (Fryer et al., 2012). In contrast, for participants in the comparison group, higher IQ was associated with lower volume z-scores in the bilateral putamen and pallidum. Together, these findings suggest smaller regional subcortical volumes in FASD may contribute to important functional outcomes such as lower IQ and may point to atypical brain-behavior relationships in youth with FASD.
5. Limitations
There are several limitations of this study that should be acknowledged. Participants in this study were recruited based on a history of PAE or being typically-developing (resulting in two distinct groups). As such, further research is needed to extend this approach in samples with greater generalizability (e.g., samples including youth with other neurodevelopmental conditions such as ADHD and autism spectrum disorder). Polysubstance exposure is common in individuals who were exposed to alcohol prenatally and was, therefore, not exclusionary in this study. We did, however, conduct analyses which showed that other substance exposure was not a confounding variable in relation to our outcomes of interest (subcortical volumetrics). We did not have access to detailed information about tobacco exposures and, therefore, we were unable to determine if prenatal tobacco exposure could have influenced the results. Also, there was a higher proportion of White participants in the comparison group vs. the FASD group, potentially introducing bias into the analyses. For example, lower socioeconomic status (SES),which is associated with race in the United States, has been shown to be associated with smaller brain volumes including subcortical volumes (Jenkins et al., 2020). We did not attempt to characterize the participants’ SES because we did not have access to details of the birth parents’ races, education levels, incomes, occupations, or other socioeconomic details. Furthermore, children were with their birth parents for different lengths of time, and some children also lived with foster parents (introducing a third set of socioeconomic variables). Therefore, it is important to acknowledge that the neurodevelopmental anomalies observed here are not the sole result of PAE but, rather, likely represent the combined impact of numerous factors including PAE, other prenatal substance exposures, SES, and other associated factors. It is worth noting that at least two previous studies have examined the relationship between SES and subcortical volumes in children with FASD and comparison groups (McLachlan et al., 2020, Uban et al., 2020). Both studies found that SES correlated positively with brain volumes in the comparison group but there were no significant correlations in the groups of children with FASD. The authors of those studies suggest that early neurodevelopmental insults like PAE may limit the ability of the environment/SES to positively influence subsequent brain growth. They also suggest that the differences in SES between birth parents and adoptive parents contributes error/variance to the quantification of SES and, therefore, may lead to lower correlations in FASD samples. Our sample was also cognitively high-functioning (i.e., mean IQ was average for the FASD group and high average for participants in the comparison group), which differs from a number of studies with other samples documenting deficits in IQ in youth with FASD (Mattson et al., 2019) and suggests that we had an acquisition bias in our sample (higher functioning families were more likely to respond to requests to participate, etc.). This may also suggest that, for some individuals in the FASD group, marginally lower IQ performance may not be the most clinically relevant functional domain in the context of other neurocognitive impairments. Our sample of youth with FASD also consisted predominantly of individuals with ARND (n = 35) and had fewer individuals with FAS (n = 1) and PFAS (n = 11). Replication of our findings with FASD samples with a wider range of clinical presentations will be important.
In the context of previous research findings suggesting a vulnerability of subcortical structures to PAE, we focused our examination on subcortical regions of interest. However, it is important to acknowledge that PAE has numerous impacts on brain development. As such, associations between any single aspect of brain structure and cognitive function are not evidence of causality and must be interpreted in the context of the existing literature. Future work using the normative modeling method we used here could benefit from network-based approaches that model the structural and functional connectivity between regions in the brain (e.g., between cortical and subcortical regions) (Grayson and Fair, 2017, Sporns, 2013). It is also important to note that we found systematically smaller norm-corrected subcortical volumes in our participants, likely reflecting differences in image acquisition and processing differences with the heterogeneous samples used by CentileBrain, which represents a limitation of this study.
6. Conclusion
We demonstrate the value of using a normative modeling approach to characterizing structural subcortical brain anomalies that occur in FASD. Youth with FASD show significantly smaller volumes across a majority of subcortical regions compared to typically-developing individuals. In addition, youth with FASD who show atypical subcortical volumes demonstrate greater impairment in neurocognitive functioning, suggesting such brain-based differences may contribute to important functional outcomes.
Data availability statement
Limited, de-identified data are available upon request; Please see https://doi.org/10.5967/ntw9-h991 for more information.
Funding information
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD, https://doi.org/10.5967/ntw9-h991), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD, including information describing available data, can be found at www.cifasd.org. This work was supported by the NIAAA (grant numbers 5U01AA026102, 5U01AA014834, 5U24AA014815, 5U24AA014811), the National Institute of Biomedical Imaging and Bioengineering (NIBIB P41 EB027061), the Biotechnology Research Center (P41 EB015 894), the NINDS Institutional Center Core Grants to Support Neuroscience Research (P30 NS076408), and the High Performance Connectome Upgrade for Human 3T MR Scanner (1S10OD017974-01).
CRediT authorship contribution statement
Blake A. Gimbel: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Donovan J. Roediger: Writing – review & editing, Visualization, Methodology, Data curation, Conceptualization. Mary E. Anthony: Writing – review & editing, Project administration, Data curation. Abigail M. Ernst: Writing – review & editing, Project administration, Data curation. Kent A. Tuominen: Writing – review & editing, Data curation. Bryon A. Mueller: Writing – review & editing, Methodology, Data curation. Erik de Water: Writing – review & editing, Formal analysis, Data curation. Madeline N. Rockhold: Writing – review & editing, Project administration, Data curation. Jeffrey R. Wozniak: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Acknowledgements
We thank the children and families who participated in this research. We acknowledge the contributions of Proof Alliance (formerly the Minnesota Organization on Fetal Alcohol Spectrum Disorders), which included assistance with participant recruitment and public awareness of the study. We also acknowledge the contributions of Kenneth Lyons Jones, Alyssa Krueger and Christopher Lindgren who assisted with study execution.
Data availability
Data will be made available on request.
References
- Archibald S.L., Fennema-Notestine C., Gamst A., Riley E.P., Mattson S.N., Jernigan T.L. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev. Med. Child Neurol. 2001;43(3):148–154. [PubMed] [Google Scholar]
- Astley S.J., Aylward E.H., Olson H.C., Kerns K., Brooks A., Coggins T.E., Davies J., Dorn S., Gendler B., Jirikowic T., Kraegel P., Maravilla K., Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem R.A.I., Seidlitz J., White S.R., Vogel J.W., Anderson K.M., Adamson C., Adler S., Alexopoulos G.S., Anagnostou E., Areces-Gonzalez A., Astle D.E., Auyeung B., Ayub M., Bae J., Ball G., Baron-Cohen S., Beare R., Bedford S.A., Benegal V., Alexander-Bloch A.F. Brain charts for the human lifespan. Nature. 2022;604(7906):525–533. doi: 10.1038/s41586-022-04554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffen S.C., Warton C.M.R., Lindinger N.M., Randall S.R., Lewis C.E., Molteno C.D., Jacobson J.L., Jacobson S.W., Meintjes E.M. Reductions in corpus callosum volume partially mediate effects of prenatal alcohol exposure on IQ. Front. Neuroanat. 2018;11:132. doi: 10.3389/fnana.2017.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D., Skiles M., Wade B.S.C., Abildskov T.J., Tustison N.J., Scheibel R.S., Newsome M.R., Mayer A.R., Stone J.R., Taylor B.A., Tate D.F., Walker W.C., Levin H.S., Wilde E.A. FreeSurfer 5.3 versus 6.0: are volumes comparable? A Chronic Effects of Neurotrauma Consortium study. Brain Imaging Behav. 2018 doi: 10.1007/s11682-018-9994-x. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A., Stoner S.A., Riley E.P., Moore E.M. Subcortical volume in middle-aged adults with fetal alcohol spectrum disorders. Brain Commun. 2024;6(5) doi: 10.1093/braincomms/fcae273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjuland K.J., Rimol L.M., Løhaugen G.C.C., Skranes J. Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. Eur. J. Paediat. Neuro.: EJPN: Off. J. Euro. Paediat. Neurol. Soc. 2014;18(5):578–590. doi: 10.1016/j.ejpn.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Boateng T., Beauchamp K., Torres F., Ruffaner-Hanson C.D., Pinner J.F.L., Vakamudi K., Cerros C., Hill D.E., Stephen J.M. Brain structural differences in children with fetal alcohol spectrum disorder and its subtypes. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1152038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein F.L., Sampson P.D., Streissguth A.P., Connor P.D. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 2001;64(1):4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Coles C.D., Goldstein F.C., Lynch M.E., Chen X., Kable J.A., Johnson K.C., Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn. 2011;75(1):67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and Surface Reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis D.C., Kaplan E., Kramer J.H. The Psychological Corporation; San Antonio, TX: 2001. Delis-Kaplan executive function system (D-KEFS) [Google Scholar]
- Dodge N.C., Thomas K.G.F., Meintjes E.M., Molteno C.D., Jacobson J.L., Jacobson S.W. Reduced Hippocampal Volumes Partially Mediate Effects of Prenatal Alcohol Exposure on Spatial Navigation on a Virtual Water Maze Task in Children. Alcohol. Clin. Exp. Res. 2020 doi: 10.1111/acer.14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas P.K., Gutman B., Anderson A., Larios C., Lawrence K.E., Narr K., Sengupta B., Cooray G., Douglas D.B., Thompson P.M., McGough J.J., Bookheimer S.Y. Hemispheric brain asymmetry differences in youths with attention-deficit/hyperactivity disorder. NeuroImage. Clinical. 2018;18:744–752. doi: 10.1016/j.nicl.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek J., Skocic J., Sheard E., Rovet J. Hippocampal abnormalities in youth with alcohol-related neurodevelopmental disorder. J. Int. Neuropsychol. Soc.: JINS. 2014;20(2):181–191. doi: 10.1017/S1355617713001343. [DOI] [PubMed] [Google Scholar]
- Fernández de Gamarra-Oca L., Kvanta H., Broström L., Nosko D., Eklöf E., Ojeda N., Zubiaurre-Elorza L., Padilla N., Ådén U. Hippocampal volumes and cognitive performance in children born extremely preterm with and without low-grade intraventricular haemorrhage. Brain Struct. Funct. 2023;228(5):1191–1200. doi: 10.1007/s00429-023-02643-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip P., Bednarik P., Eberly L.E., Moheet A., Svatkova A., Grohn H., Kumar A.F., Seaquist E.R., Mangia S. Different FreeSurfer versions might generate different statistical outcomes in case–control comparison studies. Neuroradiology. 2022;64(4):765–773. doi: 10.1007/s00234-021-02862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer S.L., Mattson S.N., Jernigan T.L., Archibald S.L., Jones K.L., Riley E.P. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2012;36(11):1932–1941. doi: 10.1111/j.1530-0277.2012.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P., Lebel C., Narr K.L., Mattson S.N., May P.A., Adnams C.M., Riley E.P., Jones K.L., Kan E.C., Sowell E.R. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Hum. Brain Mapp. 2015;36(6):2318–2329. doi: 10.1002/hbm.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R., Yu Y., Qi Y.X., Fan Y.-N., Chen S., Gao C., Haas S.S., New F., Boomsma D.I., Brodaty H., Brouwer R.M., Buckner R., Caseras X., Crivello F., Crone E.A., Erk S., Fisher S.E., Franke B., Glahn D.C., ENIGMA Lifespan Working Group Normative modelling of brain morphometry across the lifespan with CentileBrain: algorithm benchmarking and model optimisation. The Lancet. Digital Health. 2024;6(3):e211–e221. doi: 10.1016/S2589-7500(23)00250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel B.A., Roediger D.J., Ernst A.M., Anthony M.E., de Water E., Mueller B.A., Rockhold M.N., Schumacher M.J., Mattson S.N., Jones K.L., Lim K.O., Wozniak J.R. Delayed cortical thinning in children and adolescents with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2023;47(7):1312–1326. doi: 10.1111/acer.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel B.A., Roediger D.J., Ernst A.M., Anthony M.E., Mueller B.A., de Water E., Rockhold M.N., Collaborative Initiative on Fetal Alcohol Spectrum Disorders, Wozniak J.R. Normative magnetic resonance imaging data increase the sensitivity to brain volume abnormalities in the classification of fetal alcohol spectrum disorder. J. Pediatr. 2024;266 doi: 10.1016/j.jpeds.2023.113868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L.A., Moore E.M., Wozniak J.R., Coles C.D., Kable J.A., Sowell E.R., Jones K.L., Riley E.P., Mattson S.N., CIFASD Neural correlates of verbal memory in youth with heavy prenatal alcohol exposure. Brain Imaging Behav. 2018;12(3):806–822. doi: 10.1007/s11682-017-9739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummer-Strawn L.M., Reinold C., Krebs N.F., Centers for Disease Control and Prevention (CDC) Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report. Recommen. Rep. / Center Disease Cont. 2010;59(RR-9):1–15. [PubMed] [Google Scholar]
- Haddad, E., Pizzagalli, F., Zhu, A. H., Bhatt, R. R., Islam, T., Gari, I. B., Dixon, D., Thomopoulos, S. I., Thompson, P. M., Jahanshad, N., 2022. Multisite test-retest reliability and compatibility of brain metrics derived from FreeSurfer versions 7.1, 6.0, and 5.3. 10.1101/2022.04.13.488251. [DOI] [PMC free article] [PubMed]
- Hannula D.E., Duff M.C., editors. The Hippocampus from Cells to Systems. Springer International Publishing; 2018. [Google Scholar]
- Harms M.P., Somerville L.H., Ances B.M., Andersson J., Barch D.M., Bastiani M., Bookheimer S.Y., Brown T.B., Buckner R.L., Burgess G.C., Coalson T.S., Chappell M.A., Dapretto M., Douaud G., Fischl B., Glasser M.F., Greve D.N., Hodge C., Jamison K.W., Yacoub E. Extending the human connectome project across ages: imaging protocols for the lifespan development and aging projects. Neuroimage. 2018;183:972–984. doi: 10.1016/j.neuroimage.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme H.E., Kalberg W.O., Elliott A.J., Blankenship J., Buckley D., Marais A.-S., Manning M.A., Robinson L.K., Adam M.P., Abdul-Rahman O., Jewett T., Coles C.D., Chambers C., Jones K.L., Adnams C.M., Shah P.E., Riley E.P., Charness M.E., Warren K.R., May P.A. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics. 2016;138(2) doi: 10.1542/peds.2015-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janacsek K., Evans T.M., Kiss M., Shah L., Blumenfeld H., Ullman M.T. Subcortical Cognition: The Fruit Below the Rind. Annu. Rev. Neurosci. 2022;45:361–386. doi: 10.1146/annurev-neuro-110920-013544. [DOI] [PubMed] [Google Scholar]
- Jarmasz J.S., Basalah D.A., Chudley A.E., Del Bigio M.R. Human brain abnormalities associated with prenatal alcohol exposure and fetal alcohol spectrum disorder. J. Neuropathol. Exp. Neurol. 2017;76(9):813–833. doi: 10.1093/jnen/nlx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L.M., Chiang J.J., Vause K., Hoffer L., Alpert K., Parrish T.B., Wang L., Miller G.E. Subcortical structural variations associated with low socioeconomic status in adolescents. Hum. Brain Mapp. 2020;41(1):162–171. doi: 10.1002/hbm.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.L., Smith D.W., Ulleland C.N., Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Joseph J., Warton C., Jacobson S.W., Jacobson J.L., Molteno C.D., Eicher A., Marais P., Phillips O.R., Narr K.L., Meintjes E.M. Three-dimensional surface deformation-based shape analysis of hippocampus and caudate nucleus in children with fetal alcohol spectrum disorders. Hum. Brain Mapp. 2014;35(2):659–672. doi: 10.1002/hbm.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X.-Z., Postema M.C., Guadalupe T., de Kovel C., Boedhoe P.S.W., Hoogman M., Mathias S.R., van Rooij D., Schijven D., Glahn D.C., Medland S.E., Jahanshad N., Thomopoulos S.I., Turner J.A., Buitelaar J., van Erp T.G.M., Franke B., Fisher S.E., van den Heuvel O.A., Francks C. Mapping brain asymmetry in health and disease through the ENIGMA consortium. Hum. Brain Mapp. 2022;43(1):167–181. doi: 10.1002/hbm.25033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A.M., Roediger D.J., Mueller B.A., Boys C.A., Hendrickson T.J., Schumacher M.J., Mattson S.N., Jones K.L., Riley E.P., Lim K.O., Wozniak J.R. Para-limbic structural abnormalities are associated with internalizing symptoms in children with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2020;44(8):1598–1608. doi: 10.1111/acer.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Probst C., Gmel G., Rehm J., Burd L., Popova S. Global prevalence of fetal alcohol spectrum disorder among children and youth: A systematic review and meta-analysis. JAMA Pediatr. 2017;171(10):948–956. doi: 10.1001/jamapediatrics.2017.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Roussotte F., Sowell E.R. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol. Rev. 2011;21(2):102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Shen M., Stockton M.E., Zhao X. Hippocampal deficits in neurodevelopmental disorders. Neurobiol. Learn. Mem. 2019;165(106945) doi: 10.1016/j.nlm.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little G., Beaulieu C. Multivariate models of brain volume for identification of children and adolescents with fetal alcohol spectrum disorder. Hum. Brain Mapp. 2020;41(5):1181–1194. doi: 10.1002/hbm.24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Riley E.P., Jernigan T.L., Garcia A., Kaneko W.M., Ehlers C.L., Jones K.L. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol. Teratol. 1994;16(3):283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Mattson S.N., Riley E.P., Sowell E.R., Jernigan T.L., Sobel D.F., Jones K.L. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol. Clin. Exp. Res. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson S.N., Foroud T., Sowell E.R., Jones K.L., Coles C.D., Fagerlund A., Autti-Rämö I., May P.A., Adnams C.M., Konovalova V., Wetherill L., Arenson A.D., Barnett W.K., Riley E.P., CIFASD Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44(7–8):635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Bernes G.A., Doyle L.R. Fetal alcohol spectrum disorders: A review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2019;43(6):1046–1062. doi: 10.1111/acer.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P.A., Chambers C.D., Kalberg W.O., Zellner J., Feldman H., Buckley D., Kopald D., Hasken J.M., Xu R., Honerkamp-Smith G., Taras H., Manning M.A., Robinson L.K., Adam M.P., Abdul-Rahman O., Vaux K., Jewett T., Elliott A.J., Kable J.A., Hoyme H.E. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA. 2018;319(5):474–482. doi: 10.1001/jama.2017.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan K., Zhou D., Little G., Rasmussen C., Pei J., Andrew G., Reynolds J.N., Beaulieu C. Current socioeconomic status correlates with brain volumes in healthy children and adolescents but not in children with prenatal alcohol exposure. Front. Hum. Neurosci. 2020;14:223. doi: 10.3389/fnhum.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E.M., Migliorini R., Infante M.A., Riley E.P. Fetal Alcohol Spectrum Disorders: Recent Neuroimaging Findings. Curr. Dev. Disord. Rep. 2014;1(3):161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A., Lebel C., Rasmussen C., Andrew G., Beaulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2011;35(8):1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- Nellhaus G. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968;41(1):106–114. [PubMed] [Google Scholar]
- Pangelinan M.M., Zhang G., VanMeter J.W., Clark J.E., Hatfield B.D., Haufler A.J. Beyond age and gender: relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-age children. Neuroimage. 2011;54(4):3093–3100. doi: 10.1016/j.neuroimage.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli B., Weinberg J., Hicks G.G. Effects of prenatal alcohol exposure (PAE): insights into FASD using mouse models of PAE. Biochem. Cell Bio.Bioch. Biol. Cell. 2018;96(2):131–147. doi: 10.1139/bcb-2017-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (n.d.). R: A language and environment for statistical computing (Version 4.1.1). https://www.R-project.org/ (Original work published 2021).
- Rajaprakash M., Chakravarty M.M., Lerch J.P., Rovet J. Cortical morphology in children with alcohol-related neurodevelopmental disorder. Brain and Behavior. 2014;4(1):41–50. doi: 10.1002/brb3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R.S., Nokelainen P., Valkonen K., Kolehmainen A.I., Kumpulainen K.I., Könönen M., Vanninen R.-L.-S., Kuikka J.T. Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-beta-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol. Psychiatry. 2005;57(12):1565–1572. doi: 10.1016/j.biopsych.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Rimol L.M., Rise H.H., Evensen K.A.I., Yendiki A., Løhaugen G.C., Indredavik M.S., Brubakk A.-M., Bjuland K.J., Eikenes L., Weider S., Håberg A., Skranes J. Atypical brain structure mediates reduced IQ in young adults born preterm with very low birth weight. Neuroimage. 2023;266(119816) doi: 10.1016/j.neuroimage.2022.119816. [DOI] [PubMed] [Google Scholar]
- Roediger D.J., Krueger A.M., de Water E., Mueller B.A., Boys C.A., Hendrickson T.J., Schumacher M.J., Mattson S.N., Jones K.L., Lim K.O., Wozniak J.R. Hippocampal subfield abnormalities and memory functioning in children with fetal alcohol Spectrum disorders. Neurotoxicol. Teratol. 2021;83 doi: 10.1016/j.ntt.2020.106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte F.F., Sulik K.K., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., O’Connor M.J., Narr K.L., Sowell E.R. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum. Brain Mapp. 2012;33(4):920–937. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S., Kia S.M., Wolfers T., Fraza C., Zabihi M., Dinga R., Berthet P., Worker A., Verdi S., Ruhe H.G., Beckmann C.F., Marquand A.F. The normative modeling framework for computational psychiatry. Nat. Protoc. 2022;17(7):1711–1734. doi: 10.1101/2021.08.08.455583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S., Barkema P., Tso I.F., Sripada C., Beckmann C.F., Ruhe H.G., Marquand A.F. Evidence for Embracing Normative Modeling. eLife. 2023;12 doi: 10.7554/eLife.85082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr J.A. Frontal-striatal circuit functions: context, sequence, and consequence. J. Int. Neuropsychol. Soc.: JINS. 2003;9(1):103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Sporns O. Structure and function of complex brain networks. Dialogues Clin. Neurosci. 2013;15(3):247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramoney S., Joshi S.H., Wedderburn C.J., Lee D., Roos A., Woods R.P., Zar H.J., Narr K.L., Stein D.J., Donald K.A. The impact of prenatal alcohol exposure on gray matter volume and cortical surface area of 2 to 3-year-old children in a South African birth cohort. Alcohol. Clin. Exp. Res. 2022;46(7):1233–1247. doi: 10.1111/acer.14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie M., Wozniak J.R., Parnell S.E., Wetherill L., Mattson S.N., Sowell E.R., Kan E., Riley E.P., Jones K.L., Coles C., Foroud T., Hammond P., CIFASD Combined face-brain morphology and associated neurocognitive correlates in fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2018;42(9):1769–1782. doi: 10.1111/acer.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Jahanshad N., Ching C.R.K., Salminen L.E., Thomopoulos S.I., Bright J., Baune B.T., Bertolín S., Bralten J., Bruin W.B., Bülow R., Chen J., Chye Y., Dannlowski U., de Kovel C.G.F., Donohoe G., Eyler L.T., Faraone S.V., Favre P., ENIGMA Consortium ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry. 2020;10(1):100. doi: 10.1038/s41398-020-0705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003;4(1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Treit S., Zhou D., Lebel C., Rasmussen C., Andrew G., Beaulieu C. Longitudinal MRI reveals impaired cortical thinning in children and adolescents prenatally exposed to alcohol. Hum. Brain Mapp. 2014;35(9):4892–4903. doi: 10.1002/hbm.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Zhou D., Chudley A.E., Andrew G., Rasmussen C., Nikkel S.M., Samdup D., Hanlon-Dearman A., Loock C., Beaulieu C. Relationships between head circumference, brain volume and cognition in children with prenatal alcohol exposure. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0150370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Jeffery D., Beaulieu C., Emery D. Radiological findings on structural magnetic resonance imaging in fetal alcohol spectrum disorders and healthy controls. Alcohol. Clin. Exp. Res. 2020;44(2):455–462. doi: 10.1111/acer.14263. [DOI] [PubMed] [Google Scholar]
- Uban K.A., Kan E., Wozniak J.R., Mattson S.N., Coles C.D., Sowell E.R. The relationship between socioeconomic status and brain volume in children and adolescents with prenatal alcohol exposure. Front. Hum. Neurosci. 2020;14:85. doi: 10.3389/fnhum.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zuo C., Xu Q., Hao L., Zhang Y. Attention-deficit/hyperactivity disorder is characterized by a delay in subcortical maturation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;104(110044) doi: 10.1016/j.pnpbp.2020.110044. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Pearson Assessments; 2008. Wechsler adult intelligence scale-fourth edition. [Google Scholar]
- Wechsler D., Pearson Education, Inc., Psychological Corporation . NCS Pearson, Inc.; 2014. Wechsler intelligence scale for children - 5th edition. [Google Scholar]
- Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Carlozzi N.E., Slotkin J., Blitz D., Wallner-Allen K., Fox N.A., Beaumont J.L., Mungas D., Nowinski C.J., Richler J., Deocampo J.A., Anderson J.E., Manly J.J., Borosh B., Gershon R.C. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Lichter R., Dennison M., Vijayakumar N., Schwartz O., Byrne M.L., Simmons J.G., Yücel M., Pantelis C., McGorry P., Allen N.B. Structural brain development and depression onset during adolescence: a prospective longitudinal study. Am. J. Psychiatry. 2014;171(5):564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- Willoughby K.A., Sheard E.D., Nash K., Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J. Int. Neuropsychol. Soc.: JINS. 2008;14(6):1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Chang P.-N., Muetzel R.L., Caros L., Lim K.O. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2006;30(10):1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Muetzel R.L., Mueller B.A., McGee C.L., Freerks M.A., Ward E.E., Nelson M.L., Chang P.-N., Lim K.O. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: An extension of previous diffusion tensor imaging findings. Alcohol. Clin. Exp. Res. 2009;33(10):1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Limited, de-identified data are available upon request; Please see https://doi.org/10.5967/ntw9-h991 for more information.
Data will be made available on request.